Abstract
Kaposi sarcoma (KS) is an aggressive cancer caused by human herpesvirus-8, primarily seen in immunocompromised patients. As opposed to the well-described cutaneous manifestations and pulmonary complications of KS, hepatic KS is rarely reported before death as most patients with hepatic KS do not manifest symptoms or evidence of liver injury. In patients with acquired immune deficiency syndrome, hepatic involvement of KS is present in 12%-24% of the population on incidental imaging and in approximately 35% of patients with cutaneous KS if an autopsy was completed after their death. Patients with clinically significant hepatic injury due to hepatic KS usually have an aggressive course of disease with hepatic failure often progressing to multi-organ failure and death. Here we report an unusual presentation of acute liver injury due to hepatic KS and briefly review the published literature on hepatic KS.
Keywords: Herpesvirus 8, Acquired immune deficiency syndrome-related Kaposi sarcoma, Acquired immune deficiency syndrome hepatopathy, Human, Kaposi sarcoma
Core tip: Hepatic Kaposi sarcoma (KS) is a clinical presentation that disproportionately affects the human immunodeficiency virus/acquired immune deficiency syndrome (AIDS) population. Up to 34% of patients with AIDS and KS have hepatic involvement. Usually hepatic KS is clinically indolent and diagnosed during autopsy. When clinically significant, hepatic KS presents with evidence of liver injury with elevation in bilirubin and liver enzymes, has characteristic findings on imaging and may progress to liver failure and death. Treatment is indicated in patients with progressive and symptomatic hepatic disease in the absence of other etiologies.
CASE PRESENTATION
A forty-eight years old African American male with HIV and CD4 count of 8/μL presented with conjunctival icterus. Physical exam showed cachexia, icterus, a violaceous 1 cm plaque on the soft palate and similar lesion on the chest wall, and a soft, non-distended, non-tender abdomen. He denied prior treatment with antiretroviral medications. Laboratory studies were significant for AST (SPGT) 172 U/L, ALT (SGOT) 201 U/L, total bilirubin of 20.0 mg/dL, direct bilirubin 14.9 mg/dL, alkaline phosphatase 947 U/L, INR 2.5, and platelet count 52000/μL. Acute and chronic serologies for hepatitis A, B and C, histoplasma, and cytomegalovirus as well as a toxicology screen were negative. Patient did report taking cotrimoxazole for five days which he completed a month prior to presentation. Magnetic resonance imaging (MRI) of the Abdomen showed innumerable 10 mm T2 intense hepatic nodules without enhancement (Figure 1). Liver biopsy was positive for Cytokeratin 7 and human herpes virus-8, consistent with infiltrative Kaposi sarcoma (KS) (Figures 2 and 3). There was no evidence of drug induced liver injury on histopathology. There was no lymphadenopathy indicative of hemophagocytic lymphohistiocytosis or Castleman’s disease. The diagnosis was most consistent with acute liver injury (ALI) secondary to infiltrative hepatic KS, stage T1, I1, S1. The patient was not a candidate for cytotoxic therapy given progressive liver injury and was started on rituximab and ganciclovir. Liver injury progressed and was further complicated by acute kidney injury, hypoxic respiratory failure, consumptive coagulopathy and septic shock. The patient received broad-spectrum antibiotics, blood products, vasopressors and ventilator support but unfortunately expired.
Figure 1.
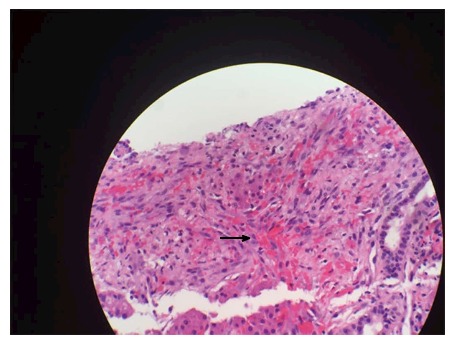
Spindle cells with cytokeratin 7 staining positive.
Figure 2.
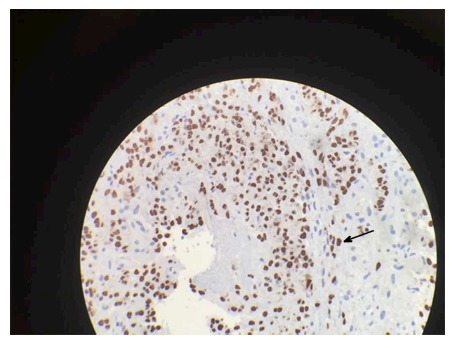
Positive human herpersvirus-8 immunohistochemical staining.
Figure 3.
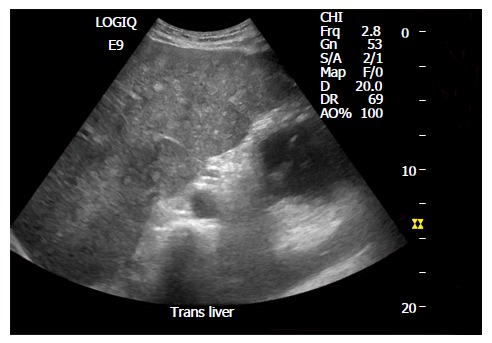
Ultrasound image with multiple small round hyperechoic nodules.
BACKGROUND
KS is an angioproliferative low-grade neoplasm that is associated with human herpesvirus-8 (HHV-8). KS can be codified into different clinical variants depending on the patient cohort and the presentation of the disease[1]. The “classical” form primarily affects men of Ashkenazic Jewish or Mediterranean background and follows an indolent cutaneous course. The “African endemic” form of the disease commonly affects Africans as the name implies, presents with lymphadenopathy and is usually fatal within 1-3 years. The “iatrogenic” form is due to HHV-8 activation caused by medical immunosuppression from treatment of autoimmune disorders or post-organ transplantation. The fourth and most common variant, acquired immune deficiency syndrome (AIDS)-related KS, is rapidly progressive and holds the highest rate of hepatic involvement[2].
The most common presentation of KS is a cutaneous papular disease with lesions on the legs, oral cavity, and genitalia. However, the most common site of visceral organ involvement is the gastrointestinal tract[3]. First described by Moritz Kaposi in 1872, hepatic KS was an autopsy diagnosis that rarely resulted in clinically significant disease or ALI[4]. To further understand hepatic KS, a systematic search of the literature was conducted on PubMed (1954 to 2015), EBSCO HOST (1956 to 2015), the Cochrane Database of Systematic Review, and the OVID interface (1946 to 2015) with comprehensive search terms as documented in Table 1.
Table 1.
MeSH search terms
| Liver |
| Hepatopathy |
| Hepatitis |
| Hepatology |
| Cholestatic injury |
| Hepatocellular injury |
| Kaposi sarcoma |
| Herpesvirus 8, human |
| AIDS-related Kaposi sarcoma |
| Non-AIDS-related Kaposi sarcoma |
| Liver neoplasms |
AIDS: Acquired immune deficiency syndrome.
EPIDEMIOLOGY
While most herpesviruses are widespread in the adult population, the prevalence of HHV-8 varies with human immunodeficiency virus (HIV) status and exposure risk factors. In the United States, only 5% of HIV uninfected men are seropositive for HHV-8 compared to 25%-60% of HIV-positive men who have sex with men (MSM)[5]. These rates are reflective of HHV-8 and HIV co-infection in MSM in other countries as well[6].
AIDS-patients have 20000 times greater risk of developing KS than the general population. Patients on HAART with a CD4 count of < 200 cells/μL are 18.9 times more likely to have KS than those with CD4 ≥ 500 cells/μL[7]. In the era of ARV therapy, improved control of HIV viremia and preserved CD4 T-cell function has lead to an 80% decreased incidence of AIDS-associated KS[8], AIDS-related KS currently affects < 1% of AIDS patients, compared to 15% in the pre-HAART era[9].
MODES OF TRANSMISSION
Behavioral risk factors for HHV-8 transmission are incompletely understood. Saliva exchange appears to an important factor with HHV-8 DNA detected in the saliva of 61% of HHV-8-infected MSM[10,11]. With HHV-8 seropositivity higher in the MSM population, commercial sex workers and those with other sexually transmitted infections, a sexual route of transmission has also been proposed. HHV-8 DNA can be isolated from semen and vaginal secretions, but viral load is lower than that found in saliva, calling into question the clinical significance of sexual transmission[12]. HHV-8 seroprevalence in HIV-infected injection drug users is substantially lower than hepatitis B and C rates in HIV-infected MSM[13]. This finding is suggestive that blood exchange through contaminated needle sharing is a less significant route of HHV-8 transmission compared to salivary or sexual contact[14].
HEPATIC INVOLVEMENT
Hepatic KS is typically asymptomatic and rarely diagnosed in life. Therefore the true incidence of hepatic KS is not well-documented and is limited to small case series and reports. Prevalence of hepatic KS has primarily been determined from autopsy series with small sample sizes, which accounts for the wide variation in prevalence reported. In one autopsy series, approximately 34% of AIDS-related KS cases involved the liver while in another report, 8.3% had liver involvement[15,16]. In another retrospective review, hepatic involvement was present in 9 of 41 patients or 22% of cases of AIDS-related KS in post mortem dissection[17]. In this study ante-mortem hepatic KS was suspected in only one patient, in whom a computerized tomography (CT) scan demonstrated hepatosplenomegaly with a confluence of hypodense lesions in the left hepatic lobe. Autopsy confirmed disseminated KS. Schneiderman et al[18] found KS on liver biopsy in 18.6% of AIDS patients making KS the most common hepatic pathological diagnosis caused by AIDS. All of these patients already had a diagnosis of extrahepatic KS at time of biopsy. In contrast, 66% of patients with extrahepatic KS did not manifest hepatic involvement.
As mentioned above, most cases of hepatic KS were not clinically significant. In the study by Schneiderman et al[18], there were no statistically significant differences in transaminases, lactic dehydrogenase, alkaline phosphatase or bilirubin based on liver involvement with KS. However, in the few reported patients with clinically significant disease, a rapid progression to liver and multi-organ failure has been reported, usually with fatal outcomes (Table 2).
Table 2.
Outcomes in patients with clinically symptomatic hepatic Kaposi sarcoma
| Age (yr) | Sex | HIV status | CD4 count (cells/mm3) | Liver chemistry profile | Pathology | Treatment | Hospital course + complications |
| 45[63] | M | (+) | 192 | T Bil 19.35 ALP 1309 AST 204 ALT 188 GGT 827 | HHV-8 PCR VL (+) 24000 copies/mL. Liver biopsy revealed features of KS with spindle cells, extravasation of red blood cells and haemosiderin deposition. IHC staining HHV8 (+) | Paclitaxel, Montelukast | Continued on chemotherapy. Subsequently developed respiratory and renal failure, anemia and thrombocytopenia from aggressive metastatic KS |
| 36[64] | M | (+) | 17 | PTT 70 (s) ALT 185 T Bil 23 | Necroscopy showed bile duct proliferation with diffuse fibrosis with lymphohistiocytic infiltration | Liposomal doxorubicin | Jaundice, renal failure, fulminant liver failure |
| 28[65] | M | (+) | NR | NR | Biopsy residues of spindle cells lining portal tracts. Immunoperoxidase staining factor VIII (+) | Palliative care | Liver function continued to decline and patient died from respiratory failure two weeks later |
| 38[66] | M | (+) | < 2001 | AST 147 ALT 180 ALP 573 | Gross specimen with fibrous thickening of portal tracts and dark red nodules in periportal areas and diffusely infiltrating liver parenchyma | Chemotherapy, NOS | Partial cutaneous response, died several weeks later |
| 40[4] | M | (+) | NR | Reportedly, “normal” | KS present on biopsy of lymph nodes. US with three 7-12 mm hyperechoic nodules. Periportal groups of dilated blood filled cavernous spaces lined by flat endothelial cells and interspersed of spindle cells. Extravasated erythrocytes and minimal hemosiderin deposits | Combination Chemotherapy, NOS | Complete remission of cutaneous lesions and reduction in size of two of the lesions with the third not visible. Readmitted six months later for severe relapse of cutaneous KS. Reinitiated chemotherapy with rapid deterioration and death within one month |
| 48[67] | M | (+) | 8 | TBili 20.0 ALP 947 AST 186 ALT 155 INR 1.9 | Liver biopsy was Cytokeratin-7 and HHV-8 staining positive | Ganciclovir and Rituximab | Presented with jaundice and acute liver injury with a cholestatic pattern, progressed to fulminant hepatic failure and ultimately death |
| 44[68] | M | (+) | CD4/CD8 ratio 0.08 | AST 153 ALT 124 ALP 1228 | Laproscopy demonstrated enlarged liver with multiple purple 2-3 mm nodules Biopsy demonstrated spindle cells, vascular slits, extravasated red cells and lymphocytic infiltration | Platinum based chemotherapy, NOS | Primary hepatic manifestations without cutaneous lesions. Persistent abdominal pain after treatment. Progressed to cutaneous lesions six weeks after treatment. Lost to follow-up |
Less than 200, not otherwise reported. M: Male; F: Female; TBili: Total Bilirubin (units, mg/dL); ALP: Alkaline phosphatase (units, IU/L); AST: Aspartate transaminase (IU/L); ALT: Alanine transaminase (IU/L); GGT: Gamma glutamyl transpeptidase (units, IU/L); HHV-8: Human herpes virus-8; PCR: Polymerase chain reaction; VL: Viral load; IHC: Immunohistochemistry; NR: Not reported; NOS: Not otherwise specified; US: Ultrasound; KS: Kaposi sarcoma.
In the non-HIV population, the incidence of KS is 0.2% in liver transplant patients from the United States, but the prevalence is higher in patients from Africa, the Middle East or the Mediterranean[19]. KS affected 4.7% of renal transplant patients in Saudi Arabia, 2.4% of recipients in Israel and 0.52% of recipients in France. While there is a well described clinical burden of post-transplant lymphoproliferative disorder including cutaneous and visceral manifestations of KS, there is no described literature of post-transplant hepatic KS.
PATHOGENESIS OF HHV-8 ASSOCIATED TUMORS
HHV-8 consists of a large double stranded DNA genome that includes approximately 145 kilobases (kbases) long region encodinging all the expressed viral genes, flanked by approximately 20-30 kbases that encode a number of mimicked human genes, several of which have immunologic or angiogenic properties[20,21]. HHV-8 has a tropism for both hematopoietic and non-hematopoietic cells including monocytes, B cells, endothelial cells and also hepatocytes[22]. Endothelial cells appear to be the most important host cells for oncogenic transformation as HHV-8 infection of these cells leads to their long-term proliferation and survival[23]. Similar to other herpesviruses, HHV-8 alternates between two metabolic cycles: Latent infection, where few genes are expressed, and the active lytic infection, where viral replication and multiple gene expression occurs. Lytic replication can be induced by oxidative stress, hypoxia, inflammatory cytokines, chemical exposure or concomitant infections, including HIV[24-27].
In hepatocytes infected with HHV8 genome by DNA polymerase chain reaction amplification, immunohistochemistry demonstrates expression of the transcriptional regulator, latency-associated nuclear antigen-1 (LANA-1)[28]. It has also been directly implicated in oncogenesis because of its ability to bind to the tumour-suppressing protein p53[29]. Furthermore, hepatocyte growth factor/scatter factor, a kinase that mediates epithelial cell proliferation and angiogenesis[30] has been demonstrated to induce HHV-8 lytic replication, providing a means of KS progression in the liver[31].
DIAGNOSIS OF KS
A full integumentary survey including oral and rectal examination quantifying extent of disease should be completed in patients with suspected KS. Cutaneous or visceral biopsy is required for diagnosis. On gross pathology, there are usually multiple, grossly irregular, variable sized red-brown spongiform nodules seen in the periportal connective tissue[32]. Histopathologic features of disease include thin walled vascular formations and inflammatory infiltration. Spindle cell formation is also characteristic of angioproliferative HHV-8-infected cells that have undergone reprogramming of vascular endothelium and tumorigenesis[33]. Immunohistochemical staining is characteristic for HHV-8 LANA expression within the spindle cell formation[34]. Immunohistochemistry staining for endothelial cell markers factor VIII, CD31, CD34 and lymphatic vessel endothelial receptor 1 further corroborate a diagnosis of KS[35].
Further investigation of visceral KS is warranted in the presence of adenopathy or occult bleeding. Patients with cutaneous KS and iron deficiency anemia, fecal occult blood or gastrointestinal symptoms warrant GI endoscopic evaluation. Patients with cutaneous KS and concurrent adenopathy should receive CT of the chest, abdomen, and pelvis to evaluate for visceral KS and HHV-8-related lymphoproliferative disorders including primary effusion lymphoma, Castleman disease, and plasmablastic lymphoma[36].
KS STAGING AND PROGNOSIS
As KS is a disseminated angioproliferative virally mediated malignancy, classic tumor, node, metastasis staging as used in other cancers does not accurately prognosticate disease or dictate treatment. AIDS Clinical Trials Group (ACTG) Oncology Committee has codified staging of AIDS-associated KS[37]. The ACTG staging system risk stratifies patients low risk (0) or high risk (1) based on three criteria: Tumor burden (T), immune status (I), and systemic illness (S). For tumor burden, poor risk (T1) is defined by presence of extensive cutaneous, oral disease or visceral disease. For immune status, poor risk (I1) is defined by CD4 count of less than 150 cells/μL. For systemic illness, poor risk (S1) is defined by the presence of constitutional symptoms, poor performance status, or other opportunistic infections. While these criteria were validated in the pre-ART era, post-ART therapy, a CD4 cutoff of 100 cells/mm3 has an unclear role in predicting mortality[38,39].
IMAGING IN HEPATIC KS
Hepatic KS has characteristic findings on individual imaging modalities that can help delineate clinically significant disease. Abdominal ultrasound imaging of the liver can demonstrate inhomogeneous cystic lesions with hyperechoic bands and nodules along the peripheral branches of portal veins. Likewise, computed tomography of the abdomen is characteristic for inhomogeneous hepatomegaly with multiple small hypodense nodules, often in the periportal area[40] (Figure 4). Mild hepatomegaly is a non-specific finding in 19% of patients with AIDS-related KS[41]. MRI shows hyperintense nodules on T1-weighted in-phase imaging and hypointense nodules on T1- weighted out-of-phase imaging (Figure 5). Neither T2-weighted imaging nor late hepatobiliary volumetric interpolated breath hold examination have any specific findings in hepatic KS[42].
Figure 4.
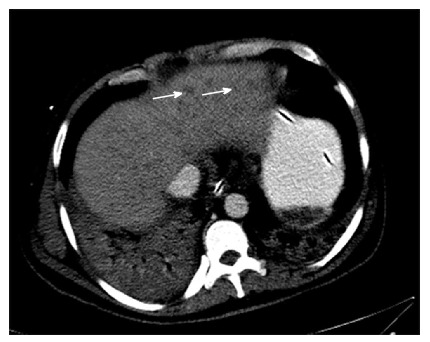
Computerized tomography scan enlarged inhomogeneous liver with multiple hypodense lesions.
Figure 5.
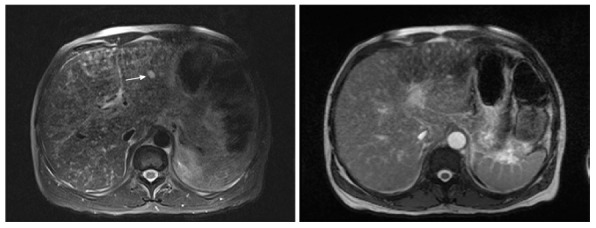
SPAIR and T2 images Kaposi sarcoma on magnetic resonance imaging.
Image guided biopsy of hepatic nodules in patients suspected to have liver involvement demonstrate hyaline globules, hemosiderin accumulation, macrovaculoar steatosis, large fibrotic portal spaces, bile duct ectasia, neoductogenesis and spindle cells with large, irregular nuclei. Staining of the perinodular tissues is positive for CD31, CD34 and factor VIII as can be seen in extrahepatic KS as well.
TREATMENT
As shown in Table 2, hepatic KS is predominantly manifested in patients with HIV/AIDS. While overall HIV mortality is improving in the era of ARV therapy, patients with AIDS-associated KS have an increased risk of death, compared to HIV controls, irrespective of CD4 count[43]. HIV-infected patients initiating ARV commonly have progression of their KS lesions[44]. However, long term ARV therapy is associated with a reduced incidence of KS. Guidelines currently recommend correcting underlying immunodeficiency by treating AIDS with ARV therapy. Studies indicate that control of KS progression is related to the degree of control of HIV, rather than the specific cART regimen utilized[45]. Beyond ARVs, a variety of systemic therapies may be used in KS. Usually systemic therapy is indicated in progressive disease, with symptomatic visceral involvement, in the presence of immune reconstitution inflammatory syndrome (IRIS) or with extensive cutaneous involvement. These strategies are not specific to hepatic dysfunction in the setting of KS.
Radiotherapy is a well-established treatment and has a robust clinical response for classic nodular KS but tends to be a palliative approach. While it may be a good modality for superficial lesions, electron beam radiation therapy (EBRT) has limited penetration below the dermis; deeper or unresponsive KS may be treated with standard non-EBRT approaches[46].
Retinoid products appear to inhibit IL-6, a cytokine implicated in KS pathogenesis, and have an antiproliferative effect on KS lesions[47]. Application of alitretinoin can reduce cutaneous lesions of both classic and HIV-KS but has no role in systemic disease[48].
The role of chemotherapy in addition to standard antiretroviral therapy has been explored. A meta-analysis of studies demonstrated that although chemotherapy in addition to ARVs did not have a mortality benefit, it did reduce disease progression[49]. Current first-line therapy for advanced AIDS-KS is liposomal anthracyclines, including pegylated liposomal doxorubicin (PLD). In a randomized control trial (RCT), PLD demonstrated superiority to previous conventional chemotherapy, bleomycin and vincristine with 58.7% vs 23.3% (P < 0.001) response rate and a decreased adverse event rate (10.7% vs 26.7%)[50]. Another RCT of liposomal daunorubicin verses doxorubicin, bleomycin and vincristine showed no statistical difference in response rate or disease progression (25% vs 28%)[51]. When the analysis was restricted to patients receiving prior zidovudine, however, survival was improved in the liposomal daunorubicin group. Another non-randomized study showed a trend toward mortality benefit with liposomal doxorubicin as compared to bleomycin plus vinblastine, vincristine or ARV monotherapy alone, although this did not reach statistical significance[52].
Interferon-alpha, has an array of antiviral and antiangiogenic properties with efficacy in AIDS-KS, but its use is limited due to hepatotoxicity[48].
Paclitaxel has systemic response rates from 59%-71% and is approved as second-line treatment for KS. In randomized controls, paclitaxel does not demonstrate benefit over PLD in complete or partial remission and no mortality data were available according to KS staging[53]. Less well tolerated than doxorubicin, adverse events include peripheral neuropathies, cytopenias, and gastrointestinal upset. Third line agents for AIDS related visceral KS include etoposide, bleomycin, vinblastine, and vincristine with overall response rates ranging from 23% to 36%. The median survival times are 11 (6 to 20) mo in the bleomycin only group and 13 (7 to 36) mo in the ABV group. With extensive side effect profiles, including secondary malignancies, these treatment modalities are maintained in resource-limited settings[54,55].
Although HAART with or without chemotherapy is the current recommended treatment, novel targets are being explored including inhibitors of angiogenesis and matrix metalloproteinases. These drugs are currently in various phases of clinical trials[56]. Inhibition of HHV-8 replication with agents such as foscarnet and ganciclovir have also been explored[57].
Finally, it bears mentioning that treatment for HIV/AIDS in patients co-infected with HHV-8 can cause a paradoxical worsening of disease. In the KS AIDS AntiRetroviral Therapy Trial, 23/112 (21%) of co-infected patients receiving ARV therapy developed KS-IRIS, which was defined as a rapid worsening of KS beyond its natural course within 12 wk of initiating ARV therapy. Of those 23 patients, 10 died, 9 of which had visceral KS. Eighteen patients in the study overall (16%) had worsening elevation in their liver enzymes and two patients (1.8%) died of liver failure. In this study, exclusion criteria included HIV-KS patients with direct serum bilirubin > 85 μmol/L or aspartate aminotransferase or alanine aminotransferase > 2.5 times the normal range[39].
Biologic and targeted molecular therapies may have a supplementary or alternative role in AIDS-KS, but are currently in early stages of clinical trials. In the AIDS Malignancy Consortium, a phase II trial of imantinib with a small sample size showed a partial response in approximately one third of patients[58]. In another study focusing on patients who did not respond to chemotherapy and chimeric antigen receptor T cell therapy, bevacizumab, an anti-vascular endothelial growth-factor monoclonal antibody, had a response rate in again approximately one third of patients[59]. The cytokine, interleukin-12 had a response rate of 71% in small phase I and phase II trials. However, patients were ineligible if they had transaminitis or a history of hepatic disease[60]. Ongoing studies include a phase II trial for the utility of combined PLD and bevacizumab in the treatment of advanced AIDS-KS[61] and a phase I study for dosing and side effect profile of combination therapy with ipilimumab, a cytotoxic T-lymphocyte antigen 4 antibody and nivolumab, an antibody against programmed cell death 1 for the treatment of advanced KS solid tumors[62]. These trials show that biologic and molecular therapies may have a role in the future as alternative treatment therapy for some patients with AIDS-KS.
Currently, HHV-8 infection cannot be eradicated but long-term remission is feasible. Treatment is indicated in patients with progressive hepatic disease in the absence of other etiologies.
CONCLUSION
Hepatic KS is a clinical presentation that disproportionately effects the HIV/AIDS population. Up to 34% of patients with AIDS and KS have hepatic involvement. It is rarely clinically significant and often diagnosed during autopsy, but can cause liver injury or even fatal liver failure, as demonstrated in the case presentation above and case series in Table 2. In an immune compromised patient with ALI or failure, a thorough skin exam in addition to abdominal imaging and biochemical testing should be pursued and a diagnosis of hepatic KS should be considered. Treatment of hepatic KS does not differ from systemic treatment of other KS manifestations with HAART and chemotherapy, and should be considered within the context of medical comorbidities and severity of disease. Due to wide population prevalence, a lack of clinically significant disease and variable presentations, there is little clinical data or dedicated clinical trials for liver specific disease, and further investigation is warranted.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No conflicts of interest for all authors in the study.
Peer-review started: August 16, 2016
First decision: October 20, 2016
Article in press: December 14, 2016
P- Reviewer: Baddour N, Coban M, Zhang Q S- Editor: Ji FF L- Editor: A E- Editor: Li D
References
- 1.Tacconi D, Vergori A, Lapini L, Magnolfi A, Carnevali A, Caremani M. Hepatic Kaposi’s sarcoma in a patient affected by AIDS: Correlation between histology and imaging. J Ultrasound. 2012;15:215–219. doi: 10.1016/j.jus.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin RW, Hood AF, Farmer ER. Kaposi sarcoma. Medicine (Baltimore) 1993;72:245–261. doi: 10.1097/00005792-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Restrepo CS, Martínez S, Lemos JA, Carrillo JA, Lemos DF, Ojeda P, Koshy P. Imaging manifestations of Kaposi sarcoma. Radiographics. 2006;26:1169–1185. doi: 10.1148/rg.264055129. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien PH, Brasfield RD. Kaposi’s sarcoma. Cancer. 1966;19:1497–1502. doi: 10.1002/1097-0142(196611)19:11<1497::aid-cncr2820191106>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 5.Casper C, Wald A, Pauk J, Tabet SR, Corey L, Celum CL. Correlates of prevalent and incident Kaposi’s sarcoma-associated herpesvirus infection in men who have sex with men. J Infect Dis. 2002;185:990–993. doi: 10.1086/339605. [DOI] [PubMed] [Google Scholar]
- 6.Giuliani M, Cordiali-Fei P, Castilletti C, Di Carlo A, Palamara G, Boros S, Rezza G. Incidence of human herpesvirus 8 (HHV-8) infection among HIV-uninfected individuals at high risk for sexually transmitted infections. BMC Infect Dis. 2007;7:143. doi: 10.1186/1471-2334-7-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lodi S, Guiguet M, Costagliola D, Fisher M, de Luca A, Porter K. Kaposi sarcoma incidence and survival among HIV-infected homosexual men after HIV seroconversion. J Natl Cancer Inst. 2010;102:784–792. doi: 10.1093/jnci/djq134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhutani M, Polizzotto MN, Uldrick TS, Yarchoan R. Kaposi sarcoma-associated herpesvirus-associated malignancies: epidemiology, pathogenesis, and advances in treatment. Semin Oncol. 2015;42:223–246. doi: 10.1053/j.seminoncol.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiels MS, Pfeiffer RM, Gail MH, Hall HI, Li J, Chaturvedi AK, Bhatia K, Uldrick TS, Yarchoan R, Goedert JJ, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103:753–762. doi: 10.1093/jnci/djr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casper C, Krantz E, Selke S, Kuntz SR, Wang J, Huang ML, Pauk JS, Corey L, Wald A. Frequent and asymptomatic oropharyngeal shedding of human herpesvirus 8 among immunocompetent men. J Infect Dis. 2007;195:30–36. doi: 10.1086/509621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pauk J, Huang ML, Brodie SJ, Wald A, Koelle DM, Schacker T, Celum C, Selke S, Corey L. Mucosal shedding of human herpesvirus 8 in men. N Engl J Med. 2000;343:1369–1377. doi: 10.1056/NEJM200011093431904. [DOI] [PubMed] [Google Scholar]
- 12.Pellett PE, Spira TJ, Bagasra O, Boshoff C, Corey L, de Lellis L, Huang ML, Lin JC, Matthews S, Monini P, et al. Multicenter comparison of PCR assays for detection of human herpesvirus 8 DNA in semen. J Clin Microbiol. 1999;37:1298–1301. doi: 10.1128/jcm.37.5.1298-1301.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renwick N, Halaby T, Weverling GJ, Dukers NH, Simpson GR, Coutinho RA, Lange JM, Schulz TF, Goudsmit J. Seroconversion for human herpesvirus 8 during HIV infection is highly predictive of Kaposi’s sarcoma. AIDS. 1998;12:2481–2488. doi: 10.1097/00002030-199818000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Hladik W, Dollard SC, Mermin J, Fowlkes AL, Downing R, Amin MM, Banage F, Nzaro E, Kataaha P, Dondero TJ, et al. Transmission of human herpesvirus 8 by blood transfusion. N Engl J Med. 2006;355:1331–1338. doi: 10.1056/NEJMoa055009. [DOI] [PubMed] [Google Scholar]
- 15.Niedt GW, Schinella RA. Acquired immunodeficiency syndrome. Clinicopathologic study of 56 autopsies. Arch Pathol Lab Med. 1985;109:727–734. [PubMed] [Google Scholar]
- 16.Lemlich G, Schwam L, Lebwohl M. Kaposi’s sarcoma and acquired immunodeficiency syndrome. Postmortem findings in twenty-four cases. J Am Acad Dermatol. 1987;16:319–325. doi: 10.1016/s0190-9622(87)70043-7. [DOI] [PubMed] [Google Scholar]
- 17.Antinori S, Ridolfo AL, Esposito R, Vago L, Moroni M. Liver involvement in AIDS-associated malignancies. J Hepatol. 1994;21:1145–1146. doi: 10.1016/s0168-8278(05)80634-8. [DOI] [PubMed] [Google Scholar]
- 18.Schneiderman DJ, Arenson DM, Cello JP, Margaretten W, Weber TE. Hepatic disease in patients with the acquired immune deficiency syndrome (AIDS) Hepatology. 1987;7:925–930. doi: 10.1002/hep.1840070522. [DOI] [PubMed] [Google Scholar]
- 19.Sharma S, Gurakar A, Camci C, Jabbour N. Avoiding pitfalls: what an endoscopist should know in liver transplantation--part II. Dig Dis Sci. 2009;54:1386–1402. doi: 10.1007/s10620-008-0520-7. [DOI] [PubMed] [Google Scholar]
- 20.Russo JJ, Bohenzky RA, Chien MC, Chen J, Yan M, Maddalena D, Parry JP, Peruzzi D, Edelman IS, Chang Y, et al. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore PS, Boshoff C, Weiss RA, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996;274:1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- 22.Antman K, Chang Y. Kaposi’s sarcoma. N Engl J Med. 2000;342:1027–1038. doi: 10.1056/NEJM200004063421407. [DOI] [PubMed] [Google Scholar]
- 23.Flore O, Rafii S, Ely S, O’Leary JJ, Hyjek EM, Cesarman E. Transformation of primary human endothelial cells by Kaposi’s sarcoma-associated herpesvirus. Nature. 1998;394:588–592. doi: 10.1038/29093. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Feng J, Sun R. Oxidative stress induces reactivation of Kaposi’s sarcoma-associated herpesvirus and death of primary effusion lymphoma cells. J Virol. 2011;85:715–724. doi: 10.1128/JVI.01742-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis DA, Rinderknecht AS, Zoeteweij JP, Aoki Y, Read-Connole EL, Tosato G, Blauvelt A, Yarchoan R. Hypoxia induces lytic replication of Kaposi sarcoma-associated herpesvirus. Blood. 2001;97:3244–3250. doi: 10.1182/blood.v97.10.3244. [DOI] [PubMed] [Google Scholar]
- 26.Shin HJ, DeCotiis J, Giron M, Palmeri D, Lukac DM. Histone deacetylase classes I and II regulate Kaposi’s sarcoma-associated herpesvirus reactivation. J Virol. 2014;88:1281–1292. doi: 10.1128/JVI.02665-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng Y, Zhang X, Huang Z, Cheng L, Yao S, Qin D, Chen X, Tang Q, Lv Z, Zhang L, et al. Intracellular Tat of human immunodeficiency virus type 1 activates lytic cycle replication of Kaposi’s sarcoma-associated herpesvirus: role of JAK/STAT signaling. J Virol. 2007;81:2401–2417. doi: 10.1128/JVI.02024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wheat WH, Cool CD, Morimoto Y, Rai PR, Kirkpatrick CH, Lindenbaum BA, Bates CA, Ellison MC, Serls AE, Brown KK, et al. Possible role of human herpesvirus 8 in the lymphoproliferative disorders in common variable immunodeficiency. J Exp Med. 2005;202:479–484. doi: 10.1084/jem.20050381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friborg J, Kong W, Hottiger MO, Nabel GJ. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature. 1999;402:889–894. doi: 10.1038/47266. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura T, Mizuno S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:588–610. doi: 10.2183/pjab.86.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mercader M, Taddeo B, Panella JR, Chandran B, Nickoloff BJ, Foreman KE. Induction of HHV-8 lytic cycle replication by inflammatory cytokines produced by HIV-1-infected T cells. Am J Pathol. 2000;156:1961–1971. doi: 10.1016/S0002-9440(10)65069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buetow PC, Buck JL, Ros PR, Goodman ZD. Malignant vascular tumors of the liver: radiologic-pathologic correlation. Radiographics. 1994;14:153–166; quiz 167-168. doi: 10.1148/radiographics.14.1.8128048. [DOI] [PubMed] [Google Scholar]
- 33.Hong YK, Foreman K, Shin JW, Hirakawa S, Curry CL, Sage DR, Libermann T, Dezube BJ, Fingeroth JD, Detmar M. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat Genet. 2004;36:683–685. doi: 10.1038/ng1383. [DOI] [PubMed] [Google Scholar]
- 34.Hammock L, Reisenauer A, Wang W, Cohen C, Birdsong G, Folpe AL. Latency-associated nuclear antigen expression and human herpesvirus-8 polymerase chain reaction in the evaluation of Kaposi sarcoma and other vascular tumors in HIV-positive patients. Mod Pathol. 2005;18:463–468. doi: 10.1038/modpathol.3800221. [DOI] [PubMed] [Google Scholar]
- 35.Pantanowitz L, Otis CN, Dezube BJ. Immunohistochemistry in Kaposi’s sarcoma. Clin Exp Dermatol. 2010;35:68–72. doi: 10.1111/j.1365-2230.2009.03707.x. [DOI] [PubMed] [Google Scholar]
- 36.Du MQ, Diss TC, Liu H, Ye H, Hamoudi RA, Cabeçadas J, Dong HY, Harris NL, Chan JK, Rees JW, et al. KSHV- and EBV-associated germinotropic lymphoproliferative disorder. Blood. 2002;100:3415–3418. doi: 10.1182/blood-2002-02-0487. [DOI] [PubMed] [Google Scholar]
- 37.Krown SE, Metroka C, Wernz JC. Kaposi’s sarcoma in the acquired immune deficiency syndrome: a proposal for uniform evaluation, response, and staging criteria. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol. 1989;7:1201–1207. doi: 10.1200/JCO.1989.7.9.1201. [DOI] [PubMed] [Google Scholar]
- 38.Nasti G, Talamini R, Antinori A, Martellotta F, Jacchetti G, Chiodo F, Ballardini G, Stoppini L, Di Perri G, Mena M, et al. AIDS-related Kaposi’s Sarcoma: evaluation of potential new prognostic factors and assessment of the AIDS Clinical Trial Group Staging System in the Haart Era--the Italian Cooperative Group on AIDS and Tumors and the Italian Cohort of Patients Naive From Antiretrovirals. J Clin Oncol. 2003;21:2876–2882. doi: 10.1200/JCO.2003.10.162. [DOI] [PubMed] [Google Scholar]
- 39.Mosam A, Shaik F, Uldrick TS, Esterhuizen T, Friedland GH, Scadden DT, Aboobaker J, Coovadia HM. A randomized controlled trial of highly active antiretroviral therapy versus highly active antiretroviral therapy and chemotherapy in therapy-naive patients with HIV-associated Kaposi sarcoma in South Africa. J Acquir Immune Defic Syndr. 2012;60:150–157. doi: 10.1097/QAI.0b013e318251aedd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hammerman AM, Kotner LM, Doyle TB. Periportal contrast enhancement on CT scans of the liver. AJR Am J Roentgenol. 1991;156:313–315. doi: 10.2214/ajr.156.2.1898805. [DOI] [PubMed] [Google Scholar]
- 41.Moon KL, Federle MP, Abrams DI, Volberding P, Lewis BJ. Kaposi sarcoma and lymphadenopathy syndrome: limitations of abdominal CT in acquired immunodeficiency syndrome. Radiology. 1984;150:479–483. doi: 10.1148/radiology.150.2.6691105. [DOI] [PubMed] [Google Scholar]
- 42.Valls C, Cañas C, Turell LG, Pruna X. Hepatosplenic AIDS-related Kaposi’s sarcoma. Gastrointest Radiol. 1991;16:342–344. doi: 10.1007/BF01887385. [DOI] [PubMed] [Google Scholar]
- 43.Maskew M, Fox MP, van Cutsem G, Chu K, Macphail P, Boulle A, Egger M, Africa FI. Treatment response and mortality among patients starting antiretroviral therapy with and without Kaposi sarcoma: a cohort study. PLoS One. 2013;8:e64392. doi: 10.1371/journal.pone.0064392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bower M, Fox P, Fife K, Gill J, Nelson M, Gazzard B. Highly active anti-retroviral therapy (HAART) prolongs time to treatment failure in Kaposi’s sarcoma. AIDS. 1999;13:2105–2111. doi: 10.1097/00002030-199910220-00014. [DOI] [PubMed] [Google Scholar]
- 45.Martinez V, Caumes E, Gambotti L, Ittah H, Morini JP, Deleuze J, Gorin I, Katlama C, Bricaire F, Dupin N. Remission from Kaposi’s sarcoma on HAART is associated with suppression of HIV replication and is independent of protease inhibitor therapy. Br J Cancer. 2006;94:1000–1006. doi: 10.1038/sj.bjc.6603056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donato V, Guarnaccia R, Dognini J, de Pascalis G, Caruso C, Bellagamba R, Morrone A. Radiation therapy in the treatment of HIV-related Kaposi’s sarcoma. Anticancer Res. 2013;33:2153–2157. [PubMed] [Google Scholar]
- 47.Vanni T, Sprinz E, Machado MW, Santana Rde C, Fonseca BA, Schwartsmann G. Systemic treatment of AIDS-related Kaposi sarcoma: current status and perspectives. Cancer Treat Rev. 2006;32:445–455. doi: 10.1016/j.ctrv.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Martellotta F, Berretta M, Vaccher E, Schioppa O, Zanet E, Tirelli U. AIDS-related Kaposi’s sarcoma: state of the art and therapeutic strategies. Curr HIV Res. 2009;7:634–638. doi: 10.2174/157016209789973619. [DOI] [PubMed] [Google Scholar]
- 49.Gbabe OF, Okwundu CI, Dedicoat M, Freeman EE. Treatment of severe or progressive Kaposi’s sarcoma in HIV-infected adults. Cochrane Database Syst Rev. 2014;(8):CD003256. doi: 10.1002/14651858.CD003256.pub2. [DOI] [PubMed] [Google Scholar]
- 50.Stewart S, Jablonowski H, Goebel FD, Arasteh K, Spittle M, Rios A, Aboulafia D, Galleshaw J, Dezube BJ. Randomized comparative trial of pegylated liposomal doxorubicin versus bleomycin and vincristine in the treatment of AIDS-related Kaposi’s sarcoma. International Pegylated Liposomal Doxorubicin Study Group. J Clin Oncol. 1998;16:683–691. doi: 10.1200/JCO.1998.16.2.683. [DOI] [PubMed] [Google Scholar]
- 51.Gill PS, Wernz J, Scadden DT, Cohen P, Mukwaya GM, von Roenn JH, Jacobs M, Kempin S, Silverberg I, Gonzales G, et al. Randomized phase III trial of liposomal daunorubicin versus doxorubicin, bleomycin, and vincristine in AIDS-related Kaposi’s sarcoma. J Clin Oncol. 1996;14:2353–2364. doi: 10.1200/JCO.1996.14.8.2353. [DOI] [PubMed] [Google Scholar]
- 52.Grünaug M, Bogner JR, Loch O, Goebel FD. Liposomal doxorubicin in pulmonary Kaposi’s sarcoma: improved survival as compared to patients without liposomal doxorubicin. Eur J Med Res. 1998;3:13–19. [PubMed] [Google Scholar]
- 53.Cianfrocca M, Lee S, Von Roenn J, Tulpule A, Dezube BJ, Aboulafia DM, Ambinder RF, Lee JY, Krown SE, Sparano JA. Randomized trial of paclitaxel versus pegylated liposomal doxorubicin for advanced human immunodeficiency virus-associated Kaposi sarcoma: evidence of symptom palliation from chemotherapy. Cancer. 2010;116:3969–3977. doi: 10.1002/cncr.25362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Evans SR, Krown SE, Testa MA, Cooley TP, Von Roenn JH. Phase II evaluation of low-dose oral etoposide for the treatment of relapsed or progressive AIDS-related Kaposi’s sarcoma: an AIDS Clinical Trials Group clinical study. J Clin Oncol. 2002;20:3236–3241. doi: 10.1200/JCO.2002.12.038. [DOI] [PubMed] [Google Scholar]
- 55.Northfelt DW, Dezube BJ, Thommes JA, Miller BJ, Fischl MA, Friedman-Kien A, Kaplan LD, Du Mond C, Mamelok RD, Henry DH. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: results of a randomized phase III clinical trial. J Clin Oncol. 1998;16:2445–2451. doi: 10.1200/JCO.1998.16.7.2445. [DOI] [PubMed] [Google Scholar]
- 56.La Ferla L, Pinzone MR, Nunnari G, Martellotta F, Lleshi A, Tirelli U, De Paoli P, Berretta M, Cacopardo B. Kaposi’ s sarcoma in HIV-positive patients: the state of art in the HAART-era. Eur Rev Med Pharmacol Sci. 2013;17:2354–2365. [PubMed] [Google Scholar]
- 57.Gantt S, Casper C. Human herpesvirus 8-associated neoplasms: the roles of viral replication and antiviral treatment. Curr Opin Infect Dis. 2011;24:295–301. doi: 10.1097/QCO.0b013e3283486d04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koon HB, Krown SE, Lee JY, Honda K, Rapisuwon S, Wang Z, Aboulafia D, Reid EG, Rudek MA, Dezube BJ, et al. Phase II trial of imatinib in AIDS-associated Kaposi’s sarcoma: AIDS Malignancy Consortium Protocol 042. J Clin Oncol. 2014;32:402–408. doi: 10.1200/JCO.2012.48.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uldrick TS, Wyvill KM, Kumar P, O’Mahony D, Bernstein W, Aleman K, Polizzotto MN, Steinberg SM, Pittaluga S, Marshall V, et al. Phase II study of bevacizumab in patients with HIV-associated Kaposi’s sarcoma receiving antiretroviral therapy. J Clin Oncol. 2012;30:1476–1483. doi: 10.1200/JCO.2011.39.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Little RF, Pluda JM, Wyvill KM, Rodriguez-Chavez IR, Tosato G, Catanzaro AT, Steinberg SM, Yarchoan R. Activity of subcutaneous interleukin-12 in AIDS-related Kaposi sarcoma. Blood. 2006;107:4650–4657. doi: 10.1182/blood-2005-11-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.National Cancer Institute. Pilot Study of Liposomal Doxorubicin Combined With Bevacizumab Followed by Bevacizumab Monotherapy in Adults With Advanced Kaposis Sarcoma. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US) Available from: https://clinicaltrials.gov/show/NCT00923936 NLM Identifier: NCT00923936.
- 62.National Cancer Institute. Nivolumab and Ipilimumab in Treating Patients With HIV Associated Solid Tumors That Are Metastatic or Cannot Be Removed by Surgery. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US) Available from: https://clinicaltrials.gov/show/NCT02408861 NLM Identifier: NCT02408861.
- 63.Read PJ, Lucas S, Morris S, Kulasegaram R. Immune reconstitution inflammatory syndrome Kaposi sarcoma in the liver manifesting as acute obstructive hepatitis: another potential role for montelukast? Int J STD AIDS. 2013;24:156–158. doi: 10.1177/0956462412472819. [DOI] [PubMed] [Google Scholar]
- 64.Hengge UR, Brockmeyer NH, Baumann M, Reimann G, Goos M. Liposomal doxorubicin in AIDS-related Kaposi’s sarcoma. Lancet. 1993;342:497. doi: 10.1016/0140-6736(93)91624-u. [DOI] [PubMed] [Google Scholar]
- 65.Towers MJ, Withers CE, Rachlis AR, Pappas SC, Kolin A. Ultrasound diagnosis of hepatic Kaposi sarcoma. J Ultrasound Med. 1991;10:701–703. doi: 10.7863/jum.1991.10.12.701. [DOI] [PubMed] [Google Scholar]
- 66.Luburich P, Bru C, Ayuso MC, Azón A, Condom E. Hepatic Kaposi sarcoma in AIDS: US and CT findings. Radiology. 1990;175:172–174. doi: 10.1148/radiology.175.1.2179988. [DOI] [PubMed] [Google Scholar]
- 67.Van Leer-Greenberg B, Pham T, Spence L, Berger S, Chawla S. Hepatic Kaposi Sarcoma: An Uncommon Etiology of Acute Liver Injury in AIDS. Georgia Gastroenterology and Endoscopy Society Meeting, 2015 [Google Scholar]
- 68.Hasan FA, Jeffers LJ, Welsh SW, Reddy KR, Schiff ER. Hepatic involvement as the primary manifestation of Kaposi’s sarcoma in the acquired immune deficiency syndrome. Am J Gastroenterol. 1989;84:1449–1451. [PubMed] [Google Scholar]


