Abstract
The association between cheese consumption and risk for major health endpoints has been investigated in many epidemiologic studies, but findings are inconsistent. As all-cause mortality can be viewed as the final net health effect of dietary intakes, we conducted a meta-analysis to examine the long-term association of cheese consumption with all-cause mortality. Relevant studies were identified by a search of the PubMed database through May 2016. Reference lists from retrieved articles were also reviewed. Summary relative risks (RR) and 95% confidence intervals (CI) were calculated using a random-effects model. Pre-specified stratified and dose-response analyses were also performed. The final analysis included nine prospective cohort studies involving 21,365 deaths. The summary RR of all-cause mortality for the highest compared with the lowest cheese consumption was 1.02 (95% CI: 0.97, 1.06), and little evidence of heterogeneity was observed. The association between cheese consumption and risk of all-cause mortality did not significantly differ by study location, sex, age, number of events, study quality score or baseline diseases excluded. There was no dose-response relationship between cheese consumption and risk of all-cause mortality (RR per 43 g/day = 1.03, 95% CI: 0.99–1.07). No significant publication bias was observed. Our findings suggest that long-term cheese consumption was not associated with an increased risk of all-cause mortality.
Keywords: cheese, dairy, fermented food, mortality, meta-analysis
1. Introduction
Cheese, a fermented dairy product and traditionally part of the Mediterranean diet [1], is consumed by billions of people around the world. A healthy eating pattern in 2015–2020 Dietary Guidelines for Americans includes milk, yogurt, and cheese; however, the guidelines also point out that because most cheese contains more sodium and saturated fats, increased intake of dairy products would be most beneficial if more fat-free or low-fat milk products were selected rather than cheese [2]. Until now the effects of long-term cheese consumption on human health has been investigated in many epidemiologic studies, but findings are inconsistent. The scientific evidence of previous systematic review or meta-analysis indicated that the consumption of dairy foods including cheese was inversely associated with risk of type 2 diabetes (T2DM) [3]. In addition, an up-to-date meta-analysis has suggested a nonlinear inverse association between cheese consumption and risk of cardiovascular disease (CVD) [4]. On the other hand, there is some evidence that cheese consumption may be detrimental to certain diseases. A meta-analysis of prospective studies showed that a high intake of cheese was associated with increased prostate cancer risk [5], and another meta-analysis found that consumption of dairy foods, especially of milk and cheese, was associated with increased risk of Parkinson’s disease [6].
It seems that the associations between cheese consumption and risks of multiple chronic diseases have not been totally consistent, with both positive and negative associations reported. Considering the widespread consumption of cheese, a slight reduction or increase in disease risk may represent significant public health implications. All-cause mortality is used as an indicator of safety or hazard of an intervention and can be considered as the final net influence of dietary intakes on health [7]. Furthermore, four chronic diseases—cardiovascular disease, chronic respiratory disease, diabetes mellitus, and cancer—account for over 60% of all deaths globally [8]. However, a number of prospective studies that investigated the relationship between long-term cheese consumption and risk of all-cause mortality have yielded inconsistent findings. Therefore, we conducted a meta-analysis to quantify these prospective studies.
2. Methods
2.1. Literature Search
This study was conducted using a predefined protocol and in accordance with the proposed MOOSE (Meta-Analysis of Observational Studies in Epidemiology) [9]. A literature search was performed independently by two authors (XT and G-CC) on the PubMed database up to May 2016 for prospective studies of cheese consumption and risk of all-cause mortality. The following search terms were used: (1) cheese, dairy, or fermented food; (2) mortality, or death; and (3) cohort study, prospective study, or follow-up study. In addition, reference lists of relevant articles and published systematic reviews and meta-analyses were hand searched. No language restrictions were imposed.
2.2. Study Selection
Studies were considered eligible if they met the following criteria: (1) the study design was a prospective study; (2) relative risks (RRs) or hazard ratios (HRs) with 95% confidence intervals (CIs) for the association between cheese consumption and all-cause mortality were reported.
2.3. Data Extraction and Quality Assessment
Data extraction was performed using a standardized data collection form, and the information extracted was as follows: the first author’s last name, publication year, study name, duration of follow-up, number of deaths and total participants, sex and age, quantity of intake, the most fully adjusted RRs and 95% CIs, prevalent diseases excluded at baseline, and variables adjusted for in the analysis. Data were extracted by two authors (XT and G-CC). Discrepancies were resolved by discussion and, if agreement could not be achieved, by adjudication of a third reviewer (L-QQ).
2.4. Statistical Analysis
A random-effects model was used to calculate summary RRs and 95% CIs for the highest compared with the lowest category of cheese consumption. RRs and corresponding SEs were logarithmically transformed to stabilize variance and normalize the distribution [10]. For one study [7] that reported the RRs stratified by sex, results were treated as two separate reports.
Heterogeneity in studies was tested using the Q test [11] at a significant level of p < 0.1 and the I2 statistic, a quantitative measure of inconsistency across studies. To explore potential sources of heterogeneity, subgroup and meta-regression analyses were performed according to study and population characteristics including: study location, sex, age, duration of follow-up, number of events, the study quality score and baseline diseases excluded. To test the robustness of the results, a sensitivity analysis was carried out by omitting one cohort in each turn to examine the impacts of a single study on the overall effect estimate.
In addition, we quantified the dose-response relationship of cheese consumption with risk of all-cause mortality based on the method proposed by Greenland and Longnecker [12]. To perform this analysis, we estimated the distribution of cases and person-years and the RRs with the variance estimates for at least three quantitative exposure categories. When cheese consumption was reported in servings or other units, the consumption was converted into grams per day (g/day) using 43 g as a serving size, as reported in the ‘United States Department of Agriculture Food and Nutrient Database for Dietary Studies’ [13]. We further examined a potential nonlinear relationship between cheese consumption and risk of all-cause mortality by modeling exposure levels using restricted cubic splines with three knots at fixed percentiles (10%, 50%, and 95%) of the distribution [14,15]. The p-value for nonlinearity was calculated by testing the null hypothesis that the coefficient of the second spline was equal to zero. Potential publication bias was assessed with Egger’s test and Begg’s test [16,17] in which the log RRs were plotted against their SEs. All analyses were performed using STATA version 11.0 (StataCorp, College Station, TX, USA). p values < 0.05 were considered to be statistically significant.
3. Results
3.1. Literature Search
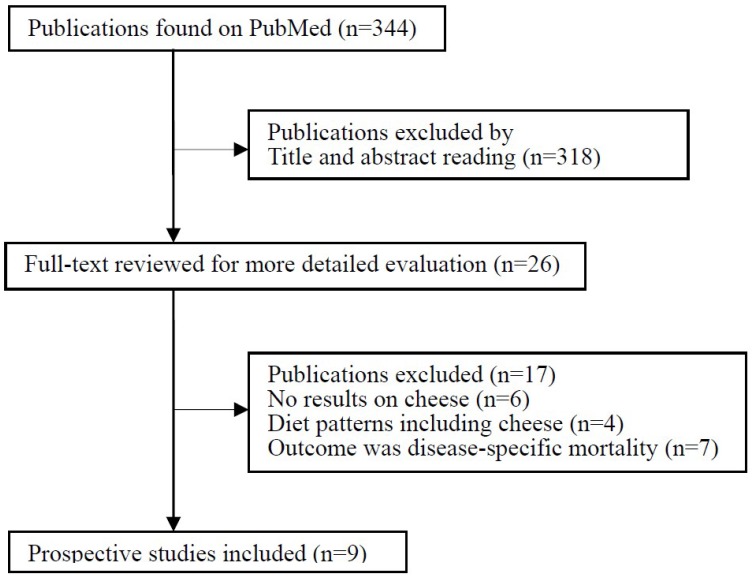
The flow chart of the literature search is presented in Figure 1. The initial search of the PubMed database identified 344 records, most of which were excluded because they were not prospective studies or because the exposure or endpoint was not relevant to our analysis, leaving 26 potentially eligible papers for full-text review. Publications were further excluded mainly because the exposure of interest was dairy intake with no data on cheese consumption (n = 6) or was specific diet patterns including cheese (n = 4). Seven studies were excluded because the outcome was CVD mortality [18,19], coronary heart disease (CHD) mortality [20,21] or stroke mortality [22,23,24] without data on all-cause mortality. Finally, nine independent prospective studies were included in the meta-analysis of cheese consumption and risk of all-cause mortality [7,25,26,27,28,29,30,31,32].
Figure 1.
Flow chart of literature search and study selection.
3.2. Study Characteristics
The characteristics of the included studies are shown in Table 1. These studies were published between 1997 and 2015. Three of the studies were conducted in The Netherlands, two in the UK, two in the United States, and one each in Italy and Australia. Overall, these studies included 21,365 deaths among 177,655 participants during mean lengths of follow-up of 5 to 15 years. All studies provided adjusted risk estimates. The quality scores assigned to each study are presented in Table 2.
Table 1.
Characteristics of included prospective studies that investigated the association of cheese consumption with risk of all-cause mortality.
| Author, Year (Country) | Study Name, Duration | Subjects | Age Range (Mean/Median), Years | No. of Deaths | Reported Results | Baseline Disease Excluded | Adjustments |
|---|---|---|---|---|---|---|---|
| Mann, 1997 [25] (UK) | Oxford Vegetarian Study, 13.3 years | 10,802 M/F | 16–79 (34.0 in M, 33.0 in F) | 383 | Cheese excluding cottage: ≥5 (T3) vs. <1 (T1) ser/week RR = 1.02 (95% CI: 0.76–1.37) |
Cancer, angina, hypertension, HD, stroke, and diabetes | Age, sex, smoking, and social class. |
| Fraser (white), 1997 [26] (USA) | Adventists Health Study, 12 years | NA (11,828 person-years) | ≥85 | 1387 | Cheese: ≥3 (T3) vs. <1 (T1) ser/week RR = 1.07 (95% CI: 0.90–1.27) (M/F) RR = 1.23 (95% CI: 0.91–1.66) (M) RR = 1.01 (95% CI: 0.82–1.24) (F) |
Cancer and HD | Age and sex. |
| Fraser (black), 1997 [27] (USA) | Black Adventists Health Study, 8 years | 1668 M/F | NA (52.0 in M, 53.4 in F) | 153 | Cheese: ≥3 (T3) vs. <1 (T1) ser/week RR = 1.70 (95% CI: 1.00–2.90) (M/F) RR = 1.20 (95% CI: 0.50–2.80) (M) RR = 2.20 (95% CI: 1.10–4.30) (F) |
None | Age, smoking and exercise. |
| Fortes, 1999 [28] (Italy) | NA, 5 years | 162 M/F | ≥65 (80) | 53 | Cheese: ≥2 (High) vs. <1 (Low) ser/week RR = 1.30 (95% CI: 0.51–3.34) |
Mental impairment and severe disability | Age, gender, number of years of education, BMI, cigarette smoking, cognitive status and presence of chronic disease. |
| Bonthuis, 2010 [29] (Australia) | NA, 14.4 years | 1529 M/F | 25–78 (49.8) | 177 | Full-fat cheese: 30 (T3) vs. 4 (T1) g/day RR = 0.91 (95% CI: 0.57–1.45) |
None | Age, sex, BMI, smoking, physical activity, school leaving age, dietary supplement use, β-carotene treatment during trial, use of medications for hypertension, diabetes mellitus, cardiac disorder, use of β-adrenergic blocking agents, and intakes of energy, alcohol and calcium. |
| Goldbohm, 2011 [7] (The Netherlands) | The Netherlands Cohort Study, 10 years | 120,852 M/F | 55–69 (NA) | 10,658 in M; 5478 in F | Cheese: 56 (Q5) vs. 1 (Q1) g/day RR = 1.04 (95% CI: 0.96–1.12) (M) RR = 0.98 (95% CI: 0.88–1.77) (F) |
Angina, MI, and stroke | Age, BMI, smoking, physical activity, education, multivitamin use, and intakes of energy, alcohol, energy-adjusted mono- and polyunsaturated fat, vegetable, and fruit. |
| Soedamah-Muthu, 2012 [30] (UK) | Whitehall II study, 11.7 years | 4526 M/F | NA (56) | 237 | Cheese: 31 (T3) vs. 6 (T1) g/day RR = 1.00 (95% CI: 0.72–1.37) |
CHD | Age, sex, BMI, smoking, physical activity, ethnicity, employment grade, family history of CHD/hypertension, and intakes of energy, alcohol, fruit and vegetables, bread, meat, fish, coffee, and tea. |
| van Aerde, 2012 [31] (The Netherlands) | Hoorn Study, 12.4 years | 1956 M/F | 50–75 (61.6) | 403 | Cheese: per 24 g/day RR = 0.96 (95% CI: 0.84–1.09) |
CVD | Age, sex, physical activity, and intakes of meat, fish, bread, vegetables, fruit, coffee, and tea. |
| Praagman, 2015 [32] (The Netherlands) | EPIC-Netherlands, 15 years | 34,409 M/F | 20–70 (43.0 in M, 51.0 in F) | 2436 | Cheese: 53.2 (Q4) vs. 6.6 (Q1) g/day RR = 1.00 (95% CI: 0.89–1.12) |
Cancer and CVD | Age, sex, smoking, BMI, physical activity, education, hypertension at baseline, and intakes of total energy, alcohol and energy-adjusted fruit and vegetables. |
BMI, body mass index; CHD, coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; EPIC, European Prospective Investigation into Cancer and Nutrition; F, female; HD, heart disease; M, male; MI, myocardial infarction; NA, not available; RR, relative risk.
Table 2.
The quality of included studies assessed by the Newcastle Ottawa Scale a.
| Selection | Comparability | Outcome | Total Stars | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Representativeness of Exposed Cohort | Selection of the Non-Exposed Cohort | Ascertainment of Exposure | Demonstration That Outcome of Interest Was Not Present at Start of Study | Comparability of Cohorts on the Basis of the Design or Analysis | Assessment of Outcome | Was Follow-Up Long Enough for Outcomes to Occur | Adequacy of Follow-Up of Cohorts | |
| Mann, 1997 [25] | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 0 | 6 |
| Fraser (white), 1997 [26] | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 5 |
| Fraser (black), 1997 [27] | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 5 |
| Fortes, 1999 [28] | 0 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 6 |
| Bonthuis, 2010 [29] | 0 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 7 |
| Goldbohm, 2011 [7] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Soedamah-Muthu, 2012 [30] | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 |
| van Aerde, 2012 [31] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Praagman, 2015 [32] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
a A study can be awarded a maximum of one star for each numbered item within the Selection and Outcome categories and a maximum of two stars for Comparability.
3.3. Cheese Consumption and Risk of All-Cause Mortality
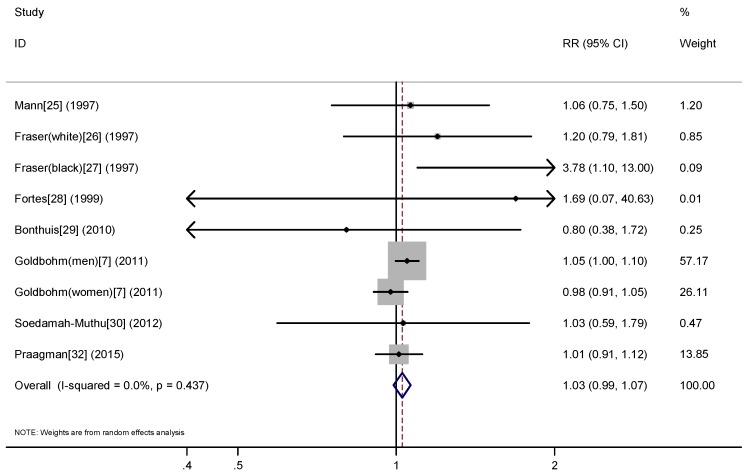
A meta-analysis of nine prospective studies yielded a summary RR of 1.02 (95% CI: 0.97–1.06) for the highest compared with the lowest cheese consumption (Figure 2), with no evidence for heterogeneity (Pheterogeneity = 0.74, I2 = 0%). There was no evidence of publication bias with Egger’s (p = 0.37) or with Begg’s test (p = 0.59).
Figure 2.
Meta-analysis of prospective studies on cheese consumption (high vs. low) and risk of all-cause mortality.
Table 3 presents the results of subgroup analyses according to study location, sex, age, duration of follow-up, number of events, the study quality score and baseline diseases excluded. The null results were consistently observed in most of the subgroups. We found evidence of a duration-specific difference in the association (p = 0.09), with a marginally significant increased risk of all-cause mortality associated high cheese consumption among two studies with a duration of <10 years (RR = 1.59, 95% CI: 1.00–2.53), but not among those with a duration of ≥10 years (RR = 1.01, 95% CI: 0.96–1.06). The overall result remained stable in a sensitivity analysis in which one study at a time was omitted and the rest were analyzed, with a range from 1.00 (95% CI: 0.94–1.06) to 1.02 (95% CI: 0.97–1.08).
Table 3.
Subgroup meta-analysis for the association of cheese consumption with risk of all-cause mortality.
| Pre-Defined Factors | Subgroups | Number of Studies | RR (95% CI) | Pheterogeneity | I2 (%) | Pinteraction |
|---|---|---|---|---|---|---|
| Study location | Europe | 7 | 1.01 (0.96–1.06) | 0.94 | 0 | 0.27 |
| USA | 2 | 1.26 (0.82–1.93) | 0.11 | 0.62 | ||
| Sex | Men | 3 | 1.05 (0.98–1.13) | 0.55 | 0 | 0.581 |
| Women | 3 | 1.07 (0.85–1.33) | 0.07 | 0.62 | ||
| Mean/median baseline age | ≥60 years | 5 | 1.02 (0.92–1.12) | 0.42 | 0 | 0.989 |
| <60 years | 5 | 1.02 (0.96–1.07) | 0.72 | 0 | ||
| Duration of follow-up | ≥10 years | 8 | 1.01 (0.96–1.06) | 0.96 | 0 | 0.09 |
| <10 years | 2 | 1.59 (1.00–2.53) | 0.63 | 0 | ||
| Number of events | ≥500 | 4 | 1.00 (0.90–1.11) | 0.46 | 0 | 0.69 |
| <500 | 6 | 1.02 (0.97–1.08) | 0.77 | 0 | ||
| Quality score | ≥8 | 4 | 1.02 (0.96–1.07) | 0.84 | 0 | 0.98 |
| <8 | 6 | 1.02 (0.92–1.12) | 0.40 | 0.03 | ||
| Baseline diseases excluded: CVD | Yes | 7 | 1.01 (0.96–1.06) | 0.93 | 80 | 0.33 |
| No | 3 | 1.23 (0.80–1.88) | 0.22 | 0.34 | ||
| Cancer | Yes | 3 | 1.02 (0.93–1.12) | 0.82 | 0 | 0.89 |
| No | 7 | 1.01 (0.96–1.07) | 0.48 | 0 |
CVD, cardiovascular disease; Pheterogeneity value for heterogeneity among studies; Pinteraction value for heterogeneity between subgroups by meta-regression analysis.
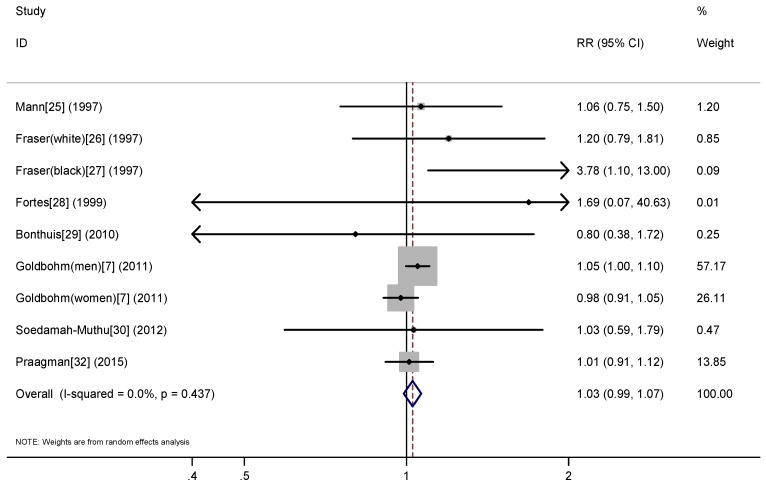
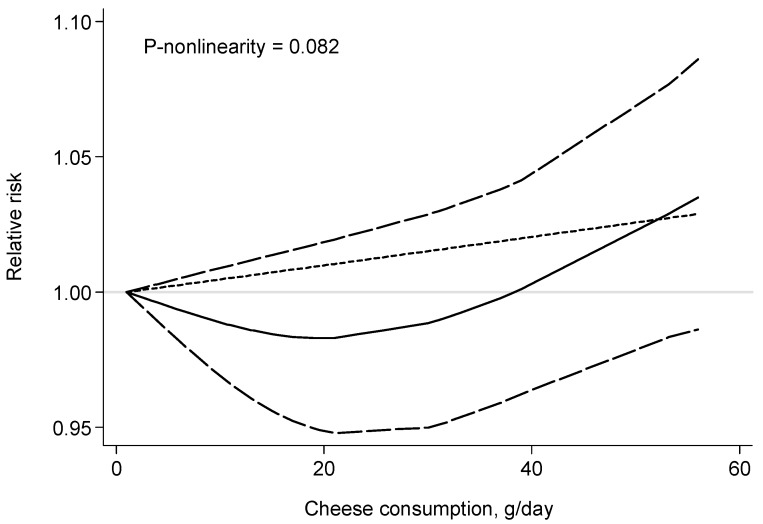
Eight prospective studies [6,23,24,25,26,27,28,30] were included in the dose-response analysis of cheese consumption and risk of all-cause mortality. The summary RR (for a 43 g/day increase in the cheese consumption) was 1.03 (95% CI: 0.99–1.07) with no evidence of heterogeneity (I2 = 0%) (Figure 3). There was no evidence for a nonlinear association between cheese consumption and risk of all-cause mortality (p for nonlinearity = 0.082) (Figure 4).
Figure 3.
Dose-response association between cheese consumption and risk of all-cause mortality, per 50 g/day.
Figure 4.
Relative risk with 95% confidence interval for the relationship between cheese consumption and risk of all-cause mortality in a restricted cubic spline random-effects meta-analysis.
4. Discussion
In this meta-analysis, we found no significant association between cheese consumption and all-cause mortality. Such a null association was consistent in stratified analysis by a number of study and population characteristics such as sex, geographical areas, baseline disease excluded, and the study quality. Dose-response analysis also found no significant association between cheese consumption and all-cause mortality. We found limited evidence for between-study heterogeneity or publication bias, and almost all studies had high quality scores (scores ≥ 8). The finding of this meta-analysis is similar with previous meta-analysis [33], which found no consistent association between milk consumption and all-cause mortality or cause-specific mortality.
Subgroup analysis based on limited numbers of studies suggested increased risk of all-cause mortality among studies followed up for <10 years but not among those followed up for ≥10 years. The result may be explained by the fact that subjects may change their diet over time and baseline measurements would not be representative for intakes during follow-up. On the other hand, those with chronic disease such as CVD and T2DM at baseline may eat more cheese in order to have good nutrition given that cheese consumption has been reported to be inversely associated with these diseases. Unfortunately, the studies with short follow-up duration were less adjusted for dietary factors.
Although we found no association between cheese consumption and risk of all-cause mortality, there are multiple nutrients in cheese that may contribute to health benefits, such as minerals, whey protein, vitamin K2 and specific types of fatty acids. Evidence suggests a neutral effect of cheese consumption on blood lipids [34,35,36], which may be due to the high content of calcium in cheese. Calcium could bind with fatty acids in the intestine to form insoluble soap and lead to reduced absorption of fat, promoting a higher excretion of fecal fat [37]. Cheese is also rich in whey protein, which has been shown to reduce weight gain and blood pressure, as well as expression of inflammation and oxidative stress markers, and ultimately reduce CVD [38]. Vitamin K2, which is exclusively synthesized by bacteria and present in cheese, has been shown to inhibit vascular calcification and plays an important role in preventing CVD and T2DM [39,40,41].
Cheese is also one of the major food sources of saturated fat, contributing to 7.7% of total solid fat intake [42]. Saturated fat intake is reported to increase plasma levels of low-density lipoprotein-cholesterol (LDL-C) [43], a well-established risk factor for CVD [44]. The 2015–2020 Dietary Guidelines for Americans [2] pointed out that intake of saturated fats in the diet should be limited to less than 10% of energy per day and replaced with unsaturated fats, which is associated with reduced blood levels of LDL-cholesterol, and a reduced risk of CVD events and CVD-related deaths. However, a recent meta-analysis [38] reported that saturated fats were not associated with all-cause mortality, CVD, CHD, ischemic stroke, or T2DM. Furthermore, not all foods rich in saturated fats are equivalent. A randomized controlled cross-over study [45] reported that saturated fat in the form of a cheese matrix decreased postprandial inflammation compared with plant sources of saturated fat in overweight and obese individuals. Cheese is one of a number of fermented dairy products in the market. For some time now, fermented foods have been popular in the nutrition industry for their purported health benefits. In a cohort study included in the present research, total fermented food intake was not found to be associated with mortality due to all causes [32].
While dairy fat does have appreciable levels of saturated fatty acids, it is made up of nearly 400 different fatty acids, including trans-fatty acids and branched-chain fatty acids [46]. All of these unique fatty acids have a biological significance [47]. In dairy fat, conjugated linoleic acids are associated with anti-atherogenic and anti-carcinogenic effects [48,49,50], while branched-chain fatty acids are resistant to oxidation [51]. Besides the levels of saturated fats, the levels of sodium in most cheeses should be considered. A meta-analysis with 23 cohort studies and two follow-up studies of RCTs showed both low sodium intakes and high sodium intakes are associated with increased all-cause mortality [52].
These findings suggest a complexity of components in cheese. Therefore, when providing dietary recommendations on high-fat dairy or cheese, overall health effects would be more informative when comparing effects of specific components in these foods.
Our study has several strengths. The present meta-analysis is based on prospective studies, so we have effectively avoided recall and selection bias. It also included multiple country-specific studies, and the total numbers of nation-specific participants and events appear reasonably robust. In addition, we conducted a further dose-response analysis to include data across different categories of exposure by converting cheese consumption from servings into a visualized unit (g/day), and therefore largely strengthened the statistical power of the analysis. Furthermore, there was little evidence of heterogeneity in the analysis. We also noticed the individual study whose characteristics differ from others, i.e., Fraser’s study, which enrolled Seventh-Day Adventists [26,27]. Because of the unique lifestyle characteristics in this population, no dietary factors were adjusted for in the cohort. In Forbes’ study, the subjects were followed up for only five years, which is the shortest among all studies [28]. Because the subjects were elderly with a mean age of 80 years old, the overall five-year survival was assessed. On the other hand, the largest [7] or lengthiest [32] studies also provided no evidence that cheese consumption is associated with mortality due to all causes, which is highly consistent with the summary RR of the meta-analysis. Sensitivity analysis demonstrated no impacts of any single study on the overall effect estimate.
Several limitations in this meta-analysis should be considered. First, the major potential confounders adjusted for in original studies differed and residual confounders may still exist. Second, measurement errors could affect our findings as assessment of cheese consumption largely depended on self-administered questionnaires. Third, the association with high-fat cheese or low-fat cheese was not addressed because few studies reported these findings [6]. Finally, although there was no evidence of publication bias, we cannot exclude such bias because of low statistical power due to limited number of studies.
5. Conclusions
In conclusion, findings of the present meta-analysis indicate that cheese consumption is not significantly associated with risk of all-cause mortality. Future large prospective studies that distinguish between high-fat and low-fat cheese are warranted.
Acknowledgments
The study was supported by grants from the National Natural Science Foundation of China (No. 81673101, 81472974, 81273067) and the ‘Blue Project’ of Jiangsu Province.
Author Contributions
Xing Tong designed the research, interpreted the data, and drafted the manuscript. Guo-Chong Chen performed the statistical analysis and made critical revision of the manuscript. Zheng Zhang and Yu-Lu Wei made critical revision of the manuscript. Jia-Ying Xu and Li-Qiang Qin handled funding and made critical revision of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hinrichs J. Mediterranean milk and milk products. Eur. J. Nutr. 2004;43(Suppl. 1):i12–i17. doi: 10.1007/s00394-004-1104-8. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services and U.S. Department of Agriculture 2015–2020 Dietary Guidelines for Americans. [(accessed on 20 July 2016)];2015 Dec; Available online: http://health.Gov/dietaryguidelines/2015/guidelines/
- 3.Aune D., Norat T., Romundstad P., Vatten L.J. Dairy products and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Am. J. Clin. Nutr. 2013;98:1066–1083. doi: 10.3945/ajcn.113.059030. [DOI] [PubMed] [Google Scholar]
- 4.Chen G.C., Wang Y., Tong X., Szeto I.M., Smit G., Li Z.N., Qin L.Q. Cheese consumption and risk of cardiovascular disease: A meta-analysis of prospective studies. Eur. J. Nutr. 2016 doi: 10.1007/s00394-016-1292-z. [DOI] [PubMed] [Google Scholar]
- 5.Aune D., Navarro Rosenblatt D.A., Chan D.S., Vieira A.R., Vieira R., Greenwood D.C., Vatten L.J., Norat T. Dairy products, calcium, and prostate cancer risk: A systematic review and meta-analysis of cohort studies. Am. J. Clin. Nutr. 2015;101:87–117. doi: 10.3945/ajcn.113.067157. [DOI] [PubMed] [Google Scholar]
- 6.Jiang W., Ju C., Jiang H., Zhang D. Dairy foods intake and risk of Parkinson’s disease: A dose-response meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2014;29:613–619. doi: 10.1007/s10654-014-9921-4. [DOI] [PubMed] [Google Scholar]
- 7.Goldbohm R.A., Chorus A.M., Galindo Garre F., Schouten L.J., van den Brandt P.A. Dairy consumption and 10-y total and cardiovascular mortality: A prospective cohort study in The Netherlands. Am. J. Clin. Nutr. 2011;93:615–627. doi: 10.3945/ajcn.110.000430. [DOI] [PubMed] [Google Scholar]
- 8.Dugani S., Gaziano T.A. 25 by 25: Achieving global reduction in cardiovascular mortality. Curr. Cardiol. Rep. 2016;18:10. doi: 10.1007/s11886-015-0679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., Moher D., Becker B.J., Sipe T.A., Thacker S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (moose) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 10.Walter S.D., Cook R.J. A comparison of several point estimators of the odds ratio in a single 2 × 2 contingency table. Biometrics. 1991;47:795–811. doi: 10.2307/2532640. [DOI] [PubMed] [Google Scholar]
- 11.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 12.Greenland S., Longnecker M.P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am. J. Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 13.Astrup A., Rice Bradley B.H., Brenna J.T., Delplanque B., Ferry M., Torres-Gonzalez M. Regular-fat dairy and human health: A synopsis of symposia presented in Europe and north America (2014–2015) Nutrients. 2016;8:463. doi: 10.3390/nu8080463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsson S.C., Orsini N., Wolk A. Dietary magnesium intake and risk of stroke: A meta-analysis of prospective studies. Am. J. Clin. Nutr. 2012;95:362–366. doi: 10.3945/ajcn.111.022376. [DOI] [PubMed] [Google Scholar]
- 15.Orsini N., Li R., Wolk A., Khudyakov P., Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: Examples, an evaluation of approximations, and software. Am. J. Epidemiol. 2012;175:66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 18.De Oliveira Otto M.C., Mozaffarian D., Kromhout D., Bertoni A.G., Sibley C.T., Jacobs D.R., Jr., Nettleton J.A. Dietary intake of saturated fat by food source and incident cardiovascular disease: The multi-ethnic study of atherosclerosis. Am. J. Clin. Nutr. 2012;96:397–404. doi: 10.3945/ajcn.112.037770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Von Ruesten A., Feller S., Bergmann M.M., Boeing H. Diet and risk of chronic diseases: Results from the first 8 years of follow-up in the epic-potsdam study. Eur. J. Clin. Nutr. 2013;67:412–419. doi: 10.1038/ejcn.2013.7. [DOI] [PubMed] [Google Scholar]
- 20.Sonestedt E., Wirfalt E., Wallstrom P., Gullberg B., Orho-Melander M., Hedblad B. Dairy products and its association with incidence of cardiovascular disease: The malmo diet and cancer cohort. Eur. J. Epidemiol. 2011;26:609–618. doi: 10.1007/s10654-011-9589-y. [DOI] [PubMed] [Google Scholar]
- 21.Avalos E.E., Barrett-Connor E., Kritz-Silverstein D., Wingard D.L., Bergstrom J.N., Al-Delaimy W.K. Is dairy product consumption associated with the incidence of CHD? Public Health Nutr. 2013;16:2055–2063. doi: 10.1017/S1368980012004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iso H., Stampfer M.J., Manson J.E., Rexrode K., Hennekens C.H., Colditz G.A., Speizer F.E., Willett W.C. Prospective study of calcium, potassium, and magnesium intake and risk of stroke in women. Stroke. 1999;30:1772–1779. doi: 10.1161/01.STR.30.9.1772. [DOI] [PubMed] [Google Scholar]
- 23.Larsson S.C., Virtamo J., Wolk A. Dairy consumption and risk of stroke in Swedish women and men. Stroke. 2012;43:1775–1780. doi: 10.1161/STROKEAHA.111.641944. [DOI] [PubMed] [Google Scholar]
- 24.Larsson S.C., Mannisto S., Virtanen M.J., Kontto J., Albanes D., Virtamo J. Dairy foods and risk of stroke. Epidemiology. 2009;20:355–360. doi: 10.1097/EDE.0b013e3181935dd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mann J.I., Appleby P.N., Key T.J., Thorogood M. Dietary determinants of ischaemic heart disease in health conscious individuals. Heart. 1997;78:450–455. doi: 10.1136/hrt.78.5.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fraser G.E., Shavlik D.J. Risk factors for all-cause and coronary heart disease mortality in the oldest-old. The Adventist Health Study. Arch. Intern. Med. 1997;157:2249–2258. doi: 10.1001/archinte.1997.00440400099012. [DOI] [PubMed] [Google Scholar]
- 27.Fraser G.E., Sumbureru D., Pribis P., Neil R.L., Frankson M.A. Association among health habits, risk factors, and all-cause mortality in a black California population. Epidemiology. 1997;8:168–174. doi: 10.1097/00001648-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Fortes C., Forastiere F., Farchi S., Rapiti E., Pastori G., Perucci C.A. Diet and overall survival in a cohort of very elderly people. Epidemiology. 2000;11:440–445. doi: 10.1097/00001648-200007000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Bonthuis M., Hughes M.C., Ibiebele T.I., Green A.C., van der Pols J.C. Dairy consumption and patterns of mortality of Australian adults. Eur. J. Clin. Nutr. 2010;64:569–577. doi: 10.1038/ejcn.2010.45. [DOI] [PubMed] [Google Scholar]
- 30.Soedamah-Muthu S.S., Masset G., Verberne L., Geleijnse J.M., Brunner E.J. Consumption of dairy products and associations with incident diabetes, CHD and mortality in the Whitehall II study. Br. J. Nutr. 2013;109:718–726. doi: 10.1017/S0007114512001845. [DOI] [PubMed] [Google Scholar]
- 31.Van Aerde M.A., Soedamah-Muthu S.S., Geleijnse J.M., Snijder M.B., Nijpels G., Stehouwer C.D., Dekker J.M. Dairy intake in relation to cardiovascular disease mortality and all-cause mortality: The Hoorn study. Eur. J. Nutr. 2013;52:609–616. doi: 10.1007/s00394-012-0363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Praagman J., Dalmeijer G.W., van der Schouw Y.T., Soedamah-Muthu S.S., Monique Verschuren W.M., Bas Bueno-de-Mesquita H., Geleijnse J.M., Beulens J.W. The relationship between fermented food intake and mortality risk in the European prospective investigation into cancer and nutrition-Netherlands cohort. Br. J. Nutr. 2015;113:498–506. doi: 10.1017/S0007114514003766. [DOI] [PubMed] [Google Scholar]
- 33.Larsson S.C., Crippa A., Orsini N., Wolk A., Michaelsson K. Milk consumption and mortality from all causes, cardiovascular disease, and cancer: A systematic review and meta-analysis. Nutrients. 2015;7:7749–7763. doi: 10.3390/nu7095363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Goede J., Geleijnse J.M., Ding E.L., Soedamah-Muthu S.S. Effect of cheese consumption on blood lipids: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2015;73:259–275. doi: 10.1093/nutrit/nuu060. [DOI] [PubMed] [Google Scholar]
- 35.Nilsen R., Hostmark A.T., Haug A., Skeie S. Effect of a high intake of cheese on cholesterol and metabolic syndrome: Results of a randomized trial. Food Nutr. Res. 2015;59:27651. doi: 10.3402/fnr.v59.27651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huth P.J., Park K.M. Influence of dairy product and milk fat consumption on cardiovascular disease risk: A review of the evidence. Adv. Nutr. 2012;3:266–285. doi: 10.3945/an.112.002030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christensen R., Lorenzen J.K., Svith C.R., Bartels E.M., Melanson E.L., Saris W.H., Tremblay A., Astrup A. Effect of calcium from dairy and dietary supplements on faecal fat excretion: A meta-analysis of randomized controlled trials. Obes. Rev. 2009;10:475–486. doi: 10.1111/j.1467-789X.2009.00599.x. [DOI] [PubMed] [Google Scholar]
- 38.Sousa G.T., Lira F.S., Rosa J.C., de Oliveira E.P., Oyama L.M., Santos R.V., Pimentel G.D. Dietary whey protein lessens several risk factors for metabolic diseases: A review. Lipids Health Dis. 2012;11:67. doi: 10.1186/1476-511X-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gast G.C., de Roos N.M., Sluijs I., Bots M.L., Beulens J.W., Geleijnse J.M., Witteman J.C., Grobbee D.E., Peeters P.H., van der Schouw Y.T. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr. Metab. Cardiovasc. Dis. NMCD. 2009;19:504–510. doi: 10.1016/j.numecd.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Beulens J.W., van der A.D., Grobbee D.E., Sluijs I., Spijkerman A.M., van der Schouw Y.T. Dietary phylloquinone and menaquinones intakes and risk of type 2 diabetes. Diabetes Care. 2010;33:1699–1705. doi: 10.2337/dc09-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao D., Ning N., Wang C., Wang Y., Li Q., Meng Z., Liu Y., Li Q. Dairy products consumption and risk of type 2 diabetes: Systematic review and dose-response meta-analysis. PLoS ONE. 2013;8:e73965. doi: 10.1371/journal.pone.0073965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dietary Guidelines Advisory Committee . Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 2010. USDA, Agricultural Research Service; Washington, DC, USA: 2011. [Google Scholar]
- 43.Mensink R.P., Zock P.L., Kester A.D., Katan M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 44.Steinberg D. Thematic review series: The pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy: Part II: The early evidence linking hypercholesterolemia to coronary disease in humans. J. Lipid Res. 2005;46:179–190. doi: 10.1194/jlr.R400012-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Demmer E., van Loan M.D., Rivera N., Rogers T.S., Gertz E.R., German J.B., Zivkovic A.M., Smilowitz J.T. Consumption of a high-fat meal containing cheese compared with a vegan alternative lowers postprandial C-reactive protein in overweight and obese individuals with metabolic abnormalities: A randomised controlled cross-over study. J. Nutr. Sci. 2016;5:e9. doi: 10.1017/jns.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Donnell A.M., Spatny K.P., Vicini J.L., Bauman D.E. Survey of the fatty acid composition of retail milk differing in label claims based on production management practices. J. Dairy Sci. 2010;93:1918–1925. doi: 10.3168/jds.2009-2799. [DOI] [PubMed] [Google Scholar]
- 47.German J.B., Gibson R.A., Krauss R.M., Nestel P., Lamarche B., van Staveren W.A., Steijns J.M., de Groot L.C., Lock A.L., Destaillats F. A reappraisal of the impact of dairy foods and milk fat on cardiovascular disease risk. Eur. J. Nutr. 2009;48:191–203. doi: 10.1007/s00394-009-0002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lock A.L., Horne C.A., Bauman D.E., Salter A.M. Butter naturally enriched in conjugated linoleic acid and vaccenic acid alters tissue fatty acids and improves the plasma lipoprotein profile in cholesterol-fed hamsters. J. Nutr. 2005;135:1934–1939. doi: 10.1093/jn/135.8.1934. [DOI] [PubMed] [Google Scholar]
- 49.Ip C., Scimeca J.A., Thompson H.J. Conjugated linoleic acid. A powerful anticarcinogen from animal fat sources. Cancer. 1994;74:1050–1054. doi: 10.1002/1097-0142(19940801)74:3+<1050::aid-cncr2820741512>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 50.Dong J.Y., Zhang L., He K., Qin L.Q. Dairy consumption and risk of breast cancer: A meta-analysis of prospective cohort studies. Breast Cancer Res. Treat. 2011;127:23–31. doi: 10.1007/s10549-011-1467-5. [DOI] [PubMed] [Google Scholar]
- 51.Kaneda T. Iso- and anteiso-fatty acids in bacteria: Biosynthesis, function, and taxonomic significance. Microbiol. Rev. 1991;55:288–302. doi: 10.1128/mr.55.2.288-302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Graudal N., Jürgens G., Baslund B., Alderman M.H. Compared with usual sodium intake, low- and excessive-sodium diets are associated with increased mortality: A meta-analysis. Am. J. Hypertens. 2014;27:1129–1137. doi: 10.1093/ajh/hpu028. [DOI] [PubMed] [Google Scholar]