Abstract
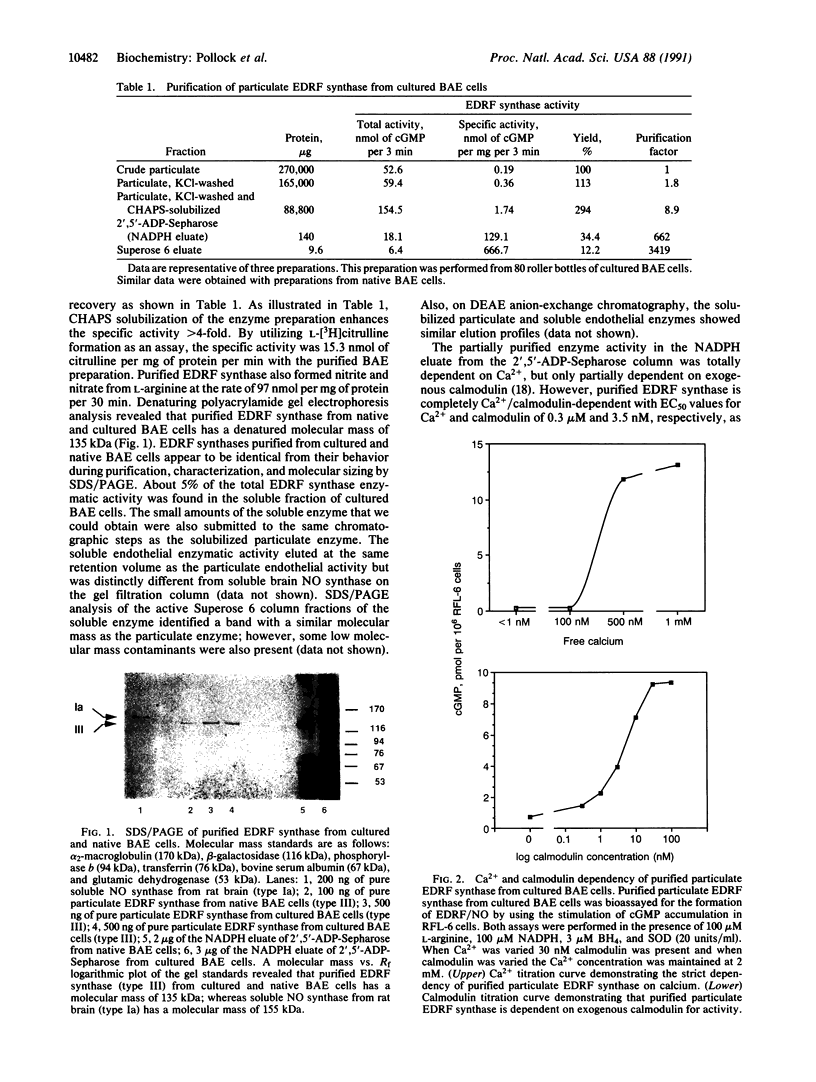
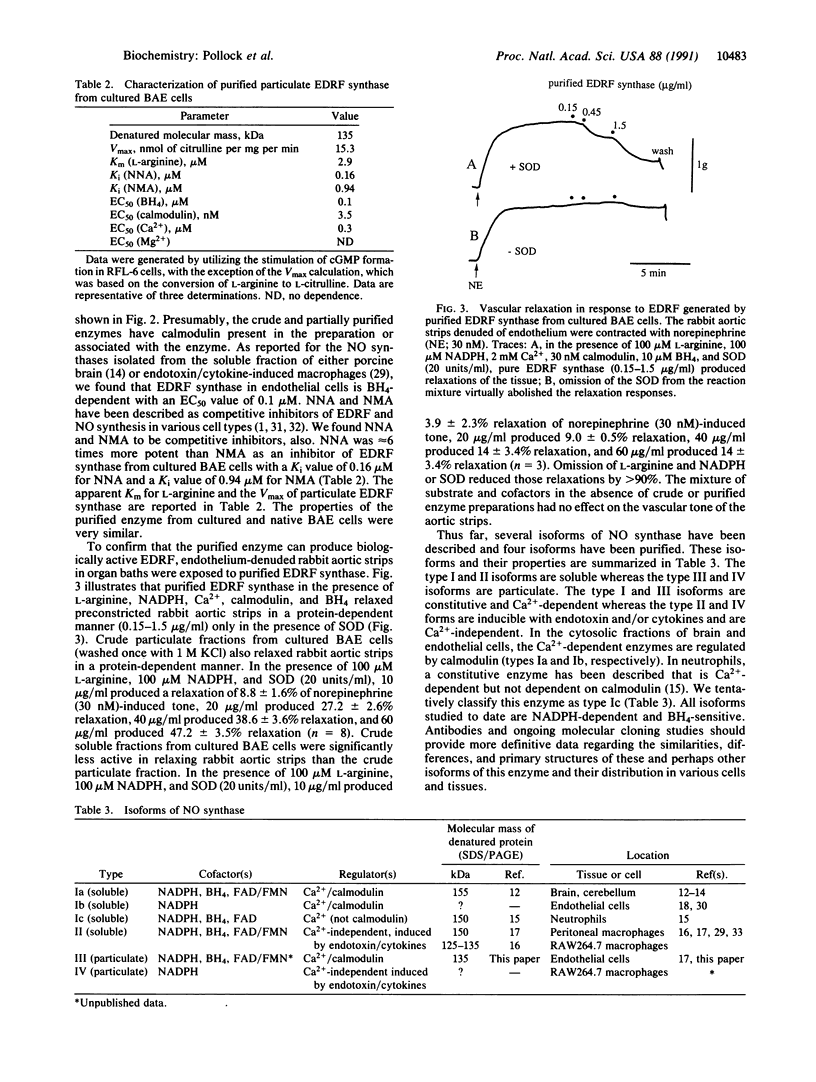
The particulate enzyme responsible for the synthesis of endothelium-derived relaxing factor has been purified from cultured and native (noncultured) bovine aortic endothelial cells. Purification of the solubilized particulate enzyme preparation by affinity chromatography on adenosine 2',5'-bisphosphate coupled to Sepharose followed by Superose 6 gel filtration chromatography resulted in a single protein band after denaturing polyacrylamide gel electrophoresis that corresponded to approximately 135 kDa. The enzyme activity in the various fractions was assayed by its stimulatory effect on soluble guanylyl cyclase of rat fetal lung fibroblasts (RFL-6 cells), by the formation of L-citrulline from L-arginine, by measuring nitrite/nitrate formation, and by bioassay on endothelium-denuded vascular strips. Endothelium-derived relaxing factor synthase was purified 3419-fold from the crude particulate fraction of cultured bovine aortic endothelial cells with a 12% recovery (RFL-6 assay). Purified endothelium-derived relaxing factor synthase required L-arginine, NADPH, Ca2+, calmodulin, and 5,6,7,8-tetrahydrobiopterin for full activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alheid U., Frölich J. C., Förstermann U. Endothelium-derived relaxing factor from cultured human endothelial cells inhibits aggregation of human platelets. Thromb Res. 1987 Sep 1;47(5):561–571. doi: 10.1016/0049-3848(87)90361-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Mülsch A. Calcium-dependent nitric oxide synthesis in endothelial cytosol is mediated by calmodulin. FEBS Lett. 1990 Jun 4;265(1-2):133–136. doi: 10.1016/0014-5793(90)80902-u. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Mülsch A., Böhme E., Busse R. Stimulation of soluble guanylate cyclase by an acetylcholine-induced endothelium-derived factor from rabbit and canine arteries. Circ Res. 1986 Apr;58(4):531–538. doi: 10.1161/01.res.58.4.531. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Pollock J. S., Schmidt H. H., Heller M., Murad F. Calmodulin-dependent endothelium-derived relaxing factor/nitric oxide synthase activity is present in the particulate and cytosolic fractions of bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1788–1792. doi: 10.1073/pnas.88.5.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Harbison R. G., Wood K. S., Kadowitz P. J. Activation of purified soluble guanylate cyclase by endothelium-derived relaxing factor from intrapulmonary artery and vein: stimulation by acetylcholine, bradykinin and arachidonic acid. J Pharmacol Exp Ther. 1986 Jun;237(3):893–900. [PubMed] [Google Scholar]
- Ishii K., Chang B., Kerwin J. F., Jr, Huang Z. J., Murad F. N omega-nitro-L-arginine: a potent inhibitor of endothelium-derived relaxing factor formation. Eur J Pharmacol. 1990 Feb 6;176(2):219–223. doi: 10.1016/0014-2999(90)90531-a. [DOI] [PubMed] [Google Scholar]
- Ishii K., Sheng H., Warner T. D., Förstermann U., Murad F. A simple and sensitive bioassay method for detection of EDRF with RFL-6 rat lung fibroblasts. Am J Physiol. 1991 Aug;261(2 Pt 2):H598–H603. doi: 10.1152/ajpheart.1991.261.2.H598. [DOI] [PubMed] [Google Scholar]
- Kwon N. S., Nathan C. F., Stuehr D. J. Reduced biopterin as a cofactor in the generation of nitrogen oxides by murine macrophages. J Biol Chem. 1989 Dec 5;264(34):20496–20501. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leitman D. C., Waldman S. A., Rapoport R. M., Murad F. Specific atrial natriuretic factor receptors mediate increased cyclic GMP accumulation in cultured bovine aortic endothelial and smooth muscle cells. Trans Assoc Am Physicians. 1985;98:243–252. [PubMed] [Google Scholar]
- Mayer B., John M., Böhme E. Purification of a Ca2+/calmodulin-dependent nitric oxide synthase from porcine cerebellum. Cofactor-role of tetrahydrobiopterin. FEBS Lett. 1990 Dec 17;277(1-2):215–219. doi: 10.1016/0014-5793(90)80848-d. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., Förstermann U., Warner T. D., Pollock J. S., Schmidt H. H., Heller M., Murad F. Endothelial cells have a particulate enzyme system responsible for EDRF formation: measurement by vascular relaxation. Biochem Biophys Res Commun. 1991 May 15;176(3):1417–1423. doi: 10.1016/0006-291x(91)90444-c. [DOI] [PubMed] [Google Scholar]
- Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest. 1986 Jul;78(1):1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Nau H., Wittfoht W., Gerlach J., Prescher K. E., Klein M. M., Niroomand F., Böhme E. Arginine is a physiological precursor of endothelium-derived nitric oxide. Eur J Pharmacol. 1988 Sep 13;154(2):213–216. doi: 10.1016/0014-2999(88)90101-x. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Pollock J. S., Nakane M., Gorsky L. D., Förstermann U., Murad F. Purification of a soluble isoform of guanylyl cyclase-activating-factor synthase. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):365–369. doi: 10.1073/pnas.88.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal J. Cation chelators and their utilization in the preparation of low concentrations of calcium. Caution of use in biological systems with high affinity to calcium. Biotechnol Appl Biochem. 1986 Oct;8(5):423–429. [PubMed] [Google Scholar]
- Stuehr D. J., Cho H. J., Kwon N. S., Weise M. F., Nathan C. F. Purification and characterization of the cytokine-induced macrophage nitric oxide synthase: an FAD- and FMN-containing flavoprotein. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7773–7777. doi: 10.1073/pnas.88.17.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Kwon N. S., Nathan C. F. FAD and GSH participate in macrophage synthesis of nitric oxide. Biochem Biophys Res Commun. 1990 Apr 30;168(2):558–565. doi: 10.1016/0006-291x(90)92357-6. [DOI] [PubMed] [Google Scholar]
- Waldman S. A., Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987 Sep;39(3):163–196. [PubMed] [Google Scholar]
- Yui Y., Hattori R., Kosuga K., Eizawa H., Hiki K., Kawai C. Purification of nitric oxide synthase from rat macrophages. J Biol Chem. 1991 Jul 5;266(19):12544–12547. [PubMed] [Google Scholar]
- Yui Y., Hattori R., Kosuga K., Eizawa H., Hiki K., Ohkawa S., Ohnishi K., Terao S., Kawai C. Calmodulin-independent nitric oxide synthase from rat polymorphonuclear neutrophils. J Biol Chem. 1991 Feb 25;266(6):3369–3371. [PubMed] [Google Scholar]




