Abstract
Objective
Consider all metabolic syndrome (MetS) components [systolic (SBP) and diastolic (DBP) blood pressures, waist circumference, HDL cholesterol, triglycerides (TG), and fasting glucose] and gender/race differential risk when assessing cardiovascular disease (CVD) risk.
Methods
We estimated a gender- and race-specific continuous MetS score using structural equation modeling and tested its association with CVD mortality using data from National Health and Nutrition Examination Survey III linked with the National Death Index. Cox proportional hazard regression tested the association adjusted for sociodemographic and behavior characteristics.
Results
For men, continuous MetS components associated with CVD mortality were SBP (hazard ratio =1.50, 95% confidence interval =1.14–1.96), DBP (1.48, 1.16–1.90), and TG (1.15, 1.12–1.16). In women, SBP (1.44, 1.27–1.63) and DBP (1.24, 1.02–1.51) were associated with CVD mortality. MetS score was not significantly associated with CVD mortality in men; but significant associations were found for all women (1.34, 1.06–1.68), non-Hispanic white women (1.29, 1.01–1.64), non-Hispanic black women (2.03, 1.12–3.69), and Mexican-American women (3.57, 2.21–5.76). Goodness-of-fit and concordance were overall better for models with the MetS score than MetS (yes/no).
Conclusions
When assessing CVD mortality risk, MetS score provided additional information than MetS (yes/no).
Introduction
Cardiovascular disease (CVD) is the leading cause of death in the US and worldwide (1). A cluster of risk factors commonly found among individuals with CVD (dyslipidemia, hypertension, hyperglycemia, and excess abdominal fat) led to the development of a condition known as metabolic syndrome (MetS) (2). Most recently, a harmonized MetS definition was presented as having abnormal values for three of the five metabolic components: blood pressure, fasting glucose, waist circumference, HDL cholesterol, or triglycerides (3), based on established cut points.
Although MetS predicts CVD events (4,5), there are some major limitations to the most recent harmonized definition of MetS (3). First, the established cut points of each metabolic component may not be most effective in predicting CVD risk for certain subgroups or populations. One example is the MetS paradox among African-Americans who have greater prevalence of hypertension and better cholesterol profiles than other races/ethnicities (6,7), and cut points may need to be adapted to identify early CVD risk. Additionally, the current MetS definition does not distinguish between which components are present, and there may be interaction between combinations of components that result in greater CVD risk than others. Additional limitations raised by a joint statement from the American Diabetes Association and the European Association for the Study of Diabetes includes ill-defined cut points with possible loss of information, lack of basis for the inclusion or exclusion of other CVD risk factors, and treatment of MetS is no different than the treatment of its components (8). Overall, the medical value of diagnosing MetS was questioned. However, CVD events and mortality have been found to be driven by MetS independently from the components (9). Additionally, individuals with MetS are at increased risk of CVD mortality and all-cause mortality compared with those without MetS (10–13). Even though these studies have consistently found a positive relationship between MetS and CVD mortality, MetS is criticized due to limitations of the definition (yes/no) and the inflexibility of evaluating abnormal MetS components differently for race and gender subgroups.
Even though the harmonized definition has provided country- and gender-specific cut points for a couple of components, ideally we would use the measured value of each component when assessing CVD risk while acknowledging differential risk among certain populations. In this study, we tested independent associations between each metabolic component and CVD mortality. Then, we used a method that addresses the limitations of MetS by estimating an individual continuous MetS score based on the actual value of all components and tested its association with CVD mortality.
Methods
Study population
Data from the National Health and Nutrition Examination Survey III (NHANES III) was used where participants were selected from a complex, multistage, probability sampling design to represent the non-institutionalized US population (14). NHANES III was conducted from 1988 to 1994 and contains data on 19,288 nonpregnant adults aged 18 years or older. Data on participants from NHANES III were linked to death certificates from the National Death Index to obtain mortality status through December 31, 2006 (15). CVD mortality was classified as cause of death from Major Cardiovascular Diseases or ICD-10 codes of I00 to I78.
Of the 19,288 nonpregnant adults, 25 were ineligible for mortality linkage resulting in a remaining 19,263 participants. Participants were then excluded if there were: no fasting lab data available (n = 11,157); no measurements for blood pressure (n = 2345) or anthropometric (n = 3154); medical history of cancer (n = 775), heart failure (n = 747), stroke (n = 646), or heart attack (n = 932); or missing covariate data (n = 772). These numbers are not mutually exclusive and 5759 participants remained in this study. Morning sample weights, also known as fasting weights, were used to account for non-response due to not fasting or missing laboratory data.
Measurements
Participants underwent interviews and detailed physical exams. Waist circumference was measured to the nearest 0.1 cm from the top of the iliac crest with the tape measure parallel to the floor. Blood pressure was determined based on an average of three blood pressure measurements. Blood samples were collected to obtain measures of plasma glucose and lipid profiles (HDL cholesterol and triglycerides).
MetS was based on the latest harmonized definition of having three or more abnormal values of any of the following components: blood pressure, fasting glucose, waist circumference, HDL cholesterol, and triglycerides (3). Presence of the blood pressure component was a systolic blood pressure ≥130 mm Hg, diastolic blood pressure ≥85 mm Hg, or prescribed medication use for high blood pressure. Abnormal fasting glucose was defined as ≥100 mg/dl or the use of glucose altering medication (insulin/diabetic pills). Gender-specific cut points were specified for waist circumference (men: ≥102 cm and women: ≥88 cm) and HDL cholesterol components (men: <40 mg/dl and women: <50 mg/dl). Abnormal triglycerides were ≥150 mg/dl. Participants meeting three or more of these criteria were categorized as having MetS.
Statistical analysis
All analyses used sampling weights and adjusted variance estimates to account for the complex sampling design. Demographic characteristics were described as count and percent for discrete variable and mean with standard errors for continuous variables. Structural equation modeling, a statistical estimation method, was used to calculate a metabolic score for each participant based on the values of the following metabolic components: systolic blood pressure, diastolic blood pressure, fasting glucose, waist circumference, HDL cholesterol, and triglycerides. During the estimation process, correlated errors were specified between systolic and diastolic blood pressure measures as well as between HDL cholesterol and triglycerides due to the relationship these variables have with each other. For the purpose of comparing components’ contribution to the metabolic score, factor loadings were standardized based on the variance of the fitted model. Because gender and race/ethnic differences in the distribution of some of these components may exist, path diagrams were estimated separately for each gender-race subgroup, and differences were tested using Score and Wald tests. Goodness-of-fit of the specified path diagrams were assessed by the standardized root mean squared residuals (SRMR; good fit ≤0.08) and by the coefficient of determination (CD; good fit >0.56 which is equivalent to an R2 of 0.75).
Cox proportional hazards regression analysis was used to test the association between time to CVD death in five ways: (1) each continuous metabolic component independently, (2) harmonized MetS (yes/no), (3) the number of metabolic components present based on harmonized cut points and (4) the metabolic score calculated from structural equation modeling. When testing the independent association between CVD mortality and each metabolic component, components were standardized to have a distribution of mean zero and a standard deviation of one for the purpose of coefficient comparison across models. Meaning, the greatest coefficient with significance would be considered a more important predictor. Person-time used in these analyses was the date from the NHANES in-person exam to the day of death or December 31, 2006 for those assumed alive. Since metabolic scores are gender and race specific, hazard ratios were stratified by gender and race/ethnicity. Models were adjusted for: age (years), education (highest grade or year of school completed), physical activity [active (moderated physical activity ≥5 times per week or vigorous physical activity ≥3 times per week) or inactive], smoking status (never, former, or current), alcohol consumption (none, less than three drinks per week, or three or more per week), and self-reported medication use for hypertension, diabetes, or high cholesterol. Statistical significance was denoted as P value less than 0.05.
Comparison between models using the harmonized MetS definition, number of abnormal components, and metabolic score were based on predictability of the models from concordance analysis (Harrell’s C coefficient and Gonen and Heller’s K coefficient) and goodness-of-fit (Akaike Information Criterion, Bayesian Information Criterion, and Royston’s R2). These models were also tested against a model having all the MetS components in the model as continuous variables. All analyses were performed using STATA 13.0 (StataCorp LP, College Station, TX).
Results
Demographic, anthropometric, and laboratory characteristic by gender and race/ethnicity are presented in Table 1. Women had an older age distribution than men with 15% being 65 years or older compared with 11% of men. Mexican-Americans were a younger group with 76% between the ages of 18 and 44 years compared with African-Americans (69%) and non-Hispanic whites (57%). About 47% of men and 43% of women had some college education or college degree. Education varied between race/ethnic groups with 18% of Mexican-Americans, 34% of African-Americans, and 48% of non-Hispanic whites with some college education or college degree attainment. The prevalence of MetS did not greatly vary by gender, but there was some variability among race/ethnic groups with 22% of non-Hispanic blacks meeting harmonized MetS compared with 28% non-Hispanic whites and 31% of Mexican-Americans. Person-time of follow-up was similar between gender and race/ethnic groups.
TABLE 1.
Prevalence of characteristics among adults aged ≥18 yearsa and by sex and race/ethnicity—National Health and Nutrition Examination Survey III, United States
| All (N = 5759), % (95% CI) | Men (N = 2721), % (95% CI) | Women (N = 3038), % (95% CI) | Non-Hispanic white (N = 2444), % (95% CI) | Non-Hispanic black (N = 1664), % (95% CI) | Mexican-American (N = 1651), % (95% CI) | |
|---|---|---|---|---|---|---|
| Age group (years) | ||||||
| 18–44 | 59.5 (56.4–62.6) | 62.3 (59.1–65.6) | 56.8 (53.1–60.5) | 57.1 (53.5–60.8) | 68.8 (65.5–72.1) | 76.0 (72.7–79.4) |
| 45–64 | 27.6 (25.2–29.9) | 26.6 (24.1–29.0) | 28.6 (25.5–31.6) | 28.9 (26.1–31.7) | 22.5 (19.6–25.5) | 18.5 (15.6–21.4) |
| ≥65 | 12.9 (11.4–14.4) | 11.1 (9.5–12.7) | 14.6 (12.7–16.6) | 14.0 (12.3–15.7) | 8.7 (7.1–10.3) | 5.5 (4.5–6.4) |
| Education (respondents aged ≥25 years) | ||||||
| <High school diploma | 20.9 (18.6–23.2) | 21.8 (18.7–24.9) | 20.1 (17.9–22.3) | 17.5 (15.0–20.1) | 29.3 (26.2–32.3) | 58.5 (55.3–61.7) |
| High school diploma | 34.1 (31.8–36.5) | 30.8 (27.7–33.8) | 37.2 (34.2–40.3) | 34.4 (31.7–37.1) | 37.1 (33.2–41.0) | 23.3 (20.3–26.3) |
| Some college | 21.6 (19.4–23.9) | 21.6 (18.2–25.0) | 21.7 (18.6–24.8) | 22.4 (19.9–24.9) | 20.2 (17.4–23.0) | 12.3 (9.3–15.4) |
| ≥College degree | 23.3 (20.9–25.8) | 25.9 (22.7–29.1) | 21.0 (18.2–23.8) | 25.7 (23.0–28.4) | 13.4 (10.8–16.0) | 5.9 (4.2–7.6) |
| Smoking statusb | ||||||
| Current | 28.8 (26.8–30.8) | 31.5 (29.4–33.6) | 26.3 (23.2–29.3) | 28.6 (26.2–31.1) | 33.1 (30.3–35.9) | 22.5 (19.4–25.7) |
| Former | 26.4 (24.6–28.2) | 32.1 (29.2–35.0) | 21.1 (18.8–23.3) | 28.4 (26.4–30.3) | 16.0 (13.3–18.7) | 18.6 (15.6–21.5) |
| Never | 44.8 (43.0–46.6) | 36.4 (33.6–39.2) | 52.6 (49.9–55.4) | 43.0 (40.9–45.1) | 50.9 (47.3–54.4) | 58.9 (55.5–62.3) |
| Alcohol consumption categoriesc | ||||||
| None | 41.6 (37.9–45.2) | 30.6 (25.9–35.2) | 51.9 (48.1–55.8) | 39.7 (35.5–44.0) | 52.8 (50.2–55.4) | 46.6 (42.4–50.7) |
| <3 drinks per week | 26.7 (24.3–29.1) | 25.5 (22.6–28.4) | 27.7 (24.3–31.2) | 27.6 (24.8–30.4) | 21.1 (19.0–23.1) | 24.3 (20.8–27.7) |
| ≥3 drinks per week | 31.8 (29.1–34.5) | 43.9 (39.9–47.9) | 20.4 (17.4–23.3) | 32.7 (29.5–35.9) | 26.2 (24.0–28.4) | 29.2 (26.2–32.2) |
| Physical activityd | ||||||
| Active | 44.2 (40.9–47.4) | 48.1 (44.0–52.2) | 40.5 (36.7–44.2) | 45.4 (41.8–49.1) | 40.9 (37.9–44.0) | 31.9 (27.9–35.9) |
| Inactive | 55.8 (52.6–59.1) | 51.9 (47.8–56.0) | 59.5 (55.8–63.3) | 54.6 (50.9–58.2) | 59.1 (56.0–62.1) | 68.1 (64.1–72.1) |
| Medication use fore: | ||||||
| High cholesterol | 2.5 (1.9–3.1) | 2.3 (1.6–3.0) | 2.7 (1.8–3.5) | 2.7 (1.9–3.4) | 1.8 (1.2–2.4) | 1.0 (0.5–1.4) |
| Hypertension | 10.3 (9.1–11.6) | 8.2 (6.7–9.7) | 12.3 (10.7–13.9) | 10.2 (8.7–11.6) | 13.9 (11.5–16.3) | 5.5 (4.3–6.6) |
| Diabetes | 2.4 (1.8–3.0) | 2.8 (2.0–3.7) | 2.0 (1.4–2.7) | 2.2 (1.5–2.9) | 3.9 (3.0–4.8) | 3.1 (2.3–3.9) |
| Metabolic syndrome (harmonized definition)f | 27.3 (25.2–29.3) | 28.4 (25.0–31.8) | 26.2 (23.6–28.8) | 27.7 (25.3–30.2) | 22.1 (19.9–24.2) | 30.7 (27.7–33.6) |
| Waist circumference (cm) | 91.3 (0.3) | 95.1 (0.4) | 87.7 (0.5) | 91.1 (0.4) | 92.1 (0.4) | 92.2 (0.5) |
| Systolic blood pressure (mm Hg) | 120.9 (0.4) | 123.8 (0.5) | 118.2 (0.6) | 120.8 (0.5) | 122.6 (0.7) | 118.8 (0.5) |
| Diastolic blood pressure (mm Hg) | 73.9 (0.2) | 76.4 (0.4) | 71.5 (0.2) | 73.8 (0.3) | 75.3 (0.4) | 73.0 (0.4) |
| HDL cholesterol (mg/dl) | 50.6 (0.4) | 45.7 (0.5) | 55.2 (0.5) | 50.2 (0.5) | 54.9 (0.6) | 47.9 (0.5) |
| Triglycerides (mg/dl) | 252.5 (31.0) | 294.8 (39.4) | 212.6 (34.9) | 258.7 (33.2) | 203.5 (31.1) | 262.8 (35.7) |
| Plasma glucose (mg/dl) | 99.5 (0.5) | 102.6 (0.6) | 96.6 (0.7) | 99.1 (0.5) | 101.1 (1.3) | 102.4 (0.8) |
| Person-years of follow-up | 14.3 (0.2) | 14.2 (0.2) | 14.3 (0.2) | 14.2 (0.3) | 14.2 (0.2) | 14.5 (0.3) |
Participants were excluded if they were: pregnant women; missing anthropometric, laboratory, or blood pressure measurements; fasting less than 8 h; or medical history of cancer (all cancers except skin cancer) or CVD (coronary heart disease, heart attack, or stroke).
Smoking status defined as: current smokers (self-reported current smoker), former smoker (100+ cigarettes in lifetime and not current smoker), and never smoker (has not smoked 100+ cigarettes in lifetime and not current smoker).
Alcohol consumption defined as first having at least 12 alcohol drinks in the past 12 months to distinguish between drinkers and nondrinkers. Among those who had at least 12 alcoholic drinks, an average number of drinks per week was calculated and categorized as <3 or ≥3 drinks per week.
Physical activity was categorized as active or inactive. Active was defined as at least 30 min of: moderate physical activity at least five times per week or vigorous physical activity at least three times per week.
Self-reported medication use.
Metabolic syndrome NHLBI definition is defined as having three or more of the following characteristics: (1) systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg, (2) fasting glucose ≥100 mg/dl, (3) triglycerides ≥150 mg/dl, (4) HDL cholesterol <40 mg/dl for men or <50 mg/dl for women, or (5) waist circumference ≥102 cm for men or ≥88 cm for women.
CI, confidence interval; SE, standard error.
Systolic blood pressure, diastolic blood pressure and triglycerides were independently associated with CVD mortality in non-Hispanic white men (Table 2). In these associations, the strength of association was greatest for systolic blood pressure (adjusted standardized hazard ratio =1.51, 95% confidence interval: 1.06–2.16) and diastolic blood pressure (1.51, 1.10–2.07) compared with triglycerides (1.16, 1.13–1.19). Among non-Hispanic black men, systolic (1.58, 1.17–2.13) and diastolic (1.53, 1.21–1.93) blood pressures as well as HDL cholesterol (1.43, 1.14–1.79) were independently associated with CVD mortality. None of the metabolic components were independently associated with CVD mortality among Mexican-American men.
TABLE 2.
Cox proportional hazard ratio for CVD mortality associated with each standardizeda metabolic component individually by gender—National Health and Nutrition Examination Survey III, United States
| Men
|
Women
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | No. events | HRb | 95% CI | P | N | No. events | HRb | 95% CI | P | |
| All | 2721 | 220 | 3038 | 203 | ||||||
| Waist circumference | 1.02 | (0.779–1.344) | 0.870 | 1.14 | (0.937–1.389) | 0.189 | ||||
| Systolic blood pressure | 1.50 | (1.141–1.961) | 0.004 | 1.44 | (1.268–1.630) | <0.0001 | ||||
| Diastolic blood pressure | 1.48 | (1.155–1.897) | 0.002 | 1.24 | (1.024–1.510) | 0.028 | ||||
| HDL cholesterol | 1.09 | (0.827–1.435) | 0.542 | 1.01 | (0.765–1.334) | 0.944 | ||||
| Triglycerides | 1.15 | (1.122–1.168) | <0.0001 | 1.18 | (0.951–1.454) | 0.134 | ||||
| Plasma glucose | 1.03 | (0.796–1.326) | 0.834 | 1.09 | (0.872–1.358) | 0.455 | ||||
| Non-Hispanic white | 1126 | 122 | 1318 | 125 | ||||||
| Waist circumference | 1.06 | (0.759–1.474) | 0.740 | 1.10 | (0.875–1.390) | 0.406 | ||||
| Systolic blood pressure | 1.51 | (1.060–2.155) | 0.023 | 1.43 | (1.221–1.680) | <0.0001 | ||||
| Diastolic blood pressure | 1.51 | (1.096–2.072) | 0.012 | 1.23 | (0.962–1.572) | 0.099 | ||||
| HDL cholesterol | 1.01 | (0.728–1.414) | 0.933 | 1.03 | (0.743–1.428) | 0.859 | ||||
| Triglycerides | 1.16 | (1.129–1.186) | <0.0001 | 1.19 | (0.938–1.510) | 0.153 | ||||
| Plasma glucose | 0.97 | (0.656–1.443) | 0.891 | 1.12 | (0.892–1.418) | 0.321 | ||||
| Non-Hispanic black | 762 | 55 | 902 | 54 | ||||||
| Waist circumference | 0.88 | (0.601–1.299) | 0.529 | 1.29 | (0.919–1.803) | 0.141 | ||||
| Systolic blood pressure | 1.58 | (1.166–2.131) | 0.003 | 1.44 | (1.177–1.753) | 0.0004 | ||||
| Diastolic blood pressure | 1.53 | (1.212–1.930) | 0.0004 | 1.26 | (0.951–1.658) | 0.108 | ||||
| HDL cholesterol | 1.43 | (1.137–1.786) | 0.002 | 0.90 | (0.634–1.288) | 0.575 | ||||
| Triglycerides | 0.73 | (0.413–1.280) | 0.270 | 1.46 | (0.927–2.290) | 0.103 | ||||
| Plasma glucose | 1.11 | (0.938–1.306) | 0.228 | 0.92 | (0.701–1.195) | 0.516 | ||||
| Mexican-American | 833 | 43 | 818 | 24 | ||||||
| Waist circumference | 1.01 | (0.535–1.906) | 0.975 | 1.36 | (0.807–2.282) | 0.249 | ||||
| Systolic blood pressure | 1.61 | (0.901–2.859) | 0.108 | 1.97 | (1.203–3.228) | 0.007 | ||||
| Diastolic blood pressure | 1.13 | (0.739–1.716) | 0.581 | 1.67 | (0.799–3.494) | 0.173 | ||||
| HDL cholesterol | 0.86 | (0.391–1.888) | 0.706 | 0.87 | (0.569–1.329) | 0.519 | ||||
| Triglycerides | 1.32 | (0.907–1.913) | 0.148 | 1.15 | (0.763–1.726) | 0.508 | ||||
| Plasma glucose | 1.15 | (0.793–1.665) | 0.462 | 1.68 | (1.404–2.014) | <0.0001 | ||||
Each hazard ratio is a model.
All metabolic components were standardized to the normal distribution (mean =0 and standard deviation =1) for the purpose of coefficient comparison across models.
Models adjusted for age, smoking status, education, physical activity, alcohol consumption, and medication use for diabetes, hypertension, or high cholesterol.
HR, hazard ratio; CI, confidence interval.
Systolic blood pressure was associated with CVD mortality within all race/ethnic groups in women and the strongest association was observed among Mexican-American (1.97, 1.20–3.23) compared with non-Hispanic whites (1.43, 1.22–1.68) and non-Hispanic blacks (1.44, 1.18–1.75) (Table 2, unadjusted estimates Supporting Information Table S1). Diastolic blood pressure was associated with CVD mortality among all women (1.24, 1.02–1.51). Other significant independent association with CVD mortality was fasting glucose (1.68, 1.40–2.01) in Mexican-American women.
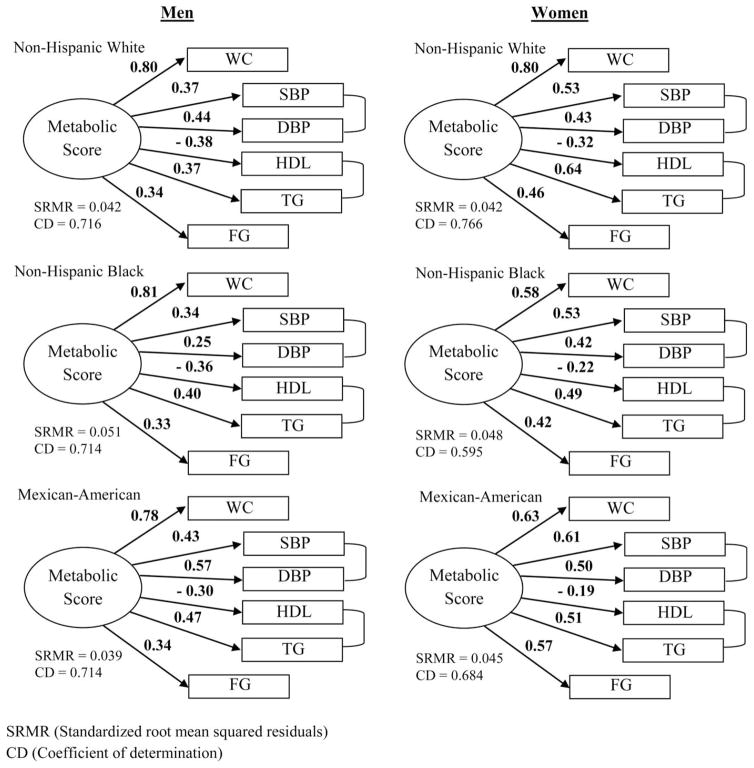
There was slight variation in the standardized factor loadings from the structural equation modeling by gender and race/ethnicity (Figure 1). Based on the Score and Wald tests (Supporting Information Table S2), factor loadings in the path diagrams were significantly different across gender and race groups. Overall, the standardized factor loadings were greatest for waist circumference (ranging from 0.58 to 0.81) for all subgroups. In men, standardized factor loading absolute values for all other metabolic components were close to half that of waist circumference, with HDL having a negative value. The greatest variation in factor loadings across race/ethnic groups in men was between systolic (ranging from 0.34 to 0.43) and diastolic (ranging from 0.25 to 0.57) blood pressures. In women, standardized factor loadings for metabolic components varied more across race/ethnic groups compared with men with the largest range observed for waist circumference (0.58–0.80). Standardized factor loadings estimated without correlated errors for SBP and DBP as well as HDL and TG are shown on Supporting Information Figure S1. Since estimated covariance between SBP and DBP as well as HDL and TG were significantly different than zero (P value <0.05, and in most cases P value <0.001), all further results only considered MetS score derived from the path diagrams with specified correlated error. Based on the SRMR and CD, all path diagrams had good fit.
Figure 1.
Metabolic score path diagrams with standardized factor loadings for each gender-race subgroup among US adults—National Health and Nutrition Examination Survey III, 1988–2006. WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; TG, triglycerides; FG, fasting glucose.
The metabolic score derived from the structural equation modeling was associated with CVD mortality for: non-Hispanic white women (1.29, 1.01–1.64), non-Hispanic black women (2.03, 1.12–3.69), and Mexican-American women (3.57, 2.21–5.76) (Table 3, unadjusted estimates Supporting Information Table S3). In this study, the harmonized defined MetS (yes/no) was not significantly associated with CVD mortality in almost all of the gender and race/ethnic subgroups with the exception among non-Hispanic black women (2.69, 1.45–4.97). The number of abnormal metabolic components present based on the harmonized definition was associated with CVD mortality for: non-Hispanic white women (1.15, 1.04–1.27), non-Hispanic black women (1.40, 1.10–1.77), and Mexican-American women (1.32, 1.07–1.61). When comparing the models using the metabolic score with those using harmonized MetS or the number of abnormal metabolic components present, the models with the metabolic score were a better fit based on the Akaike Information Criterion and Bayesian Information Criterion. Using concordance analysis, for the most part, the models with better predictability were those with the metabolic score based on the Harrell’s C coefficient and Gönen and Heller’s K coefficient (Supporting Information Table S4). However, having all metabolic components as continuous variables in the model had the best fit.
TABLE 3.
Cox proportional hazard ratioa for the association between metabolic syndrome (score and traditional definition) and CVD mortality stratified by gender and race—National Health and Nutrition Examination Survey III, United States
| N | No. events | Metabolic scoreb
|
Metabolic syndrome
|
No. metabolic components
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |||
| Men | 2721 | 220 | 1.28 | (0.961–1.701) | 0.092 | 1.08 | (0.780–1.491) | 0.648 | 1.02 | (0.884–1.181) | 0.770 |
| Non-Hispanic white | 1126 | 122 | 1.41 | (0.978–2.023) | 0.066 | 1.13 | (0.758–1.674) | 0.555 | 1.02 | (0.856–1.226) | 0.793 |
| Non-Hispanic black | 762 | 55 | 0.90 | (0.617–1.323) | 0.602 | 0.89 | (0.491–1.616) | 0.704 | 0.98 | (0.777–1.243) | 0.885 |
| Mexican-American | 833 | 43 | 1.36 | (0.726–2.544) | 0.337 | 0.88 | (0.372–2.067) | 0.763 | 1.04 | (0.725–1.485) | 0.842 |
| Women | 3038 | 203 | 1.34 | (1.059–1.683) | 0.015 | 1.27 | (0.899–1.806) | 0.174 | 1.16 | (1.056–1.276) | 0.002 |
| Non-Hispanic white | 1318 | 125 | 1.29 | (1.009–1.644) | 0.042 | 1.16 | (0.782–1.708) | 0.467 | 1.15 | (1.036–1.267) | 0.008 |
| Non-Hispanic black | 902 | 54 | 2.03 | (1.116–3.689) | 0.020 | 2.69 | (1.453–4.969) | 0.002 | 1.40 | (1.103–1.766) | 0.006 |
| Mexican-American | 818 | 24 | 3.57 | (2.209–5.759) | <0.0001 | 1.99 | (0.691–5.705) | 0.203 | 1.32 | (1.072–1.613) | 0.009 |
Models adjusted for age, smoking status, education, physical activity, alcohol consumption, and medication use for diabetes, hypertension, or high cholesterol.
Metabolic score used in the models was derived specifically for each individual subgroup.
HR, hazard ratio; CI, confidence interval.
Discussion
SEM to examine the associations between MetS and cardiovascular mortality has been sparsely utilized, yet it offers some advantages in assessing risk including considering actual values for each MetS component, their collective association with cardiovascular risk, and allowing the collective influence of MetS components to vary within subgroups of race/ethnicity and gender. MetS conceptually has been an information reduction approach in identifying those individuals at greater risk for CVD mortality instead of considering all components as predictors which resulted in the best fit and predictability model (Supporting Information Table S4). However, among the three forms of defining metabolic syndrome, the metabolic score estimated from SEM was a slightly better predictor for CVD mortality compared with harmonized MetS or the number of metabolic components present in this study of a representative sample of US adults.
Of all the metabolic components, systolic and diastolic blood pressure measures were repeatedly independently associated with CVD mortality across gender and race/ethnicity subgroups. Even though systolic blood pressure and diastolic blood pressure were associated with CVD mortality in this study, it is an association previously documented in other studies (16–18). Although it has been previously recognized that waist circumference (19–21), triglycerides (22), and fasting glucose (23) are independently associated with CVD risk; these findings were not consistent in this study. There were no significant associations observed with waist circumference and CVD mortality. Several significant linear associations with CVD mortality were observed within selected groups, including: fasting glucose among Mexican-American women, triglycerides among non-Hispanic white men, HDL cholesterol among non-Hispanic black men, and blood pressure among all groups except Mexican-American men. Dichotomizing these variables, as what is done in the harmonized MetS, may lose the effectiveness of quantifying CVD risk.
Many studies have found MetS to be associated with CVD events and/or mortality (13,24–32). Even though this association was not observed in this study, some reasons for the discrepancy could be due to the MetS definition used and the study population. Before the release of the harmonized definition in 2009, all MetS studies varied on the components and cut points used to define MetS relying on definitions from the World Health Organization, European Group for the Study of Insulin Resistance, National Cholesterol Education Program, American College of Endocrinology, or International Diabetes Federation. Although systematic reviews and meta-analyses on this topic have been consistent showing a positive association between MetS and CVD (13,24–26,28,32), the findings between studies were variable and the harmonized definition used in this study has been reported to attenuate results more so than the other definitions (11,12,27). Furthermore, some studies would substitute certain measures for others based on the data collected, such as using body mass index as opposed to waist circumference. In addition, the majority of the studies that investigated the association between MetS and CVD events or mortality were conducted among populations outside of the US. The few studies from the US were not very diverse or did not report results within gender or race/ethnic subgroups.
A major weakness of all the MetS definitions is that quantifying CVD risk is limited to yes/no and differentiation of CVD risk between combinations of components is ignored. Components may not weigh equally towards CVD risk and different clusters of components may increase CVD risk more so than others (27,31). In a study by Huang et al. (27), the cluster of high blood pressure, HDL, and WC appeared to have the highest risk for CVD mortality of all combinations, even compared with having all metabolic components present. They also observed that having high blood pressure, HDL, WC, and FG decreased the risk by half compared with if FG was not in the cluster. The underlying etiology of how the components interact to increase CVD risk is unknown. We do not fully understand the relationship all metabolic components have in relation to CVD risk and two-way, three-way, four-way, or a five-way interaction between metabolic components may be present. As a result, using harmonized MetS or treating metabolic components individually may not be the most effective way to assess or address CVD risk, especially among certain subgroups or populations.
Previous studies using SEM to assess MetS with CVD risk have found positive associations with atherosclerosis, coronary artery calcification, diabetes, carotid intima media thickness, and CVD mortality (33–37). Although gender and race/ethnicity subgroup differences in MetS using SEM has been noted (34,38,39); the previous studies either did not consider these differences, examined different path diagrams, or had study populations from other countries compared with this study. However, across all studies, the consensus was that assessing MetS using SEM was more effective in estimating CVD risk than MetS (yes/no).
There are a few limitations in this study. First, the follow-up time is based on linkage to death certificates from the National Death Index and it is possible that some deaths might have been missed. Second, we were unable to capture CVD events; we only had data on CVD mortality which limits our ability to assess the association with MetS and overall CVD risk. Third, the structural equation model proposed may not represent the true underlying etiology, especially if there are interactions between components. We considered each metabolic component acting independently although simultaneously in contributing to the metabolic score, but we may need to consider how the values of some components may affect the values of another to further increase CVD risk. Additionally, the structural equation model assumed a reflective approach implying that changes in the latent variable, MetS score, affect each component as opposed to the counterfactual formative approach inferring that changes in the components affect the MetS score. Potential mis-specification of the model approach can be problematic in determining which components load on a factor when performing traditional factor analysis (40). However, in our study we did not conduct any exploratory analysis to determine which variables to include as components of the MetS score but tested a pre-specified structural equation model based on predetermined risk factors and therefore potential mis-specification of the model would not affect our results. Fourth, although we were able to link to mortality data, all measured data were of cross-sectional design and only obtained at baseline. Therefore, changes may have occurred during follow-up time that changed CVD risk; such as initiation of medication use, diagnosis, or medical procedures; could not be accounted for in this study. Fifth, many statistical tests were performed and some significant results may have occurred due to chance. Finally, significant differences in the SEM analyses may have been a function of large sample sizes.
Future studies are needed to understand the etiology of metabolic components and how they may interact or relate to CVD risk. Although studies have shown CVD risk differences by gender and by race, most studies do not show results within race-gender subcategories and there is a need for more research in this area. Another research focus needed is investigations within subcategories of CVD due to the heterogeneity of this category (e.g., stroke, heart attack, or arrhythmia). Risk assessment using harmonized MetS may not capture or distinguish risk severity for CVD mortality. Although it has been previously stated that treatment for MetS is no different than the treatment of each component (8) and it may be the best current approach, treating individual risk factors independently may not be the most effective treatment method due to possible interactions between components which may require consideration of the relationship these factors have with each other. Other than diet and physical activity which may affect all components, we recognize that at this time treating each component individually and focusing on prevention are the best practices available until more is learned and the knowledge gap is narrowed. In this study, SEM to assess CVD mortality risk provided additional information than harmonized MetS or the number of MetS components present in that predictions became significant when using the metabolic score. Although the metabolic score driven from SEM has the potential to accurately estimate CVD risk tailored for different subgroups and therefore have positive clinical and public health implications, at this time more knowledge is needed on the etiology between metabolic components and CVD risk to establish the true path diagram and may be the reason that the models with all the components present predicted CVD mortality better than any of the MetS approaches. Even though calculations of the metabolic score using SEM is complex posing impractical risk assessment ability in the clinical setting, the future of electronic medical records may be able to take the actual metabolic component values and estimate more accurate CVD risk tailored for certain subgroups.
Supplementary Material
Footnotes
Disclosure: The authors declare no conflict of interest. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or any other entity of the US Government.
Author contributions: C.I.M., Q.Y., E.S.F., E.G., and A.L.V. designed this study; Q.Y., E.S.F., and E.G. provided subject matter expertise; Q.Y. provided statistical guidance for this study; A.L.V. supervised the process and completion of this project; C.I.M. analyzed the data, wrote the article, and has primary responsibility for the final content. All authors read, reviewed, and approved the final manuscript.
Additional Supporting Information may be found in the online version of this article.
References
- 1.National Center for Health. Deaths: Preliminary Data for 2011. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System; [Google Scholar]
- 2.Grundy SM, Brewer HB, Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 3.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 4.Novo S, Peritore A, Guarneri FP, et al. Metabolic syndrome (MetS) predicts cardio and cerebrovascular events in a twenty years follow-up. A prospective study. Atherosclerosis. 2012;223:468–472. doi: 10.1016/j.atherosclerosis.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt C, Bergstrom GM. The metabolic syndrome predicts cardiovascular events: results of a 13-year follow-up in initially healthy 58-year-old men. Metab Syndr Relat Disord. 2012;10:394–399. doi: 10.1089/met.2012.0048. [DOI] [PubMed] [Google Scholar]
- 6.Gaillard T, Schuster D, Osei K. Metabolic syndrome in Black people of the African diaspora: the paradox of current classification, definition and criteria. Ethn Dis. 2009;19:S2-1–7. [PubMed] [Google Scholar]
- 7.Lea JP, Greene EL, Nicholas SB, et al. Cardiorenal metabolic syndrome in the African diaspora: rationale for including chronic kidney disease in the metabolic syndrome definition. Ethn Dis. 2009;19:S2-11–14. [PubMed] [Google Scholar]
- 8.Kahn R, Buse J, Ferrannini E, et al. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 9.Simons LA, Simons J, Friedlander Y, McCallum J. Is prediction of cardiovascular disease and all-cause mortality genuinely driven by the metabolic syndrome, and independently from its component variables? The Dubbo study. Heart Lung Circ. 2011;20:214–219. doi: 10.1016/j.hlc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Ruotsalainen S, Moilanen L, et al. The metabolic syndrome predicts cardiovascular mortality: a 13-year follow-up study in elderly non-diabetic Finns. Eur Heart J. 2007;28:857–864. doi: 10.1093/eurheartj/ehl524. [DOI] [PubMed] [Google Scholar]
- 12.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 13.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 14.National Center for Health. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital Health Stat. 1994;32:1–407. [PubMed] [Google Scholar]
- 15.National Center for Health Statistics. Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination III Public-use Linked Mortality File. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2006. [Last accessed July 8, 2013]. Available from: http://www.cdc.gov/nchs/data_access/data_linkage/mortality/nhanes3_linkage.htm. [Google Scholar]
- 16.Miura K, Daviglus ML, Dyer AR, et al. Relationship of blood pressure to 25-year mortality due to coronary heart disease, cardiovascular diseases, and all causes in young adult men: the Chicago Heart Association Detection Project in Industry. Arch Intern Med. 2001;161:1501–1508. doi: 10.1001/archinte.161.12.1501. [DOI] [PubMed] [Google Scholar]
- 17.Psaty BM, Furberg CD, Kuller LH, et al. Association between blood pressure level and the risk of myocardial infarction, stroke, and total mortality: the cardiovascular health study. Arch Intern Med. 2001;161:1183–1192. doi: 10.1001/archinte.161.9.1183. [DOI] [PubMed] [Google Scholar]
- 18.Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med. 1993;153:598–615. doi: 10.1001/archinte.153.5.598. [DOI] [PubMed] [Google Scholar]
- 19.Bajaj HS, Brennan DM, Hoogwerf BJ, et al. Clinical utility of waist circumference in predicting all-cause mortality in a preventive cardiology clinic population: a PreCIS Database Study. Obesity (Silver Spring) 2009;17:1615–1620. doi: 10.1038/oby.2009.44. [DOI] [PubMed] [Google Scholar]
- 20.Fan J, Song Y, Chen Y, et al. Combined effect of obesity and cardio-metabolic abnormality on the risk of cardiovascular disease: a meta-analysis of prospective cohort studies. Int J Cardiol. 2013;168:4761–4768. doi: 10.1016/j.ijcard.2013.07.230. [DOI] [PubMed] [Google Scholar]
- 21.Thomas F, Pannier B, Benetos A, et al. Visceral obesity is not an independent risk factor of mortality in subjects over 65 years. Vasc Health Risk Manag. 2013;9:739–745. doi: 10.2147/VHRM.S49922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Zeng FF, Liu ZM, et al. Effects of blood triglycerides on cardiovascular and all-cause mortality: a systematic review and meta-analysis of 61 prospective studies. Lipids Health Dis. 2013;12:159. doi: 10.1186/1476-511X-12-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barr EL, Boyko EJ, Zimmet PZ, et al. Continuous relationships between non-diabetic hyperglycaemia and both cardiovascular disease and all-cause mortality: the Australian Diabetes, Obesity, and Lifestyle (AusDiab) study. Diabetologia. 2009;52:415–424. doi: 10.1007/s00125-008-1246-y. [DOI] [PubMed] [Google Scholar]
- 24.Ardern CI, Janssen I. Metabolic syndrome and its association with morbidity and mortality. Appl Physiol Nutr Metab. 2007;32:33–45. doi: 10.1139/h06-099. [DOI] [PubMed] [Google Scholar]
- 25.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119:812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 26.Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 27.Huang KC, Lee LT, Chen CY, et al. All-cause and cardiovascular disease mortality increased with metabolic syndrome in Taiwanese. Obesity (Silver Spring) 2008;16:684–689. doi: 10.1038/oby.2007.112. [DOI] [PubMed] [Google Scholar]
- 28.Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 29.Mozaffarian D, Kamineni A, Prineas RJ, Siscovick DS. Metabolic syndrome and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med. 2008;168:969–978. doi: 10.1001/archinte.168.9.969. [DOI] [PubMed] [Google Scholar]
- 30.Saito I, Iso H, Kokubo Y, et al. Metabolic syndrome and all-cause and cardiovascular disease mortality: Japan Public Health Center-based Prospective (JPHC) Study. Circ J. 2009;73:878–884. doi: 10.1253/circj.cj-08-1025. [DOI] [PubMed] [Google Scholar]
- 31.Wen CJ, Lee YS, Lin WY, et al. The metabolic syndrome increases cardiovascular mortality in Taiwanese elderly. Eur J Clin Invest. 2008;38:469–475. doi: 10.1111/j.1365-2362.2008.01965.x. [DOI] [PubMed] [Google Scholar]
- 32.Wu SH, Liu Z, Ho SC. Metabolic syndrome and all-cause mortality: a meta-analysis of prospective cohort studies. Eur J Epidemiol. 2010;25:375–384. doi: 10.1007/s10654-010-9459-z. [DOI] [PubMed] [Google Scholar]
- 33.Chirinos DA, Medina-Lezama J, Arguelles W, et al. Metabolic syndrome as an underlying disease entity and its relationship to subclinical atherosclerosis in Andean Hispanics. Metab Syndr Relat Disord. 2014;12:49–55. doi: 10.1089/met.2013.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurka MJ, Lilly CL, Oliver MN, et al. An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: a confirmatory factor analysis and a resulting continuous severity score. Metabolism. 2014;63:218–225. doi: 10.1016/j.metabol.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin JW, Hwang JJ, Dai DF, et al. Using structural equation model to illustrate the relationship between metabolic risk factors and cardiovascular complications in Taiwan. Eur J Cardiovasc Prev Rehabil. 2006;13:633–639. doi: 10.1097/01.hjr.0000230095.65062.a9. [DOI] [PubMed] [Google Scholar]
- 36.Povel CM, Beulens JW, van der Schouw YT, et al. Metabolic syndrome model definitions predicting type 2 diabetes and cardiovascular disease. Diabetes Care. 2013;36:362–368. doi: 10.2337/dc11-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevenson JE, Wright BR, Boydstun AS. The metabolic syndrome and coronary artery disease: a structural equation modeling approach suggestive of a common underlying pathophysiology. Metabolism. 2012;61:1582–1588. doi: 10.1016/j.metabol.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 38.Ferguson TF, Funkhouser E, Roseman J. Factor analysis of metabolic syndrome components in the Coronary Artery Risk Development in Young Adults (CARDIA) study: examination of factors by race-sex groups and across time. Ann Epidemiol. 2010;20:194–200. doi: 10.1016/j.annepidem.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gurka MJ, Ice CL, Sun SS, et al. A confirmatory factor analysis of the metabolic syndrome in adolescents: an examination of sex and racial/ethnic differences. Cardiovasc Diabetol. 2012;11:128. doi: 10.1186/1475-2840-11-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diamantopoulos A, Siguaw JA. Formative versus reflective indicators in organizational measure development: a comparison and empirical illustration. Br J Manag. 2006;17:263–282. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.