Abstract
IMPORTANCE
Many premature infants are born without exposure to antenatal steroids (ANS) or with incomplete courses. This study evaluates the dose-dependent effect of ANS on rates of neonatal morbidities and early childhood neurodevelopmental outcomes of extremely premature infants.
OBJECTIVE
To compare rates of neonatal morbidities and 18- to 22-month neurodevelopmental outcomes of extremely premature infants exposed to no ANS or partial or complete courses of ANS.
DESIGN, SETTING, AND PARTICIPANTS
In this observational cohort study, participants were extremely premature infants (birth weight range, 401–1000 g; gestational age, 22–27 weeks) who were born at participating centers of the National Institute of Child Health and Human Development Neonatal Research Network between January 2006 and December 2011. Data were analyzed between October 2013 and May 2016.
MAIN OUTCOMES AND MEASURES
Rates of death or neurodevelopmental impairment at 18 to 22 months’ corrected age. Neurodevelopmental impairment was defined as the presence of any of the following: moderate to severe cerebral palsy, a cognitive score less than 85 on the Bayley Scales of Infant and Toddler Development III, blindness, or deafness.
RESULTS
There were 848 infants in the no ANS group, 1581 in the partial ANS group, and 3692 in the complete ANS group; the mean (SD) birth weights were 725 (169), 760 (173), and 753 (170) g, respectively, and the mean (SD) gestational ages were 24.5 (1.4), 24.9 (2), and 25.1 (1.1) weeks. Of 6121 eligible infants, 4284 (70.0%) survived to 18- to 22-month follow-up, and data were available for 3892 of 4284 infants (90.8%). Among the no, partial, and complete ANS groups, there were significant differences in the rates of mortality (43.1%, 29.6%, and 25.2%, respectively), severe intracranial hemorrhage among survivors (23.3%, 19.1%, and 11.7%), death or necrotizing enterocolitis (48.1%, 37.1%, and 32.5%), and death or bronchopulmonary dysplasia (74.9%, 68.9%, and 65.5%). Additionally, death or neurodevelopmental impairment occurred in 68.1%, 54.4%, and 48.1% of patients in the no, partial, and complete ANS groups, respectively. Logistic regression analysis revealed that complete (odds ratio, 0.63; 95% CI, 0.53–0.76) and partial (odds ratio, 0.77; 95% CI, 0.63–0.95) ANS courses were associated with lower rates of death or neurodevelopmental impairment compared with the no ANS group. The reduction in the rate of death or neurodevelopmental impairment associated with exposure to a complete ANS course may be mediated through a reduction in rates of severe intracranial hemorrhage and/or cystic periventricular leukomalacia in the neonatal period.
CONCLUSIONS AND RELEVANCE
Antenatal steroid exposure was associated with a dose-dependent protective effect against death or neurodevelopmental impairment in extremely preterm infants. The effect was partly mediated by ANS-associated reductions in rates of severe intracranial hemorrhage and/or cystic periventricular leukomalacia. These results support prompt administration of ANS, with the goal of a complete course prior to delivery.
Antenatal steroids (ANS) have been associated with reductions in rates of mortality, respiratory distress syndrome, necrotizing enterocolitis (NEC), and intracranial hemorrhage (ICH) in premature neonates.1–3 A complete course of ANS is defined as 2 intramuscular doses of betamethasone administered 24 hours apart or 4 doses of dexamethasone administered 12 hours apart. Many premature neonates are born prior to the administration of a complete course of ANS because of reduced time to delivery or maternal or fetal indications for expedited delivery.4
Data are limited on the comparative effects of no ANS and partial or complete courses of ANS on neonatal and neurodevelopmental outcomes of extremely premature infants. Previous studies evaluating the role of ANS on neurodevelopmental outcomes have grouped patients who received a partial course of ANS either with those who received no ANS or a complete course of ANS.5–6
A recent study by Carlo et al7 used the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) database to evaluate the association of ANS exposure and neurodevelopment for 10 541 extremely premature infants (gestational age [GA] of 22 to 25 weeks) aged 18 to 22 months. A subgroup analysis noted the benefit of a partial course of ANS on death or neurodevelopmental impairment (NDI) compared with no ANS in a univariate model.
The current study was designed to determine if ANS exposure is associated with a dose-dependent improvement in neurodevelopmental outcomes in premature infants aged 18 to 22 months and to determine whether this effect on neurodevelopmental outcome is mediated by a reduction in severe ICH or cystic periventricular leukomalacia (cPVL) using a large cohort of extremely premature infants. Our primary objective was to compare the rates of death or NDI among extremely premature infants exposed to no ANS, a partial course of ANS, or a complete course of ANS. Neurodevelopmental impairment was defined as any of the following: moderate to severe cerebral palsy (CP) based on the Gross Motor Classification System (GMFCS; ie, levels 3–5),8,9 a cognitive score less than 85 on the Bayley Scales of Infant and Toddler Development (BSID-III; mean [SD], 100 [15]), blindness, or deafness. Secondarily, we also sought to compare the rates of intact survival, defined as the absence of any CP, deafness, or blindness and a BSID-III cognitive score of 85 or higher at 18 to 22 months; to compare the rates of neonatal morbidities, including severe ICH, cPVL, bronchopulmonary dysplasia (BPD), and NEC among extremely premature infants exposed to no, partial, or complete courses of ANS; and to determine whether the beneficial effect of a complete course of ANS on neurodevelopment is mediated by the effect on severe ICH and/or cPVL.
Methods
Study Design
This was a secondary analysis of prospectively collected data from the NRN generic database registry and follow-up registry of extremely preterm infants born from January 2006 to December 2011 at participating NICHD NRN sites (16 centers from 2006 to 2010 and 18 throughout 2011). In 3 centers, written or oral informed parental consent was obtained for the hospital registry according to local institutional review board requirements, while waivers of consent were approved for other centers by their institutional review boards. Written informed consent was obtained from all sites for the follow-up registry.
Study Population
All neonates born with a birth weight of 401 to 1000 g and/or a GA of 22 to 27 weeks as determined by early ultrasonography or last menstrual period were included. Infants who died within 12 hours of birth without aggressive neonatal care were excluded. Patients were classified into 3 groups; the no ANS group received no ANS, the partial ANS group received 1 dose of betamethasone or less than 4 doses of dexamethasone, and the complete ANS group received 2 doses of betamethasone or 4 doses of dexamethasone.
Maternal data including race/ethnicity, marital status, history of hypertension, acute chorioamnionitis (clinical diagnosis and/or placental pathology), delivery mode, multiple births, maternal education, and ANS administration (number of doses and betamethasone vs dexamethasone) were collected after birth. Neonatal data were collected until death or discharge or for 120 days and included birth weight, GA, sex, Apgar scores, details of resuscitation in the delivery room, severe ICH (grade 3 or 4),10 cPVL, BPD (defined as a need for supplemental oxygen at 36 weeks’ postmenstrual age), NEC (based on modified Bell staging criteria11), and mortality.
Eligibility criteria for follow-up were revised by NICHD NRN during the study period. Prior to January 2008, all extremely low-birth-weight infants (birth weight of 401 to 1000 g) were observed; after January 1, 2008, only inborn infants with GAs less than 27 weeks underwent follow-up. Results of audiologic and visual assessments were gathered from assessments performed after discharge. Blindness was defined as no useful vision in either eye (ie, bilateral visual acuity of 20/200 or less). Deafness was defined as hearing impairment despite amplification or cochlear implants. Cerebral palsy was classified into mild (GMFCS possible level 1 or level 1), moderate (GMFCS level 2 or 3), and severe (GMFCS level 4 or 5) categories. The child’s weight, length, and head circumference were plotted on age-and sex-specific growth charts.12 Developmental evaluation was performed using the BSID-III13 by trained examiners blinded to ANS exposure. The maternal medical insurance status (ie, used Medicaid or private insurance) was taken as a surrogate for the socioeconomic status of the family.
The primary outcome was death or NDI at 18 to 22 months’ corrected age. Secondary outcomes were intact survival (defined as an infant who was normal and had no evidence of CP, a BSID-III cognitive score of 85 or greater, and no neurosensory deficit at 18 to 22 months) and neonatal morbidities, including an ICH grade 3 or 4, cPVL, seizure disorder needing anticonvulsant medications, NEC, and BPD. The composite outcome of mortality and each of these selected morbidities was also assessed.
Statistical Analysis
Continuous variables were described using means and SDs or medians and interquartile ranges and categorical variables using frequency and percentage. Outcome variables were compared among study groups using χ2 test for categorical variables, analysis of variables for continuous normally distributed variables, and Kruskal-Wallis test for continuous skewed variables. Logistic regression analysis was performed to assess the association between ANS and outcomes, after controlling for GA, sex, race/ethnicity, maternal health insurance, and participating center. Statistical significance was set at P < .05.
For the third objective, 4 logistic regression models were developed to examine whether the effect of ANS was mediated by severe ICH or cPVL for the primary outcome. Each of the models were adjusted for GA, center, race/ethnicity, sex, and mother’s medical insurance. Antenatal steroid exposure was considered as a 3-level categorical variable (no, partial, and complete ANS).14 The first model examined the association of ANS with the outcome of death or NDI without the mediator (severe ICH or cystic PVL). The second model examined the association of ANS with the mediator. The third model examined the association of ANS with death or NDI in the presence of the mediator and the interaction between ANS and the mediator. The interaction was not significant, so it was excluded from the final model. The final model examined the effect of ANS on the primary outcome in the presence of the mediator. To evaluate the mediation effect of severe ICH or cystic PVL with a complete course of ANS, 95% CIs were estimated using the 6 method.15
Results
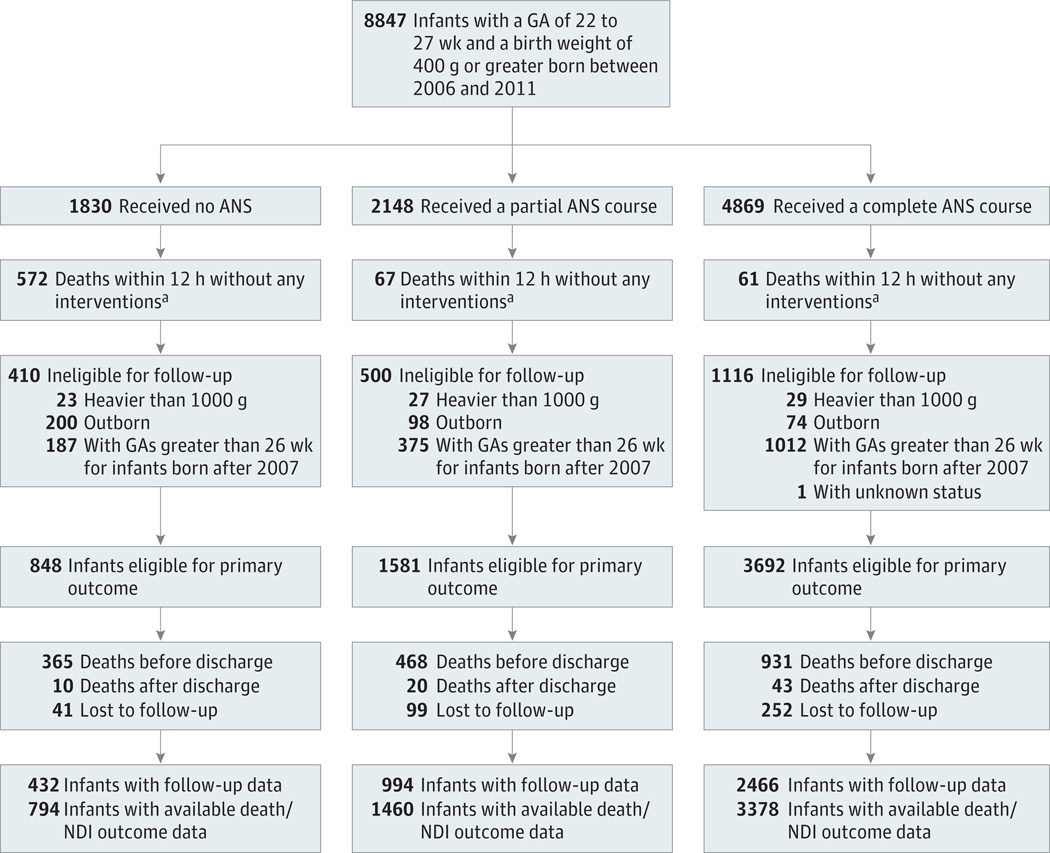
Between 2006 and 2011, 8847 infants with a GA of 27 weeks or less were born at NRN centers. Of these, 700 infants died within 12 hours without receiving aggressive neonatal care. A total of 2026 infants were not eligible for follow-up; 79 had weights greater than 1000 g, 1574 had GAs more than 26 weeks for infants born after 2007, 372 were outborn, and 1 had an unknown status. Follow-up data were available for 3892 of 4284 eligible extremely low GA neonates (90.8%) (Figure).
Figure.
Flowchart of Participants From Birth to 18- to 22-Month Follow-up
ANS indicates antenatal steroids; GA, gestational age; NDI, neurodevelopmental impairment.
a Interventions refer to chest compression, intubation, epinephrine, antibiotics, surfactant use, pressor, and volume support.
The 392 patients lost to follow-up were similar to the 3892 who underwent follow-up assessments concerning GA, birth weight, sex, and medical insurance (data not shown).
Maternal and infant characteristics for the no, partial, and complete ANS groups are described in Table 1. The complete ANS group had a higher mean GA compared with the other groups and a higher mean birth weight compared with the no ANS group. Additionally, mothers of the complete ANS group were more likely to be married and to have a high school education and less likely to be on public insurance. There were more singleton and African American infants in the no ANS group compared with the other groups (P < .001). Rates of clinical and histological chorioamnionitis were higher in the complete ANS group. More infants needed resuscitation in the delivery room and had Apgar scores less than 5 at 5 minutes in the no ANS group compared with the other groups. As there were only 142 women who received dexamethasone instead of betamethasone, the type of ANS exposure was not considered in further analysis.
Table 1.
Maternal and Neonatal Characteristics by Exposure to Antenatal Steroids
| Variable | No./Total No. (%) | P Value | ||
|---|---|---|---|---|
| No ANS Group (n = 848) |
Partial ANS Group (n = 1581) |
Complete ANS Group (n = 3692) |
||
| Maternal variable | ||||
| Race/ethnicity | ||||
| White | 394/833 (47.3) | 855/1554(55.0) | 1987/3630(54.7) | <.001 |
| African American | 399/833 (47.9) | 612/1554(39.4) | 1445/3630 (39.8) | <.001 |
| Other | 40/833 (4.8) | 87/1554(5.6) | 198/3630(5.5) | .69 |
| Married | 290/828(35.0) | 665/1563 (42.5) | 1742/3657 (47.6) | <.001 |
| Hypertensive disorder of pregnancy |
146/847 (17.2) | 329/1574(20.9) | 814/3689(22.1) | .008 |
| Chorioamnionitis | ||||
| Clinical | 107/843 (12.7) | 245/1573 (15.6) | 845/3685 (22.9) | <.001 |
| Histologic | 296/625 (47.4) | 667/1293 (51.6) | 1730/3046(56.8) | <.001 |
| Rupture of membrane >18 h | 101/807(12.5) | 257/1555 (16.5) | 1281/3662 (35.0) | <.001 |
| Cesarean delivery | 440/848 (51.9) | 967/1581 (61.2) | 2393/3692 (64.8) | <.001 |
| Education (≥high school) | 337/570(59.1) | 838/1163 (72.1) | 2055/2625 (78.3) | <.001 |
| Medicaid insurance | 495/847 (58.4) | 791/1580 (50.1) | 1799/3688 (48.8) | <.001 |
| Infant variable | ||||
| Birth weight, mean (SD), g | 725 (169) | 760(173) | 753 (170) | <.001 |
| Gestational age, mean (SD), wk | 24.5 (1.4) | 24.9 (2) | 25.1 (1.1) | <.001 |
| Male sex | 439/848 (51.8) | 813/1581 (51.4) | 1936/3692 (52.4) | .78 |
| Apgar score <5 at 5 min | 303/836(36.2) | 421/1577 (26.7) | 688/3686(18.7) | <.001 |
| Resuscitation in delivery room (intubation, chest compression, or epinephrine) |
763/847 (90.1) | 1341/1580 (84.9) | 2908/3691 (78.8) | <.001 |
| Singleton | 680/848 (80.2) | 1236/1581 (78.2) | 2702/3692 (73.2) | <.001 |
| SGA | 39/848 (4.6) | 95/1581 (6.0) | 282/3692 (7.6) | .002 |
Abbreviations: ANS, antenatal steroids; SGA, small for gestational age.
Neonatal morbidities and mortality among the 3 groups of infants are compared in Table 2. Infants who received a complete course of ANS had the lowest rates of death, death or severe ICH or cPVL, severe ICH and cPVL among survivors, death or BPD, and death or NEC.
Table 2.
Neonatal Morbidity of Infants by Exposure to Antenatal Steroids
| Variable | No/Total No. (%) |
Adjusted Odds Ratio (95% Cl)a or Estimate (95% Cl)a |
||||
|---|---|---|---|---|---|---|
| NoANS Group (n = 848) |
Partial ANS Group (n = 1581) |
Complete ANS Group (n = 3692) |
Partial vs No ANS | Complete vs No ANS | Complete vs Partial ANS | |
| Deathb | 365/847 (43.1) | 468/1580(29.6) | 931/3688(25.2) | 0.74(0.61 to 0.90) | 0.64 (0.54 to 0.77) | 0.87(0.75 to 1.00) |
| IVH grade 3 or 4b,c |
112/481 (23.3) | 212/1110(19.1) | 322/2748(11.7) | 0.82 (0.62 to 1.08) | 0.46(0.35 to 0.59) | 0.56 (0.46 to 0.68) |
| Cystic PVLb,c | 39/481 (8.1) | 86/1112 (7.7) | 114/2755 (4.1) | 1.04 (0.69 to 1.58) | 0.53 (0.36 to 0.80) | 0.51 (0.38 to 0.69) |
| IVH grade 3 or 4 or cystic PVLb,c |
121/481 (25.2) | 241/1110(21.7) | 383/2748(13.9) | 0.86 (0.66 to 1.13) | 0.50 (0.39 to 0.64) | 0.58 (0.48 to 0.70) |
| Death, IVH grade 3 or 4, or cystic PVLb |
487/847 (57.5) | 709/1578 (44.9) | 1314/3679(35.7) | 0.76(0.63 to 0.91) | 0.54(0.45 to 0.64) | 0.71 (0.62 to 0.81) |
| BPDb,c | 267/479 (55.7) | 612/1100(55.6) | 1471/2740 (53.7) | 1.02 (0.79 to 1.31) | 0.93 (0.74 to 1.17) | 0.92 (0.78 to 1.07) |
| BPD or deathb | 633/845 (74.9) | 1080/1568(68.9) | 2404/3673 (65.5) | 0.90(0.72 to 1.11) | 0.79(0.65 to 0.96) | 0.88 (0.76 to 1.02) |
| NECb,c | 42/482 (8.7) | 118/1111 (10.6) | 266/2757(9.6) | 1.52 (1.03 to 2.24) | 1.40 (0.97 to 2.0) | 0.92 (0.72 to 1.16) |
| NEC or deathb | 407/847 (48.1) | 586/1579(37.1) | 1197/3688(32.5) | 0.86(0.71 to 1.04) | 0.75 (0.63 to 0.89) | 0.87 (0.76 to 0.99) |
| Length of stay, mean(SD), dd |
116.5 (48.1) | 111.7(43.5) | 113.0(48.0) | 0.13 (−4.71 to 4.97) | 1.70 (−2.73 to 6.14) | 1.58 (−1.51 to 4.67) |
| Days on oxygen, mean(SD)d |
73.6(35.3) | 70.3 (36.2) | 68.7(37.2) | 0.55 (−2.78 to 3.88) | −0.30 (−3.35 to 2.75) | −0.86 (−2.98 to 1.27) |
| Days on ventilator, mean(SD)d |
31.8(26.9) | 28.5 (26.1) | 27.2 (25.8) | −0.44 (−2.87 to 1.99) | −1.61 (−3.84 to 0.61) | −1.18 (−2.73 to 0.38) |
Abbreviations: ANS, antenatal steroids; ESPD, bronchopulmonary dysplasia; IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis; PVL, periventricular leukomalacia.
Adjusted for gestational age, sex, race/ethnicity, maternal health insurance (Medicaid vs private insurance), and participating center.
Adjusted odds ratio (95% CI).
Among survivors.
Adjusted estimate (95% CI).
The primary outcome of death or NDI was observed in 68.1%, 54.4%, and 48.1% patients in the no, partial, and complete ANS groups, respectively (P < .001) (Table 3). Death and NDI were both separately higher in the no ANS group compared with the other groups. Intact survival was highest in the complete ANS group and lowest in the no ANS group. Weight and head circumference at follow-up were comparable in the 3 groups, but the proportion of infants with head circumference lower than the 10th percentile was significantly lower in the complete ANS group compared with the other groups.
Table 3.
18- to 22-Month Follow-up Among Infants by Exposure to Antenatal Steroids
| Variable | No./TotalNo. (%) |
Adjusted Odds Ratio (95 %CI)a or Estimate (95% CI)a |
||||
|---|---|---|---|---|---|---|
| No ANS Group (n = 794) |
Partial ANS Group (n = 1460) |
Complete ANS Group (n = 3378) |
Partial vs No ANS | Complete vs No ANS | Complete vs Partial ANS | |
| Death or NDIb | 541/794(68.1) | 794/1460 (54.4) | 1624/3378 (48.1) | 0.77(0.63 to 0.95) | 0.63 (0.53 to 0.76) | 0.82 (0.71 to 0.93) |
| Deathb | 375/794(47.2) | 488/1460 (33.4) | 974/3378 (28.8) | 0.76(0.62 to 0.93) | 0.66(0.55 to 0.79) | 0.87(0.75 to 1.01) |
| NDIb | 166/419(39.6) | 306/972 (31.5) | 651/2405 (27.1) | 0.87 (0.67 to 1.13) | 0.70(0.55 to 0.89) | 0.80 (0.68 to 0.95) |
| Intact survivalb | 233/419(55.6) | 635/972 (65.3) | 1670/2405 (69.4) | 1.25 (0.97 to 1.61) | 1.51 (1.20 to 1.90) | 1.21 (1.02 to 1.43) |
| Cognitive scoreb | ||||||
| <70 | 53/419(12.6) | 109/967(11.3) | 192/2396(8.01) | 1.04(0.72 to 1.52) | 0.72 (0.51 to 1.01) | 0.60(0.53 to 0.89) |
| 70–84 | 109/419(26.0) | 183/967(18.9) | 423/2396(17.7) | 0.82 (0.61 to 1.09) | 0.76 (0.59 to 0.99) | 0.93 (0.76 to 1.14) |
| <85 | 162/419(38.7) | 292/967(30.2) | 615/2396(25.7) | 0.86 (0.66 to 1.12) | 0.69 (0.54 to 0.87) | 0.80 (0.67 to 0.95) |
| Any CPb | 73/418(17.5) | 128/972 (13.2) | 252/2404(10.5) | 0.67 (0.48 to 0.94) | 0.51 (0.38 to 0.70) | 0.76 (0.60 to 0.97) |
| Moderate or severe CPb |
26/418(6.22) | 76/972 (7.82) | 126/2404(5.24) | 1.35 (0.83 to 2.20) | 0.87(0.55 to 1.37) | 0.64 (0.47 to 0.87) |
| Seizures after hospital dischargeb |
25/418 (6) | 34/970 (3.5) | 89/2404(3.7) | 0.57(0.33 to 1.0) | 0.66(0.41 to 1.08) | 1.16 (0.77 to 1.76) |
| Deafnessc | 10/418(2.39) | 14/972 (1.44) | 31/2405 (1.29) | 0.60 (0.26 to 1.41) | 0.58 (0.28 to 1.23) | 0.96 (0.50 to 1.85) |
| Blindnessc | 1/417 (0.24) | 2/971 (0.21) | 12/2402 (0.50) | 1.04 (0.09 to 11.70) | 2.73 (0.34 to 21.76) | 2.62 (0.58 to 11.80) |
| Weight at follow-up, mean (SD), kgd,e |
10.76(1.57) | 10.81 (1.60) | 10.80(1.57) | 0.03 (−0.16 to 0.21) | 0.02 (−0.15 to 0.19) | −0.01 (−0.12 to 0.11) |
| Weight <10th percentileb |
75/416(18.0) | 184/969 (19.0) | 455/2402 (18.9) | 1.05 (0.77 to 1.43) | 1.08(0.81 to 1.44) | 1.03 (0.85 to 1.25) |
| Head circumference at follow-up, mean (SD), cmd,f |
46.8(3.2) | 46.7 (2.2) | 46.9(2.0) | −0.20 (−0.45 to 0.05) | −0.05 (−0.28 to 0.18) | 0.15 (−0.01 to 0.31) |
| Head circumference <10th percentileb |
90/416(21.6) | 221/969(22.8) | 417/2387(17.5) | 1.23 (0.91 to 1.65) | 0.87 (0.66 to 1.14) | 0.71 (0.58 to 0.86) |
| Corrected age at follow-up, mean (SD), mod,g |
20.5 (3.1) | 20.5 (3.0) | 20.3 (2.5) | −0.05 (0.39 to 0.29) | −0.30 (−0.62 to 0.01) | −0.25 (−0.47 to-0.04) |
Abbreviations: ANS, antenatal steroids; CP, cerebral palsy; NDI, neurodevelopmental impairment.
Adjusted for gestational age, sex, race/ethnicity, maternal health insurance (Medicaid vs private insurance), and participating center.
adjusted odds ratio (95% CI).
Participating center was not adjusted for because of low prevalence.
Adjusted estimate (95% CI).
Included 416,969, and 2402 infants in the no, partial, and complete ANS groups, respectively.
Included 413,957, and 2345 infants in the no, partial, and complete ANS groups, respectively.
Included 419,969, and 2399 infants in the no, partial, and complete ANS groups, respectively.
Logistic regression analysis revealed that there was a decreased risk of death or NDI at 18 to 22 months for the complete ANS group compared with the no ANS group (OR, 0.63; 95% CI, 0.53–0.76) and the partial ANS group (OR, 0.77; 95% CI, 0.63–0.95) as well as for female vs male sex (odds ratio [OR], 0.60; 95% CI, 0.53–0.67), private insurance vs Medicaid (OR, 0.74; 95% CI, 0.65–0.85), and every additional week of gestation (OR, 0.59; 95% CI, 0.56–0.62).
The mediation analysis for death or NDI is shown in Table 4. The protective effect of complete ANS on death or NDI seems to be partly mediated through a reduction in severe ICH and/or cPVL, as the parameter estimate of the effect of complete ANS vs no ANS in the absence of the mediator was larger than in the presence of the mediator. We estimated the most conservative (widest) confidence interval for the mediation effect of severe ICH or cPVL on complete ANS given by the product of the estimates a2 and b (Table 4), using the 6 method because we had a large enough sample size.16 We assumed a correlation of −1 between the estimates of a2 and b to get the widest possible confidence interval.15 Because the 95% CI of the mediation effect of the product of a2 and b (−1.197 to −0.509) did not include 0, it indicated that the primary outcome was mediated by severe ICH or cPVL for the complete ANS group, and it was a partial mediation (Table 4).
Table 4.
Mediation Analysis for Death or Neurodevelopmental Impairment With Antenatal Steroids Exposure With Severe Intracranial Hemorrhage and Cystic Periventricular Leukomalacia As Mediatorsa
| Effect | Estimateb | SE | P Value |
|---|---|---|---|
| Association of ANS with death or NDI without the presence of severe ICH or cPVL | |||
| ANS | |||
| Partial | c1 = −0.2550 | 0.1034 | .01 |
| Complete | c2 = −0.4574 | 0.0946 | <.001 |
| Association of ANS with severe ICH or cPVL | |||
| ANS | |||
| Partial | a1 = −0.2111 | 0.1108 | .06 |
| Complete | a2 = −0.7582 | 0.1032 | <.001 |
| Association of ANS with death or NDI after controlling for severe ICH or cPVLc | |||
| Severe ICH/cPVL | b = 1.1251 | 0.0784 | <.001 |
| ANS | |||
| Partial | c’1 = −0.1332 | 0.1113 | .23 |
| Complete | c’2 = −0.2260 | 0.1020 | .03 |
Abbreviations: ANS, antenatal steroids; cPVL, cystic periventricular leukomalacia; ICH, intracranial hemorrhage; NDI, neurodevelopmental impairment.
All models were adjusted for variables (gestational age, center, race/ethnicity, sex, and mother’s medical insurance).
c1 and c2 indicate the parameter estimates of the effect of a partial and complete ANS course, respectively, on the occurrence of death or NDI in the absence of severe ICH or cPVL. a1 and a2 indicate the parameter estimates of the effect of a partial and complete ANS course, respectively, on the occurrence of severe ICH or cPVL. b indicates the parameter estimate of the effect of ICH or cPVL on the occurrence of death or NDI. c'1 and c'2 indicate the parameter estimates of the effect of a partial and complete ANS course, respectively, on death or NDI in the presence of severe ICH or cPVL.
The interaction between severe ICH or cPVL and ANS was not significant, so it was not included in the final model.
The outcomes of neonates born at 22 and 23 weeks’ GA are presented in eTable 1 and eTable 2 in the Supplement. There were 77 patients in the 22 weeks’ GA group, limiting the interpretation of data. For neonates born at 23 weeks’ GA, complete ANS was associated with a significant improvement in rates of severe ICH, death or severe ICH, BPD or death, NEC or death, NDI or death, and death compared with the no ANS group.
Discussion
In this large multicenter cohort study of extremely preterm infants born at participating NRN centers, we noted that no, partial, and complete ANS courses were associated with differential benefits on survival and neonatal morbidities as well as neurodevelopmental outcomes at 18 to 22 months. Mediation analysis supports that the improved neurodevelopmental outcomes at 18 to 22 months following a complete course of ANS is in part mediated by a reduction in severe ICH and/or cPVL. We also noted the dose-dependent beneficial effects of ANS on neonatal morbidities as well as neurodevelopmental outcomes for neonates born at 23 weeks’ GA.
Studies evaluating the long-term neurodevelopmental effects of ANS in premature infants have reported conflicting results; these differences could be because of variability in the combination of partial ANS courses with either complete ANS courses or no ANS courses. Three randomized clinical trials2,17,18 have reported the comparative effects of a partial ANS course vs no steroid exposure on neonatal outcomes. There was no decrease in the incidence of respiratory distress syndrome among infants exposed to a partial course of ANS (24.1%) compared with infants born without ANS (31.8%).2 Kari et al17 noted a lower incidence of ICH or PVL in the partial course group compared with the placebo group (OR, 0.07; 95% CI, 0.01–0.65) but did not find a reduction in chronic lung disease or death (OR, 0.77; 95% CI, 0.08–7.47). A recent meta-analysis19 reported that a single course of ANS was associated with reduced risk for CP (relative risk, 0.68; 95% CI, 0.56–0.82), psychomotor development index score less than 70 (relative risk, 0.83; 95% CI, 0.74–0.93), and severe disability (relative risk, 0.79; 95% CI, 0.73–0.85), while rates of intact survival were higher (relative risk, 1.19; 95% CI, 1.06–1.33). The meta-analysis included only 2 studies that discriminated between partial and complete ANS. However, the previous Cochrane meta-analysis by Roberts et al3 had shown no benefit of ANS on childhood outcomes. A single-center study20 noted that a complete course of ANS was associated with an increased likelihood of intact survival (31%) at a corrected age of 18 to 22 months among extremely low-birth-weight infants compared with a partial course (5%). The NICHD NRN study by Carlo et al7 noted the benefits of a partial course of ANS on death or NDI (68%) compared with no ANS (81%) in a univariate model.
Another large multicenter study21 involving 2549 children born premature (GA less than 29 weeks) noted that any ANS exposure was associated with a reduction in moderate CP compared with no ANS exposure (OR, 0.39; 95% CI, 0.18–0.84). However, other neurodevelopmental outcomes for children aged 2 to 3 years were not significantly different. Follow-up data were included for 647 infants at less than 26 weeks’ GA. The no ANS group included only 150 infants.
To our knowledge, the current study differs from other follow-up studies with respect to details on dose of ANS exposure and comparison of outcomes in relation to no, partial, and complete ANS courses. We also evaluated the mediating effects of reductions in severe ICH or cPVL with ANS exposure on neurodevelopmental outcome. There were significantly more African American infants in the no ANS group in our study. Whether this relates to the racial differences noted in incidence of ICH remains to be determined.22
In the current study, the rate of microcephaly was significantly lower at 18 to 22 months’ corrected age in infants who were exposed to a complete course of ANS. The mechanisms of the beneficial effect of ANS on neurodevelopmental outcomes are not clearly known. Unpreparedness of the immature vascular wall to handle the fluctuation in the cerebral blood flow associated with an increased blood pressure post partum, hypercapnia, or fluctuations in the partial pressure of carbon dioxide render the preterm brain susceptible to hemorrhage.23,24 Exposure to ANS has been shown to reduce the cerebral blood flow by increasing cerebrovascular resistance in preclinical studies.25 Other possible mechanisms include either a direct effect on the maturation of brain tissue and/or a reduction of neonatal morbidities that are associated with poor 18- to 22-month neurodevelopmental outcomes (such as severe ICH or ventilator need).4 In a preclinical model, Stonestreet et al26 noted that a course of ANS similar to that given to women in preterm labor reduced the blood-brain barrier permeability in the ovine fetus. Maturation of the blood-brain barrier may be partly responsible for the central nervous system effects observed in preterm neonates who are administered corticosteroids. Exposure to a complete course of ANS could also be a marker of adequate time for obstetric management prior to delivery.
There were limitations to our study. There was an unequal distribution of patients in the 3 groups because of the observational design. The observational design of the study suggests associations only with differential exposure to ANS and does not demonstrate a cause-and-effect relationship. In addition, infants born prior to receipt of a complete course of ANS might have different baseline characteristics or indications for preterm delivery, which could potentially be responsible for the differences in their outcomes. However, we controlled for many known potential factors that influence discharge and early childhood outcomes. We also included all eligible extremely preterm neonates born with a GA less than 27 weeks (with birth weights of at least 400 g) delivered at participating NRN centers in a defined time frame. The effect of the duration between administration of the first dose of ANS and delivery also could not be assessed, as these data were not available. The indication for the use of magnesium sulfate was not collected during the study period.
There were also strengths to our study. We analyzed a recent cohort of infants, used prespecified definitions for outcomes, and used standardized neurological assessments and psychometric evaluations by trained examiners. The study participants were from multiple centers across the United States with different race/ethnicity and socioeconomic strata, which makes the study more generalizable. This study supports the administration of ANS even in situations when the likelihood of a complete course of ANS is low owing to insufficient time because of the benefit of even an incomplete course on outcome.
As the neonatal and early childhood neurodevelopmental outcomes are different in infants exposed to no, partial, and complete courses of ANS, this may have implications for the design of trials to evaluate neurodevelopmental outcomes. Future trials should take into account the differential associations of no, partial, and complete courses of ANS with death or NDI. Whether an accelerated course would improve neonatal outcomes when time does not permit completion of a conventional course needs to be studied. In addition, outcome estimators currently in use may be improved by using ANS exposure trichotomized as no, partial, or complete steroid administration.
Conclusions
In this large multicenter prospective data registry, we noted that the protective effect associated with ANS on early childhood neurodevelopmental outcome was dose-dependent. Improved survival without NDI was associated with reduced ICH or cPVL in the ANS groups, as noted by mediation analysis. These results may aid in better counseling for parents of extremely preterm infants shortly after delivery. Finally, and most importantly, these results support prompt administration of ANS, with the goal of a complete course prior to extremely preterm birth.
Supplementary Material
Key Points.
Question
What are the neonatal and early childhood outcomes associated with no, partial, and complete courses of antenatal steroids to preterm infants?
Finding
In this observational cohort study, antenatal steroid administration was associated with a protective effect on death or neurodevelopmental impairment. The beneficial effect of antenatal steroids was dose-dependent, with maximal benefit associated with a complete course of antenatal steroids.
Meaning
Antenatal steroids should be administered promptly, even in situations when the likelihood of a complete course of antenatal steroids is low because of insufficient time, as outcomes of premature infants born after an incomplete course of antenatal steroids are better than outcomes of infants born without exposure to any antenatal steroids.
Acknowledgments
Funding/Support: This work was conducted for and supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Center for Research Resources, and the National Center for Advancing Translational Sciences provided grant support for the 17 generic database and follow-up studies of the Neonatal Research Network through cooperative agreements.
Role of the Funder/Sponsor: The Eunice Kennedy Shriver National Institute of Child Health and Human Development program scientist had input into the design and conduct of the study; collection, analysis, and interpretation of the data; preparation, review and approval of the manuscript; and decision to submit the manuscript for publication. Data collected at participating sites of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network were transmitted to RTI International, the data coordinating center for the network, which stored, managed, and analyzed the data for this study.
Group Information
In addition to those listed as authors, the Telemedicine Approaches to Evaluating Acute-Phase Retinopathy of Prematurity study investigators included the following: Neonatal Research Network Steering Committee Chairs: Alan H. Jobe, MD, PhD, University of Cincinnati (2003–2006); Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006–2011); and Richard A. Polin, MD, Division of Neonatology, College of Physicians and Surgeons, Columbia University, (2011-present). Alpert Medical School of Brown University and Women and Infants Hospital of Rhode Island (grant U10 HD27904): Martin Keszler, MD; William Oh, MD; Betty R. Vohr, MD; Angelita M. Hensman, MS, RNC-NIC; Kristin M. Basso, BSN, MaT; Barbara Alksninis, PNP; Robert Burke, MD; Melinda Caskey, MD; Andrea Halbrook; Katharine Johnson, MD; Mary Lenore Keszler, MD; Theresa M. Leach, MEd, CAES; Bonnie E. Stephens, MD; Suzy Ventura; and Victoria E. Watson, MS, CAS. Case Western Reserve University, Rainbow Babies and Children’s Hospital (grants U10 HD21364 and M01 RR80): Michele C. Walsh, MD, MS; Avroy A. Fanaroff, MD; Anna Marie Hibbs, MD; Deanne E. Wilson-Costello, MD; Nancy S. Newman, BA, RN; Bonnie S. Siner, RN; Monika Bhola, MD; Gulgun Yalcinkaya, MD; and Harriet G. Friedman, MA. Children’s Mercy Hospital, University of Missouri Kansas City School of Medicine (grant U10 HD68284): William E. Truog, MD; Eugenia K. Pallotto, MD, MSCE; Howard W. Kilbride, MD; Cheri Gauldin, RN, MSN, CCRC; Anne Holmes, RN, MSN, MBA-HCM, CCRC; and Kathy Johnson, RN, CCRC. Cincinnati Children’s Hospital Medical Center, University Hospital, and Good Samaritan Hospital (grants U10 HD27853 and M01 RR8084): Kurt Schibler, MD; Edward F. Donovan, MD; Cathy Grisby, BSN, CCRC; Barbara Alexander, RN; Kate Bridges, MD; Estelle E. Fischer, MHSA, MBA; Teresa L. Gratton, PA; Holly L. Mincey, RN, BSN; Greg Muthig, BS; Jody Hessling, RN; Teresa L. Gratton, PA; Lenora D. Jackson, CRC; Kristin Kirker, CRC; and Kimberly Yolton, PhD. Duke University School of Medicine, University Hospital, Duke Regional Hospital, and the University of North Carolina (grants U10 HD40492 and M01 RR30): Ronald N. Goldberg, MD; C. Michael Cotten, MD, MHS; Ricki F. Goldstein, MD; Joanne Finkle, RN, JD; Patricia L. Ashley, MD, PhD; William F. Malcolm, MD; Kathy J. Auten, MSHS; Kimberley A. Fisher, PhD, FNP-BC, IBCLC; Katherine A. Foy, RN; Sandra Grimes, RN, BSN; Kathryn E. Gustafson, PhD; Melody B. Lohmeyer, RN, MSN; Matthew M. Laughon, MD, MPH; Carl L. Bose, MD; Janice Bernhardt, MS, RN; Gennie Bose, RN; and Janice K. Wereszczak, CPNP-AC/PC. Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (grants U10 HD27851, M01 RR39, and UL1 TR454): David P. Carlton, MD; Ira Adams-Chapman, MD; Ellen C. Hale, RN, BS, CCRC; Yvonne C. Loggins, RN, BSN; Maureen Mulligan LaRossa, RN; and Sheena L. Carter, PhD. Eunice Kennedy Shriver National Institute of Child Health and Human Development: Stephanie Wilson Archer, MA. Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (grants U10 HD27856, M01 RR750, and UL1 TR6): Gregory M. Sokol, MD; Brenda B. Poindexter, MD, MS; Anna M. Dusick, MD (deceased); Lu-Ann Papile, MD; Dianne E. Herron, RN; Lucy C. Miller, RN, BSN, CCRC; Carolyn Lytle, MD, MPH; Ann B. Cook, MS; Heike M. Minnich, PsyD, HSPP; Abbey C. Hines, PsyD; Leslie Dawn Wilson, BSN, CCRC; and Faithe Hamer, BS. Nationwide Children’s Hospital and the Ohio State University Medical Center (grant U10 HD68278): Pablo J. Sanchez, MD; Leif D. Nelin, MD; Sudarshan R. Jadcherla, MD; Patricia Luzader, RN; Christine A. Fortney, PhD, RN; Gail E. Besner, MD; and Nehal A. Parikh, MD. RTI International (grant U10 HD36790): Dennis Wallace, PhD; W. Kenneth Poole, PhD (deceased); Jamie E. Newman, PhD, MPH; Jeanette O’Donnell Auman, BS; Margaret M. Crawford, BS, CCRP; Marie G. Gantz, PhD; Carolyn M. Petrie Huitema, MS, CCRP; and Kristin M. Zaterka-Baxter, RN, BSN, CCRP. Stanford University, Dominican Hospital, El Camino Hospital, and Lucile Packard Children’s Hospital (grants U10 HD27880, M01 RR70, and UL1 TR93): Krisa P. Van Meurs, MD; David K. Stevenson, MD; Susan R. Hintz, MD, MS Epi; M. Bethany Ball, BS, CCRC; Marian M. Adams, MD; Barbara Bentley, PhD; Elizabeth Bruno, PhD; Maria Elena DeAnda, PhD; Anne M. DeBattista, RN, PNP; Lynne C. Huffman, MD; Jean G. Kohn, MD, MPH; Casey E. Krueger, PhD; Andrew W. Palmquist, RN; Melinda S. Proud, RCP; Brian Tang, MD; and Hali E. Weiss, MD. Tufts Medical Center, Floating Hospital for Children (grants U10 HD53119 and M01 RR54): Ivan D. Frantz III, MD; John M. Fiascone, MD; Elisabeth C. McGowan, MD; Brenda L. MacKinnon, RNC; Ana K. Brussa, MS, OTR/L; Anne Furey, MPH; Ellen Nylen, RN, BSN; and Cecelia E Sibley, PT, MHA. University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (grants U10 HD34216 and M01 RR32): Namasivayam Ambalavanan, MD; Myriam Peralta-Carcelen, MD, MPH; Monica V. Collins, RN, BSN, MaEd; Shirley S. Cosby, RN, BSN; Fred J. Biasini, PhD; Kristy Domnanovich, PhD; Kristen C. Johnston, MSN, CRNP; Carin Kiser, MD; Sara Kryzwanski, MS; Kathleen G. Nelson, MD; Cryshelle S. Patterson, PhD; Vivien A. Phillips, RN, BSN; Richard V. Rector, PhD; Leslie Rodrigues, PhD; Sarah Ryan, PhD; Leigh Ann Smith, CRNP; Amanda D. Soong, MD; and Sally Whitley, MA, OTR-L, FAOTA. University of California–Los Angeles, Mattel Children’s Hospital, Santa Monica Hospital, Los Robles Hospital and Medical Center, and Olive View Medical Center (grant U10 HD68270): Uday Devaskar, MD; Meena Garg, MD; Isabell B. Purdy, PhD, CPNP; Teresa Chanlaw, MPH; and Rachel Geller, RN, BSN. University of California-San Diego Medical Center and Sharp Mary Birch Hospital for Women and Newborns (grant U10 HD40461): Neil N. Finer, MD; Yvonne E. Vaucher, MD, MPH; David Kaegi, MD; Maynard R. Rasmussen, MD; Kathy Arnell, RNC; Clarence Demetrio, RN; Martha G. Fuller, RN, MSN; Chris Henderson, RCP, CRTT; Wade Rich, BSHS, RRT; and Radmila West, PhD. University of Iowa and Mercy Medical Center (grants U10 HD53109 and M01 RR59): Edward F. Bell, MD; Dan L. Ellsbury, MD; John A. Widness, MD; Tarah T. Colaizy, MD, MPH; Michael J. Acarregui, MD; Jane E. Brumbaugh, MD; Karen J. Johnson, RN, BSN; Donia B. Campbell, RNC-NC; Diane L. Eastman, RN, CPNP MA; and Jacky R. Walker, RN. University of Miami, Holtz Children’s Hospital (grants U10 HD21397 and M01 RR16587): Shahnaz Duara, MD; Charles R. Bauer, MD; Ruth Everett-Thomas, RN, MSN; Sylvia Fajardo-Hiriart, MD; Arielle Rigaud, MD; Maria Calejo, MS; Silvia M. Frade Eguaras, MA; Michelle Harwood Berkowits, PhD; Andrea Garcia, MS; Helina Pierre, BA; and Alexandra Stoerger, BA. University of New Mexico Health Sciences Center (grants U10 HD53089, M01 RR997, and UL1 TR41): Kristi L. Watterberg, MD; Robin K. Ohls, MD; Janell F. Fuller, MD; Conra Backstrom Lacy, RN; Sandra Brown, BSN; Andrea Freeman Duncan, MD; Carol Hartenberger, BSN, MPH; Jean R. Lowe, PhD; and Rebecca A. Montman, BSN, RNC. University of Pennsylvania, Hospital of the University of Pennsylvania, Pennsylvania Hospital, and Children’s Hospital of Philadelphia (grant U10 HD68244): Barbara Schmidt, MD, MSc; Haresh Kirpalani, MB, MSc; Sara B. DeMauro, MD, MSCE; Aasma S. Chaudhary, BS, RRT; Soraya Abbasi, MD; Toni Mancini, RN, BSN, CCRC; Dara M. Cucinotta, RN; Judy C. Bernbaum, MD; Marsha Gerdes, PhD; and Hallam Hurt, MD. University of Rochester Medical Center, Golisano Children’s Hospital, and the University of Buffalo Women’s and Children’s Hospital of Buffalo (grants U10 HD68263, U10 HD40521, UL1 RR24160, M01 RR44, and UL1 TR42): Carl T. D’Angio, MD; Dale L. Phelps, MD; Ronnie Guillet, MD, PhD; Gary J. Myers, MD; Linda J. Reubens, RN, CCRC; Erica Burnell, RN; Diane Hust, MS, RN, CS; Julie Babish Johnson, MSW; Julianne Hunn, BS; Rosemary L. Jensen; Emily Kushner, MA; Deanna Maffett, RN; Joan Merzbach, LMSW; Holly I. M. Wadkins, MA; Kelley Yost, PhD; Lauren Zwetsch, RN, MS, PNP; Satyan Lakshminrusimha, MD; Anne Marie Reynolds, MD, MPH; Farooq Osman, MD; Ashley Williams, MSEd; and Karen Wynn, RN. University of Texas Health Science Center at Houston Medical School, Children’s Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (grant U10 HD21373): Kathleen A. Kennedy, MD, MPH; Jon E. Tyson, MD, MPH; Georgia E. McDavid, RN; Nora I. Alaniz, BS; Katrina Burson, RN, BSN; Patricia W. Evans, MD; Charles Green, PhD; Beverly Foley Harris, RN, BSN; Margarita Jiminez, MD, MPH; Anna E. Lis, RN, BSN; Sarah Martin, RN, BSN; Brenda H. Morris, MD; M. Layne Poundstone, RN, BSN; Peggy Robichaux, RN, BSN; Saba Siddiki, MD; Maegan C. Simmons, RN; Patti L. Pierce Tate, RCP; and Sharon L. Wright, MT, MLS. University of Texas Southwestern Medical Center at Dallas, Parkland Health and Hospital System, and Children’s Medical Center Dallas (grants U10 HD40689 and M01 RR633): Pablo J. Sánchez, MD; Luc P. Brion, MD; Roy J. Heyne, MD; Walid A. Salhab, MD; Charles R. Rosenfeld, MD; Diana M. Vasil, RNC-NIC; Sally S. Adams, MS, RN, CPNP; Lijun Chen, PhD, RN; Alicia Guzman; Gaynelle Hensley, RN; Elizabeth T. Heyne, MS, MA, PA-C, PsyD; Melissa H. Leps, RN; Linda A. Madden, RN, CPNP; Nancy A. Miller, RN; Janet S. Morgan, RN; Lizette E. Torres, RN; and Catherine Twell Boatman, MS, CIMI. University of Utah Medical Center, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center (grants U10 HD53124, M01 RR64, and UL1 TR105): Roger G. Faix, MD; Bradley A. Yoder, MD; Anna Bodnar, MD; Karen A. Osborne, RN, BSN, CCRC; Shawna Baker, RN; Karie Bird, RN, BSN; Jill Burnett, RNC, BSN; Laura Cole, RN; Jennifer J. Jensen, RN, BSN; Cynthia Spencer, RNC; Michael Steffen, MS, CPM; Kimberlee Weaver-Lewis, RN, BSN; Sarah Winter, MD; and Karen Zanetti, RN. Wake Forest University Baptist Medical Center, Brenner Children’s Hospital, and Forsyth Medical Center (grants U10 HD40498 and M01 RR7122): T. Michael O’Shea, MD, MPH; Robert G. Dillard, MD; Lisa K. Washburn, MD; Barbara G. Jackson, RN, BSN; Nancy Peters, RN; Korinne Chiu, MA; Deborah Evans Allred, MA, LPA; Donald J. Goldstein, PhD; Raquel Halfond, MA; Carroll Peterson, MA; Ellen L. Waldrep, MS; Cherrie D. Welch, MD, MPH; Melissa Whalen Morris, MA; and Gail Wiley Hounshell, PhD. Wayne State University, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (grant U10 HD21385): John Barks, MD; Rebecca Bara, RN, BSN; Angela Argento; PhD; Martha Carlson, MD; Laura A. Goldston, MA; Mary E. Johnson, RN, BSN; Mary Christensen, RT; and Stephanie A. Wiggins, MS. Yale University, Yale-New Haven Children’s Hospital, and Bridgeport Hospital (grants U10 HD27871, UL1 RR24139, M01 RR125, and UL1 TR142): Richard A. Ehrenkranz, MD; Harris Jacobs, MD; Christine G. Butler, MD; Patricia Cervone, RN; Sheila Greisman, RN; Monica Konstantino, RN, BSN; JoAnn Poulsen, RN; Janet Taft, RN, BSN; Joanne Williams, RN, BSN; and Elaine Romano, MSN.
Footnotes
Group Information: The National Institute of Child Health and Human Development Neonatal Research Network members are listed at the end of the article.
Author Contributions: Drs Das and Saha had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chawla, Natarajan, Shankaran, Pappas, Carlo, Higgins.
Acquisition, analysis, or interpretation of data: Chawla, Shankaran, Pappas, Stoll, Carlo, Saha, Das, Laptook, Higgins.
Drafting of the manuscript: Chawla, Shankaran.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Shankaran, Saha, Das.
Obtained funding: Shankaran, Stoll.
Administrative, technical, or material support: Stoll, Carlo, Higgins.
Study supervision: Chawla, Natarajan, Shankaran, Das, Higgins.
Conflict of Interest Disclosures: None reported.
Additional Contributions: We are indebted to our medical and nursing colleagues and the parents and their infants who agreed to take part in this study.
REFERENCES
- 1.Crowley R, Chalmers I, Keirse MJ. The effects of corticosteroid administration before preterm delivery: an overview of the evidence from controlled trials. Br J Obstet Gynaecol. 1990;97(1):11–25. doi: 10.1111/j.1471-0528.1990.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 2.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50(4):515–525. [PubMed] [Google Scholar]
- 3.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3(3):CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Chawla S, Natarajan G, Rane S, Thomas R, Cortez J, Lua J. Outcomes of extremely low birth weight infants with varying doses and intervals of antenatal steroid exposure. J Perinat Med. 2010;38(4):419–423. doi: 10.1515/jpm.2010.060. [DOI] [PubMed] [Google Scholar]
- 5.Doyle LW, Kitchen WH, Ford GW, Rickards AL, Kelly EA. Antenatal steroid therapy and 5-year outcome of extremely low birth weight infants. Obstet Gynecol. 1989;73(5, pt 1):743–746. [PubMed] [Google Scholar]
- 6.Eriksson L, Haglund B, Ewald U, Odlind V, Kieler H. Short and long-term effects of antenatal corticosteroids assessed in a cohort of 7,827 children born preterm. Acta Obstet Gynecol Scand. 2009;88(8):933–938. doi: 10.1080/00016340903111542. [DOI] [PubMed] [Google Scholar]
- 7.Carlo WA, McDonald SA, Fanaroff AA, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22 to 25 weeks’ gestation. JAMA. 2011;306(21):2348–2358. doi: 10.1001/jama.2011.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palisano RJ, Cameron D, Rosenbaum PL, Walter SD, Russell D. Stability of the gross motor function classification system. Dev Med Child Neurol. 2006;48(6):424–428. doi: 10.1017/S0012162206000934. [DOI] [PubMed] [Google Scholar]
- 9.Palisano R, Rosenbaum R, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 10.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 11.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33(1):179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 13.Bayley N. Bayley Scales of Infant and Toddler Development. 3rd. San Antonio, TX: Harcourt Assessment, Inc; 2006. [Google Scholar]
- 14.Hayes AF, Preacher KJ. Statistical mediation analysis with a multicategorical independent variable. Br J Math Stat Psychol. 2014;67(3):451–470. doi: 10.1111/bmsp.12028. [DOI] [PubMed] [Google Scholar]
- 15.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fritz MS, Mackinnon DP. Required sample size to detect the mediated effect. Psychol Sci. 2007;18(3):233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kari MA, Hallman M, Eronen M, et al. Prenatal dexamethasone treatment in conjunction with rescue therapy of human surfactant: a randomized placebo-controlled multicenter study. Pediatrics. 1994;93(5):730–736. [PubMed] [Google Scholar]
- 18.Pattinson RC, Makin JD, Funk M, et al. Dexiprom Study Group. The use of dexamethasone in women with preterm premature rupture of membranes: a multicentre, double-blind, placebo-controlled, randomised trial. SAfr Med J. 1999;89(8):865–870. [PubMed] [Google Scholar]
- 19.Sotiriadis A, Tsiami A, Papatheodorou S, Baschat AA, Sarafidis K, Makrydimas G. Neurodevelopmental outcome after a single course of antenatal steroids in children born preterm: a systematic review and meta-analysis. Obstet Gynecol. 2015;125(6):1385–1396. doi: 10.1097/AOG.0000000000000748. [DOI] [PubMed] [Google Scholar]
- 20.Chawla S, Bapat R, Pappas A, Bara R, Zidan M, Natarajan G. Neurodevelopmental outcome of extremely premature infants exposed to incomplete, no or complete antenatal steroids. J Ma tern Fetal Neonatal Med. 2013;26(15):1542–1547. doi: 10.3109/14767058.2013.791273. [DOI] [PubMed] [Google Scholar]
- 21.Wong D, Abdel-Latif M, Kent A NICUS Network. Antenatal steroid exposure and outcomes of very premature infants: a regional cohort study. Arch Dis Child Fetal Neonatal Ed. 2014;99(1):F12–F20. doi: 10.1136/archdischild-2013-304705. [DOI] [PubMed] [Google Scholar]
- 22.Shankaran S, Lin A, Maller-Kesselman J, et al. Gene Targets for Intraventricular Hemorrhage Study. Maternal race, demography, and health care disparities impact risk for intraventricular hemorrhage in preterm neonates. J Pediatr. 2014;164(5):1005–1011. e3. doi: 10.1016/j.jpeds.2014.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuji M, Saul JR, du Plessis A, et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics. 2000;106(4):625–632. doi: 10.1542/peds.106.4.625. [DOI] [PubMed] [Google Scholar]
- 24.Altaany D, Natarajan G, Gupta D, Zidan M, Chawla S. Severe intraventricular hemorrhage in extremely premature infants: are high carbon dioxide pressure or fluctuations the culprit? Am J Perinatal. 2015;32(9):839–844. doi: 10.1055/s-0034-1543950. [DOI] [PubMed] [Google Scholar]
- 25.Löhle M, Müller T, Wicher C, et al. Betamethasone effects on fetal sheep cerebral blood flow are not dependent on maturation of cerebrovascular system and pituitary-adrenal axis. J Physiol. 2005;564(pt 2):575–588. doi: 10.1113/jphysiol.2004.077537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stonestreet BS, Petersson KH, Sadowska GB, Pettigrew KD, Patlak CS. Antenatal steroids decrease blood-brain barrier permeability in the ovine fetus. Am Pysiol. 1999;276(2 pt 2):R283–R289. doi: 10.1152/ajpregu.1999.276.2.R283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.