Abstract
AIM
To determine the effects of implementing an enteral feeding protocol on the nutritional delivery and outcomes of intensive care patients.
METHODS
An uncontrolled, observational before-and-after study was performed in a tertiary mixed medical-surgical intensive care unit (ICU). In 2013, a nurse-driven enteral feeding protocol was developed and implemented in the ICU. Nutrition and outcome-related data from patients who were treated in the study unit from 2011-2012 (the Before group) and 2014-2015 (the After group) were obtained from a local electronic database, the national Population Registry and the hospital’s Infection Control Service. Data from adult patients, readmissions excluded, who were treated for at least 7 d in the study unit were analysed.
RESULTS
In total, 231 patients were enrolled in the Before and 249 in the After group. The groups were comparable regarding demographics, patient profile, and severity of illness. Fewer patients were mechanically ventilated on admission in the After group (86.7% vs 93.1% in the Before group, P = 0.021). The prevalence of hospital-acquired infections, length of ICU stay and ICU, 30- and 60-d mortality did not differ between the groups. Patients in the After group had a lower 90-d (P = 0.026) and 120-d (P = 0.033) mortality. In the After group, enteral nutrition was prescribed less frequently (P = 0.039) on day 1 but significantly more frequently on all days from day 3. Implementation of the feeding protocol resulted in a higher cumulative amount of enterally (P = 0.049) and a lower cumulative amount of parenterally (P < 0.001) provided calories by day 7, with an overall reduction in caloric provision (P < 0.001). The prevalence of gastrointestinal symptoms was comparable in both groups, as was the frequency of prokinetic use. Underfeeding (total calories < 80% of caloric needs, independent of route) was observed in 59.4% of the study days Before vs 76.9% After (P < 0.001). Inclusion in the Before group, previous abdominal surgery, intra-abdominal hypertension and the sum of gastrointestinal symptoms were found to be independent predictors of insufficient enteral nutrition.
CONCLUSION
The use of a nurse-driven feeding protocol improves the delivery of enteral nutrition in ICU patients without concomitant increases in gastrointestinal symptoms or intra-abdominal hypertension.
Keywords: Gastrointestinal symptoms, Underfeeding, Nutrition protocol, Feeding protocol, Enteral feeding, Enteral nutrition, Parenteral nutrition, Critical care
Core tip: Following implementation of a nurse-driven enteral feeding protocol in a mixed medical-surgical intensive care unit (ICU) with a high baseline underfeeding rate, caloric intake via the enteral route was significantly increased during the first week in the ICU without concomitant increases in the frequency of gastrointestinal symptoms, intra-abdominal hypertension or use of prokinetic medication.
INTRODUCTION
Enteral feeding (EN) is currently considered the best option for providing nutrition to critically ill patients. The use of the enteral route may specifically reduce disease severity by attenuating the stress response[1] while avoiding the increased infectious morbidity observed with the use of parenteral nutrition (PN)[2]. Starting EN early after admission to an intensive care unit (ICU) is favoured over a delayed approach, as it reduces morbidity and mortality[3,4]. The best clinical outcomes are achieved when over 85% of the prescribed caloric intake is provided[5]. However, inadequate enteral feeding continues to exist in ICUs worldwide[6]; indeed, a previous study[7] conducted in our ICU demonstrated that insufficient enteral feeding occurred in more than half of the patients.
Guidelines issued by the American Society for Parenteral and Enteral Nutrition and the Society of Critical Care Medicine suggest the use of a feeding protocol to improve nutritional outcomes[2]. These protocols aim to standardize and automate the provision of EN, enabling bedside nurses to initiate, monitor and alter the administration of feeds without direct orders from the attending physician[8]. Several studies[9-12] have shown an increase in nutritional provision with the use of enteral feeding protocols, but the effect of these protocols on relevant patient outcomes has been shown in only a few studies[13-15].
The aim of this study was to evaluate the effect of a nurse-driven enteral feeding protocol on the amount of nutrition provided and on patient outcomes. An observational before-and-after study was conducted.
MATERIALS AND METHODS
Ethical considerations
The study was approved by the Research Ethics Committee of the University of Tartu with waived informed consent (permit no. 258/T-6).
Statistical review statement
The statistical methods of this study were reviewed by a co-author Liis Starkopf from the Department of Public Health, Section of Biostatistics, Faculty of Health and Medical Sciences, University of Copenhagen.
Patient population
The 1st Intensive Care Unit of Tartu University Hospital is a 10-bed tertiary level mixed medical-surgical ICU in a regional hospital. Data from patients treated in this department before and after the implementation of a nurse-driven feeding protocol were compared. Included in this study were adult patients (at least 18 years of age) who were treated in the ICU for at least 7 consecutive days. Readmissions were excluded.
Design of the study
An uncontrolled, observational before-and-after study was performed. In 2013, a nurse-driven feeding protocol was implemented in the study unit. The study period comprised three phases: Pre-intervention (Before), intervention and post-intervention (After). No dieticians were involved in any of the study phases.
In 2011 and 2012, an enteral feeding protocol was not in use. Decisions regarding nutrition were made daily by the attending physician, while the nursing staff was responsible for the provision of feedings. Adult patients, not including readmissions, who were treated for at least 7 d in the ICU in 2011 and 2012 were included in the Before group.
The enteral feeding protocol was implemented in 2013 (Figure 1). In our study, the year 2013 served as a learning and adaptation period, and thus patients in this period were not included in our analysis.
Figure 1.
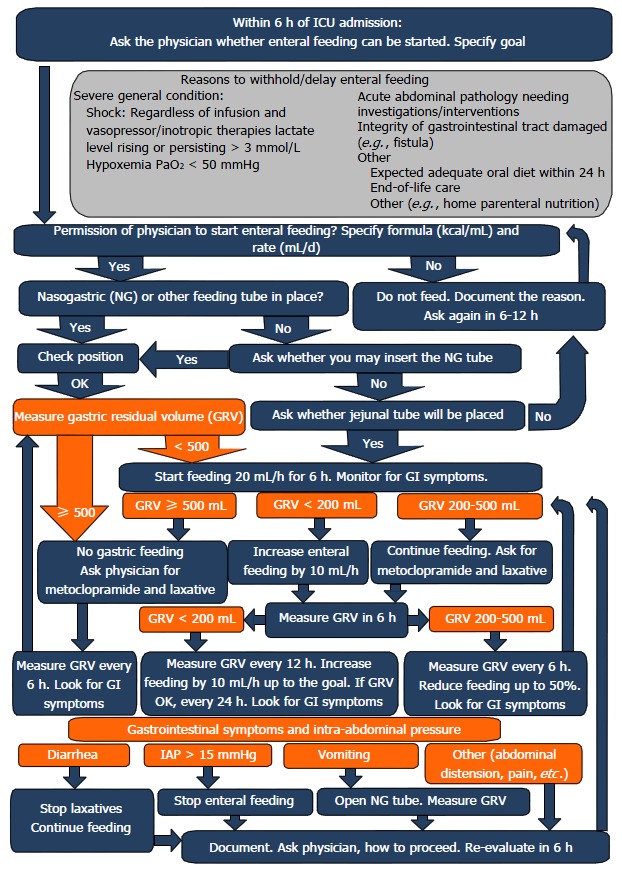
Feeding protocol. ICU: Intensive care unit; GI: Gastrointestinal.
In 2014 and 2015, the use of the enteral feeding protocol was routine. Eligible patients admitted during this period composed the After group.
In the post-intervention phase, physicians were not required to follow the feeding protocol for nutrition-related decisions. Adherence to the protocol was not assessed in the present study.
Data collection
Admission characteristics, nutritional information and outcome data were extracted from an electronic database used in the ICU, while data concerning hospital-acquired infections were provided by the Hospital Infection Control Service. Mortality data were retrieved from the national Population Register.
Enteral feeding protocol
The study authors developed the feeding protocol according to available examples in the scientific literature in 2012. The ultimate purpose was to support bedside nurses with a structural decision tree for independent decision making in enteral feeding.
Variables
Patient characteristics included age, sex, body mass index (BMI), diagnostic category, occurrence of abdominal surgery, Acute Physiology and Chronic Health Evaluation II (APACHE II)[16] and Sequential Organ Failure Assessment (SOFA)[17] scores, vasopressor or inotrope treatment and mechanical ventilation (MV) on admission to the ICU. The diagnostic category was defined as surgical or medical according to the diagnoses at ICU admission. Outcome variables were length of ICU stay and MV, prevalence of hospital-acquired infections (ventilator-associated pneumonia, urinary tract infection, blood stream infection, Clostridium difficile enterocolitis), ICU mortality and 30-, 60-, 90- and 120-d mortality. Hospital-acquired infections were defined as a diagnosis by the Hospital Infection Control Service.
Nutritional support variables included the amount of calories administered daily via enteral and parenteral routes during a patient’s ICU stay. Only data from the first 7 days were included in the analysis. Insufficient EN was defined as the provision of less than 50% of caloric needs via the enteral route and was assessed on day 4 and day 7.
Overfeeding was defined as receiving more than 110% of daily caloric needs via any route, and underfeeding as less than 80%; these variables were analysed as the total incidence during 7 d.
Dextrose-based maintenance infusions were included in the calculations of parenteral calories, whereas the nutritional value of propofol was not taken into account.
Gastrointestinal (GI) symptoms and management variables were recorded daily and were defined and calculated as follows. Absence or presence of bowel sounds was determined daily by a senior intensive care physician using auscultation in a non-protocolized manner. To measure gastric residual volume (GRV), enteral feeding was stopped, and the nasogastric tube was held closed for 30 min. The tube was then opened and remained open for 30 min with a collection bag mounted to the bed well under the level of the stomach, allowing for the free flow of gastric content. Evacuated content was discarded. Initially, after starting EN, GRV measurements were performed every 6 h. Further measurements were made every 12 h, if two consecutive measurements had yielded less than 200 mL. A large GRV was defined as a total daily volume greater than 500 mL. Bowel distension was defined when confirmed radiologically. Vomiting and GI bleeding were defined as a visible amount of vomit or the presence of a visible amount of blood in stomach contents or stool, respectively. Diarrhoea was defined as the occurrence of liquid stools more than 3 times in a day. Intra-abdominal pressure (IAP) was recorded daily in select patients who were considered at risk for intra-abdominal hypertension (IAH) according to departmental routine. In those patients, IAP was measured intermittently every 6 to 12 h (more frequently if the previous IAP was increased - 12 mmHg or higher) with a transvesical pressure measurement technique in accordance with the clinical practice guidelines of the World Society of the Abdominal Compartment Syndrome[18]. The sum of GI symptoms was defined as the sum of the daily prevalence (1) or absence (0) of previously described GI symptoms. Prescription of metoclopramide was defined as a standing order of the drug.
Statistical analysis
Categorical variables are described as the number of patients and proportions and were compared using χ2 or Fisher’s exact test. The normality of the distribution of continuous variables was evaluated by Kolmogorov-Smirnov test. Continuous variables are described as the median and inter-quartile range if not stated otherwise. Comparisons of continuous variables were performed using an independent samples median test.
Logistic regression analyses were performed to identify the independent predictors of insufficient EN by day 4 and day 7. All admission day variables that positively predicted outcomes in the univariate analysis with P < 0.2 were entered stepwise into a multiple logistic regression model. Coupling variables were added and removed with a stepwise approach to obtain a final optimal model for predicting insufficient EN. Nagelkerke R Square test was used to evaluate the power of the prediction models. The data were analysed using SPSS software (version 23.0, IBM).
RESULTS
Patient characteristics and outcome data
In total, 665 and 683 patients, respectively, were admitted to the ICU before and after the implementation of the feeding protocol. After excluding patients under 18 years of age, readmissions and patients who stayed less than 7 d in the ICU, the study population consisted of 231 patients in the Before and 249 patients in the After group.
The groups did not differ regarding patient age, sex, BMI, case-mix, APACHE II or SOFA scores nor in the frequency of vasopressor/inotrope therapy at admission. Around half of the patients had a surgical profile, and the majority of them had received abdominal surgery. The proportion of patients who were mechanically ventilated on admission was significantly smaller in the After group. No significant changes between the two groups were found in length of ICU stay, duration of mechanical ventilation, frequency of hospital-acquired infections or ICU, 30-d and 60-d mortality. However, 90-d and 120-d mortality were significantly lower in the After group (Table 1).
Table 1.
Admission characteristics and outcome data
| All | Before | After | P-value (before vs after) | |
| Admission characteristics | ||||
| n of pt | 480 | 231 | 249 | |
| Male gender, n (%) | 298 (62.1) | 151 (65.4) | 147 (59.0) | 0.159 |
| Surgical profile, n (%) | 256 (53.3) | 120 (51.9) | 136 (54.6) | 0.311 |
| Abdominal surgery, n (%) | 141 (29.4) | 72 (31.2) | 69 (27.7) | 0.232 |
| Age, mean (range) | 61.7 (18-96) | 61.5 (18-96) | 62.0 (20-93) | 0.684 |
| BMI | 27.8 (24.3-31.6) | 27.7 (24.5-31.5) | 27.8 (24.3-32.0) | 0.791 |
| APACHE II, points | 16 (11.0-22.0) | 16 (11.0-21.0) | 16 (12.0-22.0) | 0.948 |
| SOFA, points | 8 (6.0-10.0) | 8 (6.0-10.0) | 8 (7.0-10.0) | 0.504 |
| Vasopressor/inotrope, n (%) | 407 (84.8) | 195 (84.4) | 212 (85.1) | 0.462 |
| Mechanical ventilation, n (%) | 431 (89.8) | 215 (93.1) | 216 (86.7) | 0.021 |
| Outcomes | ||||
| ICU stay (d) | 13 (9-21) | 13 (9-22) | 13 (8-21) | 0.978 |
| Mechanical ventilation (d) | 10 (6-17) | 9 (6-18) | 10 (6-17) | 0.796 |
| Ventilator pneumonia, n (%) | 16 (3.3) | 7 (3.0) | 9 (3.6) | 0.461 |
| Urinary tract infection, n (%) | 31 (6.5) | 16 (6.9) | 15 (6.0) | 0.582 |
| Bloodstream infection, n (%) | 16 (3.3) | 6 (2.6) | 10 (4.0) | 0.404 |
| Cl difficile colitis, n (%) | 14 (2.9) | 6 (2.6) | 8 (3.2) | 0.450 |
| ICU mortality | 51 (10.6) | 28 (12.1) | 23 (9.2) | 0.190 |
| 30-d mortality | 121 (25.2) | 64 (27.7) | 52 (22.9) | 0.134 |
| 60-d mortality | 136 (28.3) | 73 (31.6) | 63 (25.3) | 0.076 |
| 90-d mortality | 157 (32.7) | 86 (37.2) | 71 (28.5) | 0.026 |
| 120-d mortality | 164 (34.2) | 89 (38.5) | 75 (30.1) | 0.033 |
BMI: Body mass index; APACHE II: Acute Physiology and Chronic Health Evaluation II; SOFA: Sequential Organ Failure Assessment; ICU: Intensive care unit.
Nutritional support
EN was not initiated during the ICU stay of 19 patients. After implementation of the feeding protocol, significantly fewer patients received enteral feeding on day 1 (27.7% Before vs 18.1% After; P = 0.039). On day 2, EN was administered to approximately half of the patients in both groups (48.5% Before vs 53% After; P = 0.593). The median time to EN initiation was similar between the groups [day 2 (1-5) Before vs day 2 (2-4) After (P = 0.73)]. After implementation of the feeding protocol, a larger proportion of patients received EN from day 3 onwards (Figure 2), and the median daily caloric intake via the enteral route was significantly higher on days 3, 4, 6 and 7 (Table 2).
Figure 2.
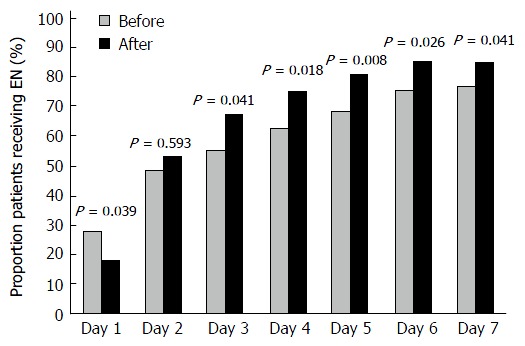
Daily proportion of patients receiving enteral nutrition. EN: Enteral feeding.
Table 2.
Daily enteral caloric intake
| Daily enteral caloric intake | Before | After | P-value |
| Median (IQR), kcal | Median (IQR), kcal | ||
| Day 1 | 0 (0-100) | 0 (0-0) | 0.016 |
| Day 2 | 0 (0-480) | 100 (0-480) | 0.409 |
| Day 3 | 160 (0-700) | 370 (0-767) | 0.031 |
| Day 4 | 340 (0-800) | 500 (10-960) | 0.003 |
| Day 5 | 400 (0-1000) | 580 (176-1100) | 0.142 |
| Day 6 | 500 (53-1000) | 695 (240-1138) | 0.003 |
| Day 7 | 500 (108-1000) | 720 (200-1155) | 0.018 |
After implementation of the feeding protocol, the cumulative amount of enterally provided calories during patients’ first week in the ICU was significantly higher (P = 0.049), while the amount of calories provided from parenteral nutrition was significantly lower (P < 0.001, Table 3). Overall, fewer calories (enteral plus parenteral) were provided during the first 7 d (P < 0.001) after the feeding protocol was implemented. The median percentage of calories administered enterally of the calculated caloric needs day-by-day is presented in Figure 3.
Table 3.
Seven-day cumulative enteral and parenteral caloric intake
| Cumulative caloric intake | All | Before | After | P-value |
| Median (IQR), kcal | Median (IQR), kcal | Median (IQR), kcal | (before vs after) | |
| Cumulative EN calories day 7 | 2870 (803-5163) | 2300 (380-5030) | 3210 (1280-5215) | 0.049 |
| Cumulative PN calories day 7 | 3100 (1338-5225) | 3977 (1775-6646) | 2600 (825-4287) | < 0.001 |
| Cumulative total calories day 7 | 6531 (5035-8140) | 7030 (5667-8970) | 6000 (4715-7498) | < 0.001 |
EN: Enteral feeding; PN: Parenteral nutrition.
Figure 3.
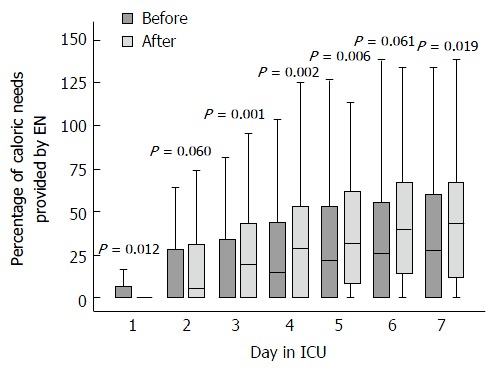
Percentage of caloric needs provided by enteral feeding before and after implementation of feeding protocol. The boxes represent interquartile range (a median value is marked with a line) and error bars 95%CI. EN: Enteral feeding.
The incidence of overfeeding in all analysed days was 8.4% in the Before and 4.5% in the After group (P < 0.001), whereas underfeeding occurred on 59.4% and 76.9% of the days in the Before and After group, respectively (P < 0.001).
The results of the regression analysis with variables predicting insufficient EN by day 4 and day 7 are shown in Table 4. The risk of insufficient EN both on day 4 and day 7 was increased in patients in the Before group.
Table 4.
Regression analysis with day of admission variables predicting insufficient enteral feeding by day 4 and day 7
| Variables predicting insufficient EN |
Day 4 |
Day 7 |
||
| OR (95%CI) | P-value | OR (95%CI) | P-value | |
| Before group | 4.02 (1.55-10.40) | 0.004 | 2.09 (1.35-3.22) | 0.001 |
| Abdominal surgery | 3.97 (1.26-12.46) | 0.018 | 3.09 (1.82-5.27) | < 0.001 |
| Sum of GI symptoms | 6.01 (2.55-14.14) | < 0.001 | 2.35 (1.60-3.44) | < 0.001 |
| IAH | 4.20 (1.32-13.34) | 0.015 | - | - |
| Nagelkerke R Square | 0.349 | 0.19 | ||
EN: Enteral feeding; GI: Gastrointestinal; IAH: Intra-abdominal hypertensio.
Gastrointestinal symptoms and treatment
We found no significant differences between the groups regarding the daily occurrence of vomiting, radiologically confirmed bowel distension, GI bleeding or large GRV (> 500 mL/d). The incidence of diarrhoea was similar and below 10% in both groups, with the only exception of day 4, when more cases were observed in the After group (19/249 vs 6/213, P < 0.05). No difference was noted in the maximal sum of GI symptoms per day [median 1 (1-2) in both groups, P = 0.112].
Half of the patients in both groups developed intra-abdominal hypertension in their first week in the ICU (51.2% Before vs 55.9% After, P = 0.218). A difference was observed only on day 5, when 29.5% of the patients in the After group had IAH compared to 20.5% in the Before group (P = 0.043).
After implementation of the feeding protocol, the prescription of metoclopramide did not increase, and on day 2, it was significantly lower (9.1% Before vs 3.6% After, P = 0.011).
DISCUSSION
This before-and-after study was designed to evaluate the effects of the implementation of a nurse-driven feeding protocol on feeding practices and on the outcomes of long-term adult patients in a mixed ICU. The amount of enterally given calories was higher after implementing the feeding protocol, without a concomitant increase in the use of a prokinetic nor in the prevalence of GI symptoms or IAH.
Our findings are in accordance with previous before-and-after studies using nurse-driven rate-based enteral feeding protocols. Studies by Arabi et al[10] and Spain et al[19] have shown an increase in the cumulative enteral caloric intake on days 7 and 3 in the ICU, respectively, while a greater proportion of patients receiving enteral nutrition after implementation of a feeding protocol was reported by Barr et al[20] and Compton et al[11]. More frequent and earlier achievement of nutritional goals have been described[11,12], as well as a reduction in the use of parenteral nutrition[12]. These were small, mostly single-centre studies and therefore lacked generalizability. A cluster randomized controlled trial (RCT) by Martin et al[13] (the ACCEPT trial) showed that evidence-based nutrition algorithms focusing on the early provision of enteral feeding and on frequent re-evaluations increased the number of days when EN was delivered and reduced both hospital length of stay and mortality. A large cluster RCT conducted by Doig et al[9] with 1118 patients and 27 enrolled ICUs showed an earlier start of both enteral and parenteral feeding and greater nutritional adequacy occurred after implementing evidence-based guidelines with a versatile practice change strategy; however, their study failed to demonstrate effects on patient outcomes. Finally, improved nutritional adequacy and reductions in infectious morbidity have been shown in studies by Heyland et al[14], using a volume-based, top-down feeding algorithm, and by Taylor et al[15], using an enhanced EN approach. The RCTs demonstrating positive effects on nutrition and clinical outcomes included a variety of interventions, meaning the effects of the feeding protocols were inseparable from those of the whole strategy. In our study, only long-term patients were included, a third of whom had abdominal surgery. Patients with complicated abdominal surgery, requiring a prolonged stay in the ICU, are undoubtedly the most challenging group of patients in terms of successful EN. This aspect needs to be taken into account when interpreting our results, which showed largely insufficient EN both before and after implementation of the feeding protocol. During the time the study was conducted, some changes in the understanding of nutrition in the acute phase of critical illness emerged, including the concept of autophagy and non-inferiority or even possible benefits of underfeeding in the early phase[21-26]. These changes may explain why EN was started less frequently on the day of admission (in most cases not the full 24-h day) in the After group compared to the Before group.
Interestingly, while the cumulative amount of enteral calories in the first week in the ICU increased, the amount of parenterally given calories decreased by a greater amount. The decreased incidence of overfeeding (considering total calories) after implementation of the feeding protocol did not explain the magnitude of the observed change. Accordingly, implementation of the enteral feeding protocol resulted in decreased total caloric intake. There are some possible explanations for this finding. Shortly before the feeding protocol was implemented, the results of the EPaNIC trial[27] were acknowledged and were potentially interpreted as an argument against early parenteral nutrition. This might have led to a reduction in PN independent of the feeding protocol. Additionally, decisions regarding the initiation of PN continued to be made by physicians in a non-protocolized way. Therefore, because more patients received EN in the After group, the physicians may have been more likely to withhold supplemental PN in these patients, whereas (full) PN was more likely to be prescribed in patients who remained without EN (larger proportion in the Before group). However, these interactions led to a negative result regarding total caloric intake. Even if significantly increased amounts of enteral calories were administered after implementation of the feeding protocol, they remained far from reaching the caloric targets. As the end-effect, the more pronounced reduction in PN resulted in an even larger caloric deficit in the After group. This is an important finding, indicating the need to plan complex nutritional interventions including EN and PN without the risk of increases in enterally provided calories resulting in an increased total caloric deficit. The presence of a dietician in the ICU would probably also help eliminate this problem. Although the optimal timing of supplemental PN is not known, a cumulative caloric deficit above 4000 kcal should likely be avoided[28-30].
It should, however, be noted that the nutritional value of propofol was not included in the caloric calculations in the present study, whereas the awareness regarding the appropriate amounts of calories provided with propofol infusions and regarding the negative impact of overfeeding[31,32] is increasing. It is not clear whether the propofol dosage influenced physicians’ decisions about the nutrition prescribed in the study period, and furthermore, whether this potential effect varied between the pre- and post-intervention phases.
The prevalence of GI symptoms is high in critically ill patients[33], and any increases in their prevalence due to more aggressive EN should be avoided. The identified differences in some of the symptoms on single days during the first week in the ICU seemed random and related to the low total number of events. However, the safety of using standard feeding protocols in certain patient groups (e.g., those at increased risk for aspiration or severe bowel distension) was not established in the current study.
The main strengths of our study are the relatively constant patient population in the study unit over the years and the daily documented data on GI symptoms. However, our study has several limitations. The single-centre design with a limited number of select (stay > 7 d) patients in a mixed ICU, with a significant proportion of patients receiving abdominal surgery, decreases the generalizability of our results. Furthermore, we studied a relatively long time span and it is possible that other non-protocolized changes in clinical routines might have occurred and influenced the outcomes. Some of the documented GI symptoms occurred very rarely, and therefore the significance of the difference in their prevalence may have changed more or less with each case.
We believe that in addition to describing the magnitude of the effect of a feeding protocol on the delivery of EN and patient-related outcomes, our study notes a possible pitfall regarding the implications of a nurse-driven feeding protocol without standardizing the use of supplemental PN.
The use of a nurse-driven feeding protocol is associated with an improved delivery of enteral nutrition without a concomitant increase in the use of prokinetics nor in the prevalence of GI symptoms or IAH in adult ICU patients with an ICU stay of at least 7 d. Increased, but still insufficient, EN may lead to the withholding of PN, resulting in an even larger total caloric deficit. Therefore, the use of an enteral nutrition protocol alone without the presence of a dietician and in absence of a standard for supplemental parenteral nutrition may not prevent severe underfeeding.
ACKNOWLEDGMENTS
We thank all the nurses of the study unit for their great work in making the implementation of the feeding protocol possible.
COMMENTS
Background
Enteral feeding is the method of choice for providing nutrition to critically ill patients, yet underfeeding continues to be a problem in intensive care units (ICUs) worldwide. This also holds true to their study unit - a mixed ICU with a high proportion of long-staying patients admitted after complicated abdominal surgery. The use of an enteral feeding protocol has been consistently shown to improve the delivery of enteral nutrition (EN) in several studies, however, only a few have reported an effect on relevant patient outcomes.
Research frontiers
Early EN has recently become a hotspot in research of nutritional support for critically ill. Yet not clearly proven, there is some data suggesting that early EN improves important patient-centred outcomes of intensive care. Implementation of a feeding protocol would be the first pragmatic step for any ICU aiming to facilitate EN. This study confirms that with protocol based approach, enteral delivery of nutrients can be significantly enhanced without an increase in gastrointestinal symptoms.
Innovations and breakthroughs
In ICU long-stayers, implementation of the enteral feeding protocol significantly improved the delivery of EN. Unlike most similar studies, the authors reported on gastrointestinal symptoms, intra-abdominal hypertension and the use of prokinetic medications and demonstrated that this improvement in EN did not increase the frequency of aforementioned problems. Importantly, after introduction of the feeding protocol, the use of parenteral nutrition decreased significantly, resulting in a reduction in both parenterally administered and total calories. Accordingly, the prevalence of underfeeding did not decrease despite implementation of the enteral feeding protocol.
Applications
This study demonstrated that use of an enteral feeding protocol was safe in terms of nutrition-related complications. However, in a nutritionally challenging patient population, it also brought along a reduction in overall caloric intake. This finding may warrant implementing a strategy of supplemental parenteral nutrition to help reduce the caloric debt seen in long-staying ICU patients.
Terminology
A nurse-driven enteral feeding protocol refers to an algorithm enabling the bedside nurse to start, monitor and adjust the delivery of enteral tube feedings to patients not capable of oral food intake.
Peer-review
This study was well conducted and nicely written. It has enough quality to be published in this journal.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Critical care medicine
Country of origin: Estonia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was approved by the institutional review board of Tartu University Hospital.
Informed consent statement: Waiver of informed consent was approved by the Ethics Committee of University of Tartu due to the observational design of the study.
Conflict-of-interest statement: ARB received honoraria for participation in the advisory board meetings of Nestlé, Fresenius and Nutricia. JS has received honoraria for advisory board participation from B. Braun Melsungen AG. The authors declare that they have no conflicts of interest regarding this particular study.
Data sharing statement: No additional data are available.
Peer-review started: September 1, 2016
First decision: October 20, 2016
Article in press: January 14, 2017
P- Reviewer: Hokama A, Joh JW S- Editor: Kong JX L- Editor: A E- Editor: Li D
References
- 1.Windsor AC, Kanwar S, Li AG, Barnes E, Guthrie JA, Spark JI, Welsh F, Guillou PJ, Reynolds JV. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut. 1998;42:431–435. doi: 10.1136/gut.42.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, McCarthy MS, Davanos E, Rice TW, Cresci GA, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) JPEN J Parenter Enteral Nutr. 2016;40:159–211. doi: 10.1177/0148607115621863. [DOI] [PubMed] [Google Scholar]
- 3.Marik PE, Zaloga GP. Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med. 2001;29:2264–2270. doi: 10.1097/00003246-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Doig GS, Heighes PT, Simpson F, Sweetman EA, Davies AR. Early enteral nutrition, provided within 24 h of injury or intensive care unit admission, significantly reduces mortality in critically ill patients: a meta-analysis of randomised controlled trials. Intensive Care Med. 2009;35:2018–2027. doi: 10.1007/s00134-009-1664-4. [DOI] [PubMed] [Google Scholar]
- 5.Heyland DK, Cahill N, Day AG. Optimal amount of calories for critically ill patients: depends on how you slice the cake! Crit Care Med. 2011;39:2619–2626. doi: 10.1097/CCM.0b013e318226641d. [DOI] [PubMed] [Google Scholar]
- 6.Heyland DK, Dhaliwal R, Wang M, Day AG. The prevalence of iatrogenic underfeeding in the nutritionally ‘at-risk’ critically ill patient: Results of an international, multicenter, prospective study. Clin Nutr. 2015;34:659–666. doi: 10.1016/j.clnu.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Kuslapuu M, Jõgela K, Starkopf J, Reintam Blaser A. The reasons for insufficient enteral feeding in an intensive care unit: A prospective observational study. Intensive Crit Care Nurs. 2015;31:309–314. doi: 10.1016/j.iccn.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Heyland DK, Cahill NE, Dhaliwal R, Sun X, Day AG, McClave SA. Impact of enteral feeding protocols on enteral nutrition delivery: results of a multicenter observational study. JPEN J Parenter Enteral Nutr. 2010;34:675–684. doi: 10.1177/0148607110364843. [DOI] [PubMed] [Google Scholar]
- 9.Doig GS, Simpson F, Finfer S, Delaney A, Davies AR, Mitchell I, Dobb G. Effect of evidence-based feeding guidelines on mortality of critically ill adults: a cluster randomized controlled trial. JAMA. 2008;300:2731–2741. doi: 10.1001/jama.2008.826. [DOI] [PubMed] [Google Scholar]
- 10.Arabi Y, Haddad S, Sakkijha M, Al Shimemeri A. The impact of implementing an enteral tube feeding protocol on caloric and protein delivery in intensive care unit patients. Nutr Clin Pract. 2004;19:523–530. doi: 10.1177/0115426504019005523. [DOI] [PubMed] [Google Scholar]
- 11.Compton F, Bojarski C, Siegmund B, van der Giet M. Use of a nutrition support protocol to increase enteral nutrition delivery in critically ill patients. Am J Crit Care. 2014;23:396–403. doi: 10.4037/ajcc2014140. [DOI] [PubMed] [Google Scholar]
- 12.Mackenzie SL, Zygun DA, Whitmore BL, Doig CJ, Hameed SM. Implementation of a nutrition support protocol increases the proportion of mechanically ventilated patients reaching enteral nutrition targets in the adult intensive care unit. JPEN J Parenter Enteral Nutr. 2005;29:74–80. doi: 10.1177/014860710502900274. [DOI] [PubMed] [Google Scholar]
- 13.Martin CM, Doig GS, Heyland DK, Morrison T, Sibbald WJ. Multicentre, cluster-randomized clinical trial of algorithms for critical-care enteral and parenteral therapy (ACCEPT) CMAJ. 2004;170:197–204. [PMC free article] [PubMed] [Google Scholar]
- 14.Heyland DK, Murch L, Cahill N, McCall M, Muscedere J, Stelfox HT, Bray T, Tanguay T, Jiang X, Day AG. Enhanced protein-energy provision via the enteral route feeding protocol in critically ill patients: results of a cluster randomized trial. Crit Care Med. 2013;41:2743–2753. doi: 10.1097/CCM.0b013e31829efef5. [DOI] [PubMed] [Google Scholar]
- 15.Taylor SJ, Fettes SB, Jewkes C, Nelson RJ. Prospective, randomized, controlled trial to determine the effect of early enhanced enteral nutrition on clinical outcome in mechanically ventilated patients suffering head injury. Crit Care Med. 1999;27:2525–2531. doi: 10.1097/00003246-199911000-00033. [DOI] [PubMed] [Google Scholar]
- 16.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 17.Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, Duchesne J, Bjorck M, Leppaniemi A, Ejike JC, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190–1206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spain DA, McClave SA, Sexton LK, Adams JL, Blanford BS, Sullins ME, Owens NA, Snider HL. Infusion protocol improves delivery of enteral tube feeding in the critical care unit. JPEN J Parenter Enteral Nutr. 1999;23:288–292. doi: 10.1177/0148607199023005288. [DOI] [PubMed] [Google Scholar]
- 20.Barr J, Hecht M, Flavin KE, Khorana A, Gould MK. Outcomes in critically ill patients before and after the implementation of an evidence-based nutritional management protocol. Chest. 2004;125:1446–1457. doi: 10.1378/chest.125.4.1446. [DOI] [PubMed] [Google Scholar]
- 21.Casaer MP, Van den Berghe G. Nutrition in the acute phase of critical illness. N Engl J Med. 2014;370:1227–1236. doi: 10.1056/NEJMra1304623. [DOI] [PubMed] [Google Scholar]
- 22.Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, Moss M, Morris A, Dong N, Rock P. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307:795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arabi YM, Aldawood AS, Haddad SH, Al-Dorzi HM, Tamim HM, Jones G, Mehta S, McIntyre L, Solaiman O, Sakkijha MH, et al. Permissive Underfeeding or Standard Enteral Feeding in Critically Ill Adults. N Engl J Med. 2015;372:2398–2408. doi: 10.1056/NEJMoa1502826. [DOI] [PubMed] [Google Scholar]
- 24.Casaer MP, Van den Berghe G. Editorial on the original article entitled “Permissive underfeeding of standard enteral feeding in critically ill adults” published in the New England Journal of Medicine on June 18, 2015. Ann Transl Med. 2015;3:226. doi: 10.3978/j.issn.2305-5839.2015.07.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermans G, Casaer MP, Clerckx B, Güiza F, Vanhullebusch T, Derde S, Meersseman P, Derese I, Mesotten D, Wouters PJ, et al. Effect of tolerating macronutrient deficit on the development of intensive-care unit acquired weakness: a subanalysis of the EPaNIC trial. Lancet Respir Med. 2013;1:621–629. doi: 10.1016/S2213-2600(13)70183-8. [DOI] [PubMed] [Google Scholar]
- 26.Braunschweig CA, Sheean PM, Peterson SJ, Gomez Perez S, Freels S, Lateef O, Gurka D, Fantuzzi G. Intensive nutrition in acute lung injury: a clinical trial (INTACT) JPEN J Parenter Enteral Nutr. 2015;39:13–20. doi: 10.1177/0148607114528541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, Van Cromphaut S, Ingels C, Meersseman P, Muller J, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365:506–517. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]
- 28.Heidegger CP, Berger MM, Graf S, Zingg W, Darmon P, Costanza MC, Thibault R, Pichard C. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet. 2013;381:385–393. doi: 10.1016/S0140-6736(12)61351-8. [DOI] [PubMed] [Google Scholar]
- 29.Villet S, Chiolero RL, Bollmann MD, Revelly JP, Cayeux R N MC, Delarue J, Berger MM. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr. 2005;24:502–509. doi: 10.1016/j.clnu.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Dvir D, Cohen J, Singer P. Computerized energy balance and complications in critically ill patients: an observational study. Clin Nutr. 2006;25:37–44. doi: 10.1016/j.clnu.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Grau T, Bonet A, Rubio M, Mateo D, Farré M, Acosta JA, Blesa A, Montejo JC, de Lorenzo AG, Mesejo A. Liver dysfunction associated with artificial nutrition in critically ill patients. Crit Care. 2007;11:R10. doi: 10.1186/cc5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulman RC, Mechanick JI. Can nutrition support interfere with recovery from acute critical illness? World Rev Nutr Diet. 2013;105:69–81. doi: 10.1159/000341272. [DOI] [PubMed] [Google Scholar]
- 33.Reintam A, Parm P, Kitus R, Kern H, Starkopf J. Gastrointestinal symptoms in intensive care patients. Acta Anaesthesiol Scand. 2009;53:318–324. doi: 10.1111/j.1399-6576.2008.01860.x. [DOI] [PubMed] [Google Scholar]


