Abstract
Type 2 diabetes (T2D) burden is increasing globally. However, evidence regarding nutrient patterns associated with the biomarkers of T2D is limited. This study set out to determine the nutrient patterns associated with fasting glucose and glycated haemoglobin the biomarkers of T2D. Factor analysis was used to derive nutrient patterns of 2010 participants stratified by urban/rural status and gender. Principal Component Analysis (PCA) was applied to 25 nutrients, computed from the quantified food frequency questionnaires (QFFQ). Three nutrient patterns per stratum, which accounted for 73% of the variation of the selected nutrients, were identified. Multivariate linear regression models adjusted for age, BMI, smoking, physical activity, education attained, alcohol intake, seasonality and total energy intake were computed. Starch, dietary fibre and B vitamins driven nutrient pattern was significantly associated with fasting glucose (β = −0.236 (−0.458; −0.014); p = 0.037) and glycated haemoglobin levels (β = −0.175 (−0.303; −0.047); p = 0.007) in rural women. Thiamine, zinc and plant protein driven nutrient pattern was associated with significant reductions in glycated haemoglobin and fasting glucose ((β = −0.288 (−0.543; −0.033); p = 0.027) and (β = −0.382 (−0.752; −0.012); p = 0.043), respectively) in rural men. Our results indicate that plant driven nutrient patterns are associated with low fasting glucose and glycated haemoglobin levels.
Keywords: plant based, fasting glucose and glycated haemoglobin, T2D, dietary patterns
1. Introduction
Type 2 diabetes (T2D) prevalence and the burden it places on populations is increasing globally, thereby making it a public health challenge which requires urgent attention [1]. For instance, the global prevalence of T2D among women increased from 5% to 7.9% from 1980 to 2014 [2]. The diabetes prevalence in Africa is projected to have the largest increase of 109% compared to other regions in the world by 2035 [3]. Urban black South Africans have not been spared from the T2D burden. The highest prevalence of T2D in Sub Saharan Africa was reported among urban black South Africans, with 60% of these cases being reported amongst women [4]. Black South African women also have the highest prevalence of obesity, which has been reported to be rising together with T2D in this population [5].
The adoption of Westernised lifestyles by urban dwellers is suggested to be among the leading factors resulting in the increase in non-communicable diseases (NCDs) such as T2D in developing countries [6]. The migration of populations from rural to urban areas has been accompanied by an increase in meat consumption as well as an increase in the consumption of sugary foods in South Africa [7]. Both of these foods are recognised as dietary risk factors for T2D [6]. However, improvements in micronutrient intake among black South African women in urban areas is suggested to be a result of the increased consumption of fruits and vegetables which are protective of T2D risk [8,9,10]. Thus, the role of diet as a risk factor for T2D is complex among the black South African women. In addition, people eat meals with a variety of nutrients which have interactive and synergistic effects on health [11]. Therefore, it is difficult to determine the separate effect of a food or nutrient on disease development as it is highly interrelated with other nutrients [11]. There is need of dietary pattern analysis methods which are able to evaluate the diet as a whole and clarify the effects of the consumption of sugary foods and meat products, together with improved intakes of fruits and vegetables, related to T2D risk amongst this population group.
Dietary pattern (or food pattern) approaches comprise data driven methods such as factor analysis, which allow the dietary information (or food intake) at hand to determine the unique dietary pattern for the population group being evaluated [10]. Although dietary patterns have been associated with disease risk, their effect is considered to be through nutrient intake; therefore, it is pivotal to determine nutrient patterns that are associated with T2D risk [12]. This information will aid in the understanding of the aetiology of T2D. The foods that people eat are governed by their cultural norms and beliefs, which vary amongst ethnic groups, making dietary patterns limited and not applicable across divergent population groups [12]. Evidence exists that the Western dietary pattern association with fasting glucose varies among different ethnic groups [13]. However, nutrients are universal, thereby making nutrient patterns associations with disease risk applicable for multiple population groups [14]. To the best of our knowledge, no study has reported on the association of the nutrient patterns with fasting glucose and glycated haemoglobin levels among apparently healthy individuals. T2D is a complex and multifactorial diseases, however glycated haemoglobin and fasting glucose levels are proxies for the development of this disease which are affected by diet [15]. Fasting glucose levels are indicative of the short term changes in glucose metabolism, while glycated haemoglobin depicts the long term changes [15]. This study seeks to evaluate the association of nutrient patterns derived by factor analysis with fasting glucose and glycated haemoglobin levels among apparently healthy black South Africans.
2. Materials and Methods
2.1. Study Population
The study participants (n = 2010) were recruited from two urban and rural areas of the North West Province into the South African arm of the Prospective urban and rural epidemiological (PURE) study using a population based sampling strategy. Apparently healthy male and female volunteers between the ages of 35 and 60 years were recruited by the fieldworkers. Individuals were considered to be apparently healthy if they were not using any medication for chronic disease and if they were not diagnosed with a chronic medical condition/disease. The international PURE study is a large-scale epidemiological study, which comprises research participants recruited from 17 low, middle, and high income countries [16]. The South African arm of the PURE study was initiated in 2005 with initial five-year follow-up intervals up to 2015. At baseline in 2005 and at the five-year intervals during the course of the study, medical history, lifestyle behaviour (physical activity and dietary intake), blood collection (for both genetic and biochemical analyses), an electrocardiogram, and anthropometric assessments were performed to determine the role of risk factors in the development of cardiovascular diseases [16]. Our study was nested in the 2005 PURE study baseline data. Stratified nutrient pattern analysis was conducted according to gender and urban/rural status among the 2010 participants of the PURE study. However, significant associations of the nutrient patterns with fasting glucose and glycated haemoglobin were noted only among the rural women and rural men. Therefore, the detailed nutrient pattern and association results of the rural participants are elaborated in the main text while results of the urban men and women are illustrated in the Supplementary Materials.
2.2. Ethical Approval
The participants gave written informed consent before participating in the study and the study was conducted according to the Declaration of Helsinki principles [17]. Ethical approval was granted by the Ethics Committee of the North-West University, Potchefstroom Campus, with ethics number NWU-00016-10-A1.
2.3. Dietary, Anthropometric and Physical Activity Assessments
The trained fieldworkers captured the dietary intake of the participants using a standardised quantitative food frequency questionnaire (QFFQ) which had been validated for the same ethnic population group of the study participants [18,19]. The reproducibility of the QFFQ was also assessed and found to be good in this specific study population [20]. The dietary intake data were coded, analysed and nutrient intakes were computed using the South African Food Composition Database [21]. Body weight measurements were performed in duplicate by the PURE research team members using a portable electronic scale (Precision Health Scale, A & D Company, Tokyo, Japan), after which the mean was recorded. The heights of the subjects were determined by the PURE study research team members using a stadiometer (IP 1465, Invicta, and London, UK). The BMI of the participants was computed using the formula: BMI = weight (kg/height (m2)). The Baecke physical activity questionnaire (BPAQ) which was validated for South Africa was used to collect the physical activity information of the participants [22]. The questionnaire was used to compute a physical activity index score as described elsewhere [22,23]. This physical activity index scores were used in the multivariate regression analysis to adjust for physical activity.
2.4. Biochemical Measurements
The research participants were required to fast (at least 8 h with no food or beverages, including water before measurements) and their blood glucose levels were measured by the PURE research team. These fasting glucose levels were measured using the SYNCHRON® System from fluoride plasma. The Bio-Rad D-10TM, HbA1c kit (Bio-Rad Laboratories, Inc., Hercules, France), which operates via cation exchange high performance liquid chromatography was used to assess HbA1c levels from whole blood ethylenediaminetetraacetic acid (EDTA) treated samples. The coefficient of variation of the glycated haemoglobin and fasting glucose tests were 1.16% and 2.1%, respectively.
2.5. Statistical Analysis
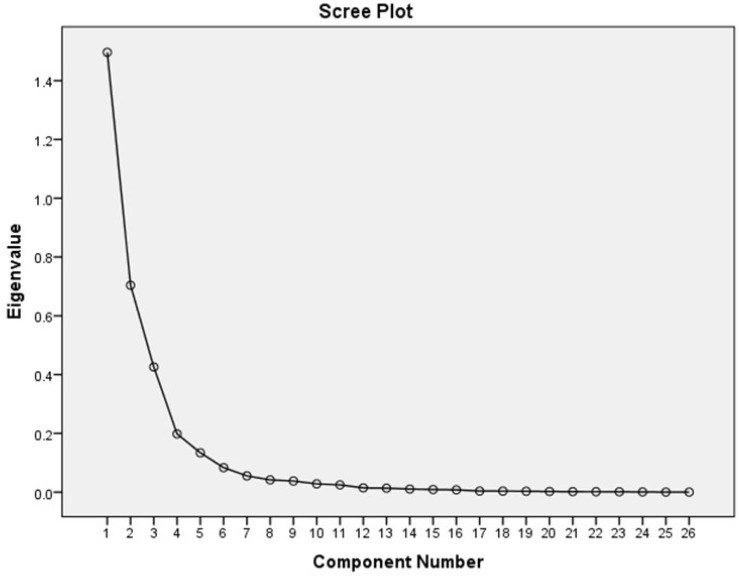
The statistical analysis was performed using the statistical package for social scientists (SPSS) version 23. Normality tests for the continuous variables were performed using the Q-Q plots. Twenty-five nutrients were used to determine the nutrient patterns as has been reported previously by Pisa et al. [24]. Among these 25 nutrients, total protein was split into animal protein and plant protein; total carbohydrates were divided into total sugar, starch, and total dietary fibre; and total fat was categorised into saturated fat, monounsaturated fat and polyunsaturated fat. The total dietary fibre comprised soluble and insoluble dietary fibre. Alcohol was not regarded as a nutrient and not included in deriving the nutrient pattern analysis. However, in view of the reported association of alcohol intake with glycated haemoglobin, it was adjusted for in the multivariate linear models for the association of the derived nutrient patterns with glycated haemoglobin and fasting glucose [25]. The nutrient intake variables from the quantitative food frequency questionnaire (QFFQ) were log transformed to remove bias due to variance as a result of the different measures of scale used to quantify the nutrients. These nutrients were adjusted for log alcohol free energy using the multivariate (standard) method [11]. The multivariate method was selected as it yielded more cumulative variance and interpretable nutrient patterns compared to the standard nutrient density method of adjusting for total energy intake [11]. Principal component analysis (PCA) was the factor reduction tool used to determine the nutrient patterns from the 25 selected nutrients. The PCA was performed with the variance based on the covariance matrix and Varimax rotation. The retained principal components (PC) were used to identify the nutrient patterns. The scree plot (Figure 1) was used to determine the number of PCs to retain. The nutrient patterns were named using the nutrients with loadings greater than ±0.47 on the PCs. The total variances explained by the retained PCs were also evaluated to determine the relevance of the extracted PCs. The PCA was a suitable data reduction approach for the nutrient data in this study as was indicated by a Kaiser–Meyer–Olkin measure of sampling adequacy of 0.911, and a Bartlett’s test of sphericity which was significant at p < 0.001. The PCs were categorised into tertiles and analysis of variance ANOVA (for continuous variables) and Chi-square (for categorical variables) tests were used to determine the descriptive characteristics of the study participants across the tertiles of the extracted PCs.
Figure 1.
Scree plot of the nutrients and the extracted principal components among rural women.
Crude and adjusted multiple linear regression models were computed to assess the association between the extracted PCs with glycated haemoglobin and fasting glucose as dependent variables separately through varied models. In these models, regression coefficients for 1 standard deviation (SD) increase in the PC scores (and their 95% confidence intervals) were computed for four models: M1: (crude); M2: (adjusted for M1 plus Log Total Energy); M3: (adjusted for M2 plus Body Mass Index); and M4: (adjusted for M3 plus age, smoking, physical activity, education level, seasonality, alcohol intake and other PCs). Seasonality was adjusted based on the months in which the dietary assessments were conducted. Thus, for the rural participants, seasonality was adjusted basing on August and September; and the months of October and November were used to adjust for seasonality in the urban participants. Partial R2 values were computed to express the variance explained by each model. Statistical significance was regarded at p value less than 0.05.
3. Results
3.1. Nutrient Patterns
Three nutrient patterns (Table S1) were extracted from the PCA, which explained 73% of the total variation of the selected nutrient factors among the rural women. The first nutrient pattern was depicted as “Magnesium, phosphorus and plant protein driven nutrients”. This pattern consisted of higher loadings for magnesium, phosphorus and plant protein. The second nutrient pattern was termed “Fat and animal protein driven nutrients” as it had higher loadings of cholesterol, monounsaturated fat, animal protein, polyunsaturated fat and saturated fat. The “Starch, dietary fibre and B vitamins driven” based nutrient pattern was the third extracted nutrient pattern. This nutrient pattern had high loadings of starch, folate, vitamin B6, dietary fibre and thiamine as illustrated in Table S1.
Three nutrient patterns were extracted among rural men and named according to the nutrients with the highest loadings as indicated in Table S1. These were the “Thiamine, zinc and plant protein driven nutrients”, “Fat and animal protein driven nutrients”, and “Retinol and vitamin B12 driven nutrients”. Three similar nutrients patterns were extracted among the rural men, urban men and urban women, which explained 76%, 77% and 76% (Tables S1 and S2) variance of the nutrients, respectively. However, some considerable differences were noted in the plant driven nutrient patterns, which accounted for the greater variation of the nutrients among the urban and rural participants (Table S3).
3.2. Descriptive Characteristics of the Study Population
The study participants included 659 rural women, 347 rural men, 605 urban women and 399 urban men. The mean fasting glucose levels and glycated haemoglobin were 4.96 ± 1.57 mmol·L−1 and 5.64% ± 0.88%, respectively [26]. The women had a mean BMI of 26.73 ± 7.21, which signified that a considerable proportion of the women were overweight. Overall, in the whole study population, 39.4% of the participants were overweight and 87.3% of these were women. The mean total energy intake was 7707.13 ± 3692.36 KJ. The high scores of magnesium, phosphorus and plant protein driven nutrient pattern were associated with high total energy intake and energy from alcohol in rural women, as indicated in Table 1. The high scores for the fat and animal protein driven nutrient pattern was significantly associated with tertiary education status, as indicated in Table 1.
Table 1.
Descriptive characteristics for the rural women of the study population according to the lowest and highest tertiles of the three nutrient patterns (PC).
| Magnesium, Phosphorus and Plant Protein Driven Nutrients | Fat and Animal Protein Driven Nutrients | Starch, Dietary Fibre and B Vitamin Driven Nutrients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T3 | p | T1 | T3 | p | T1 | T3 | p | |
| Age | 48.72 ± 10.23 | 47.51 ± 9.57 | 0.247 | 47.94 ± 8.89 | 47.73 ± 10.16 | 0.837 | 49.15 ± 10.40 | 46.94 ± 8.99 | 0.036 |
| Body Mass Index | 25.28 ± 6.63 | 25.27 ± 6.58 | 0.988 | 25.31 ± 6.75 | 26.13 ± 6.85 | 0.262 | 25.26 ± 6.41 | 25.65 ± 7.11 | 0.605 |
| Total energy | 4322.96 ± 1217.30 | 8365.01 ± 2476.92 | <0.001 | 5631.33 ± 2816.62 | 6883.88 ± 2189.38 | <0.001 | 5893.08 ± 2645.21 | 6567.51 ± 2425.60 | 0.013 |
| Alcohol (%TE) | 0.76 ± 2.56 | 7.42 ± 12.56 | <0.001 | 5.54 ± 11.70 | 1.47 ± 4.40 | <0.001 | 5.36 ± 11.91 | 1.69 ± 5.01 | <0.001 |
| Protein (%TE) | 11.26 ± 1.96 | 10.77 ± 1.64 | 0.008 | 10.27 ± 1.33 | 11.67 ± 1.81 | <0.001 | 11.23 ± 2.05 | 10.91 ± 1.31 | 0.092 |
| Current Smokers (%) | 33.5 | 36.8 | 0.168 | 38.0 | 34.7 | 0.005 | 30.6 | 36.4 | 0.595 |
| Physical Activity Index | 8.19 ± 1.42 | 8.28 ± 1.42 | 0.593 | 8.29 ± 1.29 | 8.32 ± 1.55 | 0.854 | 8.23 ± 1.37 | 8.44 ± 1.45 | 0.205 |
| Tertiary education (%) | 33.7 | 24.5 | 0.058 | 26.5 | 42.9 | 0.028 | 28.6 | 38.8 | 0.854 |
| Fasting glucose (mmol·L−1) | 4.73 ± 0.78 | 4.93 ± 1.47 | 0.219 | 4.91 ± 1.18 | 4.87 ± 1.02 | 0.784 | 5.12 ± 2.41 | 4.88 ± 0.83 | 0.160 |
| HbA1C (%) | 5.64 ± 0.53 | 5.67 ± 0.93 | 0.785 | 5.68 ± 0.91 | 5.69 ± 0.58 | 0.843 | 5.84 ± 1.23 | 5.62 ± 0.55 | 0.027 |
p = p value based on ANOVA or Chi-square test where appropriate; Alcohol (%TE) = percentage of total energy due to alcohol intake; Protein (%TE) = percentage of total energy due to protein intake; T1 = lowest tertile; T3 = highest tertile, TE = total energy; HbA1C = glycated haemoglobin.
High scores for the thiamine, zinc and plant protein nutrient pattern were associated with higher energy and alcohol intake compared to other nutrient patterns among the rural men (Table 2).
Table 2.
Descriptive characteristics for the rural men of the study population according to the lowest and highest tertiles of the three nutrient patterns (PC).
| Thiamine, Zinc and Plant Protein Driven Nutrients | Fat and Animal Protein Driven Nutrients | Retinol and Vitamin B12 Driven Nutrients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T3 | p | T1 | T3 | p | T1 | T3 | p | |
| Age | 47.95 ± 9.99 | 50.19 ± 10.69 | 0.156 | 48.43 ± 9.97 | 51.42 ± 11.45 | 0.057 | 49.66 ± 10.01 | 49.57 ± 9.95 | 0.954 |
| Body Mass Index | 20.86 ± 4.15 | 20.54 ± 4.31 | 0.606 | 20.86 ± 3.99 | 20.94 ± 4.65 | 0.894 | 20.30 ± 3.57 | 20.95 ± 4.19 | 0.297 |
| Total energy | 4693.60 ± 1584.26 | 10,637.00 ± 2887.76 | <0.001 | 6319.63 ± 3228.51 | 8220.85 ± 3228.51 | <0.001 | 7164.14 ± 2975.31 | 7855.83 ± 3672.65 | 0.159 |
| Alcohol (%TE) | 5.98 ± 9.40 | 13.82 ± 13.70 | <0.001 | 11.19 ± 13.41 | 5.70 ± 9.16 | 0.002 | 6.65 ± 11.23 | 9.19 ± 11.79 | 0.159 |
| Protein (%TE) | 11.51 ± 2.69 | 10.76 ± 1.53 | 0.014 | 10.09 ± 1.43 | 12.19 ± 2.33 | <0.001 | 10.64 ± 1.69 | 11.50 ± 2.48 | 0.005 |
| Current Smokers (%) | 32.4 | 35.9 | 0.636 | 40.0 | 26.2 | 0.003 | 31.7 | 31.7 | 0.577 |
| Physical Activity Index | 8.25 ± 1.64 | 8.02 ± 1.44 | 0.380 | 8.03 ± 1.65 | 7.99 ± 1.79 | 0.861 | 7.92 ± 1.61 | 7.97 ± 1.63 | 0.847 |
| Tertiary education (%) | 44.2 | 20.9 | 0.141 | 23.3 | 37.2 | 0.514 | 27.9 | 37.2 | 0.451 |
| Fasting glucose (mmol·L−1) | 4.90 ± 0.93 | 4.68 ± 0.90 | 0.113 | 4.75 ± 0.79 | 4.96 ± 1.24 | 0.138 | 4.94 ± 2.41 | 4.75 ± 0.78 | 0.190 |
| HbA1C (%) | 5.59 ± 0.52 | 5.51 ± 0.81 | 0.386 | 5.49 ± 0.34 | 5.62 ± 0.97 | 0.169 | 5.57 ± 0.83 | 5.53 ± 0.49 | 0.678 |
p = p value based on ANOVA or Chi-square test where appropriate; Alcohol (%TE) = percentage of total energy due to alcohol intake; Protein (%TE) = percentage of total energy due to protein intake; T1 = lowest tertile; T3 = highest tertile; TE = total energy; HbA1C = glycated haemoglobin.
3.3. Nutrient Patterns Associations with Fasting and Glycated Haemoglobin Levels
The association results of glycated haemoglobin and fasting glucose levels with 1 SD increases in the extracted nutrient patterns are shown in Table 3, Table 4, Table 5 and Table 6 for the rural women and rural men. The magnesium, phosphorus and plant protein driven nutrient pattern was associated with a consistent trend of increases in fasting glucose and glycated haemoglobin among rural women, as illustrated in Table 3.
Table 3.
Regression coefficients for fasting glucose for 1 SD increase in the derived nutrient pattern scores among rural black South African women.
| Magnesium, Phosphorus and Plant Protein Driven Nutrients | Fat and Animal Protein Driven Nutrients | Starch, Dietary Fibre and B Vitamin Driven Nutrients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B (95% CI) | p Value | R2 | B (95% CI) | p Value | R2 | B (95% CI) | p Value | R2 | |
| M1 | 0.129 (−0.014; 0.271) | 0.077 | 0.007 | 0.009 (−0.141; 0.160) | 0.902 | 0.000 | −0.164 (−0.311; −0.018) | 0.027 | 0.008 |
| M2 | 0.196 (−0.063; 0.455) | 0.138 | 0.007 | −0.020 (−0.183; 0.143) | 0.813 | 0.003 | −0.197 (−0.349; −0.049) | 0.011 | 0.016 |
| M3 | 0.278 (−0.001; 0.280) | 0.034 | 0.045 | −0.038 (−0.198; 0.123) | 0.645 | 0.037 | −0.203 (−0.351; −0.054) | 0.008 | 0.051 |
| M4 | 0.147 (−0.360; 0.655) | 0.569 | 0.086 | −0.004 (−0.290; 0.281) | 0.976 | 0.086 | −0.236 (−0.458; −0.014) | 0.037 | 0.086 |
M1: (crude); M2: (adjusted for M1 plus Log Total Energy); M3: (adjusted for M2 plus Body Mass Index); M4: (adjusted for M3 plus age, smoking, physical activity, alcohol intake, seasonality, education level, PC1, PC2 and PC3); M1 = model 1; M2 = model 2; M3 = model 3; M4 = model 4; PC1 = Magnesium, phosphorus and plant protein driven nutrients; PC2 = Fat and animal protein driven nutrients; PC3 = Starch, dietary fibre and B vitamin driven nutrients Fasting glucose units mmol·L−1 = millimoles per litre; SD = standard deviation; CI = confidence interval.
Table 4.
Regression coefficients for glycated haemoglobin for 1 SD increase in the derived nutrient pattern scores among rural black South African women.
| Magnesium, Phosphorus and Plant Protein Driven Nutrients | Fat and Animal Protein Driven Nutrients | Starch, Dietary Fibre and B Vitamin Driven Nutrients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B (95% CI) | p Value | R2 | B (95% CI) | p Value | R2 | B (95% CI) | p Value | R2 | |
| M1 | 0.029 (−0.055; 0.112) | 0.502 | 0.001 | 0.032 (−0.056; 0.120) | 0.477 | 0.001 | −0.138 (−0.224; −0.053) | 0.002 | 0.020 |
| M2 | 0.048 (−0.104; 0.199) | 0.538 | 0.001 | 0.028 (−0.067; 0.123) | 0.563 | 0.001 | −0.145 (−0.234; −0.056) | 0.001 | 0.021 |
| M3 | 0.112 (−0.036; 0.260) | 0.139 | 0.069 | 0.014 (−0.078; 0.106) | 0.766 | 0.065 | −0.151 (−0.237; −0.065) | 0.001 | 0.087 |
| M4 | 0.107 (−0.188; 0.401) | 0.478 | 0.150 | −0.011 (−0.175; 0.154) | 0.478 | 0.150 | −0.175 (−0.303; −0.047) | 0.007 | 0.150 |
M1: (crude); M2: (adjusted for M1 plus Log Total Energy); M3: (adjusted for M2 plus Body Mass Index); M4: (adjusted for M3 plus age, smoking, physical activity, alcohol intake, seasonality, education level, PC1, PC2 and PC3); M1 = model 1; M2 = model 2; M3 = model 3; M4 = model 4; PC1 = Magnesium, phosphorus and plant protein driven nutrients; PC2 = Fat and animal protein driven nutrients; PC3 = Starch, dietary fibre and B vitamin driven nutrients. Glycated haemoglobin unit = per cent; SD = standard deviation; CI = confidence interval.
Table 5.
Regression coefficients for fasting glucose for 1 SD increase in the derived nutrient pattern scores among rural black South African men.
| Thiamine, Zinc and Plant Protein Driven Nutrients | Fat and Animal Protein Driven Nutrients | Retinol and Vitamin B12 Driven Nutrients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B (95% CI) | p Value | R2 | B (95% CI) | p Value | R2 | B (95% CI) | p Value | R2 | |
| M1 | −0.057 (−0.172; 0.057) | 0.326 | 0.004 | 0.054 (−0.061; 0.169) | 0.355 | 0.003 | −0.039 (−0.156; 0.077) | 0.504 | 0.002 |
| M2 | −0.237 (−0.492; 0.019) | 0.069 | 0.013 | 0.061 (−0.064; 0.186) | 0.335 | 0.004 | −0.055 (−0.173; 0.064) | 0.363 | 0.003 |
| M3 | −0.255 (−0.496; 0.014) | 0.038 | 0.117 | 0.055 (−0.063; 0.172) | 0.363 | 0.115 | −0.082 (−0.194; 0.030) | 0.153 | 0.120 |
| M4 | −0.382 (−0.752; −0.012) | 0.043 | 0.182 | −0.051 (−0.241; 0.139) | 0.596 | 0.182 | −0.109 (−0.229; 0.032) | 0.074 | 0.182 |
M1: (crude); M2: (adjusted for M1 plus Log Total Energy); M3: (adjusted for M2 plus Body Mass Index); M4: (adjusted for M3 plus age, smoking, physical activity, alcohol intake, seasonality, education level, PC1, PC2 and PC3); M1 = model 1; M2 = model 2; M3 = model 3; M4 = model 4; PC1 = Magnesium, phosphorus and plant protein driven nutrients; PC2 = Fat and animal protein driven nutrients; PC3 = Starch, dietary fibre and B vitamin driven nutrients Fasting glucose units mmol·L−1 = millimoles per litre; SD = standard deviation; CI = confidence interval.
Table 6.
Regression coefficients for glycated haemoglobin for 1 SD increase in the derived nutrient pattern scores among rural black South African women.
| Thiamine, Zinc and Plant Protein Driven Nutrients | Fat and Animal Protein Driven Nutrients | Retinol and Vitamin B12 Driven Nutrients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B (95% CI) | p Value | R2 | B (95% CI) | p Value | R2 | B (95% CI) | p Value | R2 | |
| M1 | −0.039 (−0.117; 0.038) | 0.320 | 0.000 | 0.046 (−0.031; 0.123) | 0.241 | 0.005 | 0.012 (−0.065; 0.090) | 0.754 | 0.000 |
| M2 | −0.214 (−0.384; 0.044) | 0.014 | 0.024 | 0.053 (−0.031; 0.138) | 0.213 | 0.006 | 0.015 (−0.065; 0.094) | 0.713 | 0.001 |
| M3 | −0.230 (−0.392; −0.067) | 0.006 | 0.113 | 0.050 (−0.030; 0.131) | 0.219 | 0.092 | 0.001 (−0.075; 0.077) | 0.975 | 0.086 |
| M4 | −0.288 (−0.543; −0.033) | 0.027 | 0.174 | −0.057 (−0.189; 0.075) | 0.396 | 0.174 | −0.018 (−0.100; 0.064) | 0.662 | 0.174 |
M1: (crude); M2: (adjusted for M1 plus Log Total Energy); M3: (adjusted for M2 plus Body Mass Index); M4: (adjusted for M3 plus age, smoking, physical activity, alcohol intake, seasonality, education level, PC1, PC2 and PC3); M1 = model 1; M2 = model 2; M3 = model 3; M4 = model 4; PC1 = Magnesium, phosphorus and plant protein driven nutrients; PC2 = Fat and animal protein driven nutrients; PC3 = Starch, dietary fibre and B vitamin driven nutrients. Glycated haemoglobin unit = per cent; SD = standard deviation; CI = confidence interval.
Conversely, the starch, dietary fibre and B vitamins nutrient pattern was consistently associated with reduced glycated haemoglobin and fasting glucose levels in all evaluated linear models, for instance the M4 model: −0.175% (−0.303; −0.047); p = 0.007) and −0.236 mmol·L−1 (−0.458; −0.014); p = 0.037), respectively, as indicated in Table 3 and Table 4 among rural women.
The thiamine, zinc and plant protein nutrient pattern was associated with reduced glycated haemoglobin and fasting glucose levels in rural men as illustrated in Table 5 and Table 6. For instance, the M4 models, which explained the highest variances of the fasting glucose and glycated haemoglobin, indicated reductions of these outcome variables to be −0.382 mmol·L−1 (−0.752; −0.012; p = 0.043) and −0.288% (−0.543; −0.033; p = 0.027), respectively, as illustrated in Table 5 and Table 6.
The plant driven nutrient patterns had significant results as has been shown above among the urban and rural participants. However, some notable differences were depicted in the plant driven nutrient patterns illustrated in Table S3. The following nutrient patterns, “thiamine, zinc and plant protein driven nutrients”, “thiamine, starch and folate driven nutrients”, and “magnesium, phosphorus and plant protein driven nutrients” extracted from the urban men, urban women and rural women, respectively, had higher loadings for animal protein, saturated fat, mono-saturated fat and sugar compared to the “thiamine, zinc and plant protein driven nutrients” and “the starch, dietary fibre and B vitamin driven nutrients patterns”, which were associated with significant reductions in fasting and glycated haemoglobin among the rural men and women respectively (see factor loadings in bold in Table S3). These two nutrient patterns that had significant associations with fasting glucose and glycated haemoglobin had very low cholesterol and saturated fat as illustrated in Table S3.
4. Discussion
We set out to determine the nutrient patterns associated with fasting glucose and glycated haemoglobin levels among apparently healthy volunteers. The principal component analysis method enabled the extraction of three nutrient patterns among a black South African population which explained about 73% of the variation of the nutrient factors in the urban/rural and gender stratifications. The magnesium, phosphorus and plant protein driven nutrient pattern was associated with a trend of increasing fasting glucose and glycated haemoglobin levels per 1 SD increase in the pattern in the rural women while the thiamine, zinc and plant protein nutrient pattern was associated with a positive trend of increasing glycated haemoglobin among urban men. Notably, the starch, dietary fibre and B vitamin nutrient pattern was associated with decreases in glycated haemoglobin and fasting glucose levels, −0.175% ((−0.303; −0.047); p = 0.007) and −0.236 mmol·L−1 ((−0.458; −0.014); p = 0.037), respectively, among rural women. The thiamine, zinc and plant protein driven nutrient pattern was associated with significant reductions in fasting glucose and glycated haemoglobin of −0.382 mmol·L−1 (−0.752; −0.012; p = 0.043) and −0.288% (−0.543; −0.033; p = 0.027) in rural men. These associations were significantly maintained after adjusting for age, BMI, log total energy intake, smoking, physical activity, alcohol intake, seasonality and education level attained thus indicating an independent association of the starch, dietary fibre and B vitamin nutrient pattern and the thiamine, zinc and plant protein driven nutrient pattern with fasting glucose and glycated haemoglobin in the rural women and rural men strata’s, respectively.
Comparable studies evaluating the associations of nutrients patterns with fasting glucose and glycated haemoglobin are scarce. However, evidence exists of dietary patterns which have evaluated this phenomenon [10]. The Health/Prudent dietary pattern has been associated with decreases in fasting glucose and glycated haemoglobin levels while the Western dietary pattern has been associated with increases in these biomarkers of T2D [27,28]. However, the association of the Western dietary pattern, which is characterised with high intakes of animal proteins and snacks, with fasting glucose levels was noted to vary among ethnic groups [13]. The Western dietary pattern was reported in one study to be only significantly associated with high fasting glucose levels and glycated haemoglobin levels among Dutch and not among the Moroccans and Turkish groups [13]. Dietary patterns are known to vary among different ethnic groups, therefore nutrient patterns that were considered in our study are considered helpful in indicating a non-ethnic insight into this phenomenon.
In our study, we noted that fat and animal protein driven nutrients were also not associated with fasting glucose and glycated levels, as had been depicted in dietary pattern analysis studies among different ethnic groups [13]. This disparity from the Western populations where the animal based/Western dietary patterns are associated with increasing fasting glucose levels has been explained by the realisation that ethnic groups such as Asians may adopt the Western diets but their intake of animal products will remain lower as they also continue to consume traditional cereals and vegetables [29]. Similarly, in this study population, the intake of fat and animal protein nutrients were lower in the plant driven nutrients patterns that accounted for the greatest variance among the three nutrients patterns extracted per stratum as illustrated in Table S3. In other local studies, the protein and fat intakes as percentage of total energy intake for urban women from 1975 to 2005 did not change drastically though evidences of the adoption of Western dietary patterns were being noted and the greater proportion of the energy intake was still being contributed by the carbohydrate intake as had been noted in the Asians [6]. From 1975 to 2005, the percentage of energy of protein intake changed from 14% to 13%, while fat intake as percentage of energy changed from 21% to 30% and carbohydrate intake changed from 67% to 57% of total energy intake [6]. Thus, the intake of fat and animal protein driven nutrients in this population group might have been lower and thus not associated with increases in fasting glucose and glycated haemoglobin as was expected.
The magnesium, phosphorus and plant protein driven nutrient pattern indicated a positive trend association with increases in fasting glucose and glycated haemoglobin levels among rural women, while the thiamine, zinc and plant protein driven nutrient pattern was associated with a trend of increasing glycated haemoglobin levels in the urban men (Table S7). However, plant protein and zinc, which were high in these nutrient patterns, have been reported to independently lower fasting glucose levels [30,31,32,33]. In addition, the thiamine, zinc and plant protein driven nutrient pattern was associated with a trend of decreasing glycated haemoglobin and fasting glucose levels in the rural men. In view that the study population comprised people who consumed diets with both animal and plant based nutrients and not purely vegetarians, the discrepancies in the associations of the plant driven nutrients can be explained by the varied proportions of animal protein, saturated fat, mono-saturated fat, cholesterol and sugar among these plant driven nutrient patterns, as illustrated in Table S3. The magnesium, phosphorus and plant protein driven nutrient pattern in rural women and the thiamine, zinc and plant protein driven nutrient pattern in urban men had higher loadings of animal protein, saturated fat, mono-saturated fat, cholesterol and sugar compared to the other nutrient patterns discussed below such as the starch, dietary fibre and B vitamins driven nutrient pattern among rural women and the thiamine, zinc and plant protein nutrient pattern among rural men which associated with low fasting glucose and glycated haemoglobin levels. Animal protein, saturated fat, cholesterol and sugar are high in Western dietary patterns which have been previously associated with increased risk of T2D and this helps clarify the positive trend of association of the magnesium, phosphorus and plant protein driven nutrient pattern in rural women and the thiamine, zinc and plant protein driven nutrient pattern in urban men with the study outcome variables [34]. The comparisons of the varied constituents of the plant driven nutrients as discussed above and illustrated in Table S3 indicates the attractiveness of the PCA approach of nutrient pattern determination as it allows a whole based approach of the effect of nutrients to particular outcomes to be evaluated.
The starch, dietary fibre and B vitamins driven nutrient pattern was consistently associated with reduced fasting glucose and glycated haemoglobin levels in all the multivariate linear models which were considered in this study among rural women. The thiamine, zinc and plant protein nutrient pattern was also associated with significant reductions in glycated haemoglobin and fasting glucose among rural men. These findings are similar to the results of other studies on plant based dietary patterns and T2D risk [10,34]. It has been consistently reported that plant based diets are protective against T2D risk [10,34,35]. A number of mechanisms have been proposed to explain this phenomenon [10]. Plant based diets which are rich in dietary fibre are suggested to reduce T2D risk by reducing postprandial insulin demand, improving insulin sensitivity and the antioxidants found in these diets may help enhance β-cell function [36,37]. Dietary fibre from cereal foods which are also high in starch has been consistently shown across eight European countries to be associated with reduced T2D risk [38]. Maize meal fortified with iron, zinc and B vitamins which is largely consumed among the black South African population group is known to contain resistant starches that are partially digested and have been associated with improved insulin sensitivity which may then lead to reductions in fasting glucose levels [39,40]. Thiamine was also high in this nutrient pattern. Evidence exists that dietary fibre beneficial effects might also be due to its concomitant intake together with thiamine [41].The results of this study suggest that starch, dietary fibre and B vitamins nutrients, zinc and plant protein consumed together may lead to the lowering of fasting and glycated haemoglobin levels as has been postulated elsewhere [35].
The strengths of the current study include the use of a validated QFFQ and recruitment criteria of selecting apparently healthy individuals who were not taking chronic medications or suffering from chronic diseases. This might have helped to prevent the confounding of drugs and T2D to the nutrient pattern association results with glycated haemoglobin and fasting glucose levels. The factor analysis approach used to derive the nutrient patterns is known for depicting real-world dietary behaviours [42]. However, this approach is based on a number of subjective decisions such as naming nutrient patterns, method of rotation and selection of food groups which can lead to an overall measurement error [10,42]. Since this approach is a posteriori analysis tool, it derives nutrient patterns based on data at hand and this makes comparisons with other studies difficult [10,42]. However, regardless of the differences in the constituents of the nutrient patterns due to the data driven approaches used to derive them, consistent similarities of the dietary factors associated with T2D risk as reported in this study, has been depicted in multiple populations [10,34,37]. It might be probable that the residual confounding effect of total energy intake might have led to distortions in the association of the nutrient patterns with fasting glucose and glycated haemoglobin levels. Residual confounding is a result of measurement error in a confounder included in the model [43]. Although the nutrient and total energy intake based on the QFFQs are not precisely measured, adjustment for total energy intake should control for total energy intake confounding [44]. However, evidence exists that the control of total energy intake is not complete in epidemiological studies involving QFFQs thereby leading to residual confounding [44,45]. Therefore, there is need to further explore the association of the nutrient patterns in isocaloric clinical trials which are better designed to control for confounding for total energy intake [45].
5. Conclusions
In summary, our results indicate the beneficial associations of plant driven nutrient patterns with reductions in fasting glucose and glycated haemoglobin levels. However, the small variances that were explained by the models explored in this study are suggestive of the presence of other factors that affect the variation of fasting glucose and glycated haemoglobin which were not accounted for in this study. Thus, more studies are required to further explore the association of nutrient patterns with fasting glucose and glycated haemoglobin.
Acknowledgments
This research was supported by a grant National Research Foundation of South Africa (87970). Tinashe Chikowore is supported by the National Research Foundation of South Africa (89117). We would like to acknowledge A. Kruger and the PURE research team, funders and participants.
Supplementary Materials
The following are available online at http://www.mdpi.com/2072-6643/9/1/9/s1, Table S1. Extracted nutrient patterns and factor loadings of rural women and rural men, Table S2. Nutrient patterns and factor loadings for urban participants, Table S3. Comparison of the factors loadings in the plant driven nutrient patterns among the urban and rural participants, Table S4. Regression coefficients for fasting glucose for 1 SD increase in the derived nutrient pattern scores among urban black South African women, Table S5. Regression coefficients for glycated haemoglobin for 1 SD increase in the derived nutrient pattern scores among urban black South African women, Table S6. Regression coefficients for fasting glucose for 1 SD increase in the derived nutrient pattern scores among urban black South African men, Table S7. Regression coefficients for glycated haemoglobin for 1 SD increase in the derived nutrient pattern scores among urban black South African men.
Author Contributions
T.C. conceptualized the paper, analysed the data and wrote the draft article. P.T.P. designed the data analysis plan and contributed to the writing of the paper. E.W.-V. designed the dietary data collection tools, coded and analysed the data for nutrients and contributed to the writing of the paper. T.v.Z., E.J.M.F. and K.R.C. supervised and contributed to the writing of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chen L., Magliano D.J., Zimmet P.Z. The worldwide epidemiology of type 2 diabetes mellitus—Present and future perspectives. Nat. Rev. Endocrinol. 2012;8:228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 2.Collaboration N.C.D.R.F. Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guariguata L., Whiting D.R., Hambleton I., Beagley J., Linnenkamp U., Shaw J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Peer N., Steyn K., Lombard C., Lambert E.V., Vythilingum B., Levitt N.S. Rising diabetes prevalence among urban-dwelling black South Africans. PLoS ONE. 2012;7:e43336. doi: 10.1371/journal.pone.0043336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sartorius B., Veerman L.J., Manyema M., Chola L., Hofman K. Determinants of obesity and associated population attributability, South Africa: Empirical evidence from a national panel survey, 2008–2012. PLoS ONE. 2015;10:e0130218. doi: 10.1371/journal.pone.0130218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vorster H.H., Kruger A., Margetts B.M. The nutrition transition in Africa: Can it be steered into a more positive direction? Nutrients. 2011;3:429–441. doi: 10.3390/nu3040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vorster H.H., Kruger A., Wentzel-Viljoen E., Kruger H.S., Margetts B.M. Added sugar intake in South Africa: Findings from the adult prospective urban and rural epidemiology cohort study. Am. J. Clin. Nutr. 2014;99:1479–1486. doi: 10.3945/ajcn.113.069005. [DOI] [PubMed] [Google Scholar]
- 8.Vorster H.H., Margetts B.M., Venter C.S., Wissing M.P. Integrated nutrition science: From theory to practice in South Africa. Public Health Nutr. 2005;8:760–765. doi: 10.1079/PHN2005775. [DOI] [PubMed] [Google Scholar]
- 9.Hattingh Z., Walsh C.M., Bester C.J., Oguntibeju O.O. Evaluation of energy and macronutrient intake of black women in Bloemfontein: A cross-sectional study. Afr. J. Biotechnol. 2008;7:4019–4024. [Google Scholar]
- 10.McEvoy C.T., Cardwell C.R., Woodside J.V., Young I.S., Hunter S.J., McKinley M.C. A posteriori dietary patterns are related to risk of type 2 diabetes: Findings from a systematic review and meta-analysis. J. Acad. Nutr. Diet. 2014;114:1759–1775. doi: 10.1016/j.jand.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Hu F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Salehi-Abargouei A., Esmaillzadeh A., Azadbakht L., Keshteli A.H., Feizi A., Feinle-Bisset C., Adibi P. Nutrient patterns and their relation to general and abdominal obesity in Iranian adults: Findings from the Sepahan study. Eur. J. Nutr. 2016;55:505–518. doi: 10.1007/s00394-015-0867-4. [DOI] [PubMed] [Google Scholar]
- 13.Dekker L.H., van Dam R.M., Snijder M.B., Peters R.J., Dekker J.M., de Vries J.H., de Boer E.J., Schulze M.B., Stronks K., Nicolaou M. Comparable dietary patterns describe dietary behavior across ethnic groups in The Netherlands, but different elements in the diet are associated with glycated hemoglobin and fasting glucose concentrations. J. Nutr. 2015;145:1884–1891. doi: 10.3945/jn.114.207472. [DOI] [PubMed] [Google Scholar]
- 14.Freisling H., Fahey M.T., Moskal A., Ocke M.C., Ferrari P., Jenab M., Norat T., Naska A., Welch A.A., Navarro C., et al. Region-specific nutrient intake patterns exhibit a geographical gradient within and between European countries. J. Nutr. 2010;140:1280–1286. doi: 10.3945/jn.110.121152. [DOI] [PubMed] [Google Scholar]
- 15.Koenig R.J., Peterson C.M., Jones R.L., Saudek C., Lehrman M., Cerami A. Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N. Engl. J. Med. 1976;295:417–420. doi: 10.1056/NEJM197608192950804. [DOI] [PubMed] [Google Scholar]
- 16.Teo K., Chow C.K., Vaz M., Rangarajan S., Yusuf S. The Prospective Urban Rural Epidemiology (PURE) study: Examining the impact of societal influences on chronic noncommunicable diseases in low-, middle-, and high-income countries. Am. Heart J. 2009;158:1–7. doi: 10.1016/j.ahj.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Arie S. Revision of Helsinki declaration aims to prevent exploitation of study participants. BMJ. 2013;347:f6401. doi: 10.1136/bmj.f6401. [DOI] [PubMed] [Google Scholar]
- 18.MacIntyre U.E., Venter C.S., Vorster H.H., Steyn H.S. A combination of statistical methods for the analysis of the relative validation data of the quantitative food frequency questionnaire used in the THUSA study. Public Health Nutr. 2001;4:45–51. doi: 10.1079/PHN200039. [DOI] [PubMed] [Google Scholar]
- 19.Wolmarans P., Danster N., Dalton A., Rossouw K., Schönfeldt H. Condensed Food Composition Tables for South Africa. Medical Research Council; Cape Town, South Africa: 2010. [Google Scholar]
- 20.Wentzel-Viljoen E., Laubscher R., Kruger A. Using different approaches to assess the reproducibility of a culturally sensitive quantified food frequency questionnaire. S. Afr. J. Clin. Nutr. 2011;24:143–148. doi: 10.1080/16070658.2011.11734366. [DOI] [Google Scholar]
- 21.Wolmarans P., Chetty J., Danster-Christians N. Food composition activities in South Africa. Food Chem. 2013;140:447–450. doi: 10.1016/j.foodchem.2012.10.064. [DOI] [PubMed] [Google Scholar]
- 22.Kruger H., Venter C., Steyn H. A standardised physical activity questionnaire for a population in transition: The THUSA study. Afr. J. Phys. Health Educ. Recreat. Dance. 2000;6:54–64. [Google Scholar]
- 23.Oyeyemi A.L., Moss S.J., Monyeki M.A., Kruger H.S. Measurement of physical activity in urban and rural South African adults: A comparison of two self-report methods. BMC Public Health. 2016;16:1004. doi: 10.1186/s12889-016-3693-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pisa P.T., Pedro T.M., Kahn K., Tollman S.M., Pettifor J.M., Norris S.A. Nutrient patterns and their association with socio-demographic, lifestyle factors and obesity risk in rural South African adolescents. Nutrients. 2015;7:3464–3482. doi: 10.3390/nu7053464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boeing H., Weisgerber U.M., Jeckel A., Rose H.J., Kroke A. Association between glycated hemoglobin and diet and other lifestyle factors in a nondiabetic population: Cross-sectional evaluation of data from the Potsdam cohort of the European Prospective Investigation into Cancer and Nutrition Study. Am. J. Clin. Nutr. 2000;71:1115–1122. doi: 10.1093/ajcn/71.5.1115. [DOI] [PubMed] [Google Scholar]
- 26.Alberti K.G., Zimmet P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a who consultation. Diabet. Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 27.Kim H.S., Park S.Y., Grandinetti A., Holck P.S., Waslien C. Major dietary patterns, ethnicity, and prevalence of type 2 diabetes in rural Hawaii. Nutrition. 2008;24:1065–1072. doi: 10.1016/j.nut.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liese A.D., Weis K.E., Schulz M., Tooze J.A. Food intake patterns associated with incident type 2 diabetes: The insulin resistance atherosclerosis study. Diabetes Care. 2009;32:263–268. doi: 10.2337/dc08-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilbert P.A., Khokhar S. Changing dietary habits of ethnic groups in Europe and implications for health. Nutr. Rev. 2008;66:203–215. doi: 10.1111/j.1753-4887.2008.00025.x. [DOI] [PubMed] [Google Scholar]
- 30.Roberts-Toler C., O’Neill B.T., Cypess A.M. Diet-induced obesity causes insulin resistance in mouse brown adipose tissue. Obesity (Silver Spring) 2015;23:1765–1770. doi: 10.1002/oby.21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capdor J., Foster M., Petocz P., Samman S. Zinc and glycemic control: A meta-analysis of randomised placebo controlled supplementation trials in humans. J. Trace Elem. Med. Biol. 2013;27:137–142. doi: 10.1016/j.jtemb.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Hruby A., Ngwa J.S., Renstrom F., Wojczynski M.K., Ganna A., Hallmans G., Houston D.K., Jacques P.F., Kanoni S., Lehtimaki T., et al. Higher magnesium intake is associated with lower fasting glucose and insulin, with no evidence of interaction with select genetic loci, in a meta-analysis of 15 CHARGE Consortium Studies. J. Nutr. 2013;143:345–353. doi: 10.3945/jn.112.172049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khattab M., Abi-Rashed C., Ghattas H., Hlais S., Obeid O. Phosphorus ingestion improves oral glucose tolerance of healthy male subjects: A crossover experiment. Nutr. J. 2015;14:112. doi: 10.1186/s12937-015-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alhazmi A., Stojanovski E., McEvoy M., Garg M.L. The association between dietary patterns and type 2 diabetes: A systematic review and meta-analysis of cohort studies. J. Hum. Nutr. Diet. 2014;27:251–260. doi: 10.1111/jhn.12139. [DOI] [PubMed] [Google Scholar]
- 35.Sabate J., Wien M. A perspective on vegetarian dietary patterns and risk of metabolic syndrome. Br. J. Nutr. 2015;113(Suppl. 2):S136–143. doi: 10.1017/S0007114514004139. [DOI] [PubMed] [Google Scholar]
- 36.Barclay A.W., Petocz P., McMillan-Price J., Flood V.M., Prvan T., Mitchell P., Brand-Miller J.C. Glycemic index, glycemic load, and chronic disease risk—A meta-analysis of observational studies. Am. J. Clin. Nutr. 2008;87:627–637. doi: 10.1093/ajcn/87.3.627. [DOI] [PubMed] [Google Scholar]
- 37.Montonen J., Knekt P., Harkanen T., Jarvinen R., Heliovaara M., Aromaa A., Reunanen A. Dietary patterns and the incidence of type 2 diabetes. Am. J. Epidemiol. 2005;161:219–227. doi: 10.1093/aje/kwi039. [DOI] [PubMed] [Google Scholar]
- 38.InterAct Consortium Dietary fibre and incidence of type 2 diabetes in eight European countries: The epic-interact study and a meta-analysis of prospective studies. Diabetologia. 2015;58:1394–1408. doi: 10.1007/s00125-015-3585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brites C.M., Trigo M.J., Carrapico B., Alvina M., Bessa R.J. Maize and resistant starch enriched breads reduce postprandial glycemic responses in rats. Nutr. Res. 2011;31:302–308. doi: 10.1016/j.nutres.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Gower B.A., Bergman R., Stefanovski D., Darnell B., Ovalle F., Fisher G., Sweatt S.K., Resuehr H.S., Pelkman C. Baseline insulin sensitivity affects response to high-amylose maize resistant starch in women: A randomized, controlled trial. Nutr. Metab. (Lond.) 2016;13:2. doi: 10.1186/s12986-016-0062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bakker S.J., Hoogeveen E.K., Nijpels G., Kostense P.J., Dekker J.M., Gans R.O., Heine R.J. The association of dietary fibres with glucose tolerance is partly explained by concomitant intake of thiamine: The hoorn study. Diabetologia. 1998;41:1168–1175. doi: 10.1007/s001250051047. [DOI] [PubMed] [Google Scholar]
- 42.Moeller S.M., Reedy J., Millen A.E., Dixon L.B., Newby P.K., Tucker K.L., Krebs-Smith S.M., Guenther P.M. Dietary patterns: Challenges and opportunities in dietary patterns research an experimental biology workshop, April 1, 2006. J. Am. Diet. Assoc. 2007;107:1233–1239. doi: 10.1016/j.jada.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 43.Fewell Z., Davey Smith G., Sterne J.A. The impact of residual and unmeasured confounding in epidemiologic studies: A simulation study. Am. J. Epidemiol. 2007;166:646–655. doi: 10.1093/aje/kwm165. [DOI] [PubMed] [Google Scholar]
- 44.Willett W. Nutritional Epidemiology. Oxford University Press; New York, NY, USA: 1998. [Google Scholar]
- 45.Jakes R.W., Day N.E., Luben R., Welch A., Bingham S., Mitchell J., Hennings S., Rennie K., Wareham N.J. Adjusting for energy intake—What measure to use in nutritional epidemiological studies? Int. J. Epidemiol. 2004;33:1382–1386. doi: 10.1093/ije/dyh181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.