Abstract
Meyer et al. find that subjects lacking the AIRE gene, critical for self-tolerance in T lymphocytes, show a broad range of autoantibody specificities, which can have extremely high affinities. The data also suggest that some of these autoantibodies can, surprisingly, prevent some types of auto-immunity, particularly type I diabetes.
In molecular biology, there has been a persistent bias against the value of descriptive biology. This might go back to a remark attributed to Ernest Rutherford that “There are two kinds of science; physics and stamp collecting.” Of course, the real power in any scientific area comes with a precise knowledge of mechanisms, but it shouldn’t be forgotten that careful observation and discovering new phenomena is the starting point of every field. A case in point is the paper in this issue of Cell by Meyer et al. (2016), who take advantage of new technologies that are allowing us to get precise data about the human immune system to discover some remarkable and unexpected properties of human beings with a particular immune deficiency.
They start, innocently enough, with a straightforward enquiry into the nature of the autoantibodies in subjects that are deficient in what is known as the AIRE gene, which was originally identified in APS1—an autoimmune syndrome characterized by autoantibodies, impaired endocrine function, and chronic Candida infections (Nagamine et al., 1997; Finnish-German APECED Consortium, 1997). Later work in mice has shown that Aire has a specific role in stimulating the expression of tissue-specific genes in the thymus that wouldn’t normally be expressed in that organ and that this helps ensure T cell tolerance to self-antigens (Mathis and Benoist, 2009). There is also evidence that Aire has a role in ensuring T cell tolerance in peripheral immune organs such as the spleen and lymph nodes (Gardner et al., 2013). Tolerance is induced at least in some cases by clonal deletion of self-specific T cells (Anderson et al., 2005) but also might take the form of inhibiting activation (Davis, 2015).
In some mouse strains, Aire deficiency leads to severe autoimmunity and early death, but in other strains and in human beings, the effects are more subtle. In this study, the authors gathered specimens and data on 81 patients. Then they analyzed the autoantibodies in their serum for specificity. Remarkably, they found that each patient expresses on average approximately 100 different specificities, such that, all together, they were able to identify over 3,700 antibody specificities, showing that there was an almost random pattern of targets. However, there were some specificities that were shared, particularly anti-cytokine antibodies to cytokines in the type I interferon group. Even more remarkable is that, when they characterized some of these autoantibodies with respect to their affinity, they found that many were astonishing high, with one having a KD of 10−14M and others in the picomolar range (10−12M), much higher than the nanomolar affinities that one gets with a standard immunization. They further note that patients with this syndrome seem resistant to many autoimmune diseases (multiple sclerosis, lupus, and others) but a fraction do develop type I diabetes. While there is a growing literature correlating anti-cytokine antibodies with a susceptibility to particular infectious diseases (Kisand et al., 2010), they postulated that such autoantibodies might, in some cases, have a protective effect. In particular, α-interferons have been implicated in type I diabetes in mice, and so they looked to see whether the expression of autoantibodies to these cytokines correlated with this type of autoimmunity in their cohort. Indeed, they found that, while all the patients in their substudy had antibodies to this family of cytokines, those expressed by the 8 APS1 subjects with type I diabetes did not neutralize, whereas the 13 patients who did not have diabetes did. While not a proof, this is a seriously “smoking gun” suggesting a critical role for these cytokines in this particular (and major) autoimmune disease. It is also interesting that, while α-interferon is prominent in anti-viral responses and has been used therapeutically, APS1 patients do not seem to be particularly prone to viral infections, indicating that there is enough redundancy in other parts of the immune system to carry the load.
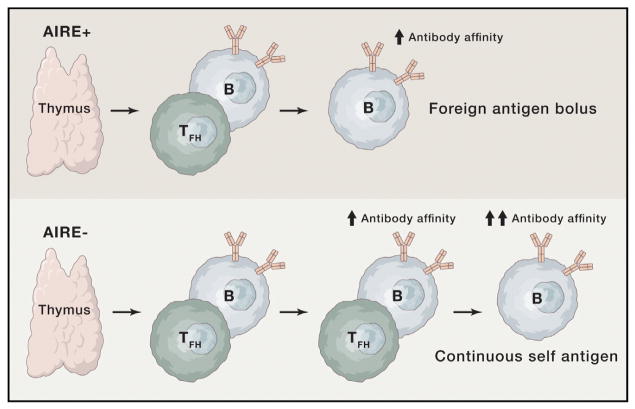
So how to summarize this study? Figure 1 attempts to do this by showing that the lack of AIRE activity in the thymus and in the periphery in patients with the APS1 syndrome leads to a failure of T cell tolerance in those T cells that are specific for the many self-antigens that AIRE is responsible for expressing. Just how many this represents is evident in the many different autoantibody specificities in this cohort.
Figure 1. A Simplified View of T-B Lymphocyte Interactions in the Presence or Absence of the AIRE Gene’s Influence on T Cell Tolerance.
Normally, follicular helper CD4+ T cells emerge from the thymus with those expressing self-specific T cell receptors either purged or suppressed (upper part of the figure). They are then able to stimulate B cells specific for the same antigen to mutate their antibody genes to achieve higher affinities after a bolus of immunizing antigen or an infection. In AIRE-deficient subjects, it is suggested that the absence of T cell tolerance allows multiple T-B interactions of this sort due to the continuous presence of self-antigen, resulting in the very high affinities seen in Meyer et al. (2016). Here, arrows denote increases in antibody affinity.
But why would these autoantibodies have such high affinities? A key factor in stimulating a given B cell to mutate its immunoglobulin genes from micromolar to nanomolar affinities in the course of an immune response cells are follicular helper T cells (TFH). The authors suggest that the lack of T cell tolerance in general and TFHs in particular could divert B cells originally having other specificities onto this self-reactive path. But an additional wrinkle could come from recent work by Goodnow and colleagues (Sabouri et al., 2014), who have found that, in addition to TFHs boosting the affinities of antibodies for foreign antigens,, autoantibody-producing B cells can be selected to reduce their affinity for self. If this reversal of affinity was dependent on input from T cells enforcing self-specific tolerance (either regulatory T cells or perhaps TFH cells that had escaped clonal deletion in the thymus), and if those cells were absent due to the lack of Aire, then the ubiquitous presence of self-antigens coupled with a selection for high affinity antibodies in germinal centers might result in the amazing affinities seen in this system. In any event, these results show how carefully analyzing particular human mutations can uncover new phenomena worthy of further study. This not only opens up new vistas regarding our understanding of immune function and dysfunction but also shows how work on the human immune system can not only inform translational work but add to our understanding of basic principles as well.
References
- Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Davis MM. Immunity. 2015;43:833–835. doi: 10.1016/j.immuni.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Finnish-German APECED Consortium. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- Gardner JM, Metzger TC, McMahon EJ, Au-Yeung BB, Krawisz AK, Lu W, Price JD, Johannes KP, Satpathy AT, Murphy KM, et al. Immunity. 2013;39:560–572. doi: 10.1016/j.immuni.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisand K, Bøe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, Perheentupa J, Erichsen MM, Bratanic N, et al. J Exp Med. 2010;207:299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D, Benoist C. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- Meyer S, Woodward M, Hertel C, Vlaicu P, Haque Y, Kärner J, Macagno A, Onuoha SC, Fishman D, Peterson H, et al. Cell. 2016;166(this issue):582–595. doi: 10.1016/j.cell.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis SE, et al. Nat Genet. 1997;17:393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- Sabouri Z, Schofield P, Horikawa K, Spierings E, Kipling D, Randall KL, Langley D, Roome B, Vazquez-Lombardi R, Rouet R, et al. Proc Natl Acad Sci USA. 2014;111:E2567–E2575. doi: 10.1073/pnas.1406974111. [DOI] [PMC free article] [PubMed] [Google Scholar]