Abstract
Background
Foam rollers, or other similar devices, are a method for acutely increasing range of motion, but in contrast to static stretching, do not appear to have detrimental effects on neuromuscular performance.
Purpose
The purpose of this study was to investigate the effects of different volumes (60 and 120 seconds) of foam rolling of the hamstrings during the inter‐set rest period on repetition performance of the knee extension exercise.
Methods
Twenty‐five recreationally active females were recruited for the study (27.8 ± 3.6 years, 168.4 ± 7.2 cm, 69.1 ± 10.2 kg, 27.2 ± 2.1 m2/kg). Initially, subjects underwent a ten‐repetition maximum testing and retesting, respectively. Thereafter, the experiment involved three sets of knee extensions with a pre‐determined 10 RM load to concentric failure with the goal of completing the maximum number of repetitions. During the inter‐set rest period, either passive rest or foam rolling of different durations (60 and 120 seconds) in a randomized order was employed.
Results
Ninety‐five percent confidence intervals revealed dose‐dependent, detrimental effects, with more time spent foam rolling resulting in fewer repetitions (Cohen's d of 2.0 and 1.2 for 120 and 60 seconds, respectively, in comparison with passive rest).
Conclusion
The results of the present study suggest that more inter‐set foam rolling applied to the antagonist muscle group is detrimental to the ability to continually produce force. The finding that inter‐set foam rolling of the antagonist muscle group decreases maximum repetition performance has implications for foam rolling prescription and implementation, in both rehabilitation and athletic populations.
Level of evidence
2b
Keywords: Fatigue, performance, self‐manual therapy, self‐myofascial release
INTRODUCTION
When producing a net joint moment, the net external force applied to the joint is proportional to the force generated by the agonist muscle minus that of the antagonist.1 Essentially, greater antagonist activation reduces net force output, but is necessary for maintaining appropriate joint stability.1 Thus, greater net joint moments could potentially be achieved by inhibiting the co‐activation of the antagonist muscle.
Static stretching of the agonist muscle group, especially of long duration, has been repeatedly shown to impede neuromuscular function2, including the repetition performance in resistance exercise.3-5 Hypothetically, stretching the antagonist muscle group could therefore cause inhibition of the antagonist and thus augment the net joint moment, as the latter represents the difference between the moment generated by the agonist and antagonist, respectively1. Three recent studies have indeed shown that to be the case.6-8 Sandberg et al8 showed that stretching the antagonist muscle group results in greater net knee extension moment production during isokinetic knee extensions at fast velocities, in addition to improvements in vertical jump height and power. However, investigators did not note any differences in electromyography (EMG) amplitude in vastus lateralis and biceps femoris muscles between static stretching and passive rest conditions. Similarly, Paz et al7 did not observe any differences in EMG amplitude of pectoralis major, latissimus dorsi, biceps brachii, and triceps brachii muscles, but noted improvements in repetition performance during the seated row exercise following proprioceptive neuromuscular facilitation (PNF) stretching of the shoulder adductors. Lastly, Miranda et al6 observed a superior effect of antagonist static stretching of pectoralis major compared to passive rest on repetition performance of the seated row exercise. Furthermore, stretching of the pectoralis major resulted in statistically greater latissimus dorsi and biceps brachii activation during the exercise.
Foam rollers, or other similar devices, are a method for acutely increasing range of motion (ROM), but in contrast to static stretching, do not appear to have detrimental effects on neuromuscular performance of the treated muscle group, as determined from net joint moments during maximum voluntary isometric contractions.9 A typical resistance training session may involve exercises of both agonist and antagonist muscle groups, or, in some cases, they may even be paired, a technique known as antagonist paired sets.10 Stretching the antagonist muscle group seems to increase the performance of the agonist muscle group.6-8 However, stretching the antagonist muscle group may also have a detrimental effect on the performance of subsequent exercises for the aforementioned antagonist muscle groups. This issue may be avoided by using foam rollers, or similar devices, during the inter‐set rest period. Therefore, the purpose of this study was to investigate the effects of different volumes of foam rolling (60 and 120 seconds) of the hamstrings during inter‐set rest periods on repetition performance of the knee extension exercise.
METHODS
Subjects
Twenty‐five recreationally active females (Table 1) were recruited for the study. The same population of subjects was used before in a different experiment.11 Females were recruited both out of convenience and to help narrow the gender disparity in sports and exercise medicine research.12 An a priori sample size calculation ( = 0.34; β = 0.95; α = 0.05) using G*Power13 found that six subjects would be adequate; however, in order to increase statistical power, 25 were recruited.14 Anthropometric data included body mass (Techline BAL – 150 digital scale, São Paulo, Brazil) and height (stadiometer ES 2030 Sanny, São Paulo, Brazil). Subjects were included if they had been involved in resistance training program for at least one year prior to the experiment and had experience with the knee extension exercise. Participants were free from any functional limitation or medical condition that could have compromised their health or confounded the results of the study. During the ten‐day period of data collection, the subjects were instructed not to engage in any lower body resistance training exercise or other strenuous activity. Prior to the study, all participants were provided verbal explanation of the study and read and signed informed consent and Physical Activity Readiness Questionnaire.15 All procedures were in accordance with Declaration of Helsinki and the study was approved by the Institutional Review Board of University Hospital Clementino Fraga Filho of the Federal University of Rio de Janeiro (57023616.7.0000.5257/16).
Table 1.
Subject characteristics.
| Age (years) | 27.8 ± 3.6 |
|---|---|
| Height (cm) | 168.4 ± 7.2 |
| Body mass (kg) | 69.1 ± 10.2 |
| BMI (m2/kg) | 24.2 ± 2.1 |
| RTE (months) | 23 ± 6.6 |
| Knee Extension 10RM (Test) (kg) | 70.7 ± 11 |
| Knee Extension 10RM (Retest) (kg) | 71.4 ± 11.2 |
| ICC | 0.981 |
BMI = Body Mass Index; RTE = Resistance Training Experience; ICC = Intraclass Correlation Coefficient for 10 RM test and retest
Procedures
Ten repetition maximum testing
Ten repetition maximum was determined similar to Maia et al10 Participants were sat on a knee extension machine (Selection Line Leg Extension, Technogym, Cesena, Italy), with the lumbar spine in contact with the back support, and ankle in slight dorsiflexion. Range‐of‐motion was between 100 degrees of knee flexion and full extension (0 degrees). Participants initially performed a standardized warm up consisting of two sets of fifteen repetitions of knee extensions with approximately 50% of normal training load. After the warm up, ten‐repetition maximum testing was performed. For the first trial, subjects increased their warm up load by 100% and adjusted the load as needed in the subsequent trials. Execution of the knee extension exercise was standardized by not allowing pauses between concentric and eccentric portions of the lift. A maximum of three trials were allowed per testing session, separated by three minutes of passive rest. Testing was then repeated on another day at least 48 hours later (retest). The higher load between the two testing days was considered as the 10 RM load. The 10 RM load was confirmed by calculating the intraclass correlation coefficient. In an effort to minimize the margin of error, the following strategies were adopted:16 a) all subjects received standardized instructions about the exercise technique and data collection, b) subjects received feedback as to their technique and were corrected if and when appropriate, and c) all subjects were always verbally encouraged. The knee extension apparatus used for 10 RM testing and during the experimental sessions was the same (Selection Line Leg Extension, Technogym, Cesena, Italy).
Foam Rolling
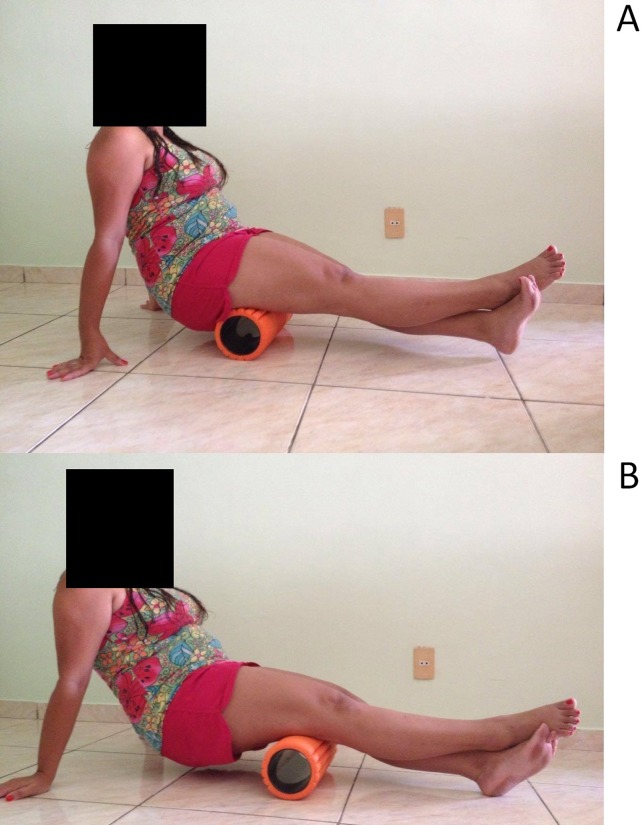
Foam rolling was performed using The Grid Foam Roller (Trigger Point Technologies, 5321 Industrial Oaks Blvd., Austin, Texas 78735, USA), which is composed of a hard inner core enclosed in a layer of ethylene vinyl acetate foam. This kind of foam roller has been shown to produce more pressure on the soft tissue than those made out of polystyrene foam.17 Foam rolling was performed bilaterally in a seated position while maintaining the knees extended but relaxed. The subjects were instructed to propel their body backward and forward on the foam roller, between ischial tuberosity and popliteal fossa in fluid, dynamic motions, while trying to exert as much pressure on the foam roller as possible. The pace of rolling was not controlled for. While it is recognized that this reduces internal validity, as the effects may potentially be pace‐dependent, not controlling the pace also enhances ecological validity of the findings, as this kind of procedure better represents situations in practice.
Experimental protocol
During the experimental sessions, participants performed knee extensions to concentric failure with the pre‐determined 10 RM load. A four‐minute rest interval was employed between each consecutive set. Both the order of visits (PR and FR) and different foam rolling volumes (FR60 and FR120) were randomized in a randomized, counterbalanced fashion. For both conditions, three sets were performed with four minutes of rest between each set. The PR condition was performed with passive rest, and the FR condition was performed during the rest period between the sets. Both FR conditions were performed in the same day, following a 10‐minute break between protocols to avoid fatigue.18,19 The number of repetitions in each set, and in total, was recorded for each condition.
Experimental Approach to the Problem
A randomized within‐subject design was used. Subjects visited the laboratory on four occasions during a ten‐day period with at least forty‐eight hours between visits. During the first two visits, the subjects underwent a ten‐repetition maximum (RM) testing and retesting, respectively. Following 10 RM testing, two experimental sessions followed in a randomized order, which included: 1) passive rest (PR), 2) in a randomized order, foam rolling for 60 seconds (FR60) and foam rolling for 120 seconds (FR120). Each experimental session consisted of three (PR condition) and six sets (FR condition) of knee extensions with 10 RM load to concentric failure, interspersed by four‐minute rest intervals, during which FR or PR were performed with the goal of completing the maximum number of repetitions.
Statistical analyses
In order to identify within‐set, between‐protocol differences, 95% confidence intervals (CI) of the differences between each protocol were calculated.20 Normality of the differences was ensured using the Shapiro‐Francia test. Rather than traditional null hypothesis statistical testing, 95% CI were used in order to prevent dichotomous interpretation of the results,21,22 to increase the likelihood of correct interpretation,21 and to allow for a more nuanced and qualitative interpretation of the data.23 For differences with a 95% CI that includes zero, the observed difference cannot be concluded to be due to chance alone; in other words, the observations are statistically different from one another when the 95% CI of differences does not include zero. Additionally, Cohen's d effect sizes were calculated using the formula , where Md is the mean difference and sd is the standard deviation of differences. This calculation differs slightly from traditional Cohen's d calculations, in that this formula better represents within‐subject differences, whereas the traditional Cohen's d formula is better for between‐subject comparisons.24-26 Cohen's d effect‐sizes were defined as small, medium, and large for 0.2, 0.5, and 0.8, respectively.27 The combination of effect‐sizes and 95% CI will therefore allow for a more nuanced and less polarizing interpretation of the results of the study.
RESULTS
The means and standard deviations of the number of repetitions performed during each set across all conditions are presented in Table 2.
Table 2.
Means ± standard deviations for repetitions in each set of each condition.
| Set 1 | Set 2 | Set 2/Set 1 | Set 3 | Set 3/Set 1 | Average | |
|---|---|---|---|---|---|---|
| PR | 10.24 ± 0.44 | 9.72 ± 0.54 | 0.95 ± 0.05 | 9.48 ± 0.51 | 0.93 ± 0.06 | 9.81 ± 0.59 |
| FR60 | 9.72 ± 0.46 | 9.32 ± 0.70 | 0.96 ± 0.06 | 8.76 ± 0.72 | 0.90 ± 0.06 | 9.27 ± 0.74 |
| FR120 | 9.72± 0.46 | 8.96± 0.73 | 0.92± 0.06 | 8.16± 0.62 | 0.84± 0.06 | 8.95± 0.88 |
PR = passive rest, FR60 = foam rolling for 60 seconds, FR120 = foam rolling for 120 seconds, Set 2/Set 1 = repetitions in set 2 normalized to repetitions in set 1; Set 3/Set 1 = repetitions in set 3 normalized to repetitions in set 1; Average = the number of repetitions across all sets for each condition
Mean differences with accompanying 95% CIs and effect sizes are reported in Table 3. On average, the number of repetitions completed in the PR condition was statistically greater than in the FR60 and FR120 conditions as 95% CIs did not include zero (Table 3). This difference was greater by 5.7% and 9.2%, respectively. Furthermore, the number of repetitions completed in FR60 was statistically greater than in the FR120 condition by 3.5%.
Table 3.
Mean differences between conditions, 95% confi dence intervals and effect sizes (Cohen's d) across all sets.
| Set 1 | Set 2 | Set 3 | Average | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean diff | 95% CI | d | Mean diff | 95% CI | d | Mean diff | 95% CI | d | Mean diff | 95% CI | d | |
| FR60 - PR | −0.52 | −0.76, −0.28* | 1.2 | −0.40 | −0.78, −0.02* | 0.6 | −0.72 | −1.11, −0.33* | 1.2 | −0.55 | −0.83, −0.26* | 1.2 |
| FR120 - PR | −0.52 | −0.79, −0.25* | 1.2 | −0.76 | −1.14, −0.38* | 1.2 | −1.32 | −1.65, −0.99* | 2.3 | −0.87 | −1.13, −0.61* | 2.0 |
| FR60 - FR120 | 0 | −0.27, 0.27 | 0 | 0.36 | 0.05, 0.67* | 0.5 | 0.60 | 0.17, 1.03* | 0.9 | 0.32 | 0.06, 0.58* | 0.6 |
(*) illustrates statistically different as CI does not include 0; ‘Mean diff’ = mean difference, ‘Average’ = between-protocol differences in the number of repetitions across all sets, ‘d’ = Cohen’s d.
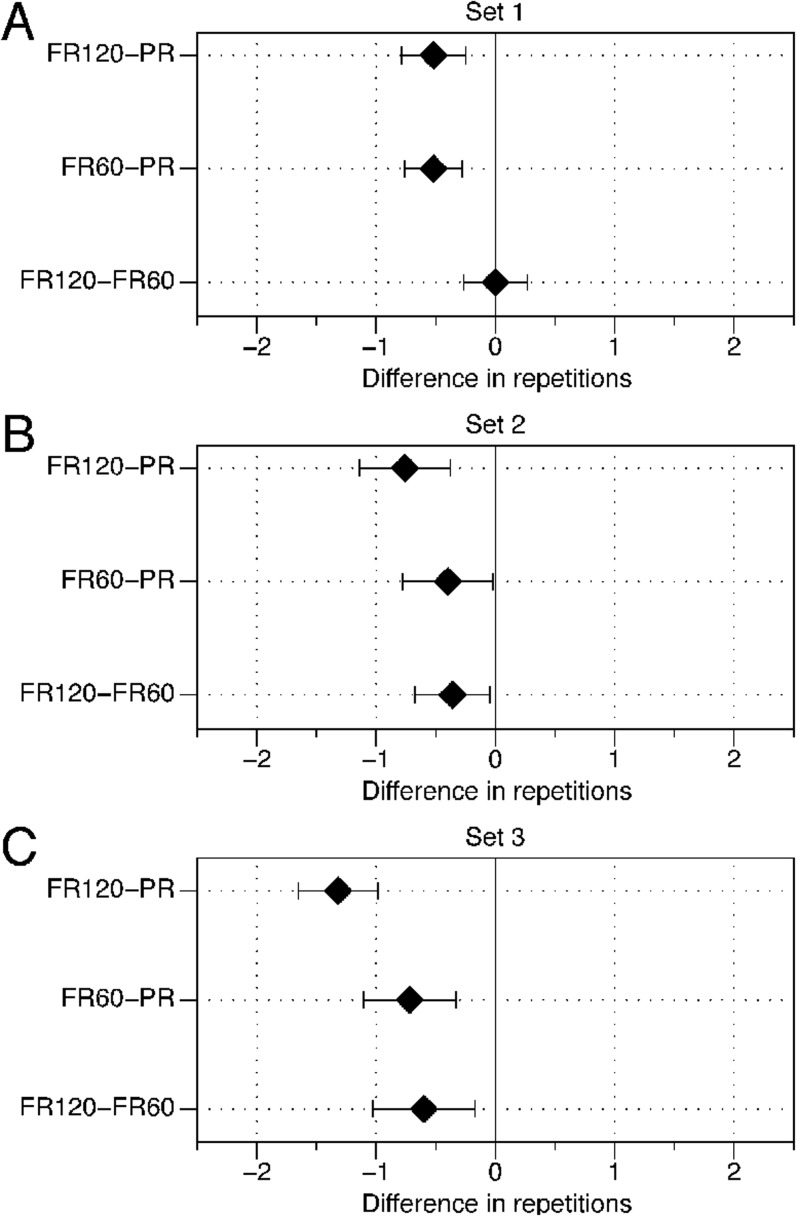
During the first set, the number of repetitions completed in the PR condition was statistically greater than the number of repetitions performed in both the FR60 and FR120 conditions by 5.2%; however, no observable statistical differences existed between the FR120 and FR60 conditions as 95% CI included zero (Table 3, Figure 1a).
Figure 1.
Foam rolling start position at ischial tuberosity (A) and foam rolling end position at popliteal fossa (B).
Figure 2.
Differences in repetitions during the first (A), the second (B) and the third set (C), including 95% confidence intervals.
During the second set, the number of repetitions completed in the PR condition was statistically greater than the number of repetitions performed in the FR60 and FR120 conditions as 95% CIs did not include zero (Table 3). This difference was greater by 4.2% and 8.1%, respectively. Furthermore, the number of repetitions performed in the FR60 condition was statistically greater than in the FR120 condition by 3.9% (Figure 1b).
During the third set, the number of repetitions completed in the PR condition was statistically greater than the number of repetitions performed in the FR60 and FR120 conditions as implied by 95% CIs not including zero (Table 3). This difference was greater by 7.9% and 15.0%, respectively. Finally, the number of repetitions completed in the FR60 condition was statistically greater than the number of repetitions performed in the FR120 condition (Figure 1c) by 7.1%.
Since the number of repetitions differed between conditions on the first set (Table 2), data for the subsequent sets were normalized accordingly (Table 4). When normalized to the performance of the first set, statistically greater number of repetitions was only performed in the FR60 when compared to the FR120 condition during the second set (Table 4). During the third set, the number of repetitions completed in the FR120 condition was statistically lower when compared to the FR60 and PR conditions (Table 4). The remaining differences were not statistically significant, as 95% CIs included zero (Table 4).
Table 4.
Mean differences between conditions normalized to the first set and the accompanying 95% confidence intervals and effect sizes (Cohen's d).
| Set 2 | Set 3 | |||||
|---|---|---|---|---|---|---|
| Mean diff | 95% CI | d | Mean diff | 95% CI | d | |
| FR60 − PR | −0.009 | −0.021, 0.040 | 0.2 | −0.026 | −0.057, 0.006 | 0.4 |
| FR120 − PR | −0.028 | −0.062, 0.006 | 0.5 | −0.087 | −0.121, −0.052* | 1.4 |
| FR60 − FR120 | 0.0337 | 0.006, 0.068* | 0.6 | 0.061 | 0.019, 0.103* | 1.0 |
(*) illustrates statistically different as CI does not include 0; ‘Mean diff = mean difference, ‘d’ = Cohen's d.
DISCUSSION
In contrast to the previous literature on static stretching of the antagonist muscle group,6-8 foam rolling the antagonist muscle group had a detrimental, dose‐dependent effect on strength endurance in knee extensions performed to momentary muscular failure. Interestingly, not only was there a dose‐dependent response, but the response was additive; that is, the magnitude of the differences between the conditions increased with each set, rather than there being a static difference with each set. However, these effects were limited to the condition with the greatest volume (FR120) when data was normalized to the performance of the first set.
While the mechanisms by which foam rolling acutely increases ROM are not fully understood, a number of mechanisms have been proposed. Briefly, these mechanisms can be divided into two categories: mechanical and neurophysiological.9 The former has been purported to be mediated by changes in fascial adhesions, piezoelectricity, cellular responses, myofascial trigger points, and/or thixotropic and viscoelastic properties of tissue, resulting in an increase in tissue compliance, and therefore, ROM.9 At present, these mechanisms are not supported by the literature. For example, Vigotsky et al28 found no changes in rectus femoris length in the modified Thomas test following a foam rolling intervention, which is a proxy measure of passive stiffness. Similar effects have been noted following massage,29,30 and the global effects following foam rolling further support this hypothesis.31 Moreover, in this study, because an increase in tissue compliance would result in a decrease in the knee flexion moment contribution from the hamstrings, one can surmise that the net knee extension moment would increase, allowing the quadriceps to perform less mechanical work over the set and thus complete more repetitions; however, this was not observed.
Neurophysiological mechanisms can be divided into two subcategories, consisting of spinal and supraspinal mediators. The former involves mechanorecptors within the muscle and fascia, which, when triggered, have inhibitory effects, such as decreasing muscle tone.9 While some studies exist to suggest that there are muscle inhibitory mechanisms with massage,32,33 the findings of Vigotsky et al28 suggest that any decrease in muscle tone following a foam rolling intervention is not enough to allow greater joint angular excursion for a given moment, thus rendering it clinically insignificant. In the case of this present study, presumably a muscle inhibitory response would have allowed subjects to perform a greater number of knee extensions following the intervention, but this was not observed. Supraspinal mediators, such as central pain modulation or descending noxious inhibitory control, have been professed to modulate perception via noxious input, resulting in an increase in stretch tolerance.31,34 It may be that some increases in ROM following foam rolling do, in fact, occur through mechanical mechanisms or spinal reflex arcs, but these are either clinically irrelevant in and of themselves, or are shadowed by supraspinal responses. In the case of manual therapy, especially more noxious variations, a descending inhibitory response is elicited via endogenous opioids and other neuropeptides acting on the periaqueductal grey and rostral ventromedial medulla.34 Opioid activity is uniquely important during fatiguing conditions, such as those in this study, as activation of opioid‐modulated pathways may attenuate afferent motor feedback from agonist musculature, resulting in greater power output in the beginning of an exercise, which eventually leads to excess peripheral muscle fatigue.35 This may have been the mechanism by which repetitions decreased with larger doses of foam rolling, but this cannot be said conclusively since work rates were not measured. While there have been a number of studies on power output following foam rolling in non‐fatiguing conditions, only one exists during fatigue, which examined the effects of foam rolling on Wingate power output,36 but those findings were unclear and equivocal. More data are needed to confirm the hypothesis that descending modulatory circuits are at play, as they may explain the findings of this present study.
Analgesia induced by manual therapies has been suggested to be at least partially mediated by the autonomic nervous system (ANS);37 that is, a shift from sympathetic to parasympathetic tone, which has been associated with increases in ROM.38 The mechanism by which these ANS shifts occur is unclear, but massage has been associated with changes in both stress hormones,39 such as cortisol, and neuropeptides,34 endogenous opioids, oxytocin, and endocannabinoids. These hormones and neuropeptides are also responsible for regulating the ANS; specifically, the aforementioned neuropeptides that are associated with a sympathetic shift also play a role in descending modulatory pathways.40,41 A sympathetic shift would likely hinder performance, as parasympathetic shifts appear to augment performance.42-44 Despite the logical basis for the aforementioned neurophysiological mechanisms, more research is needed to elucidate their presence and role following a foam rolling intervention. Additionally, the present investigation did not involve assessment of pain and biomarkers to elucidate the mechanisms of fatigue. Thus, readers should note that the discussion related to the mechanisms is speculative at this point.
There is a possibility that the effort required to foam roll was fatiguing, as foam rolling is not a passive task. This possibility was not ruled out by ways of questionnaires or any other means. Considering that adverse effects on maximum repetition performance were found in a dose‐response manner, and that these adverse effects were limited to the 120‐second condition when the data were normalized to the number of repetitions in the first set, an argument could certainly be made that the present results are confounded by the procedure in question. Furthermore, the protocol in the present study did not involve a sham group in order to exclude this possibility. However, a study has used a planking exercise as a control condition for foam rolling of the quadriceps muscle, due to similarity of isometric holds between the aforementioned activities,45 and found that perceived exertion was higher during the planking condition when compared to foam rolling. In fact, the authors argued that foam rolling may actually lower perceived ratings of fatigue.45 In the present study, an argument can also be made that comparing foam rolling to passive rest, rather than a sham or some other control condition, increases ecological validity.
There are a number of limitations to note in the interpretation of this study. First, the 60‐ and 120‐second conditions were performed on the same day, albeit randomized. While presumably randomization would have likely minimized methodological concerns, the group means likely decreased as a result. In addition, this partially convolutes the repeated‐measures, as there may have been more variation as to whether, and how much, repetitions decreased with foam rolling. Secondly, all participants were female, so caution should be exercised when trying to extrapolate these results to males. Given that females are less fatigable than males during dynamic contractions,46 females may have more room for fatigue in studies of this nature.
CONCLUSION
The finding that inter‐set foam rolling of the antagonist muscle group decreases maximum repetition performance has implications for foam rolling prescription and implementation, in both rehabilitation and athletic populations. For the purposes of performance and likely adaptation, foam rolling should not be applied to the antagonist muscle group between sets of knee extensions. Moreover, more inter‐set foam rolling, i.e. 120 seconds and likely longer, appears detrimental to the ability to continually produce force.
REFERENCES
- 1.Aagaard P Simonsen EB Andersen JL Magnusson SP Bojsen‐Møller F Dyhre‐Poulsen P. Antagonist muscle coactivation during isokinetic knee extension. Scand J Med Sci Sport. 2000;10(2):58‐67. [DOI] [PubMed] [Google Scholar]
- 2.Behm DG Blazevich AJ Kay AD McHugh M. Acute effects of muscle stretching on physical performance, range of motion, and injury incidence in healthy active individuals: a systematic review. Appl Physiol Nutr Metab. 2016;41(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 3.Franco BL Signorelli GR Trajano GS de Oliveira CG. Acute effects of different stretching exercises on muscular endurance. J Strength Cond Res. 2008;22(6):1832‐1837. [DOI] [PubMed] [Google Scholar]
- 4.Gomes TM Simão R Marques MC Costa PB da Silva Novaes J. Acute effects of two different stretching methods on local muscular endurance performance. J Strength Cond Res. 2011;25(3):745‐752. [DOI] [PubMed] [Google Scholar]
- 5.Martins A Paz G Vigario P Costa e Silva G Maia M Miranda H. Static stretching volume is associated with maximal repetition performance. J Exerc Physiol Online. 2014;17(6):24‐33. [Google Scholar]
- 6.Miranda H Maia M de F Paz GA Costa PB. Acute effects of antagonist static stretching in the inter‐set rest period on repetition performance and muscle activation. Res Sports Med. 2015;23(1):37‐50. [DOI] [PubMed] [Google Scholar]
- 7.Paz G Maia M Lima V, et al. Maximal exercise performance and electromyography responses after antagonist neuromuscular proprioceptive facilitation: A pilot study. J Exerc Physiol Online. 2012;15(6):60‐67. [Google Scholar]
- 8.Sandberg JB Wagner DR Willardson JM Smith GA. Acute effects of antagonist stretching on jump height, torque, and electromyography of agonist musculature. J Strength Cond Res. 2012;26(5):1249‐1256. [DOI] [PubMed] [Google Scholar]
- 9.Beardsley C Škarabot J. Effects of self‐myofascial release: A systematic review. J Bodyw Mov Ther. 2015;19(4):747‐758. [DOI] [PubMed] [Google Scholar]
- 10.Maia MF Willardson JM Paz GA Miranda H. Effects of different rest intervals between antagonist paired sets on repetition performance and muscle activation. J Strength Cond Res. 2014;28(9):2529‐2535. [DOI] [PubMed] [Google Scholar]
- 11.Monteiro ER Correa Neto VG. Effect of different foam rolling volumes on knee extension fatigue. Int J Sports Phys Ther. 2016;11(7):1076‐1081. [PMC free article] [PubMed] [Google Scholar]
- 12.Costello J Biuezen F Bleakley C. Where are all the female participants in Sports and Exercise Medicine research? Eur J Sport Sci. 2014;14(8):847‐851. [DOI] [PubMed] [Google Scholar]
- 13.Faul F Erdfelder E Lang A‐G Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175‐191. [DOI] [PubMed] [Google Scholar]
- 14.Beck TW. The importance of a priori sample size estimation in strength and conditioning research. J Strength Cond Res. 2013;27(8):2323‐2337. [DOI] [PubMed] [Google Scholar]
- 15.Shephard RJ. PAR‐Q, Canadian Home Fitness Test and exercise screening alternatives. Sports Med. 1988;5(3):185‐195. [DOI] [PubMed] [Google Scholar]
- 16.Miranda H Fleck SJ Simão R Barreto AC Dantas EHM Novaes J. Effect of two different rest period lengths on the number of repetitions performed during resistance training. J Strength Cond Res. 2007;21(4):1032‐1036. [DOI] [PubMed] [Google Scholar]
- 17.Curran PF Fiore RD Crisco JJ. A comparison of the pressure exerted on soft tissue by 2 myofascial rollers. J Sport Rehabil. 2008;17(4):432‐442. [DOI] [PubMed] [Google Scholar]
- 18.Larivière C Gravel D Arsenault AB Gagnon D Loisel P. Muscle recovery from a short fatigue test and consequence on the reliability of EMG indices of fatigue. Eur J Appl Physiol. 2003;89(2):171‐176. [DOI] [PubMed] [Google Scholar]
- 19.Willardson JM. A brief review: factors affecting the length of the rest interval between resistance exercise sets. J Strength Cond Res. 2006;20(4):978‐984. [PubMed] [Google Scholar]
- 20.Gardner MJ Altman DG. Confidence intervals rather than P values: Estimation rather than hypothesis testing. Br Med J (Clin Res Ed). 1986;292(6522):746‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cumming G. The new statistics: Why and how. Psychol Sci. 2014;25(1):7‐29. [DOI] [PubMed] [Google Scholar]
- 22.Kline R. Beyond Significance Testing: Reforming Data Analysis Methods in Behavioral Research. Washington, D.C: American Psychological Association; 2004. [Google Scholar]
- 23.Dragicevic P. HCI Statistics without p‐values. Inria. 2015:32. [Google Scholar]
- 24.Becker BJ. Synthesizing standardized mean‐change measures. Br J Math Stat Psychol. 1988;41(2):257‐278. [Google Scholar]
- 25.Morris SB. Estimating effect sizes from pretest‐posttest‐control group designs. Organ Res Methods. 2007;11(2):364‐386. [Google Scholar]
- 26.Smith L, Beretvas S. Estimation of the standardized mean difference for repeated measures designs. J Mod Appl Stat Methods. 2009;8(2). [Google Scholar]
- 27.Cohen J. Statistical Power Analysis for Behavioral Sciences. Routledge Academic; 1988. [Google Scholar]
- 28.Vigotsky AD Lehman GJ Contreras B Beardsley C Chung B Feser EH. Acute effects of anterior thigh foam rolling on hip angle, knee angle, and rectus femoris length in the modified Thomas test. PeerJ. 2015;3:e1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eriksson Crommert M Lacourpaille L Heales LJ Tucker K Hug F. Massage induces an immediate, albeit short‐term, reduction in muscle stiffness. Scand J Med Sci Sports. 2014;25(5):e490‐496. [DOI] [PubMed] [Google Scholar]
- 30.Thomson D Gupta A Arundell J Crosbie J. Deep soft‐tissue massage applied to healthy calf muscle has no effect on passive mechanical properties: a randomized, single‐blind, cross‐over study. BMC Sports Sci Med Rehabil. 2015;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aboodarda S Spence A Button D. Pain pressure threshold of a muscle tender spot increases following local and non‐local rolling massage. BMC Musculoskelet Disord. 2015;16(1):265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Behm DG Peach A Maddigan M, et al. Massage and stretching reduce spinal reflex excitability without affecting twitch contractile properties. J Electromyogr Kinesiol. 2013;23(5):1215‐1221. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan SJ Williams LR Seaborne DE Morelli M. Effects of massage on alpha motoneuron excitability. Phys Ther. 1991;71(8):555‐560. [DOI] [PubMed] [Google Scholar]
- 34.Vigotsky A, Bruhns R. The role of descending modulation in manual therapy and its analgesic implications: a narrative review. Pain Res Treat. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amann M Proctor LT Sebranek JJ Pegelow DF Dempsey JA. Opioid‐mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol. 2009;587(Pt 1):271‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janot J Malin B Cook R, et al. Effects of self myofascial release & static stretching on anaerobic power output. J Fit Res. 2013;2(1):2. [Google Scholar]
- 37.Bialosky JE Bishop MD Price DD Robinson ME George SZ. The mechanisms of manual therapy in the treatment of musculoskeletal pain: A comprehensive model. Man Ther. 2009;14(5):531‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bazzichi L Dini M Rossi A, et al. A combination therapy of massage and stretching increases parasympathetic nervous activity and improves joint mobility in patients affected by fibromyalgia. Health (Irvine Calif). 2010;2(8):919‐926. [Google Scholar]
- 39.Field T Hernandez‐Reif M Diego M Schanberg S Kuhn C. Cortisol decreases and serotonin and dopamine increase following massage therapy. Int J Neurosci. 2005;115(10):1397‐1413. [DOI] [PubMed] [Google Scholar]
- 40.Carter CS. Oxytocin pathways and the evolution of human behavior. Annu Rev Psychol. 2014;65:17‐39. [DOI] [PubMed] [Google Scholar]
- 41.Fields HL Heinricher MM Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219‐245. [DOI] [PubMed] [Google Scholar]
- 42.Chandler J V Blair SN. The effect of amphetamines on selected physiological components related to athletic success. Med Sci Sports Exerc. 1980;12(1):65‐69. [PubMed] [Google Scholar]
- 43.Ikai M Steinhaus A. Some factors modifying the expression of human strength. J Appl Physiol. 1961;16:157‐163. [DOI] [PubMed] [Google Scholar]
- 44.Wallin BG. Muscle sympathetic activity and plasma concentrations of noradrenaline. Acta Physiol Scand Suppl. 1984;527:21‐24. [PubMed] [Google Scholar]
- 45.Healey KC Hatfield DL Blanpied P Dorfman LR Riebe D. The effects of myofascial release with foam rolling on performance. J Strength Cond Res. 2014;28(1):61‐68. [DOI] [PubMed] [Google Scholar]
- 46.Hunter SK. Sex differences in human fatigability: mechanisms and insight to physiological responses. Acta Physiol (Oxf). 2014;210(4):768‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]