Abstract.
Graves’ disease (GD) accounts for a large proportion of pediatric hyperthyroidism, and the first-line treatment is antithyroid drug (ATD) therapy. Methimazole (MMI) is effective in most patients but is associated with significant adverse events (AEs). We reviewed the medical records of GD patients (n = 56) with onset age of <15 yr and investigated the relationship between MMI dose and AEs. The study population comprised 11 male and 45 female patients and the median age at diagnosis was 11 yr. All patients were initially treated with ATDs. Among the 52 patients initially treated with MMI, 20 received a low dose, and 32 received a high dose of MMI (< 0.7 vs ≥ 0.7 mg/kg/day, respectively). AEs occurred in 20% of the patients in the low-dose MMI group, and in 50% patients in the high-dose MMI group (p = 0.031). A greater variety of AEs was observed in the high-dose group. Neutropenia and rash were observed in both groups. With treatment transition to low-dose MMI according to the Japanese Society for Pediatric Endocrinology guidelines, we expect a decrease in the incidence of AEs in future. However, we should be careful as neutropenia and rash can occur independently of the MMI dose.
Keywords: Graves’ disease, children, antithyroid drug, methimazole, adverse event
Introduction
Graves’ disease (GD) is an acquired autoimmune hyperthyroidism with diffuse enlargement of the thyroid gland and accounts for a large proportion of pediatric hyperthyroidism. The treatment options for pediatric GD include antithyroid drugs (ATDs), thyroidectomy, and radioactive iodine therapy, same treatment options as those available to adults (1, 2). The initial treatment for GD in children is the administration of ATD, methimazole (MMI) or propylthiouracil (PTU). MMI is currently used as the first-line ATD in Japanese children and adolescents with GD according to the guidelines issued by the Japanese Society for Pediatric Endocrinology (JSPE) (3) and the Japan Thyroid Association (4). These guidelines show a relationship between the kind and dose of the ATDs and adverse events (AEs): a low dose of MMI is safer than a high dose of MMI or PTU. The guidelines also recommend the use of low-dose MMI in mild GD cases [initial serum free T4 (fT4) level < 5 ng/dL] and high-dose MMI in moderate and severe GD cases [initial serum fT4 level ≥ 5 ng/dL]. In our hospital, the MMI dose used to treat pediatric GD patients has decreased from the previously used dose, in accordance with the above-mentioned guidelines. We retrospectively investigated the relationship between MMI dose administered and the development of AEs.
Subjects and Methods
We reviewed the medical records of 56 GD patients with age of onset < 15 yr who were treated at the Osaka University Hospital between February 1979 and October 2014. We collected the following information from the patients’ medical records: sex, age at diagnosis, main reason for diagnosis, thyroid crisis, the follow-up period, underlying diseases, family history of thyroid diseases, serum fT4 level at diagnosis, initial treatment including the MMI dose, duration required for serum fT4 level normalization (≤ 1.6 ng/dL), clinical course, current clinical situation, and ATD-induced AEs. Patients initially treated with MMI were divided into low-dose (< 0.7 mg/kg/day) and high-dose (≥ 0.7 mg/kg/day or 30 mg/day) MMI groups; the dose cut-offs were chosen on the basis of the JSPE guideline (3). AEs were defined as follows: rash – developed within 1 mo of treatment initiation and/or needed antihistamine, arthritis – presence of joint pain, neutropenia – neutrophil count <1000/µL, hepatotoxicity and high serum creatine phosphokinase (CPK) – exceeding the age-related reference values, antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis – myeloperoxidase (MPO)-ANCA positivity and elevated erythrocyte sedimentation rate, and ANCA-associated glomerulonephritis – MPO-ANCA positivity and proteinuria.
Thyroid hormone measurements were performed using different assay kits depending on the date of testing. Cases that measured total T4 (TT4) levels instead of fT4 were excluded from this study about fT4. Statistical analyses were performed using the chi-square test to compare variables between the study groups. The results for categorical variables were expressed as percentages and those for continuous variables as means. This study was approved by the Ethical Review Board of the Osaka University Hospital (approval No. 14436).
Results
Patient population
The study population included 11 male (19.6%) and 45 female (80.4%) patients. The age at diagnosis was 2–15 yr (median: 11 yr, interquartile range: 10–13 yr). The follow-up duration was 1 mo–34.5 yr (mean: 9.1 yr). At the time of diagnosis, underlying diseases were identified in 12 patients (21.4%) and included: chromosomal abnormalities (one each with Down syndrome, and 22q11.2 deletion syndrome), autoimmune diseases (one each with chronic thyroiditis, idiopathic thrombocytopenic purpura, myasthenia gravis, and psoriasis vulgaris), heart diseases (two with congenital heart disease, and one with Wolff-Parkinson-White syndrome), and other diseases (two with epilepsy, two with scoliosis, one with panhypopituitarism following brain tumor surgery, and one with nephrotic syndrome). Twenty patients (35.7%) had a family history of thyroid disease, including GD in 13 patients (23.2%) [parents/siblings, n = 9; uncles/aunts, n = 4].
Clinical status at diagnosis
The reasons for the diagnosis of GD were goiter (n = 28, 50.0%), thyrotoxicosis (n = 21, 37.5%), exophthalmos (n = 6, 10.7%), and by chance (n = 1, 1.8%). Two patients experienced symptoms of thyroid crisis, one of which occurred after an 123I thyroid scintigraphy. Serum TT4 level was measured in seven patients and these cases were excluded from further analysis about fT4. For 48 patients measured the serum fT4 level at diagnosis excluding one patient whose level was unknown, the serum fT4 level at diagnosis was < 5 ng/dL in 22 patients (45.8%), 5–7 ng/dL in 17 patients (35.4%), and > 7 ng/dL in 9 patients (18.8%).
Clinical course
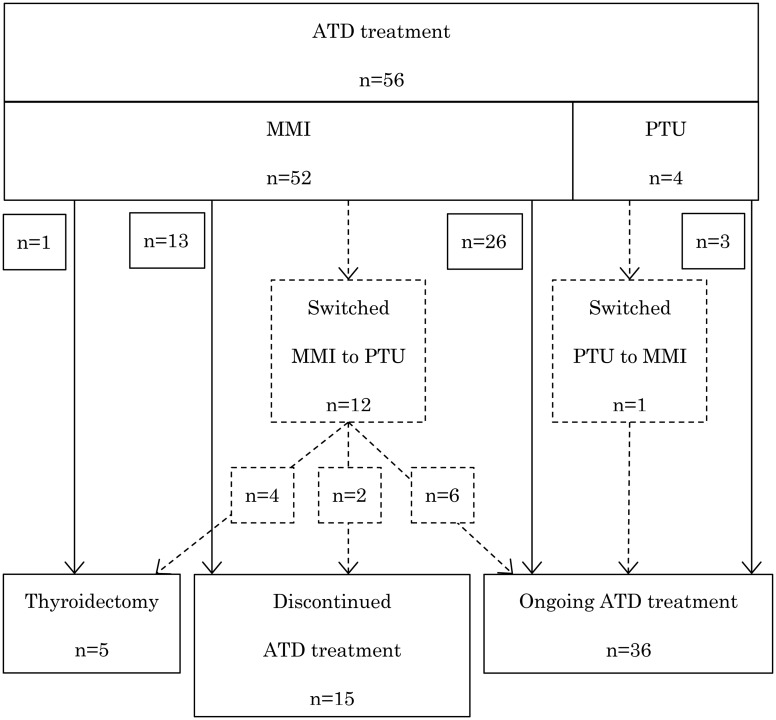
The clinical course of the patients is shown in Fig. 1. All patients were initially treated with ATDs [MMI in 52 (92.9%), and PTU in 4 (7.1%)], with the concurrent administration of propranolol in 28 patients (50.0%). Fifteen patients (26.8%) were able to discontinue ATD, and the mean duration until ATD discontinuation was 6.9 yr (range: 1.6–9.5 yr). Five patients underwent thyroidectomy either due to PTU-induced AEs (n = 2) or in accordance with the patients’ wishes (n = 3). None of the patients received radioactive iodine therapy or medication for exophthalmos. Thirty-nine patients (69.6%) were subsequently referred to endocrinologists at our hospital or a nearby clinic. Three female patients delivered four healthy babies, including two babies born to a mother treated with MMI during early pregnancy.
Fig. 1.
Patients’ clinical course. Dotted line indicates patients who were switched to another antithyroid drug.
ATD-induced AEs
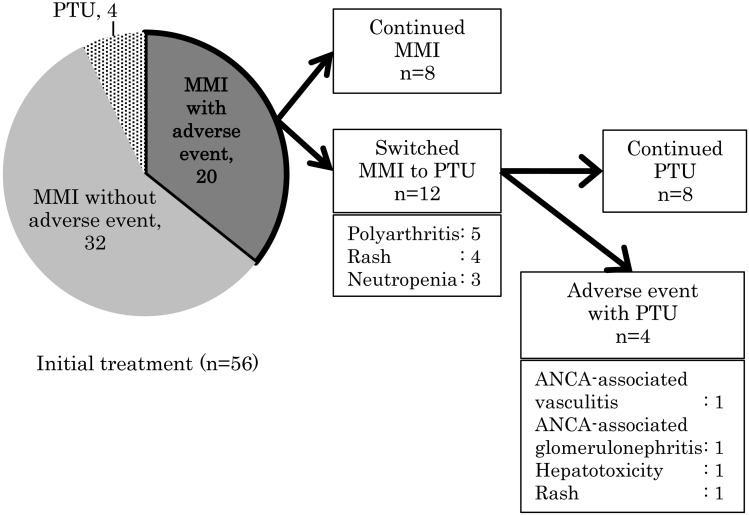
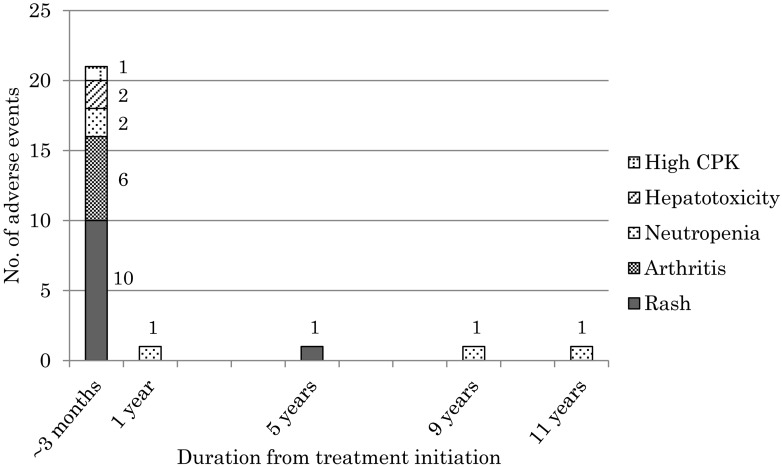
ATD-induced AEs were identified in 20 patients (35.7%) (Fig. 2), and all these patients had been initially treated with MMI. In two of these patients, one with a rash and another with ANCA-associated glomerulonephritis, AE could not be controlled by switching to PTU, and a thyroidectomy had to be performed subsequently. In 80% (16 patients, 21 events) AE-affected patients, the AE developed within 3 mo of treatment initiation, and the longest duration from treatment initiation to AE development was 11.5 yr (Fig. 3).
Fig. 2.
Antithyroid drug-induced adverse events (AEs). Four patients exhibited propylthiouracil (PTU)-induced AEs after switching to PTU due to methimazole (MMI)-induced AEs: one patient each with rash after MMI-induced neutropenia, mild hepatotoxicity after MMI-induced rash, antineutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis after MMI-induced neutropenia, and ANCA-associated vasculitis after MMI-induced arthritis.
Fig. 3.
Time to adverse event development following the initiation of antithyroid drug therapy. The neutrophil counts in patients who developed neutropenia after 1, 9, and 11 yr was 787/µL, 14/µL, and 191/µL, respectively.
Patients’ clinical status based on the administered MMI dose
Among the 52 patients initially treated with MMI, low-dose MMI (< 0.7 mg/kg/day) was administered to 20 patients (38.5%), and high-dose MMI (≥ 0.7 mg/kg/day or 30 mg/day) was administered to 32 patients (61.5%). Eight patients received treatment after the 2011 revision of the Japan Thyroid Association Guideline (4) and were all treated with a low-dose MMI. The reasons for diagnosis common to both MMI dose groups were the presence of goiter, thyrotoxicosis, and exophthalmos. The mean serum fT4 level at the time of diagnosis was 4.92 and 6.06 ng/dL in the low-dose and high-dose MMI groups, respectively. Further, the mean duration until normalization of serum fT4 levels was 0.9 and 1.1 mo in the low-dose and high-dose MMI groups, respectively, with no statistically significant intergroup difference.
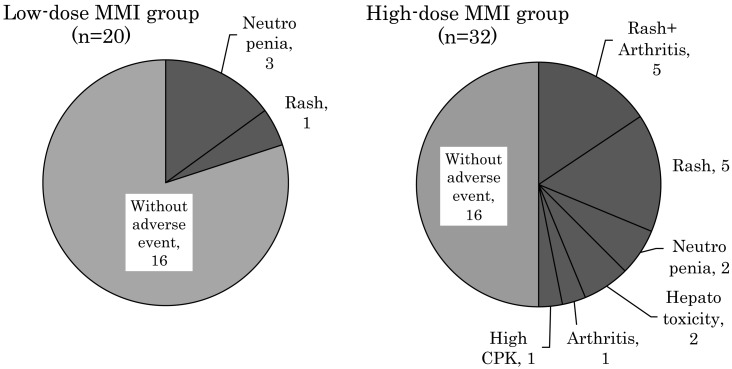
Fig. 4 shows the distribution of AEs according to the initial MMI dose, and AEs were less frequent in the low-dose group compared to the high-dose group (20% vs. 50%, p = 0.031). Of the four patients in the low-dose MMI group who developed AEs, three had neutropenia (neutrophil count: 787, 191 and 14/µL, respectively), and one had a rash. Sixteen patients in the high-dose MMI group developed AEs, ten patients had a rash, six had arthritis (five developed arthritis after a rash), two had neutropenia (neutrophil count: 947 and 342/µL, respectively), two had mild hepatotoxicity (peak serum alanine aminotransferase level: 61 and 342 IU/L, respectively), and one patient had a high serum CPK level (3050 IU/L) (Fig. 4). Thus, a greater variety of clinical symptoms was observed in the high-dose MMI group compared to the low-dose group. Although neutropenia and rash were observed in both the MMI dose groups, their severity was not statistically different between the two groups.
Fig. 4.
Comparison of the adverse events in the low-dose and high-dose MMI groups.
Discussion
We reviewed the medical records of 56 GD patients with an onset age of < 15 yr. In our study, the mean duration until normalization of the serum fT4 levels did not differ between the low-dose and the high-dose MMI groups. The frequency of MMI-induced AEs was significantly lower in the low-dose group, and a greater variety of AE symptoms was observed in the high-dose group. The new treatment guideline issued by the JSPE in 2016 (5) recommends the use of lower MMI dose in order to reduce the development of AEs. In the low-dose MMI group, agranulocytosis (neutrophil count < 500/µL) developed in two patients, and its frequency was 10%. Neutropenia and rash were observed in both the low-dose and the high-dose MMI groups. In 80% of the cases, AE developed within 3 mo of treatment initiation, while the longest duration noted in our study was 11.5 yr for MMI-induced agranulocytosis.
In a previous study on childhood-onset GD, the mean duration for serum fT4 level normalization was reported as 1.9 ± 1.2 mo and 1.4 ± 0.7 mo in the low-dose and high-dose MMI groups, respectively (not statistically significant) (6). This was similar to the results of our study. These results suggest that in children, the time to serum fT4 normalization is not related to the MMI dose. However, another study reported that the serum fT4 normalization time was shorter when high-dose MMI was administered to moderate and severe adult GD patients, rather than low-dose MMI (7). Thus, further studies are needed to clarify the therapeutic effect of different MMI doses in moderate and severe GD cases among children.
Several reports on MMI-induced AEs have shown that AEs are related to the initial MMI dose (8,9,10,11,12). It has been reported that the development of agranulocytosis depends on the ATD dose administered, and its reported frequency is 0.35% (12,13,14). Further, a study on Japanese pediatric GD patients reported that agranulocytosis developed with an MMI dose > 20 mg/day (15). In our study, however, agranulocytosis developed in 10% of the patients in the low-dose MMI group (< 0.7 mg/kg/day). Therefore, although the number of GD patients in our study was small, our results suggest that we should be careful with respect to the development of agranulocytosis, even when a low-dose MMI is administered.
Ohye et al. have reported that while most AEs (91.6%) occurred within the first 3 mo of ATD treatment (16), agranulocytosis may develop several months after ATD initiation (15, 17). In our study, while 80% of the AEs developed within 3 months of ATD treatment initiation, the longest duration for AE development was noted for MMI-induced agranulocytosis (11.5 yr from treatment initiation). These results indicate that the development of agranulocytosis should be anticipated regardless of the MMI administration period.
A few reports have observed that the development of AEs, except for agranulocytosis, depends on the ATD dose. In our study, neutropenia and rash were observed in both the low-dose and the high-dose MMI groups; therefore, it is necessary to be aware that neutropenia and rash may occur independently of the MMI dose.
The limitations of this study include its retrospective design, the small number of patients, and the non-random assignment of patients to the MMI dose-based groups. Accordingly, a long-term, double-blind prospective trial, with well-matched patient groups, needs to be conducted to confirm our findings.
In conclusion, we reviewed the medical records of GD patients with disease onset age of < 15 yr and investigated the relationship between the MMI dose and the development of AEs. AEs induced by MMI were significantly less frequent in the low-dose group, and a greater variety of AE symptoms was noted in the high-dose group. With treatment transition to low-dose MMI according to the guidelines of the JSPE, we expect that the incidence of AEs will decrease in future. However, we need to remain vigilant as neutropenia and rash can occur independently of the administered MMI dose.
References
- 1.Rivkees SA. The treatment of Graves disease in children. J Pediatr Endocrinol Metab 2006;19: 1095–111. doi: 10.1515/JPEM.2006.19.9.1095 [DOI] [PubMed] [Google Scholar]
- 2.Rivkees SA, Sklar C, Freemark M. Clinical review 99: The management of Graves disease in children, with special emphasis on radioiodine treatment. J Clin Endocrinol Metab 1998;83: 3767–76. [DOI] [PubMed] [Google Scholar]
- 3.Japanese Society for Pediatric Endocrinology2008 Guideline for the drug treatment of childhood onset Graves’ disease. Nippon Shonika Gakkai Zasshi 2008;112: 946–52 (In Japanese). [Google Scholar]
- 4.Japan Thyroid Association 2011 Guideline for the drug treatment of Graves’ diseases. 1st ed. Tokyo: Nankodo; 2011. p. 135-140. [Google Scholar]
- 5.Japanese Society for Pediatric Endocrinology2016 Guideline for the treatment of childhood onset Graves’ disease. http://jspe.umin.jp/medical/files/gravesdisease_guideline2016.pdf. (accessed: April 23, 2016).
- 6.Sato H, Minagawa M, Sasaki N, Sugihara S, Kazukawa I, Minamitani K, et al. Comparison of methimazole and propylthiouracil in the management of children and adolescents with Graves disease: efficacy and adverse reactions during initial treatment and long-term outcome. J Pediatr Endocrinol Metab 2011;24: 257–63. doi: 10.1515/jpem.2011.194 [DOI] [PubMed] [Google Scholar]
- 7.Japan Thyroid Association 2011 Guideline for the drug Treatment of Graves’ diseases. 1st ed. Tokyo: Nankodo; 2011. p. 41-50. [Google Scholar]
- 8.Sato H, Sasaki N, Minamitani K, Minagawa M, Kazukawa I, Sugihara S, et al. Higher dose of methimazole causes frequent adverse effects in the management of Graves disease in children and adolescents. J Pediatr Endocrinol Metab 2012;25: 863–7. doi: 10.1515/jpem-2012-0138 [DOI] [PubMed] [Google Scholar]
- 9.Nakamura H, Noh JY, Itoh K, Fukata S, Miyauchi A, Hamada N. Comparison of methimazole and propylthiouracil in patients with hyperthyroidism caused by Graves disease. J Clin Endocrinol Metab 2007;92: 2157–62. doi: 10.1210/jc.2006-2135 [DOI] [PubMed] [Google Scholar]
- 10.Sato S, Noh JY, Sato S, Suzuki M, Yasuda S, Matsumoto M, et al. Comparison of efficacy and adverse effects between methimazole 15 mg+inorganic iodine 38 mg/day and methimazole 30 mg/day as initial therapy for Graves disease patients with moderate to severe hyperthyroidism. Thyroid 2015;25: 43–50. doi: 10.1089/thy.2014.0084 [DOI] [PubMed] [Google Scholar]
- 11.Reinwein D, Benker G, Lazarus JH, Alexander WD, European Multicenter Study Group on Antithyroid Drug TreatmentA prospective randomized trial of antithyroid drug dose in Graves disease therapy. J Clin Endocrinol Metab 1993;76: 1516–21. [DOI] [PubMed] [Google Scholar]
- 12.Takata K, Kubota S, Fukata S, Kudo T, Nishihara E, Ito M, et al. Methimazole-induced agranulocytosis in patients with Graves disease is more frequent with an initial dose of 30 mg daily than with 15 mg daily. Thyroid 2009;19: 559–63. doi: 10.1089/thy.2008.0364 [DOI] [PubMed] [Google Scholar]
- 13.Cooper DS, Goldminz D, Levin AA, Ladenson PW, Daniels GH, Molitch ME, et al. Agranulocytosis associated with antithyroid drugs. Effects of patient age and drug dose. Ann Intern Med 1983;98: 26–9. doi: 10.7326/0003-4819-98-1-26 [DOI] [PubMed] [Google Scholar]
- 14.Hamerschlak N, Maluf E, Biasi Cavalcanti A, Avezum Júnior A, Eluf-Neto J, Passeto Falcão R, et al. Incidence and risk factors for agranulocytosis in Latin American countriesthe Latin Study: a multicenter study. Eur J Clin Pharmacol 2008;64: 921–9. doi: 10.1007/s00228-008-0513-7 [DOI] [PubMed] [Google Scholar]
- 15.Minamitani K, Oikawa J, Wataki K, Kashima K, Hoshi M, Inomata H, et al. A report of three girls with antithyroid drug-induced agranulocytosis: retrospective analysis of 18 cases aged 15 years or younger reported between 1995 and 2009. Clin Pediatr Endocrinol 2011;20: 39–46. doi: 10.1297/cpe.20.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohye H, Minagawa A, Noh JY, Mukasa K, Kunii Y, Watanabe N, et al. Antithyroid drug treatment for graves disease in children: a long-term retrospective study at a single institution. Thyroid 2014;24: 200–7. doi: 10.1089/thy.2012.0612 [DOI] [PubMed] [Google Scholar]
- 17.Tamai H, Takaichi Y, Morita T, Komaki G, Matsubayashi S, Kuma K, et al. Methimazole-induced agranulocytosis in Japanese patients with Graves disease. Clin Endocrinol (Oxf) 1989;30: 525–30. doi: 10.1111/j.1365-2265.1989.tb01424.x [DOI] [PubMed] [Google Scholar]