Abstract
Background
The Functional Movement Screen (FMS™) is a battery of tests designed to assess movement competency; the overhead deep squat test, specifically, has been shown to be an accurate predictor of overall FMS™ scores. Self‐massage (SM) is a ubiquitous warm‐up utilized to increase joint range of motion and, therefore, may be effective for improving performance of the overhead deep squat test.
Purpose
To examine how different doses (30, 60, 90, and 120 seconds) of SM of different areas of the body (plantar fascia, latissimus dorsi, and lateral thigh) affects the score obtained on an overhead deep squat test.
Methods
Twenty recreationally active females were recruited to be tested on four occasions: sessions one and two consisted of baseline testing, session three consisted of SM applied to the lateral thigh, and session four consisted of SM applied to the lateral torso and plantar fascia.
Results
In all SM conditions, at least 90 seconds was required for a change in deep squat score from baseline; therefore, it is concluded that SM the lateral torso, plantar fascia, and lateral thigh for 90 seconds or more are effective interventions for acutely improving overhead deep squat scores.
Conclusion
Self‐massage appears to be an effective modality for inducing acute improvements in the performance of the FMS™ overhead deep squat in all conditions tested.
Level of evidence
2b
Keywords: Flexibility, foam rolling, self‐manual therapy, self‐myofascial release, tennis ball
INTRODUCTION
The squat is a fundamental movement pattern that is required for numerous activities of daily living, such as sitting, lifting, and most sporting activities.1 Furthermore, it is a staple exercise in strength and conditioning programs that has been shown to increase performance,2 as well as being used in clinical rehabilitation programs.3,4 A prerequisite for more intense – that is, loaded – squat activities is the correct and consistent performance of a bodyweight squat.5
The Functional Movement Screen (FMS™) is a pre‐participation screening system comprised of seven “fundamental movement patterns” that require both stability and mobility, such as the overhead deep squat.6 Each movement pattern is given a score from 0 to 3, with 0 being given in the presence of pain; 1 if the individual is unable to perform the movement; 2 if the individual is able to perform the movement, but needs to compensate in some way; and 3 if the individual is able to perform the movement without any compensations.6
The overhead deep squat component of the FMS™ was designed to assess bilateral symmetry (or lack thereof) and functional mobility of the hips, knees, and ankles. By adding the dowel held overhead, it also assesses bilateral, symmetrical mobility of the shoulders and thoracic spine, in addition to stability and motor control of the core musculature.6 Poor performance of this test can be the result of several factors including, but not limited to, poor glenohumeral and thoracic spine mobility, limited mobility in the lower extremity – e.g., poor closed kinetic chain dorsiflexion or poor hip flexion – and limited stability and/or motor control of the core musculature.6 Some evidence suggests that the overhead deep squat test can predict the overall FMS™ score and thus may provide a time‐efficient assessment of individuals who may require further screening.7
One of the potential deficits in the performance of the squat is limited mobility.1,6 More specifically, because the overhead deep squat involves large angular excursions of the ankles (dorsiflexion), knees (flexion), hips (flexion), and shoulders (flexion), competent performance of the overhead deep squat is predicated on the mobility of the aforementioned joints to flex and extend through large angular excursions. Restrictions to these movements may be attributable to extensors around these joints, in addition to ligaments, connective tissue, and, in some cases, structural variability, which provide resistive moments or endpoints; as such, warmups that increase the extensibility of these soft‐tissues may be efficacious for improving the performance of the overhead deep squat. It is important to note, however, that such interventions may only be effective for limitations in mobility, but not stability.
Acutely, improvements in mobility can be achieved by self‐massage (SM); i.e., using a foam roller,8,9 a roller massager,10,11 or tennis ball.12 Current literature on the dose‐response of the acute effects of SM on changes in range‐of‐motion (ROM) remains equivocal. Sullivan et al.11 and Bradbury‐Squires et al.10 did not find statistically significant differences between different volumes of SM, but noted a trend for greater increases with greater volumes. Couture et al.13 did not find any difference in ROM changes between different volumes. However, in contrast to previous studies,10,11 Couture et al.13 also did not observe changes in ROM in comparison to baseline. This discrepancy may have been a function of the tool used, intensity (pressure) of SM, method used for ROM assessment, or pace.
At present, there are two primary, competing hypotheses as to the mechanisms by which self‐massage improves ROM. The first is a mechanical perspective, which states that the act of self‐massage deforms the tissues to which it is applied, which in turn will allow for greater extensibility of those tissues.14 The second is a neurophysiological perspective, which states that the sensory input provided by self‐massage, such as noxious stimuli, elicits a central neurophysiological response, which either modulates perception, allowing for a greater ROM,14,15 or, alternatively, that the sensory input may decrease efferent drive and neural tone.14 If the central neurophysiological mechanism is indeed at least partially a driver of changes in joint ROM, then changes in ROM will occur at joints foreign to the ones being targeted, which has recently been shown to occur.16 Implicated by these findings is that mobility limitations during the overhead deep squat can be improved, no matter the structure to which self‐massage is applied.
Therefore, the purpose of this study was to investigate the acute effects of different volumes of self‐massage applied to the lateral thigh (Experiment 1) and lateral torso and plantar surface of the foot (Experiment 2) on the performance of the overhead deep squat test of FMS™. The lateral thigh was chosen because the underlying areas, i.e., iliotibial band and tensor fascia latae, have been suggested to be one of the common hindrances in the performance of the squat;1 the plantar surface of the foot and lateral torso were chosen because the latissimus dorsi may limit shoulder flexion, while, from a fascial perspective, the plantar surface of the foot may be related to ankle dorsiflexion ability. However, from a central, neurophysiological perspective, the area to which self‐massage is applied should not matter. Therefore, limited extensibility of these areas may be associated with decreased hip flexion and/or extension, shoulder flexion, and dorsiflexion, respectively, and thus, increasing the extensibility of these areas may help individuals achieve greater scores on the overhead deep squat test. If shown to be effective, SM could be incorporated into corrective exercise programs that are designed to improve fundamental movement competency.
METHODS
Subjects
A convenience sample of twenty recreationally active, resistance‐trained females were recruited and participated in both experiments (Table 1). No a priori sample size calculation was conducted due to the ordinal nature of FMSTM scores, so a convenience sample of 20 females was recruited. Anthropometric data included body mass (Techline BAL – 150 digital scale, São Paulo, Brazil) and height (Stadiometer ES 2030 Sanny, São Paulo, Brazil). Subjects were included if they had been involved in resistance training program for at least one year prior to the experiment, for an average of 40‐50 minutes per session, 3‐4 sessions per week, using loads with 8‐12 repetitions maximum, and rest intervals between one and three minutes between sets. That way, familiarity with the squatting movement pattern was ensured in order to minimize the effect of learning. Participants were excluded if they had any experience with SM or a musculoskeletal or neuromuscular injury that could have affected their ability to perform the overhead squat; furthermore, participants were excluded if they scored 0 or 3 on the overhead deep squat test. Prior to the study, participants were provided a verbal explanation of the procedures and signed informed consent and Physical Activity Readiness Questionnaire.17 All procedures were in accordance with Declaration of Helsinki and the study was approved by the Institutional Review Board of University Hospital Clementino Fraga Filho of the Federal University of Rio de Janeiro.
Table 1.
Subject characteristics. Data expressed as means ± SD.
| Experiment 1* | Experiment 2* | |
|---|---|---|
| N | 20 | 20 |
| Age (years) | 26.2 ± 6.4 | 26.3 ± 6.3 |
| BM (kg) | 63.6 ± 10.2 | 63.4 ± 9.9 |
| Height (cm) | 164.0 ± 6.9 | 164.0 ± 6.9 |
| BMI | 23.4 ± 2.0 | 23.5 ± 2.1 |
| RTE (months) | 17.7 ± 3.7 | 20.0 ± 3.5 |
BM = body mass; BMI = body mass index, RTE = resistance training experience.
all subjects were females.
Procedures
Self‐massage
SM was performed using The Grid Foam Roller (Trigger Point Technologies, 5321 Industrial Oaks Blvd., Austin, Texas 78735, USA) and a tennis ball (Head Master, Belo Horizonte, Minas Gerais, Brazil). In Experiment 1, foam rolling was performed in a decubitus lateral position with the foam roller placed under the lateral side of the thigh. The leg being treated was extended, while the other was crossed over the treated leg in a flexed position. Participants were instructed to roll the lateral part of their thigh up and down on the foam roller, between the greater trochanter and lateral epicondyle of the knee, in dynamic motions, while trying to exert as much pressure on the foam roller as possible.
In Experiment 2, the lateral side of the torso and plantar surface of the foot were treated. For the former, foam rolling was performed in a decubitus lateral position. Participants were instructed to roll their lateral trunk up and down on the foam roller, between the proximal third of the arm and inferior part of the ribcage, while trying to exert as much pressure on the foam roller as possible. SM on the plantar surface of the foot was performed with a tennis ball in a standing position. These methods differ slightly from Grieve et al.12, in that Grieve et al.12 had participants perform SM while seated rather than standing, but standing likely results in greater pressure and, presumably, a greater effect. Participants were instructed to roll the tennis ball on the sole of the foot between the midfoot and proximal phalanges, while trying to exert as much pressure on the ball as possible. In both experiments, the order of treatment between the left and right limbs was randomized.
FMS™ Overhead Deep Squat
A full description of the overhead deep squat test (Figure 1) has been provided previously.18 Briefly, the individual begins standing with his/her feet approximately shoulder width apart with the dowel pressed overhead while keeping the elbows extended. The individual then descends as far into a squat as they can while maintaining an upright torso, heels on the floor, and the dowel pressed overhead. The descended position is then maintained for a count of one second before the individual is allowed to return to the starting position. If the score of ‘3’ has not been achieved (Figure 1, E and F), the individual proceeds to perform the same test with a 2x6 block under his or her heels. If a competent movement pattern is demonstrated, the individual receives a score of ‘2’ (Figure 1, C and D). Screening was always performed by the same experienced rater in both experiments. Experienced raters are expected to achieve acceptable reliability, particularly for overhead deep squat test.19 In order to ensure that the rater in this present experiment was adequate, test‐retest reliability and minimum detectable change scores were calculated. Standardized test instructions were provided.18 Participants were allowed three trials and the best trial was recorded.
Figure 1.
FMS™ for overhead deep squat test.6 A and B = score 1; C and D = score 2; E and F = score 3.
Experimental approach to the problem
A randomized (aleatory entry in latin square format) within‐subject design was used for treatment (i.e., lateral thigh, lateral torso and plantar surface), as well as the experimental protocols. Two experiments were conducted, separated by roughly two to three months. For each experiment, the same participants visited the laboratory on four occasions at similar times during the day to avoid diurnal variations, with a minimum of ninety‐six hours between visits. All procedures were performed barefoot and no additional warm‐up was performed beforehand.
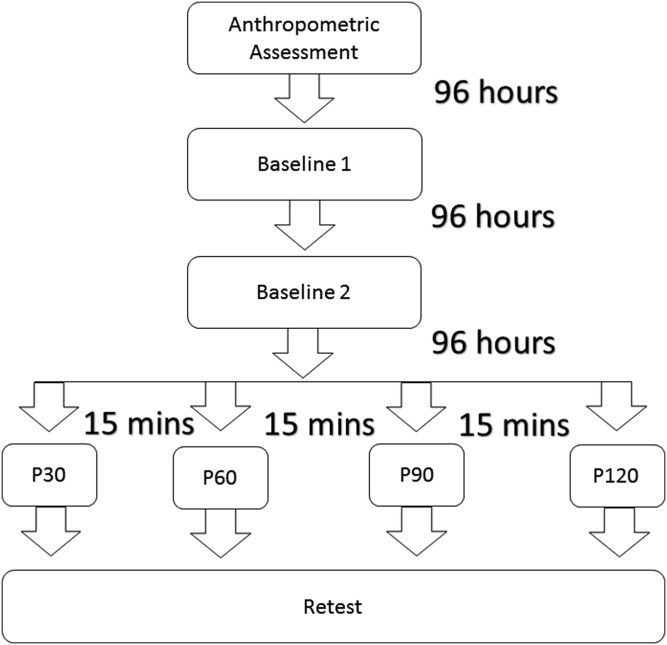
In Experiment 1 (Figure 2), participants visit the laboratory four times with 96‐hours between visits. Anthropometric data was collected during the first visit. On the second and third visits, the participants underwent overhead deep squat testing (baseline 1) and retesting (baseline 2), respectively. The experimental visit followed and consisted of four different, single‐set SM with foam rolling protocols treating both lateral thighs unilaterally in a randomized order: P30 – thirty seconds of foam rolling per side, P60 – sixty seconds of foam rolling per side, P90 – ninety seconds of foam rolling per side, P120 – 120 seconds of foam rolling per side. A fifteen‐minute interval was employed between each protocol, based on the findings that acute increases in ROM following SM are persistent for 10 minutes,8 but not longer.9 After each protocol, participants were scored on their performance of the overhead deep squat test.
Figure 2.
Study design for Experiment 1 – self‐massage of the lateral thigh. P30 – thirty seconds of foam rolling per side, P60 – sixty seconds of foam rolling per side, P90 – ninety seconds of foam rolling per side, P120 – 120 seconds of foam rolling per side.
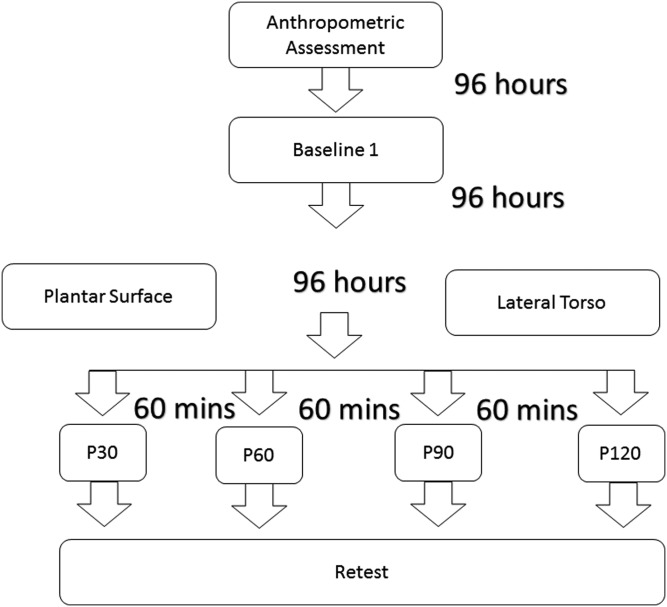
In Experiment 2 (Figure 3), anthropometric data was collected during the first visit followed by assessment of the overhead deep squat performance on the second visit (baseline). Only a single day of testing for the overhead deep squat was performed for the second experiment because the data from the first experiment showed similar effects of different SM protocols when compared to baseline 1 and baseline 2 (see Results). On the third and fourth visits, participants performed SM of lateral torso and plantar surface of the foot, respectively, as per randomization. During each of these two visits, four different, single‐set SM protocols (P30, P60, P90 and P120) were employed in a randomized order with a sixty‐minute rest interval between each protocol: P30 – thirty seconds of self‐massage per side, P60 – sixty seconds of self‐massage per side, P90 – ninety seconds of self‐massage per side, P120 – 120 seconds of self‐massage per side. After each protocol, participants were scored on their performance of the overhead deep squat test.
Figure 3.
Study design for Experiment 2 – self‐massage of lateral torso and plantar surface of the foot. P30 – thirty seconds of self‐massage per side, P60 – sixty seconds of self‐massage per side, P90 – ninety seconds of self‐massage per side, P120 – 120 seconds of self‐massage per side.
Statistical analyses
Reliability was established using the baseline measures from the first two days of the first experiment and calculating test‐retest intraclass correlation coefficients (ICC). From the ICCs, a minimum detectable change (MDC) at the 95% level was calculated. A Friedman nonparametric test was used to determine the effects of different experimental conditions on the dependent variable. If the null hypothesis was not accepted, pairwise comparisons were performed with a Bonferroni correction for multiple comparisons. All analyses were performed using SPSS (version 21, SPSS Inc., Chicago, IL, USA). Statistical significance was set at an alpha level of 0.05.
RESULTS
Subject details are provided in Table 1. Using the baseline measures on the first two days, a test‐retest ICC of 0.481 was found, which corresponds to a MDC of 1.00.
Experiment 1 – Lateral Thigh
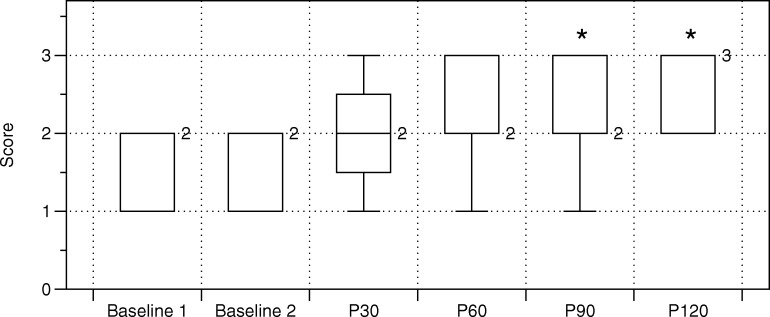
Participants achieved a statistically greater score on the overhead deep squat test with P90 and P120 as compared to both the baseline 1 (p = 0.004 and p < 0.001, respectively) and baseline 2 (p = 0.020 and p = 0.001, respectively) values (Table 2, Figure 4). No other statistically significant differences were observed.
Table 2.
Differences between protocols for Experiment 1. Data expressed as medians with interquartile ranges.
| Condition | Volume | Median | Interquartile Range | Min | Max |
|---|---|---|---|---|---|
| Baseline 1 | 2.0 | 1–2 | 1 | 2 | |
| Baseline 2 | 2.0 | 1–2 | 1 | 2 | |
| SM | P30 | 2.0 | 1.75–2.25 | 1 | 3 |
| P60 | 2.0 | 2–3 | 1 | 3 | |
| P90*† | 2.0 | 2–3 | 1 | 3 | |
| P120*† | 3.0 | 2–3 | 2 | 3 | |
| Δ from Baseline 1 | P30 | 0.0 | 0–1 | 0 | 1 |
| P60 | 1.0 | 0–1 | 0 | 2 | |
| P90* | 1.0 | 0–1 | 0 | 2 | |
| P120* | 1.0 | 1–1 | 0 | 2 | |
| Δ from Baseline 2 | P30 | 0.0 | 0–1 | −1 | 1 |
| P60 | 1.0 | 0–1 | −1 | 2 | |
| P90† | 1.0 | 0–1 | −1 | 2 | |
| P120† | 1.0 | 0.75–1 | 0 | 2 |
SM – self‐massage of the lateral thigh; P30 – thirty seconds of foam rolling for each side; P60 – sixty seconds of foam rolling for each side; P90 – ninety seconds of foam rolling for each side; P120 – 120 seconds of foam rolling for each side.
Statistically different from baseline 1;
Statistically different from baseline 2.
Figure 4.
Box and whisker plots of the effects of foam rolling the lateral thigh on FMS™ squat score. * denotes a statistical difference from both Baseline 1 and Baseline 2.
Experiment 2 – Lateral Torso and Plantar Surface of the Foot
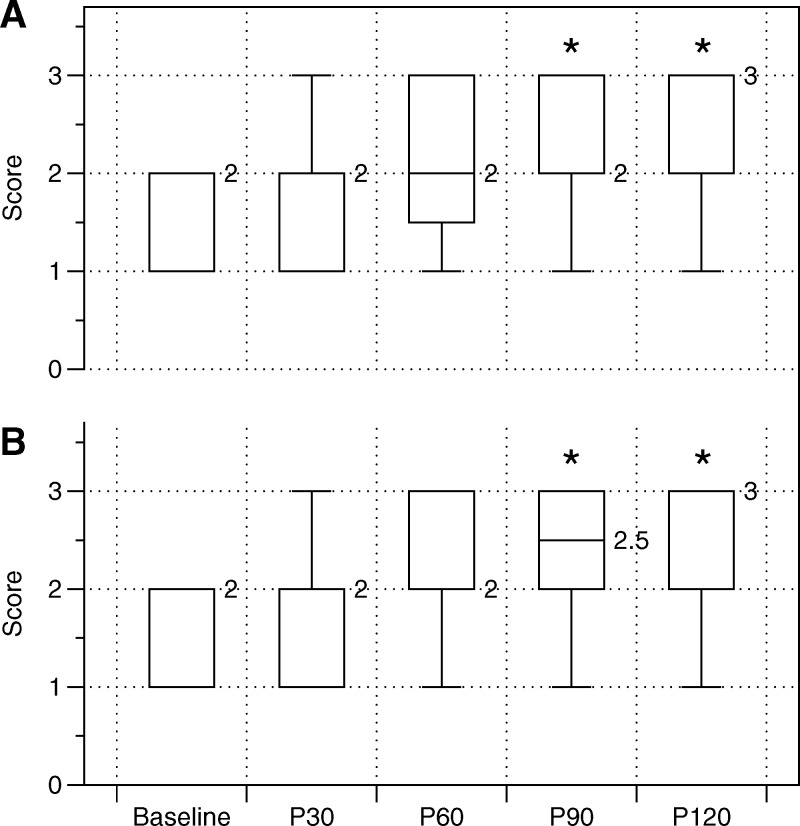
For both conditions – that is, the plantar surface of the foot and lateral torso – participants achieved statistically greater scores on the overhead deep squat test with P90 (p = 0.040 and p = 0.029, respectively) and P120 (p = 0.001 and p < 0.001, respectively) as compared to the baseline value (Table 3, Figure 5). Furthermore, participants achieved statistically greater scores with P120 as compared to P30 (p = 0.019 and p = 0.032, respectively). A greater score was also achieved when the lateral torso was treated for 120 seconds as compared to when the plantar surface of the foot was treated for 30 seconds (p = 0.011). There were no other statistically significant differences between conditions.
Table 3.
Differences between conditions and protocols for Experiment 2. Data expressed as medians with interquartile ranges.
| Condition | Volume | Median | Interquartile Range | Min | Max |
|---|---|---|---|---|---|
| Baseline | 2.0 | 1–2 | 1 | 2 | |
| PF | P30 | 2.0 | 1–2 | 1 | 3 |
| P60 | 2.0 | 1.75–3 | 1 | 3 | |
| P90* | 2.0 | 2–3 | 1 | 3 | |
| P120*†‡ | 3.0 | 2–3 | 1 | 3 | |
| Δ PF from Baseline | P30 | 0.0 | 0–0 | −1 | 1 |
| P60 | 0.0 | 0–1 | 0 | 1 | |
| P90* | 1.0 | 0–1 | −1 | 1 | |
| P120*†‡ | 1.0 | 1–1 | 0 | 2 | |
| LTO | P30 | 2.0 | 1–2 | 1 | 3 |
| P60 | 2.0 | 2–3 | 1 | 3 | |
| P90* | 2.5 | 2–3 | 1 | 3 | |
| P120*†‡ | 3.0 | 2–3 | 1 | 3 | |
| Δ LTO from Baseline | P30 | 0.0 | 0–1 | −1 | 1 |
| P60 | 1.0 | 0–1 | −1 | 2 | |
| P90* | 1.0 | 0–1 | −1 | 2 | |
| P120*†‡ | 1.0 | 0.75–1 | 0 | 2 |
PF – plantar surface of the foot; LTO – lateral torso; P30 – thirty seconds of self‐massage for each side; P60 – sixty seconds of self‐massage for each side; P90 – ninety seconds of self‐massage for each side; P120 – 120 seconds of self‐massage for each side.
Statistically different from baseline;
Statistically different from P30;
Statistically different from PF P30.
Figure 5.
Box and whisker plots of the effects of foam rolling the lateral torso (A) and plantar surface of the foot (B) on FMS™ squat score. * denotes a statistical difference from Baseline.
All statistical changes observed exceeded the calculated MDC.
DISCUSSION
The main findings of the present study were: 1) higher volumes ( > 90 seconds) appear to be superior to lower volumes ( < 90 seconds) for acutely improving performance of the FMS™ overhead deep squat test; 2) 90 seconds appears to be the necessary threshold to achieve these beneficial changes; 3) treatment of lateral side of the torso and plantar surface of the foot seem to be equally effective in inducing acute improvements in overhead deep squat test if done for sufficient time; i.e., 90 seconds.
Previously, research has not shown there to be a statistical difference between different volumes of SM for increasing ROM, but has noted a ‘trend’ for greater doses potentially inducing greater changes in ROM.10,11 The present study extends this observation by showing that greater volumes seem to be superior for inducing movement performance, which may be a result of acute changes in ROM. Moreover, the present study showed that 90 seconds appears to be the volume‐threshold necessary to induce these changes, as 30‐ and 60‐seconds were not found to result in a statistically different performance of the overhead deep squat test as compared to baseline. Interestingly, Bradbury‐Squires et al.10 also found improvements in ‘movement efficiency’ following SM, as assessed by a decrease in electromyography amplitude during a lunge. The current findings could be considered in agreement with Bradbury‐Squires et al.10 in the context that the FMS™ test battery has been suggested as an assessment tool for movement competency.18 In contrast, not only did Couture et al.13 show no statistical difference between SM performed for two sets of 10 seconds and four sets of 30 seconds, but there were also no statistical differences in ROM as compared to baseline. This discrepancy could possibly be a function of the tool and intensity (pressure) used as well as methodology of ROM assessment. The latter was tightly controlled, as knee extension ROM was measured by restricting any contribution from other joints.13 Conversely, other studies have assessed ROM wherein contribution from other joints cannot be excluded.10,11 Similarly, in the present study, a complex, multi‐joint movement was used, and it is unclear as to whether or not the same results would have been observed had the experimental procedure included a single‐joint ROM assessment. With regard to the type of tool used, both Sullivan et al.11 and Bradbury‐Squires et al.10 used a roller massager, while Couture et al.13 used a commercially‐available foam roller made out of polystyrene foam. In contrast, in the present study, a uniformly cylindrical foam roller composed of a hard and hollow inner core enclosed with a layer of ethylene vinyl acetate foam was used. This type of foam roller has been shown to produce more pressure on area to which it is applied as opposed to those made of polystyrene foam.20 Sullivan et al.11 and Bradbury‐Squires et al.10 also used a specifically‐designed apparatus to control for pressure applied to the underlying tissue, and in doing so, increased internal validity of the results. On the other hand, both Couture et al.13 and the present study did not use a special device, but provided participants with similar instructions as to how pressure should be applied. While pressure relative to body weight was not measured in this present study, as opposed to Couture et al.13, it is possible that, as a function of different body parts being treated, the participants in the current study were able to induce greater pressure on the underlying tissue. However, it should to be noted that, to date, the effect of intensity (pressure) has not been explored in a tightly controlled setting and thus its effects remain unclear and require further exploration. However, preliminary evidence suggests that variations in pressure may exert different effects on active and passive ROM21.
At present, the mechanisms by which SM induces acute changes in ROM have not been fully elucidated, but many have been proposed, including both mechanical and neurophysiological mechanisms.14 The former has been associated with changes in fascial adhesions, piezoelectricity, cellular responses, myofascial trigger points, and/or thixotropic and viscoelastic properties of the tissue, resulting in increased tissue compliance.14 However, Vigotsky et al.15 did not find any changes in rectus femoris length in the modified Thomas test, a proxy measure of passive stiffness,22 following SM intervention. Granted, participants were also taken through a rigorous dynamic warm‐up which could have maximized the potential acute extensibility gains prior to testing.15 In addition, similar effects have also been observed following massage.23 Furthermore, the available evidence suggests that SM exerts global effects.12,16,24,25 The latter is also supported by the findings of this present study, as similar improvements in the overhead deep squat test were noted in all conditions tested. Taken together, findings from the aforementioned studies suggest that the mechanical contribution to changes in ROM following SM intervention needs to be reconsidered.
With regard to neurophysiological mechanisms, both spinal and supraspinal mediators have been considered. The former has been associated with mechanoreceptors within muscle and fascia and are suggested to have inhibitory effects when triggered, such as decreasing muscle tone.14,26 However, the findings of Vigotsky et al.15 suggest that any decrease in muscle tone following SM is insufficient to allow for greater ROM, thus rendering these mechanisms clinically insignificant. It may be that some increases in ROM following SM are a result of mechanical mechanisms or modulation of a spinal reflex arc, but these are likely clinically insignificant by themselves, or are predominated by supraspinal mediators. The latter, such as central pain modulation or descending noxious inhibitory control, have been asserted to mediate perception via noxious input and thus increase stretch tolerance.15,27
Analgesic effects and relaxation following manual therapies have been suggested to be mediated by autonomic nervous system activity (ANS);28 i.e., a shift from sympathetic to parasympathetic tone, which has been associated with increases in ROM.29 The exact mechanism of this ANS shift is unclear; however, massage has been associated with changes in both stress hormones – for example, cortisol30 – and neuropeptides – for example, endogenous opioids, oxytocin, and endocannabinoids.27 Both of these appear to be responsible for regulating the ANS, and specifically, neuropeptides may play a role in descending modulatory pathways.31 Similarly, proxies of ANS activity have been found to change following SM.32,33 Given that SM has been shown to induce a noxious stimuli10 and that the participants were instructed to exert as much pressure as possible on the area under treatment, it is probable that changes observed in the present experiments were elicited as a result of a descending inhibitory response. It is equally probable that the observed changes were due to an ANS shift to parasympathetic tone. Considering that this present study found that there was a minimum volume‐threshold to induce beneficial changes, it would seem prudent to hypothesize that a certain amount of time is needed for the response to take place. Despite the logical basis for the aforementioned mechanisms, it has to be noted that they are based solely on explanations available from the existing literature, and the present study was not designed to assess these mechanisms, and more research is required to elucidate their existence and role in SM, specifically, rather than extrapolating from the massage and manual therapy literature.
There are a number of limitations to note when interpreting the result of the present study. Firstly, no pre‐intervention screen was performed on the day of testing and thus the comparisons were performed between days. Therefore, between‐day variability in test scores could have confounded the results. Secondly, different SM conditions were performed on the same day interspersed by a 15‐minute and 60‐minute interval of passive rest during Experiments 1 and 2, respectively. While presumably, randomization would have likely minimized methodological concerns, it is possible that there was a learning effect to the performance of the test. Indeed, previous research has shown that the ‘control’ group can exhibit movement screen changes after 12 weeks without being subjected to the intervention program designed to improve the outcome scores.34 However, given that the participants were resistance trained and thus familiar with the squat pattern, the learning effect was likely smaller. In Experiment 1, a 15‐minute rest‐interval had been chosen based on the findings that acute increases in ROM following SM are persistent for 10 minutes,8 but not longer.9 However, a recent study suggested that changes can last up to 20 minutes,16 but this evidence was not available until after the experiment had been completed. For the second experiment, it was decided to extend the rest‐interval to 60‐minutes as to decrease the risk of carryover from previous trials. Nevertheless, no statistical differences were observed between protocols that would indicate a 15‐minute interval to be insufficient and a confounding factor for repeated measures. Thirdly, while the overhead deep squat test has been shown to predict the overall FMS™ score, it is limited as to its ability to assess overall asymmetry.7 Moreover, the fact that the trials were not investigator‐blinded should be taken into account, as FMS™ scores are a subjective, qualitative outcome that may be subject to bias; therefore, by knowing what and how much each subject foam rolled before scoring her, there may have been inherent bias. Lastly, the pace of rolling was not controlled for, thereby reducing internal validity of the results due to the possibility of pace‐dependent outcomes.15 Nonetheless, not controlling for pace enhances ecological validity of the findings, as it is a better representation of the scenario in practice.
CONCLUSION
In conclusion, SM appears to be an effective modality for acute improvements in the FMS™ overhead deep squat performance, in all conditions tested; i.e., SM to the lateral thigh, plantar surface of the foot, and lateral side of the trunk. Furthermore, treatment lasting 90 seconds may be needed for beneficial changes to occur, with a slight possibility that longer durations may provide additional benefits. Whether the same modality is beneficial for chronic – that is, long‐term – improvements in the overhead deep squat test or the full FMS™ test battery is a matter for future research.
REFERENCES
- 1.Myer GD Kushner AM Brent JL, et al. The back squat: A proposed assessment of functional deficits and technical factors that limit performance. Strength Cond J. 2014;36(6):4‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duehring MD Feldmann CR Ebben WP. Strength and conditioning practices of United States high school strength and conditioning coaches. J Strength Cond Res. 2009;23(8):2188‐2203. [DOI] [PubMed] [Google Scholar]
- 3.Myer GD Ford KR Hewett TE. Rationale and clinical techniques for anterior cruciate ligament injury prevention among female athletes. J Athl Train. 2004;39(4):352‐364. [PMC free article] [PubMed] [Google Scholar]
- 4.Schoenfeld BJ. Squatting kinematics and kinetics and their application to exercise performance. J Strength Cond Res. 2010;24(12):3497‐3506. [DOI] [PubMed] [Google Scholar]
- 5.Myer GD Faigenbaum AD Chu DA, et al. Integrative training for children and adolescents: techniques and practices for reducing sports‐related injuries and enhancing athletic performance. Phys Sportsmed. 2011;39(1):74‐84. [DOI] [PubMed] [Google Scholar]
- 6.Cook G Burton L Hoogenboom BJ Voight M. Functional movement screening: The use of fundamental movements as an assessment of function ‐ Part 1. Int J Sports Phys Ther. 2014;9(3):396‐409. [PMC free article] [PubMed] [Google Scholar]
- 7.Clifton DR Grooms DR Onate JA. Overhead deep squat performance predicts Functional Movement Screen™ score. Int J Sports Phys Ther. 2015;10(5):622‐627. [PMC free article] [PubMed] [Google Scholar]
- 8.MacDonald GZ Penney MD Mullaley ME, et al. An acute bout of self‐myofascial release increases range of motion without a subsequent decrease in muscle activation or force. J Strength Cond Res. 2013;27(3):812‐821. [DOI] [PubMed] [Google Scholar]
- 9.Škarabot J Beardsley C Štirn I. Comparing the effects of self‐myofascial release with static stretching on ankle range‐of‐motion in adolescent athletes. Int J Sports Phys Ther. 2015;10(2):203‐212. [PMC free article] [PubMed] [Google Scholar]
- 10.Bradbury‐Squires DJ Noftall JC Sullivan KM Behm DG Power KE Button DC. Roller‐massager application to the quadriceps and knee‐joint range of motion and neuromuscular efficiency during a lunge. J Athl Train. 2015;50(2):133‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan KM Silvey DBJ Button DC Behm DG. Roller‐massager application to the hamstrings increases sit‐and‐reach range of motion within five to ten seconds without performance impairments. Int J Sports Phys Ther. 2013;8(3):228‐236. [PMC free article] [PubMed] [Google Scholar]
- 12.Grieve R Goodwin F Alfaki M Bourton A‐J Jeffries C Scott H. The immediate effect of bilateral self myofascial release on the plantar surface of the feet on hamstring and lumbar spine flexibility: A pilot randomised controlled trial. J Bodyw Mov Ther. 2015;19(3):544‐552. [DOI] [PubMed] [Google Scholar]
- 13.Couture G Karlik D Glass SC Hatzel BM. The effect of foam rolling duration on hamstring range of motion. Open Orthop J. 2015;9:450‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beardsley C Škarabot J. Effects of self‐myofascial release: a systematic review. J Bodyw Mov Ther. 2015;19:747‐758. [DOI] [PubMed] [Google Scholar]
- 15.Vigotsky AD Lehman GJ Contreras B Beardsley C Chung B Feser EH. Acute effects of anterior thigh foam rolling on hip angle, knee angle, and rectus femoris length in the modified Thomas test. PeerJ. 2015;3:e1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly S Beardsley C. Specific and cross‐over effects of foam rolling on ankle dorsiflexion range of motion. Int J Sport Phys Ther. 2016;11(4):544‐551. [PMC free article] [PubMed] [Google Scholar]
- 17.Shephard RJ. PAR‐Q, Canadian Home Fitness Test and exercise screening alternatives. Sports Med. 1988;5(3):185‐195. [DOI] [PubMed] [Google Scholar]
- 18.Cook G Burton L Hoogenboom BJ Voight M. Functional movement screening: The use of fundamental movements as an assessment of function‐Part 2. Int J Sports Phys Ther. 2014;9(4):549‐563. [PMC free article] [PubMed] [Google Scholar]
- 19.Moran RW Schneiders AG Major KM Sullivan SJ. How reliable are Functional Movement Screening scores?. A systematic review of rater reliability. Br J Sports Med. 2016;50(9):527‐536. [DOI] [PubMed] [Google Scholar]
- 20.Curran PF Fiore RD Crisco JJ. A comparison of the pressure exerted on soft tissue by 2 myofascial rollers. J Sport Rehabil. 2008;17(4):432‐442. [DOI] [PubMed] [Google Scholar]
- 21.Gabrow L Young J Alcock L Škarabot J Behm D The effect of varied force applications with self‐manual therapy on range of motion and voluntary contractile properties. In: Proceedings of the 18th Annual TRAC Meeting. Copenhagen, Denmark; 2016. [Google Scholar]
- 22.Vigotsky AD Lehman GJ Beardsley C Contreras B Chung B Feser EH. The modified Thomas test is not a valid measure of hip extension unless pelvic tilt is controlled. PeerJ. 2016;4:e2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomson D Gupta A Arundell J Crosbie J. Deep soft‐tissue massage applied to healthy calf muscle has no effect on passive mechanical properties: a randomized, single‐blind, cross‐over study. BMC Sports Sci Med Rehabil. 2015;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monteiro E Škarabot J Vigotsky A Brown A Gomes T da Silva Novaes J. Acute effects of different foam rolling volumes of antagonist muscle group in the inter‐set rest period on maximum repetition performance. Manuscript Submitted for Publication; 2016. [Google Scholar]
- 25.Aboodarda S Spence A Button D. Pain pressure threshold of a muscle tender spot increases following local and non‐local rolling massage. BMC Musculoskelet Disord. 2015;16(1):265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behm DG Peach A Maddigan M, et al. Massage and stretching reduce spinal reflex excitability without affecting twitch contractile properties. J Electromyogr Kinesiol. 2013;23(5):1215‐1221. [DOI] [PubMed] [Google Scholar]
- 27.Vigotsky A, Bruhns R. The role of descending modulation in manual therapy and its analgesic implications: a narrative review. Pain Res Treat. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bialosky JE Bishop MD Price DD Robinson ME George SZ. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther. 2009;14(5):531‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bazzichi L Dini M Rossi A, et al. A combination therapy of massage and stretching increases parasympathetic nervous activity and improves joint mobility in patients affected by fibromyalgia. Health (Irvine Calif). 2010;2(8):919‐926. [Google Scholar]
- 30.Field T Hernandez‐Reif M Diego M Schanberg S Kuhn C. Cortisol decreases and serotonin and dopamine increase following massage therapy. Int J Neurosci. 2005;115(10):1397‐1413. [DOI] [PubMed] [Google Scholar]
- 31.Fields HL Heinricher MM Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219‐245. [DOI] [PubMed] [Google Scholar]
- 32.Chan Y‐C Wang T‐J Chang C‐C, et al. Short‐term effects of self‐massage combined with home exercise on pain, daily activity, and autonomic function in patients with myofascial pain dysfunction syndrome. J Phys Ther Sci. 2015;27(1):217‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim K Park S Goo B‐O Choi S‐C. Effect of self‐myofascial release on reduction of physical stress: a pilot study. J Phys Ther Sci. 2014;26(11):1779‐1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frost DM Beach TAC Callaghan JP McGill SM. Exercise‐based performance enhancement and injury prevention for firefighters: contrasting the fitness‐ and movement‐related adaptations to two training methodologies. J Strength Cond Res. 2015;29(9):2441‐2459. [DOI] [PubMed] [Google Scholar]