Abstract
Background
Cyclophosphamide chemotherapy is a mainstay of adjuvant breast cancer treatment. Unfortunately, this drug is associated with cognitive impairments in cancer patients that may accelerate cognitive aging. Memory is particularly affected in many patients. In order to better understand the precise cognitive impairments caused by this chemotherapy agent, we investigated a clinically relevant dose and administration paradigm on delayed spatial memory abilities in C57BL/6 mice. We utilized a delayed alternation paradigm similar to a delayed match to sample paradigm reported to be sensitive in neurotoxicology research.
Methods
A dose of 200 mg/kg cyclophosphamide was administered intravenously (at weekly intervals) for 4 weeks to C57BL/6 mice starting at 6 ½ months of age. Memory was tested in mice using a reward-based delayed spatial alternation paradigm with delay values of 1.5, 3, 6.1, 12.4 and 25 seconds presented randomly over 80 sessions (16 reinforcers per session), and testing began at the initiation of chemotherapy through 3 months.
Results
At the longest delay, i.e., that requiring the greatest memory, mice treated with chemotherapy exhibited a significant decline over time in proportion correct which leveled off compared to controls that continued to improve slightly.
Conclusions
Our clinically relevant model shows cyclophosphamide chemotherapy causes a slight decline in delayed spatial memories at the longest delay that is sustained over time as mice age.
Keywords: cancer-related cognitive impairment, cancer treatments, “chemo brain”, memory, delayed alternation
Introduction
Cancer survivorship has increased substantially over the past 3 decades with over 13 million survivors currently living in the US today; treatment-related issues accompanying cancer survivorship produce a tremendous impact on an aging population during and after treatments (Ganz, 2005, 2008). Unfortunately, cancer treatments, particularly chemotherapy, leads to a host of side effects including cognitive impairments (Janelsins et al., 2014). Neuropsychological and self-report assessments during and following chemotherapy reveal deficits including memory lapses, trouble concentrating, difficulty in word association, confusion, trouble multi-tasking, and slow thinking and information processing (Vardy et al., 2007, Bower, 2008). Chemotherapy-related cognitive impairment (CRCI) is most common and most severe during and just after treatment (Wefel et al., 2004) and more pronounced in those who received high-dose chemotherapy, and importantly, these deficits can continue for several years following treatments (van Dam et al., 1998, Schultz et al., 2003). These observations suggest that managing the adverse neurobehavioral effects of chemotherapy during the course of treatment and early after treatment could prevent the onset or persistence of cognitive complications in cancer patients and survivors.
We need clinically relevant animal models that will help us understand the etiology of CRCI and to develop and test interventions to alleviate or prevent CRCI. Deficits in learning and memory are among the most frequently reported by cancer patients, and patients’ performance is reduced on a number of objective memory measures. Our group has interest in assessing CRCI using computerized batteries derived from experimental psychology which have shown that chemotherapy and other treatments impair cognitive performance in cancer patients (Capuron et al., 2001, Vardy et al., 2014, Vardy et al., 2015). In order to allow for forward and backward translation in research, we decided to assess the cognitive impact of a clinically relevant course of cyclophosphamide chemotherapy using a delayed spatial alternation paradigm that is similar to delayed spatial memory assessment paradigms used in clinical research that have shown to be sensitive in neurotoxicology research and have been used in investigations of cognitive effects in breast cancer patients (Fray and Robbins, 1996, Minton and Stone, 2012). Delayed spatial alternation allows for the assessment of learning and memory over multiple delay intervals and has been used in several experimental psychology models and has been suggested as a possible assessment tool for investigating chemotherapy toxicity (Cory-Slechta et al., 1991, Weiss, 2010). We hypothesized that chemotherapy, given in a clinically relevant manner at weekly intervals and in a human-to-mouse dose translatable fashion delivered intravenously, would lead to worse performance on the delayed alternation paradigm, particularly as delay values and thus required remembering time increased, as compared to controls administered equivalent volumes of saline.
Materials and Methods
Mice
Male C57BL/6 mice were purchased through Taconic (Germantown, NY) at 23-25g at 6-8 weeks of age. Upon arrival, all mice were allowed to acclimate to their home cage for two weeks prior to initiation of experiments. After this acclimation period, the mice were weighed and maintained at 80% of that weight via caloric regulation throughout the rest of the experiment. Mice remained in general good health throughout the experiment. The food reward used in behavioral testing food type was BioServe dustless precision pellets (20mg) rodent grain-based diet, and the mice were given supplemental food (Lab Diet 5001 rodent diet) if necessary to maintain the 80% weight.
All animal housing and procedures were performed in compliance with guidelines established by the University Committee of Animal Resources at the University of Rochester.
Behavioral Testing – Delayed Spatial Alternation
We followed a standard delayed alternation protocol adapted from Cory-Slechta et al. (Cory-Slechta et al., 1991). Behavioral testing was carried out in operant chambers, each of which contained two response levers, with a single light emitting diode over each lever, a houselight, and a pellet trough located midway between the two levers that was used to deliver food reward. Mice were first trained to lever press using an auto-shaping procedure developed in our laboratory that began with a variable time schedule during which food pellets were dispensed into the food trough at variable time (VT) intervals averaging 60 sec, independently of responding, for a period of 20 min. Any responses on either lever during that period produced additional food reward; if 10 responses occurred during that period, the VT component was terminated and a fixed ratio (FR) 1 schedule of reward was in effect in which a single response on either lever was required for food delivery. If 10 responses did not occur during the VT component, it terminated after 20 min and the FR component began. Sessions were terminated with the delivery of 50 food rewards. During autoshaping, lights above each lever were illuminated.
Following autoshaping, a response alternation training program was implemented in which mice were required to alternate responses on the two levers, with the houselight illuminated above the correct lever at the end of a constant delay interval. The correct lever press produced a pellet and the light was then extinguished, at which time the other lever became the “correct” lever and the next delay interval was started.
Following simple response alternation training, the full delayed alternation program was implemented. In this paradigm, alternating responses were again required, but no lever lights signaled which lever was correct; mice had to remember which lever they had pressed previously and subsequently move to the alternate lever. In addition, delays were imposed between the opportunities to alternate responses, using delay values that were sufficiently long to produce essentially chance responding. During the delayed spatial alternation procedure, mice were presented randomly within each session with 5 different delays at 1.5, 3, 6.1, 12.4 and 25 seconds allowing a delay by accuracy function to be generated for each session. Responses on either lever during the delay reset the delay value and the correct alternate response was required before another trial began.
Training procedures were conducted for approximately 3 months to achieve stable delay functions, after which chemotherapy was implemented. Baseline data included the 10 sessions prior to the initiation of chemotherapy. Behavioral testing was carried out 5 days per week (Monday through Friday) on the delayed spatial alternation starting after the first chemotherapy administration and continuing for 3 months.
Chemotherapeutic Paradigms
The chemotherapeutic agent was purchased from Sigma Aldrich (St. Louis, MO). Cyclophosphamide (Cat # C-0768) was stored according to manufacturer's instructions until use, at which time it was diluted in sterile saline. The C57BL/6 mice were given four intravenous tail vein injections (approximately 6 ½ months of age) of chemotherapeutic agent (cyclophosphamide at 200 mg/Kg; n=5) or saline equivalent (n=4) on 4 consecutive Mondays. We followed the dose translation calculations of Reagan-Shaw et al (Reagan-Shaw et al., 2008). The clinically relevant dose our mouse equivalent dose was translated from is 600 mg/m2 which is the dose that this chemotherapy agent is commonly administered in breast cancer therapy.
Statistical Analyses
MedAssociates software was used to collect data in the delayed spatial alternation which were then exported into RS1. Data were analyzed with R, SAS and JMP. Percent correct for each delay and number of responses per reinforce (i.e. reward) are reported for each day and delay. In all models, we assessed the cumulative effect of chemotherapy vs saline over time. To visualize the trajectories of the percent correct and responses/reward response over time, we plotted the data versus time, color coding by group. Then we superimposed nonparametric regression estimates of the average trajectories for each group. See Figure(s) 1 and 2. The LOWESS smoother (span=2/3, robust Tukey biweight with 3 iterations) was used to fit the nonparametric estimates (Cleveland, 1979). To test for statistically significant differences in trajectory shape between the groups, we used mixed models with shape (represented with a third-order polynomial) as the main effect, and the interaction between group and shape as the key measure of differences in shape over time. The mouse-specific random effect was also a third order polynomial. Maximum likelihood estimation was used, and a likelihood ratio test was used to evaluate the significance of the group by shape interaction. We found that third order polynomials gave a faithful representation of the curves visualized via the LOWESS smoother. This analysis was performed for each delay time. In order to combine delay times, we calculated the area under the percent correct (or responses/reward) versus delay time data (Area under the Curve, AUC) for each mouse using the trapezoidal rule. The AUC was then modeled using the same mixed modeling methodology as detailed above. All calculations were performed using R,(R Core Team, 2013) and the mixed models were fit using the nlme package (Pinheiro et al., 2015). See Figures 4 and 5. In all cases, p≤0.05 was considered significant.
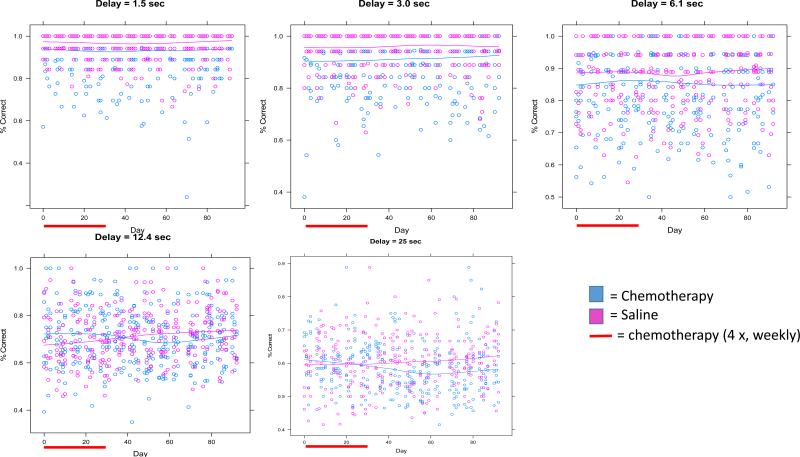
Figure 1. Percent Correct Across Delays.
Each figure represents the percent correct for each delay with each day of delayed spatial alternation. Individual data points represent individual mice..The red bar represents the period of chemotherapy or saline administration (1 × per week for each of 4 weeks). Lines represent fitted lines for chemotherapy-treated mice and controls. Significant effects between groups and group by time interactions are found at the 12.4 and 25 second delays as noted in the text.
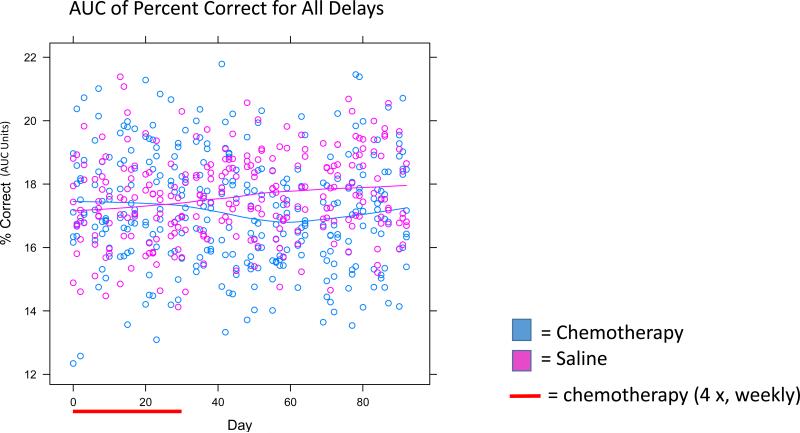
Figure 2. Area Under the Curve (AUC) Analysis for Percent Correct for All Delays.
All data points from Figure 1 were combined together to represent an AUC. Individual data points represent individual mice. Significant effects were seen between groups as noted in the text.
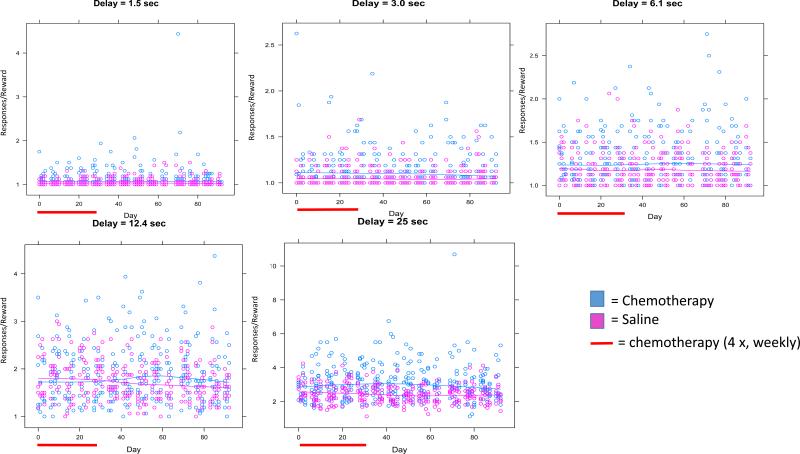
Figure 4. Responses/Reward Across Delays.
Each figure represents the responses per reward for each delay for each day of delayed spatial alternation. Individual data points represent individual mice..The red bar represents the period of chemotherapy or saline administration (1 × per week for each of 4 weeks). Lines represent fitted lines for chemotherapy-treated mice and controls. Significant effects between groups and group by time interactions are found at the 12.4 and 25 second delays as noted in the text.
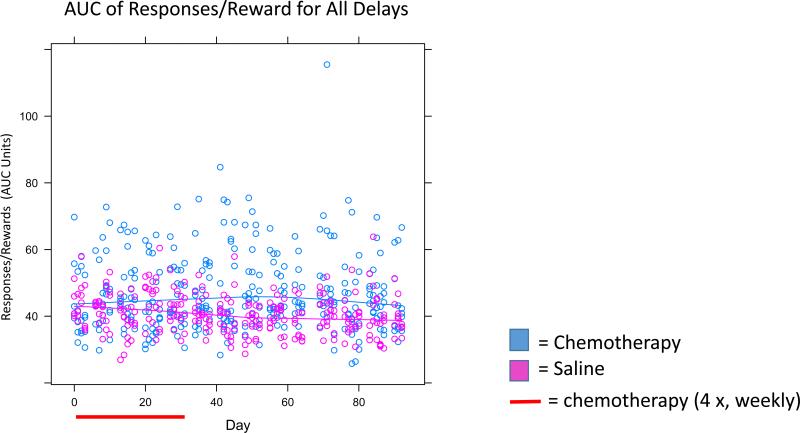
Figure 5. AUC Analysis for Responses/Reward for All Delays.
All data points from Figure 4 were combined together to represent an AUC. Individual data points represent individual mice. Significant effects were seen between groups as noted in the text.
Results
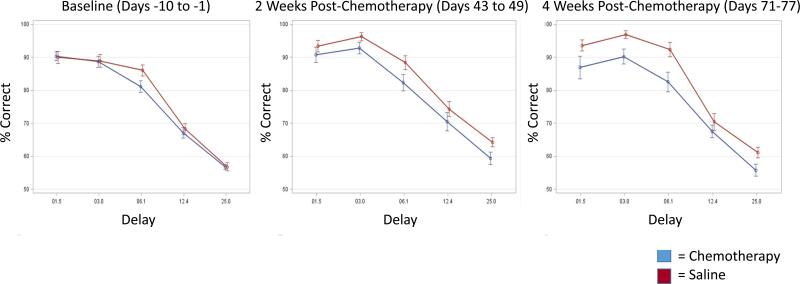
Baseline short delay percent correct values at 1.5, 3 and 6.1 seconds were 90%, 88%, and 83%, respectively. At delays of 1.5, 3.0, and 6.1 seconds, chemotherapy-treated mice generally had lower proportions of percent correct compared to saline-treated mice; however, there were no significant group or group × time interaction differences (p>0.05; Figure 1). At the 12.4 second delay, baseline percent correct values were 68%. Chemotherapy-treated mice initially started the study with slightly greater percent correct than saline-treated mice at this delay but during and following chemotherapy, chemotherapy-treated mice showed gradual decline in percent correct compared to saline-treated mice who gradually improved resulting in a lower percent correct in chemotherapy-treated mice compared to saline-treated mice and representing a significant group difference (p<0.05) as well as a significant group × time interaction (p<0.005; Figure 1). The 25 second delay value produced a baseline percent correct value of 57%, consistent with the fact that memory was most taxed at this long delay value. At this delay, while mice in both groups started off with similar percent correct values; the chemotherapy-injected mice continued to gradually decline over the course of the 3 months post-treatment, while the saline-injected mice continued to gradually increase over the post-treatment interval as evidenced by a statistically significant group × time interaction (p<0.05; Figure 1). We also performed an AUC analysis across percent correct on all delays, which revealed significant effects by group (p<0.005; Figure 2) and group × time interaction (p<0.005; Figure 2). To assess percent correct differences at key points in time between chemotherapy-treated and saline-treated mice we created forgetting functions (showing average percent correct as a function of delay on the same graph) for the baseline interval as well as the 2 and 4 weeks post-chemotherapy intervals (Figure 3). At baseline, mice in both groups performed similarly as a function of delay but in the post-treatment period, chemotherapy-treated mice performed less well than saline-treated mice as a function of delay.
Figure 3. Forgetting functions of Percent Correct Across Delays.
Forgetting functions of percent correct × delay were created for key time intervals including 10 days of baseline (day −10 to day −1), 2 weeks post-chemotherapy (Days 43-49) and 4 weeks post-chemotherapy (Days 71-77) as an alternate way of viewing the data in Figures 1 and 2. Data points represent averages across the data interval. Error bars are +/− SEM.
The ratio of responses per reward was higher in chemotherapy-treated mice compared to controls at all delays; however the effects were only statistically significant at the 12.4 and 25 second delays (p<0.05; Figure 4). At the 12.4 second delay, the ratio of responses to reward delivery was higher in chemotherapy-treated mice compared to controls, and this difference was statistically significant (p<0.05; Figure 4) as well as the group × time interaction at both the 12.4 and 25 second delays (p<0.05; Figure 4) meaning that chemotherapy-treated mice made slightly more responses/reward over time and controls made slightly less responses/reward over time. The AUC analysis on all delays for responses/reward revealed a trend for overall group difference (p=0.06) and a significant group × time interaction (p<0.05; Figure 5).
There were no significant differences in weight in chemotherapy- and saline-treated mice by groups, and this lack of an anticipated difference is likely due to the caloric regulation required for motivation of responding (p>0.05). Overall, mice in both groups slightly increased in weights over the course of the experiment (from 19.4 to 20.4 on average), but did not differ more than 5% from their baseline.
Conclusions
Utilizing a delayed spatial alternation paradigm, we found, for what appears to be the first time, that chemotherapy leads to a subtle but sustained gradual memory decline over time in C57BL6 mice compared to controls receiving saline. These findings provide what may be a useful animal model of aspects of this phenomenon which is highly relevant clinically, as our paradigm uses a clinically relevant dose translated from the dosage given in breast cancer treatments as well as a clinically relevant route of administration. Moreover, the delayed spatial alternation task used is very similar to cognitive tests being used in clinical research (e.g. CANTAB delayed matching to sample and other similar tests) with cancer patients and other populations (Capuron et al., 2001, Egerhazi et al., 2007, Minton and Stone, 2012). Lastly, our results show a cumulative effect of chemotherapy treatments leading to a gradual and sustained subtle decline of cognitive function representing the long-term effects of treatment as mice age. The subtle nature of these effects with this model is somewhat akin to the subtle effects that are often observed on some clinical neuropsychological tests in humans.
The results at the 25 second delay support our hypothesis that chemotherapy-treated mice would perform less well at the longest delay (i.e. the delay where memory is most taxed), and it is interesting that the effects of treatment are gradual and sustained over time in our model. It is not surprising that chemotherapeutic treatment did not induce deficits at the shorter delays, since these delays involve less sustained memory compared to the longest delay. Thus, these shorter delays may not be sensitive enough to detect chemotherapy effects. It is less clear at present why the chemotherapy-treated mice initially performed better than saline-treated mice at the 12.4 second delay. However, with time post-treatment, as controls continued to show improvement at this delay, accuracy levels of chemotherapy-treated mice showed no improvement, but actually declined to levels similar to the performance curve at the 25 second delay. Correspondingly, the number of responses required for each reward earned was significantly higher. Interestingly, there is wide variation in the number of responses per reward illustrating the individual susceptibility of the mice to perform on this task upon chemotherapy exposure.
While our results provide novel data on long-term memory changes over time in a delayed spatial alternation paradigm, several other studies have shown behavioral deficits on other cognitive tasks in other chemotherapy paradigms. Primarily, aspects of spatial and non-spatial memory, as well as executive functions have shown to be impaired in rodents treated with chemotherapy (reviewed in (Dietrich et al., 2015) and (Seigers and Fardell, 2011).
It is still unclear whether the mechanisms leading to this memory decline are direct or indirect; however, several studies have shown that impaired neurogenesis in the hippocampus may be involved (summarized in (Dietrich et al., 2015)). Adult hippocampal neurogenesis—a process by which new neurons are generated from newly birthed neural precursors initiating from the subgranular zone (SGZ) of the dentate gyrus— is involved in maintenance of learning and memory as well as brain plasticity (Zhao et al., 2008). Mechanisms related to diminished neurogenesis are the most widely studied; however, evidence from other pre-clinical research (and clinical research) suggests that many mechanisms play a role in the development of CRCI, including inflammation, hormonal changes, DNA damage, oxidative stress, mitochondrial dysfunction, and altered growth factor levels (Chen et al., 2006, Dietrich et al., 2006, Ahles and Saykin, 2007, Han et al., 2008, Janelsins and Gross, 2010, Kesler et al., 2013, Vichaya et al., 2016). Supporting the animal literature of cognitive impairments in chemotherapy models and brain inherent alterations in several molecular indicators of central nervous system abnormalities are an accumulating number of neuroimaging studies in cancer patients that show the impact of chemotherapy on brain structure and function, revealing white and gray matter loss, altered resting state metabolism changes and altered white matter integrity, and brain activation upon cognitive task challenge (Deprez et al., Inagaki et al., 2007, Silverman et al., 2007, Swayampakula et al., 2007, Abraham et al., 2008, de Ruiter et al., 2011, de Ruiter et al., 2012, Hosseini and Kesler, 2014, Dietrich et al., 2015). Lastly, the interactions between these factors and genetic factors (such as specific alleles in genes APOE, TNF and COMT) may exacerbate CRCI in the human condition and these relationships needs to be further investigated in animal studies (Ahles et al., 2003, Small et al., 2011).
Our study adds to the field by the usage of clinically relevant doses of chemotherapy and administration paradigms in a memory task not yet studied, as well as assessment of cognitive effects during chemotherapy and continuing over a long period of time after treatments have stopped and mice have aged.
Future studies need to address limitations to this work including assessing multiple agents simultaneously, assessing female mice and comparing young versus older mice. Additionally, conducting studies with multiple agents in relevant tumor models will allow us to differentiate cognitive effects of chemotherapy, aging, and cancer as well as to further elucidate the possible mechanisms discussed herein.
Highlights.
Delayed spatial alternation detects subtle but sustained cognitive effects of cyclophosphamide chemotherapy
Utilization of a chemotherapy model with clinically relevant features
Acknowledgements
This work was primarily supported by NCI K07CA168886 (MCJ) and NIH DP2CA195765 (MCJ). We are grateful for technical advice of Dr. Sander Stern and Dr. Troy Zarcone and initial financial support of Dr. Gary Morrow through NCI R25CA102618.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cancer Facts and Figures 2008. American Cancer Society; Atlanta: 2008. [Google Scholar]
- Abraham J, Haut MW, Moran MT, Filburn S, Lemiuex S, Kuwabara H. Adjuvant chemotherapy for breast cancer: effects on cerebral white matter seen in diffusion tensor imaging. Clinical breast cancer. 2008;8:88–91. doi: 10.3816/CBC.2008.n.007. [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B, Mott LA. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 2003;12:612–619. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- Bower JE. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. 2008;26:768–777. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Dantzer R. Timing and specificity of the cognitive changes induced by interleukin-2 and interferon-alpha treatments in cancer patients. Psychosom Med. 2001;63:376–386. doi: 10.1097/00006842-200105000-00007. [DOI] [PubMed] [Google Scholar]
- Chen Y, Daosukho C, Opii WO, Turner DM, Pierce WM, Klein JB, Vore M, Butterfield DA, St Clair DK. Redox proteomic identification of oxidized cardiac proteins in adriamycin-treated mice. Free Radic Biol Med. 2006;41:1470–1477. doi: 10.1016/j.freeradbiomed.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Cleveland WS. Robust locally weighted regression and smoothing scatterplots. JASA. 1979;74:829–836. [Google Scholar]
- Cory-Slechta DA, Pokora MJ, Widzowski DV. Behavioral manifestations of prolonged lead exposure initiated at different stages of the life cycle: II. Delayed spatial alternation. Neurotoxicology. 1991;12:761–776. [PubMed] [Google Scholar]
- de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, Caan M, Douaud G, Lavini C, Linn SC, Boven E, van Dam FS, Schagen SB. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Human brain mapping. 2012;33:2971–2983. doi: 10.1002/hbm.21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, van Dam FS, Nederveen AJ, Boven E, Schagen SB. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Human brain mapping. 2011;32:1206–1219. doi: 10.1002/hbm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprez S, Amant F, Yigit R, Porke K, Verhoeven J, Van den Stock J, Smeets A, Christiaens MR, Leemans A, Van Hecke W, Vandenberghe J, Vandenbulcke M. Sunaert S Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Human brain mapping. doi: 10.1002/hbm.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5:22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J, Prust M, Kaiser J. Chemotherapy, cognitive impairment and hippocampal toxicity. Neuroscience. 2015;309:224–232. doi: 10.1016/j.neuroscience.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Egerhazi A, Berecz R, Bartok E, Degrell I. Automated Neuropsychological Test Battery (CANTAB) in mild cognitive impairment and in Alzheimer's disease. Progress in neuro-psychopharmacology & biological psychiatry. 2007;31:746–751. doi: 10.1016/j.pnpbp.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Fray PJ, Robbins TW. CANTAB battery: proposed utility in neurotoxicology. Neurotoxicol Teratol. 1996;18:499–504. doi: 10.1016/0892-0362(96)00027-x. [DOI] [PubMed] [Google Scholar]
- Ganz PA. A teachable moment for oncologists: cancer survivors, 10 million strong and growing! Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:5458–5460. doi: 10.1200/JCO.2005.04.916. [DOI] [PubMed] [Google Scholar]
- Han R, Yang YM, Dietrich J, Luebke A, Mayer-Proschel M, Noble M. Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. J Biol. 2008;7:12. doi: 10.1186/jbiol69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini SM, Kesler SR. Multivariate pattern analysis of FMRI in breast cancer survivors and healthy women. Journal of the International Neuropsychological Society : JINS. 2014;20:391–401. doi: 10.1017/S1355617713001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki M, Yoshikawa E, Matsuoka Y, Sugawara Y, Nakano T, Akechi T, Wada N, Imoto S, Murakami K, Uchitomi Y. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109:146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry. 2014;26:102–113. doi: 10.3109/09540261.2013.864260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Roscoe JA, Berg MJ, Thompson BD, Gallagher MJ, Morrow GR, Opanashuk LA, Gross RA. IGF-1 Partially Restores Chemotherapy-induced Reductions in Neural Cell Proliferation in Adult C57BL/6 mice. Cancer investigation. 2010;28:292–298. doi: 10.3109/07357900903405942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, Dhabhar FS. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain, behavior, and immunity. 2013;30(Suppl):S109–116. doi: 10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton O, Stone PC. A comparison of cognitive function, sleep and activity levels in disease-free breast cancer patients with or without cancer-related fatigue syndrome. BMJ supportive & palliative care. 2012;2:231–238. doi: 10.1136/bmjspcare-2011-000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-122. 2015 [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2013. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Schultz PN, Beck ML, Stava C, Vassilopoulou-Sellin R. Health profiles in 5836 long-term cancer survivors. Int J Cancer. 2003;104:488–495. doi: 10.1002/ijc.10981. [DOI] [PubMed] [Google Scholar]
- Seigers R, Fardell JE. Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neuroscience and biobehavioral reviews. 2011;35:729–741. doi: 10.1016/j.neubiorev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Silverman DH, Dy CJ, Castellon SA, Lai J, Pio BS, Abraham L, Waddell K, Petersen L, Phelps ME, Ganz PA. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5-10 years after chemotherapy. Breast cancer research and treatment. 2007;103:303–311. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- Small BJ, Rawson KS, Walsh E, Jim HS, Hughes TF, Iser L, Andrykowski MA, Jacobsen PB. Catechol-O-methyltransferase genotype modulates cancer treatment-related cognitive deficits in breast cancer survivors. Cancer. 2011;117:1369–1376. doi: 10.1002/cncr.25685. [DOI] [PubMed] [Google Scholar]
- Swayampakula AK, Alkhouri N, Haut MW, Abraham J. Cognitive impairment with significant brain parenchymal volume loss following standard adjuvant chemotherapy in a patient with breast cancer. Clin Adv Hematol Oncol. 2007;5:985–987. discussion 987-988. [PubMed] [Google Scholar]
- van Dam FS, Schagen SB, Muller MJ, Boogerd W, vd Wall E, Droogleever Fortuyn ME, Rodenhuis S. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst. 1998;90:210–218. doi: 10.1093/jnci/90.3.210. [DOI] [PubMed] [Google Scholar]
- Vardy J, Dhillon HM, Pond GR, Rourke SB, Xu W, Dodd A, Renton C, Park A, Bekele T, Ringash J, Zhang H, Burkes R, Clarke SJ, Tannock IF. Cognitive function and fatigue after diagnosis of colorectal cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2014;25:2404–2412. doi: 10.1093/annonc/mdu448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy J, Rourke S, Tannock IF. Evaluation of cognitive function associated with chemotherapy: a review of published studies and recommendations for future research. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:2455–2463. doi: 10.1200/JCO.2006.08.1604. [DOI] [PubMed] [Google Scholar]
- Vardy JL, Dhillon HM, Pond GR, Rourke SB, Bekele T, Renton C, Dodd A, Zhang H, Beale P, Clarke S, Tannock IF. Cognitive Function in Patients With Colorectal Cancer Who Do and Do Not Receive Chemotherapy: A Prospective, Longitudinal, Controlled Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:4085–4092. doi: 10.1200/JCO.2015.63.0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichaya EG, Molkentine JM, Vermeer DW, Walker AK, Feng R, Holder G, Luu K, Mason RM, Saligan L, Heijnen CJ, Kavelaars A, Mason KA, Lee JH, Dantzer R. Sickness behavior induced by cisplatin chemotherapy and radiotherapy in a murine head and neck cancer model is associated with altered mitochondrial gene expression. Behavioural brain research. 2016;297:241–250. doi: 10.1016/j.bbr.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100:2292–2299. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- Weiss B. Evaluation of multiple neurotoxic outcomes in cancer chemotherapy. Advances in experimental medicine and biology. 2010;678:96–112. doi: 10.1007/978-1-4419-6306-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]