Abstract
Climate change has been predicted to increase the global mean temperature and to alter the ecological interactions among organisms. These changes may play critical roles in influencing the life history traits of the intermediate hosts (IHs). This review focused on studies and disease models that evaluate the potential effect of temperature rise on the ecology of IH snails and the development of parasites within them. The main focus was on IH snails of schistosome parasites that cause schistosomiasis in humans. A literature search was conducted on Google Scholar, EBSCOhost and PubMed databases using predefined medical subject heading terms, Boolean operators and truncation symbols in combinations with direct key words. The final synthesis included nineteen published articles. The studies reviewed indicated that temperature rise may alter the distribution, optimal conditions for breeding, growth and survival of IH snails which may eventually increase the spread and/or transmission of schistosomiasis. The literature also confirmed that the life history traits of IH snails and their interaction with the schistosome parasites are affected by temperature and hence a change in climate may have profound outcomes on the population size of snails, parasite density and disease epidemiology. We concluded that understanding the impact of temperature on the growth, fecundity and survival of IH snails may broaden the knowledge on the possible effects of climate change and hence inform schistosomiasis control programmes.
Keywords: growth, fecundity, survival, intermediate host snails, schistosomes, schistosomiasis, temperature
1. Introduction
Schistosomiasis is associated with high morbidity and mortality in sub-Saharan Africa (SSA) [1]. There are two main forms of the disease in SSA, urogenital and intestinal schistosomiasis [2], mainly caused by trematode parasites Schistosoma haematobium and Schistosoma mansoni, respectively [3,4]. Transmission of these parasites is dependent on many factors. They range from climatic factors such as rainfall and temperature [5,6] to IH (intermediate host) snails, parasites and human related factors that may maintain or increase the transmission of the disease [7]. Although praziquantel has been used to reduce infection among infected individuals, cases of re-infection due to exposure to infected water as well as tolerance of the parasite to praziquantel, have been reported in endemic areas [8,9,10]. Furthermore, a rise in temperature due to climate change may modify the incidences of schistosomiasis by altering the snail fecundity, growth and survival rates, their distribution patterns and parasite development rate [11,12]. It is therefore important to understand the temperature-driven changes on the snail and snail-parasite system to enhance our knowledge on the climate-disease theory.
Growing evidence suggests that climate change will lead to an increase in temperature [13,14] and incidences of extreme events [15]. The predicted changes in temperature are expected to impact on the ecological interactions between organisms thereby affecting possible outcomes of host-parasite interactions [16,17]. Studies on the implications of climate change on diseases have mainly been done for malaria [18,19,20] with only a few focusing on schistosomiasis [2,21,22]. Thus, there has been a continuous debate on the extent to which schistosomiasis burden will be influenced by climate change. Therefore, this necessitates further investigations to identify the major stages within the IH life cycle that are likely to be impacted most.
Significant progress has been made towards understanding the epidemiology and control of schistosomiasis [23,24]. This has led to initiatives such as mass drug administration [25,26], the development of schistosomiasis control programmes [27,28] and disease epidemiology and GIS (Geographic Information System) models [29,30,31,32]. However, the projected impact of climate driven changes on the epidemiology of schistosomiasis may compromise the control efforts. Although some snail interruptive measures such as the use of molluscicides and biological control have been used [33,34], the influence of temperature on snail population size and parasite distribution is not well understood for the efficient management, surveillance, control and prevention of the disease [32]. Understanding the role of temperature in driving changes in the ecology and population dynamics of both infected and non-infected snails is essential for predicting the possible impacts of climate change on future schistosomiasis dynamics [35]. This information can be obtained from evidence based field and laboratory studies. The present review therefore explored the effects of temperature on the growth, fecundity and survival of IH snail species from the genus Bulinus and Biomphalaria.
2. Materials and Methods
2.1. Search Strategy
A systematic search of literature on Google Scholar, PubMed and EBSCOhost databases was conducted using the following terms and Boolean operators (OR, AND): Schistosomiasis AND Temperature, Bulinus OR Biomphalaria AND growth, fecundity AND survival in sub-Saharan Africa. Articles that were identified were screened by reading through the titles and abstracts. In addition, reference and bibliographic lists of the selected articles were screened as potential leads to any additional relevant studies for inclusion. Full text articles were retrieved and managed in Endnote reference manager version X7 (Clarivate Analytics, Philadelphia, PA, USA). The review included literature from 1980 to 2016.
2.2. Inclusion Criteria and Exclusion Criteria
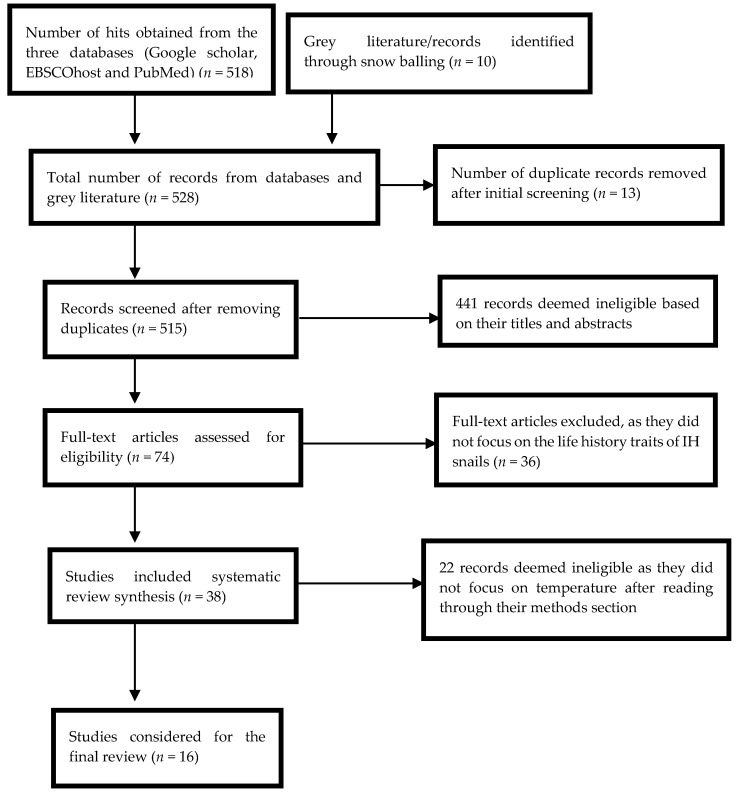
Studies were included in the review if they were published in peer-reviewed journals and explicitly reported on (1) temperature and IH snails of schistosomes that cause human schistosomiasis and (2) the genus Bulinus or Biomphalaria (Figure 1, Table 1).
Figure 1.
PRISMA diagram.
Table 1.
Summary of laboratory studies, field studies and models that assessed the effect of temperature on Bulinus and Biomphalaria snail species between 1980 and 2016.
| Author (Reference) | Objective | Snail Species Studied | Methods | Outcome |
|---|---|---|---|---|
| Appleton and Eriksson [36] | To determine the influence of fluctuating above-optimal temperature regimes on the fecundity of Bi. pfeifferi | Biomphalaria pfeifferi | Laboratory experiment |
|
| Dagal et al. [37] | To determine the effect of some physico-chemical factors (temperature, pH and salinity) on the hatchability of egg masses and survival of juvenile and adult snails | Bulinus (Physopsis) abyssinucus | Laboratory experiment |
|
| El-Emam and Madsen [38] | To compare the effect of temperature on the growth, survival and fecundity of Bu. truncatus and Bi. alexandrina | Biomphalaria alexandrina and Bulinus truncatus | Laboratory experiment |
|
| Joubert et al. [39] | To determine the survival of Bu. africanus (Krauss), Bu. globosus and Bi. pfeifferi at constant high temperatures of 34 °C to 40 °C | Bulinus africanus (Krauss), Bulinus globosus (Morelet) and Biomphalaria pfeifferi (Krauss) | Laboratory experiment |
|
| Kubiriza et al. [40] | To compare the performance (survival, growth, hatchability and reproduction) of Bu. nyassanus to other Bulinus spp. when exposed to different constant temperatures | Bulinus nyassanus (Smith, 1877) | Laboratory experiment |
|
| McCreesh et al. [41] | To determine the effects of water temperature on the mortality, fecundity, and growth rates of Bi. sudanica | Biomphalaria sudanica | Laboratory experiment |
|
| Pflüger et al. [42] | To evaluate the effect of temperature on the development rate of Schistosoma haematobium development in Bulinus snails | Bulinus truncatus | Laboratory experiment |
|
| Pflüger [43] | To determine the effect of temperature on the length of the prepatent period in infected Bi. glabrata snails | Biomphalaria glabrata | Laboratory experiment |
|
| Mofolusho and Benson [44] | To access the influence of acclimatization (to laboratory conditions) on the fecundity and fertility of field collected Bi. pfeifferi | Biomphalaria pfeifferi (Krauss, 1848) | Laboratory experiment |
|
| Joubert et al. [45] | To determine the survival of Bu. africanus (Krauss), Bu. globosus (Morelet) and Bi. pfeifferi (Krauss) at constant low temperatures (0 °C to 8 °C) | Bulinus africanus (Krauss), Bulinus globosus (Morelet) and Biomphalaria pfeifferi (Krauss) | Laboratory experiment |
|
| Barbosa et al. [46] | To determine the effect of seasonal temperature variation on egg production during the year | Biomphalaria glabrata | Laboratory experiment |
|
| O’keeffe [47] | To evaluate the effect of seasonal climatic changes on the natural populations of Bu. globosus | Bulinus globosus | Field experiment |
|
| Woolhouse and Chandiwana [48] | To determine the factors that influence the abundance of Bu. globosus in space and time for a critical assessment of the possible effectiveness of different snail control strategies | Bulinus globosus | Field experiment |
|
| Mangal et al. [49] | To determine the impact of temperature on the worm burden and prevalence of schistosomiasis for optimal disease control strategies | Non specific Biomphalaria spp. | Modelling |
|
| McCreesh and Booth [50] | To simulate all temperature-sensitive stages of S. mansoni and the life cycle of its intermediate host snail Bi. pfeifferi | Biomphalaria pfeifferi | Modelling |
|
| Ngarakana-Gwasira et al. [51] | To develop an epidemiological model for improved predictions of the impact of climatic factors on the dynamics and variation of schistosomiasis intensity in Zimbabwe | Non-specific snail species | Modelling |
|
3. Results
The systematic literature search yielded a total of five hundred and twenty-eight hits which included abstracts, reports, books and duplicate articles (Figure 1). Four hundred and ninety articles were excluded due to duplication and did not explicitly report on temperature in relation to Bulinus (hereafter abbreviated as Bu.) and Biomphalaria (hereafter abbreviated as Bi.) snail species as the IH of schistosomes that cause human schistosomiasis. A further twenty-two articles were excluded as they did not focus on temperature related studies although they looked at the life history traits of IH snails. Sixteen publications were considered for this review (Table 1). The results from the publications that were eligible for the review were classified into the following themes: temperature and timing of sexual maturity and fecundity, temperature and growth, temperature and survival and temperature and parasite development. These are discussed below in relation to potential change in the disease risk with a possible rise in temperature.
3.1. Temperature and Timing of Sexual Maturity and Fecundity
Temperature has been observed to have variable effects of the timing of sexual maturity and fecundity of both Bulinus and Biomphalaria snail species. According to Barbosa et al. [46], the reproductive rate of Bi. glabrata varied inversely with temperature. At 24 °C, the reproductive rate was reduced while it was maximal at 19.9 °C. El-Emam and Madsen [38] working independently on Bi. alexandrina reported that the net reproductive rate was optimal at 26 °C. A study by Appleton and Eriksson [36] suggested that above 27 °C, Bi. pfeifferi had reduced egg mass production. In another study, McCreesh et al. [41] concluded that at 21.6 °C, the fecundity of Bi. sudanica was at its optimal. Bulinus species have been observed to tolerate higher fecundity temperature levels. According to Kubiriza et al. [40], the number of eggs laid by Bu. nyassanus maintained at 22, 25, 28 and 31 °C were not significantly different. However, at 22 °C, the net reproductive rate of the snails was greatly reduced (Table 2).
Table 2.
Some important terms that have been used in the review.
3.2. Temperature and Growth
Growth has been observed to be influenced by temperature. According to Kubiriza et al. [40], the shell heights of Bu. nyassanus maintained at 25, 28 and 31 °C were not significantly different. However, snail growth was reduced at 22 °C. Woolhouse and Chandiwana [48] working on Bu. globosus in Zimbabwe observed that the shell height of snails increased linearly with temperature. Snails that were maintained at 22.5–23.5 °C had longer shell heights than those maintained at 14–16 °C. Furthermore, O’keeffe [47] suggested that their intrinsic growth rate reduced if these snails were maintained at temperatures above 28.5 °C. Although increased snail growth rate had been observed to increase with temperature, a study by McCreesh et al. [41] suggested that the relationship between water temperature and the growth rate of Bi. sudanica was not clear.
3.3. Temperature and Survival
The survival of snails at various temperatures may be key to the establishment and invasion of snails into new areas. Bulinus species have been observed to tolerate higher survival temperatures than Biomphalaria species. According to Dagal et al. [37], the maximal survival of Bu. abyssinucus was observed to be in the temperature ranges between 20 and 35 °C while no snails survived at 40 °C. Kubiriza et al. [40] suggested that the survival of Bu. nyassanus was optimal at 25 °C. However, this was not significantly different from the survival of snails at 28 and 31 °C. Joubert et al. [39] observed that Bu. globosus had marked increases in survival at higher temperatures (34 and 36 °C) as compared to Bi. pfeifferi and Bu. africanus. El-Emam and Madsen [38] observed that the survival of Bi. alexandrina and Bu. truncatus was reduced at temperatures above 33 °C and below 10 °C. According to McCreesh and Booth [50], the survival of Bi. pfeifferi outside the temperature ranges of 14.0–31.5 °C is greatly reduced, leading to a possible reduction in disease risks. Infected Bi. glabrata maintained at 16 °C were observed to have a reduced survival rate [43], while the survival of infected Bu. truncatus was also observed to be reduced at 17 and 33 °C [42].
3.4. Temperature and Parasite Development
Temperature has been observed to influence the parasite development rate. According to McCreesh and Booth [50], the prepatent period of Bi. pfeifferi reduced from 130 days when maintained at 14 °C to 18 days when maintained at 32 °C. On the other hand, Pflüger [43] observed that Bi. glabrata snails maintained at 32 and 33 °C had a prepatent period of 15 days. Furthermore, the study suggested that below 14.2 °C, the development of cercariae in Bi. glabrata comes to a standstill. Pflüger et al. [42] also observed that Bu. truncatus snails maintained at 18 °C had the longest prepatent period of 106–113 days while it was 17–19 days for snails maintained at 30–31 °C.
3.5. Temperature Dependent Model Formulation
A number of schistosomiasis models, mathematical and statistical have been developed to understand the transmission dynamics of schistosomiasis. Most of these models synthesize information from experimental studies and extrapolate to give future projections.
3.5.1. Statistical Models
Prediction of the distribution of snails has been used to potentially indicate the risks of disease transmission. Studies by Pedersen et al. [52] and Stensgaard et al. [53] have combined aspects of temperature and Geographic Information System (GIS) to predict the spatial distribution of the disease. These studies reveal that areas that will be suitable for both snail and parasite distribution. The studies further suggest that variable habitats may be created due to rise in temperature.
3.5.2. Mathematical Models
A model by McCreesh and Booth [50] suggested that in the two main schistosome transmission sites, rivers and lakes, transmission may be high at 15–19 °C and 20–25 °C, respectively. The model further suggested that a rise in temperature may reduce the disease risks in some transmission zones such as lakes, ponds, reservoirs and dams. Furthermore, that temperatures around 20 °C may lead to an increase in disease risks in rivers and streams while the risk of infection is likely to reduce at temperatures above 25 °C.
Ngarakana-Gwasira et al. [51] suggested that the temperature range between 18 and 28 °C was the most ideal for disease transmission. The model further suggested that 23 °C was the most ideal temperature for disease transmission. The model by Mangal et al. [49] suggested that schistosomiasis prevalence would be stable in the temperature range of 20–35 °C. The model also suggested that the increase in the survival of the parasite at 20 °C indicated that this was the most ideal temperature for applying parasite control measures.
4. Discussion
4.1. Growth and Fecundity Components: Do Temperature Levels Matter?
Snails allocate their resources towards growth, fecundity and survival [54] and the allocation of resource to any of these traits comes as a trade-off between growth, fecundity and survival [55,56]. The allocation of resources to fecundity means a reduction in resource allocation to the other physiological functions, growth and survival. Temperature on the other hand affects the physiological function of organisms [17,57]. It plays an important role in the timing of sexual maturity in IH snails [21,36]. High temperatures promote increased egg mass output and hastenens gametogenesis [58]. Therefore, this suggests that a rise in temperature may have a positive effect on snail fecundity as well as snail population growth. On the other hand, temperature levels above 30 °C have been observed to affect gametogenesis [22], suggesting that the positive effect of temperature on snail fecundity can only occur within a certain temperature range thermally tolerable to snails. Determination of this temperature range is important for understanding the likely impact of climate change on the population dynamics of snails in order to inform snail control programmes [49].
Studies done by McCreesh et al. [41], Kubiriza et al. [40] and El-Emam and Madsen [38] suggested that temperature strongly enhanced the fecundity of IH snails. In another study, Barbosa et al. [46] suggested that the relationship between reproductive rate of Bi. glabrata and temperature was non-linear. These studies have shown that different snails species have different optimal temperatures for fecundity. The output of egg masses at various temperature levels may also suggest that some IH snail species may be well adapted to low or high temperatures. Production of more egg masses at high temperature may also suggest that a possible rise in temperature may lead to an increase in the population size of IH snails especially during the post-rainy and cold dry seasons [59]. This may have implications on the incidences and prevalence of schistosomiasis. McCreesh et al. [35] have also suggested that a rise in temperature may potentially increase habitats suitable for transmission of S. mansoni. The study further observed that this may lead to increased risks of disease transmission in Rwanda, Burundi, south-west Kenya and the eastern parts of Zambia. This may be attributed to an increase in the population size of IH snails. Earlier studies by Appleton [60] and Michelson [22] while working on Bi. pfeifferi and Bi. glabrata, respectively, observed that the optimal fecundity temperature range for these snails was higher than that observed by McCreesh et al. [41]. This suggests that Biomphalaria species may have a slightly higher optimal temperature for fecundity and hence rise in temperature may alter the population dynamics and schistosomiasis incidences.
A recent snail survey in Uganda observed that snails may be found at high altitudes [61]. This may suggest that temperature may have increased, thus creating new habitats for snail invasion in places previously observed to be unsuitable for snails. This may also translate into the spread of the disease to areas that previously were unsuitable for disease occurrence.
High temperatures have also been associated with altered membrane permeability, increased accumulation of toxic metabolites and reduced immunity defences of IH snails [37,62]. This may increase the vulnerability of snails to infection which may later affect the survival and egg mass output [54,63,64]. Studies exploring the fecundity responses of Bulinus species when exposed to different temperature levels concluded that these IH snails maintained maximal reproductive output at 25 °C [65,66]. Bulinus globosus snails have also been observed to be highly fecund, tolerant to high temperatures and to re-populate quickly [39,47,65]. Thus, the results from these studies suggest that egg mass output at supra-optimal temperatures and the optimal fecundity temperature for Bulinus species may be lower than that observed by Kubiriza et al. [40].
The results from the reviewed studies may be insightful in predicting the possible impacts of temperature rise on snail fecundity. However, besides differences in the snail species used in the laboratory experiments, methodological differences ranging from the source of IH snails used (field or laboratory bred snails) and their acclimatization period to laboratory conditions (field collected snails) may have affected the performance of snails in the various studies. For example, the studies by Kubiriza et al. [40] and McCreesh et al. [41] both used field collected snails while Pflüger et al. [42] used a cohort of both field collected and laboratory bred snails. The likely exposure of field collected IH snails to more than two temperature regimes (field temperature, acclimatization temperature and experimental temperature) may have had an impact on the physiology of the IH snails and ultimately their performance. Mofolusho and Benson [44] conducted a study to evaluate the effect of acclimatization of field collected Bi. pfeifferi (Krauss, 1848) to laboratory conditions on snail survival and fecundity. The study observed that the survival and fecundity of acclimatized field collected IH snails were much lower than laboratory bred snails. Furthermore, Paull et al. [14] suggested that the use of field collected IH snails in laboratory temperature driven experiments after their prior exposure to field temperatures (high or low) may affect their future physiological functions. This is because temperature is energetically stressful on IH snails and may affect their physico-chemical conditions [14,67]. This may therefore lead to a reduction in their performance due to depleted energy resources in a new environment or an increase in their performance as a result of increased energy allocation to one trait due to change in temperature. This also suggests that the use of results from laboratory studies based on field collected snails should be done with caution, taking into account the limitations.
Besides fecundity, snail growth is another important life history trait that can determine the invasion and adaptation of snails to new areas. Growth affects the fecundity, ability of IH snails to withstand infection and availability of resource to trematodes during intramolluscan trematode development [21,48,68]. According to McCreesh et al. [41], the relationship between water temperature and IH snail growth was unclear. Other studies [38,40] observed an increase in the growth of IH snails with a rise in temperature (Table 1). Although the initial sizes of IH snails used by McCreesh et al. [41] varied and snails of different sizes were also used in the study by Kubiriza et al. [40], it is evident that temperature enhances snail growth. Studies conducted by Appleton [60] and Michelson [22] suggested that higher temperatures accelerate the growth of IH snails compared to lower temperatures. It was observed that Bi. pfeifferi had optimal growth at temperatures between 22.8 and 28 °C while Bi. glabrata snails maintained at 30 °C had accelerated growth compared to those at 25 °C. Furthermore, the optimal temperature ranges observed from these studies coincide with the optimal disease transmission temperature range (22–27 °C) [66]. This may suggest that increased snail population and growth within this temperature range may lead to a possible rise in the prevalence of schistosomiasis although this can only happen within temperature ranges tolerable by snails. Growth is a physiological trait; hence it can only increase within a thermally favourable temperature. Above this temperature range, no further growth may be observed. At extreme temperatures, the growth of snails diminishes and mortality rates are high [51,64].
4.2. Snail Survival and Temperature Rise: Its Role in Schistosomiasis Incidence
Host survival is an important aspect for the success of completing the disease transmission cycle. The survival time of IH snails is affected by both biotic and abiotic factors [5]. Infection of IHs has been observed to lead to phenotypic modifications which affect their survival time [69]. The development of schistosomes and the completion of their life cycle within the IH snails is greatly dependent on the survival of snails [70]. Survival of various IH snails has been observed to occur at various temperatures. Non-infected Bi. sudanica had optimal survival at 20 °C [41]. Temperatures below or above this increased snail mortality. For non-infected Bu. globosus, low and high temperature studies [39,45,47] concluded that these snails were well adapted to higher and lower temperatures compared to Bi. pfeifferi. O’keeffe [47] observed that Bu. globosus could survive at 30 °C and this was corroborated by the findings of Joubert et al. [39]. A study conducted by Appleton [60] concluded that the survival time of Bi. pfeifferi was significantly reduced when maintained above 27 °C. According to Joubert et al. [39], Bu. globosus had a lower mortality rate at 34 °C compared to Bi. pfeifferi suggesting that in some places, urogenital schistosomiasis may be more prevalent than intestinal schistosomiasis.
The role of trematode infection on snail survival time has had variable conclusions. Studies have shown that at high temperatures, mortality is high for both infected and non-infected IH snails [37,42,64]. Other studies [63,64] observed that infection had no effect on the survival time of IH snails, while Stirewalt [11] proposed that the mortality rates of infected IH snails rose with increasing temperature. This suggests a possible reduction in the amount of cercariae produced and in disease risks during dry hot seasons when the population size of snails is reduced [59].
Studies [38,39,40,41,45] have given insightful results on the effect of temperature on the survival of IH snails and its implications for disease transmission. However, most of the studies were done on non-infected snails. Understanding the thermal behaviour of both infected and non-infected snails may be important in predicting the overall effect of climate change on schistosomiasis. For example, within certain temperature levels, Studer et al. [64] observed that infected IH snails had higher survival rates compared to non-infected snails. A study by Seppälä and Jokela [62] suggested that temperatures above 30 °C affect the immunity defences of IH snails and increases their vulnerability to infection and infection induced mortality [71]. The observed variations may suggest the need for further studies on the interaction of temperature and infection on IH snail mortality. This may also assist in providing evidence to show if the alterations in the development of snails at high temperature is a result of phenotypic adaptation [62] and to estimate the possible effects of climate change on disease risks. This may also help develop more generalizable effects of temperature change on the population dynamics of IH snails and contribute to refined parameter estimations that may be included in disease models.
4.3. Schistosoma Development in Infected Snails: Does Temperature Matter?
The life cycle of Schistosoma parasites is complex as it involves molluscs and warm blooded mammals. The parasite also undergoes both asexual and sexual reproduction [2]. Once the parasite has been released into an aquatic environment by a mammal, the parasite must find a compatible IH snail species or it dies [2]. Temperature is key in that it affects the infectivity and the size of snails [63] influencing the amount of resources that may be available during the intramolluscan stages [54,68]. The intramolluscan parasite development stages are dependent on temperature [11,72,73] although Morley and Lewis [74] suggest that temperature may have no effect on the rate of cercariae development in infected hosts. Studies by Paull and Johnson [63] and Stirewalt [11] suggested that temperature influenced the rate of intramolluscan cercariae development. High temperatures have been observed to shorten the prepatent period [11,72]. Although low temperature may inhibit the development of cercariae [11,43,63], a rise in temperature to levels above the minimum cercariae development threshold temperature may lead to the production of cercariae [63]. This may also suggest that disease risks may be reduced during cold seasons despite the high IH snail population size. On the other hand, resumption of suitable conditions for cercariae development may lead to an increase in disease risks in such areas.
High temperatures elevate the output of cercariae [75] suggesting a possible increase in disease risks with a rise in temperature. On the other hand, very high temperature (i.e., 33 °C) and low temperatures (i.e., 17 °C) may lead to a reduction in disease risk due to increased snail mortality and a reduction in the development of cercariae within the IH snails [42,76]. This suggests that at extremely high temperatures such as those observed during the dry hot seasons, a reduction in disease incidence because of an increase in the mortality rate of both infected and non-infected IH snails may be experienced [64,70].
4.4. Disease Models Parameterization: Does Experimental Data Source Matter?
Following the initial model by Macdonald [77], a number of models have been developed to understand the transmission dynamics of schistosomiasis. Such models mainly focused on predicting disease transmission and intensity as well as providing solutions towards the application of chemotherapy [78,79,80].
4.4.1. Statistical Models
Statistical models have been used to understand the risk factors of schistosomiasis and in developing relationships between various factors related to disease transmission [81]. This may contribute to improving disease monitoring and control through well designed disease and IH snail control programmes [78,79,80]. Parameterized statistical models have also been used to predict the possible estimates for disease threshold cases, snail population and worm burden that can be used in mathematical models to develop possible control measures [29,78]. Keeling and Rohani [82] proposed that variations in the ecology of IH snails that may be caused by temperature, may need to be accounted for in modelling. These variations may range from the growth, fecundity and survival rates to parasite production rates. For example, a schistosomiasis model by Liang et al. [83] fitted using least-squares modelled snail recruitment and showed how this is dependent on temperature. The model however assumed a constant snail mortality rate which may have led to the under-estimation or over-estimation of the effect of temperature on the snail population size. This assumption may also have disregarded the possible heterogeneity in the survival of IH snails maintained at different temperature with different infection statuses. Modelling has incorporated aspects of GIS to understand the effects of temperature on the transmission of schistosomiasis. Models by Pedersen et al. [52] and by Stensgaard et al. [32] concluded that temperature is an important factor that can limit the occurrence of schistosomiasis and creation of new snail habitats. Studies [39,47,84] observed that snail species such as Bu. globosus survived at 30 °C while Bi. pfeifferi exposed to temperatures above 29 °C experienced hyperthermia [60].
4.4.2. Mathematical Models
The use of mathematical models has increased the understanding of the dynamics of schistosomiasis transmission and the implementation of IH control programmes [80]. It is however important that parameterized models take into account the important life history components of both the IH snails and the schistosome. A mechanistic model by Mangal et al. [49] describes the parasite burden, density of uninfected snails and those in the prepatent and patent stages. The model suggested that the mean worm burden was lowest at 20 °C, highest at 30 °C and diminished at 35 °C. The observed reduction in the worm burden at 35 °C suggests that high temperatures, especially those observed during dry hot summers may reduce prevalence of schistosomiasis [59]. A sensitivity analysis conducted on the model parameters showed the effectiveness of carrying out parasite control measures when temperature was about 20 °C. The model further suggested that application of chemotherapy at this temperature may reduce the parasite burden and prevalence of the disease. The model also shows how slight increases in temperature can affect the prevalence of schistosomiasis. However, the development and parameterization of the mechanistic model was based on non-species-specific Biomphalaria snail population.
Like the mechanistic model, a mathematical and GIS model for Zimbabwe has been developed by Ngarakana-Gwasira et al. [51]. The model explores the impact of temperature and other climatic factors such as rainfall on schistosomiasis transmission. The model suggests the ideal temperature range for schistosomiasis transmission and incorporates GIS to predict areas that may be vulnerable to disease spread with a rise in temperature. The conclusion from the mathematical and GIS model by Ngarakana-Gwasira et al. [51] has been based on non-specific snail species. Although the model was parameterized based on the works done on Bulinus (Physopsis) abyssinucus [37], this snail is little known as an IH of trematodes in Zimbabwe. The model however has observed that certain areas in Zimbabwe may need special attention owing to their potential for increased disease incidences. Areas such as Chiredzi and Mushandike have been observed to be high risk areas for disease transmission.
The agent based model by McCreesh and Booth [50] reported infection risks from river and lake scenarios based on three different temperature ranges. The study suggested the optimal temperature ranges for human infections at two possible schistosome infested points (lake or river). The model also showed the times of day at which cercariae shedding would be at its maximal level, thus demonstrating the importance of temperature in schistosome transmission. Furthermore, the study suggested that a rise in temperature may increase disease risks owing to increased cercariae output. This model agrees with the conclusion reached through a meta-analysis by Poulin [75]. The agent based model quantifies disease risk based on the amount of cercariae shed by snails into the natural environment. However, it does not take into consideration the effect of temperature on snail mortality and its eventual impact on the amount of cercariae that can be produced. This is because a rise in temperature increases snail mortality which may later impact on the amount of cercariae produced [64,74]. Furthermore, increased parasite induced mortality among infected snails [85] has been observed and this leads to a reduction in the population density of parasitized IH snails and possible amount of cercariae produced. However, some of the shortcomings of such models have been accounted for through the development of Bayesian models which include geostatistical and spatial scan statistics [81]. This makes statistical models useful in estimating parameters that can be used for the development of mathematical models.
4.5. Potential Future Research Areas
This review has shown that differences in the IH snail species used and in the methodological approaches may explain the variations observed from the various temperature driven studies. The impact of climate change on schistosomiasis still remains unclear owing to the many factors that may be at play. The distribution of schistosomiasis may not only be affected by temperature but also by the availability of water. Other factors such as IH snails, parasites and human related [7] may also affect disease distribution. This suggests that differences in the rainfall patterns and temperature in different areas may affect the fecundity, survival and growth of IH snails which ultimately impact on disease incidence. It is possible to evaluate the effects of temperature rise on disease incidences and snail ecology. However, confounding factors in different environments, exposure of IH snails to multiple variables, such as turbidity and pH among others, may also have an influence on the data obtained. Nevertheless, carefully designed laboratory experiments can provide data for parameterization of disease models.
There is a need to understand the effect of temperature on all the development stages of the snail-parasite system as well as the survival rates of IH snails over time. There may also be a need to evaluate the tolerance of successive snail generations to various temperature levels. This is because over time, snails may adapt to higher temperatures thus affecting the parameters used in disease models based on the performance of a single generation. To achieve this, the use of experimental data coupled with field studies may prove valuable in observing the changes in the environment. Temperature driven experiments based on infected and non-infected snails may assist in understanding the life stages that are likely to be affected the most by increasing temperature and how this will affect the dynamics of schistosomiasis. The use of GIS in models will also assist in projecting the likely areas that can be future disease hot spots owing to temperature increase.
5. Conclusions
The review shows that temperature is an important factor in the spread of schistosomiasis. It also shows that it may continue to play a critical role in designing schistosomiasis control programmes. Climate change may lead to a rise in temperature and this may lead to a complex relationship between IH snails and schistosomes. Although a rise in temperature may most likely lead to the disappearance of schistosomiasis in certain areas, a possible increase in the snail population due to a rise in fecundity and a reduction in the parasite development rate may increase schistosome infective stages in other areas. Disease modelling, mathematical and statistical, may however be a useful tool in understating the epidemiology of schistosomiasis. However, these models may need to include most temperature dependent stages and the biology of the IH snails and schistosomes for improved predictions, analysis and application. The use of data from well-designed laboratory and field experiments that systematically assess the effect of temperature at various stages of the snail and parasite development, will also improve the precision of models.
Acknowledgments
The study was done as part of the PhD work for the first author. The student received financial support from University of KwaZulu Natal College of Health Sciences through the CoHS student scholarship programme and from UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) and the Canadian International Development Research Centre (IDRC) through their support towards a Malaria and Bilharzia in Southern Africa (MABISA) project. The authors further acknowledge the four reviewers for their comments which improved the manuscript.
Author Contributions
Chester Kalinda, Moses Chimbari and Samson Mukaratirwa developed the concept; Chester Kalinda did literature searches, analysis and reporting. Moses Chimbari and Samson Mukaratirwa guided the process of the literature search, and manuscript writing. All the authors have read and approved the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Van der Werf M.J., de Vlas S.J., Brooker S., Looman C.W., Nagelkerke N.J., Habbema J.D.F., Engels D. Quantification of clinical morbidity associated with schistosome infection in Sub-Saharan Africa. Acta Trop. 2003;86:125–139. doi: 10.1016/S0001-706X(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 2.Appleton C., Madsen H. Human schistosomiasis in wetlands in Southern Africa. Wetlands Ecol. Manag. 2012;20:253–269. doi: 10.1007/s11273-012-9266-2. [DOI] [Google Scholar]
- 3.Mintsa-Nguéma R., Moné H., Ibikounlé M., Mengué-Ngou-Milama K., Kombila M., Mouahid G. Cercarial emergence pattern of Schistosoma haematobium from Libreville, Gabon. Parasite. 2013;21 doi: 10.1051/parasite/2014004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McManus D.P., Loukas A. Current status of vaccines for schistosomiasis. Clin. Microbiol. Rev. 2008;21:225–242. doi: 10.1128/CMR.00046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appleton C. Review of literature on abiotic factors influencing the distribution and life cycles of bilharziasis intermediate host snails. Malacol. Rev. 1978;11:1–25. [Google Scholar]
- 6.Brown D. Freshwater Snails of Africa and Their Medical Importance. Taylor and Francis; London, UK: 1994. [Google Scholar]
- 7.Walz Y., Wegmann M., Dech S., Raso G., Utzinger J. Risk profiling of schistosomiasis using remote sensing: Approaches, challenges and outlook. Parasites Vectors. 2015;8 doi: 10.1186/s13071-015-0732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tchuenté L.-A.T., Momo S.C., Stothard J.R., Rollinson D. Efficacy of praziquantel and reinfection patterns in single and mixed infection foci for intestinal and urogenital schistosomiasis in Cameroon. Acta Trop. 2013;128:275–283. doi: 10.1016/j.actatropica.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Bennett J.L., Day T., Feng-Tao L., Ismail M., Farghaly A. The development of resistance to anthelmintics: A perspective with an emphasis on the antischistosomal drug praziquantel. Exp. Parasitol. 1997;87:260–267. doi: 10.1006/expr.1997.4229. [DOI] [PubMed] [Google Scholar]
- 10.Fallon P., Tao L., Ismail M., Bennett J. Schistosome resistance to praziquantel: Fact or artifact? Parasitol. Today. 1996;12:316–320. doi: 10.1016/0169-4758(96)10029-6. [DOI] [PubMed] [Google Scholar]
- 11.Stirewalt M. Effect of snail maintenance temperatures on development of Schistosoma mansoni. Exp. Parasitol. 1954;3:504–516. doi: 10.1016/0014-4894(54)90046-6. [DOI] [PubMed] [Google Scholar]
- 12.Paull S.H., Johnson P.T. Experimental warming drives a seasonal shift in the timing of host-parasite dynamics with consequences for disease risk. Ecol. Lett. 2014;17:445–453. doi: 10.1111/ele.12244. [DOI] [PubMed] [Google Scholar]
- 13.Karl T.R., Trenberth K.E. Modern global climate change. Science. 2003;302:1719–1723. doi: 10.1126/science.1090228. [DOI] [PubMed] [Google Scholar]
- 14.Paull S.H., Raffel T.R., LaFonte B.E., Johnson P.T.J. How temperature shifts affect parasite production: Testing the roles of thermal stress and acclimation. Funct. Ecol. 2015;29:1–10. doi: 10.1111/1365-2435.12401. [DOI] [Google Scholar]
- 15.Meehl G.A., Tebaldi C. More intense, more frequent, and longer lasting heat waves in the 21st century. Science. 2004;305:994–997. doi: 10.1126/science.1098704. [DOI] [PubMed] [Google Scholar]
- 16.Kutz S., Hoberg E., Polley L., Jenkins E. Global warming is changing the dynamics of arctic host-parasite systems. Biol. Sci. 2005;272:2571–2576. doi: 10.1098/rspb.2005.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rohr J.R., Raffel T.R., Blaustein A.R., Johnson P.T., Paull S.H., Young S. Using physiology to understand climate-driven changes in disease and their implications for conservation. Conserv. Physiol. 2013;1 doi: 10.1093/conphys/cot022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paaijmans K., Blanford S., Bell A., Blanford J.I., Read A., Thomas M. Influence of climate on malaria transmission depends on daily temperature variation. Proc. Natl. Acad. Sci. USA. 2010;107:15135–15139. doi: 10.1073/pnas.1006422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pascual M., Ahumada J., Chaves L., Rodo X., Bouma M. Malaria resurgence in the east African highlands: Temperature trends revisited. Proc. Natl. Acad. Sci. USA. 2006;103:5829–5834. doi: 10.1073/pnas.0508929103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chirebvu E., Chimbari M.J., Ngwenya B.N., Sartorius B. Clinical malaria transmission trends and its association with climatic variables in Tubu Village, Botswana: A retrospective analysis. PLoS ONE. 2016;11:e0139843. doi: 10.1371/journal.pone.0139843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marti H. Field observations on the population dynamics Bulinus globosus, the intermediate host of Schistosoma haematobium in the Ifakara area, Tanzania. J. Parasitol. 1986;72:119–124. doi: 10.2307/3281803. [DOI] [PubMed] [Google Scholar]
- 22.Michelson E.M. The effects of temperature on growth and reproduction of Bi. glabrata in the laboratory. Am. J. Hyg. 1961;73:66–74. doi: 10.1093/oxfordjournals.aje.a120166. [DOI] [PubMed] [Google Scholar]
- 23.Kazibwe F., Makanga B., Rubaire-Akiiki C., Ouma J., Kariuki C., Kabatereine N., Vennervald B.J., Rollinson D., Stothard J. Transmission studies of intestinal schistosomiasis in Lake Albert, Uganda and experimental compatibility of local Biomphalaria spp. Parasitol. Int. 2010;59:49–53. doi: 10.1016/j.parint.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Lardans V., Dissous C. Snail control strategies for reduction of schistosomiasis transmission. Parasitol. Today. 1998;14:413–417. doi: 10.1016/S0169-4758(98)01320-9. [DOI] [PubMed] [Google Scholar]
- 25.Koukounari A., Gabrielli A.F., Touré S., Bosqué-Oliva E., Zhang Y., Sellin B., Donnelly C.A., Fenwick A., Webster J.P. Schistosoma haematobium infection and morbidity before and after large-scale administration of praziquantel in Burkina Faso. J. Infect. Dis. 2007;196:659–669. doi: 10.1086/520515. [DOI] [PubMed] [Google Scholar]
- 26.Hodges M.H., Dada N., Warmsley A., Paye J., Bangura M.M., Nyorkor E., Sonnie M., Zhang Y. Mass drug administration significantly reduces infection of Schistosoma mansoni and hookworm in school children in the national control program in Sierra Leone. BMC Infect. Dis. 2012;12 doi: 10.1186/1471-2334-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Utzinger J., Bergquist R., Shu-Hua X., Singer B.H., Tanner M. Sustainable schistosomiasis control—The way forward. Lancet. 2003;362:1932–1934. doi: 10.1016/S0140-6736(03)14968-9. [DOI] [PubMed] [Google Scholar]
- 28.Fenwick A., Webster J.P., Bosque-Oliva E., Blair L., Fleming F., Zhang Y., Garba A., Stothard J., Gabrielli A.F., Clements A. The schistosomiasis control initiative (SCI): Rationale, development and implementation from 2002–2008. Parasitology. 2009;136:1719–1730. doi: 10.1017/S0031182009990400. [DOI] [PubMed] [Google Scholar]
- 29.Anderson R.M., May R.M. Helminth infections of humans: Mathematical models, population dynamics, and control. Adv. Parasitol. 1985;24:1–101. doi: 10.1016/s0065-308x(08)60561-8. [DOI] [PubMed] [Google Scholar]
- 30.Wang X. Ph.D. Thesis. Case Western Reserve University; Cleveland, OH, USA: 2012. Mathematical Models of Schistosomiasis Transmission, Morbidity and Control with Applications to Endemic Communities in Coastal Kenya. [Google Scholar]
- 31.Mukaratirwa S., Malone J., McCarroll J., Kristensen T. Satellite surveillance, geographical information systems and the seasonal suitability of environment for the development of the snail-parasite system of urinary and intestinal schistosomiasis in Zimbabwe; Proceedings of the Workshop on Medical and Veterinary Malacology in Africa; Harare, Zimbabwe. 8–12 November 1999; pp. 265–271. [Google Scholar]
- 32.Stensgaard A., Jorgensen A., Kabatereine N.B., Malone J.B., Kristensen T.K. Modelling the distribution of schistosoma mansoni and host snails in Uganda using satellite sensor data and geographical information systems. Parassitologia. 2005;47:115–125. [PubMed] [Google Scholar]
- 33.Fenwick A., Webster J.P. Schistosomiasis—Challenges for control, treatment and drug resistance. Curr. Opin. Infect. Dis. 2006;19:577–582. doi: 10.1097/01.qco.0000247591.13671.6a. [DOI] [PubMed] [Google Scholar]
- 34.Chimbari M.J., Ndamba J., Madsen H. Food selection behaviour of potential biological agents to control intermediate host snails of schistosomiasis: Sargochromis codringtoni and Tilapia rendalli. Acta Trop. 1996;61:191–199. doi: 10.1016/0001-706X(95)00144-4. [DOI] [PubMed] [Google Scholar]
- 35.McCreesh N., Booth M. The effect of simulating different intermediate host snail species on the link between water temperature and schistosomiasis risk. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0087892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Appleton C., Eriksson I.M. The influence of fluctuating above-optimal temperature regimes on the fecundity of Biomphalaria pfeifferi (Mollusca: Planorbidae) Trans. Royal Soc. Trop. Med. Hyg. 1984;78:49–54. doi: 10.1016/0035-9203(84)90171-8. [DOI] [PubMed] [Google Scholar]
- 37.Dagal M.A., Upatham E.S., Kruatrachue M., Viyanant V. Effects of some physico-chemical factors on the hatching of egg masses and on the survival of juvenile and adult snails Bulinus (Physopsis) abyssinucus. J. Sci. Soc. Thail. 1986;12:23–30. doi: 10.2306/scienceasia1513-1874.1986.12.023. [DOI] [Google Scholar]
- 38.El-Emam M., Madsen H. The effect of temperature, darkness, starvation and various food types on growth, survival and reproduction of Helisoma duryi, Biomphalaria alexandrina and Bulinus truncatus (Gastropoda: Planorbidae) Hydrobiologia. 1982;88:265–275. doi: 10.1007/BF00008506. [DOI] [Google Scholar]
- 39.Joubert P.H., Pretorius S.J., DeKock K.N., Vaneeden J.A. Survival of Bulinus-Africanus (Krauss), Bulinus-globosus (Morelet) and Biomphalaria-pfeifferi (Krauss) at constant high-temperatures. S. Afr. J. Zool. 1986;21:85–88. doi: 10.1080/02541858.1986.11447963. [DOI] [Google Scholar]
- 40.Kubiriza G.K., Madsen H., Likongwe J.S., Stauffer J.R., Kang’Ombe J., Kapute F. Effect of temperature on growth, survival and reproduction of Bulinus nyassanus (Smith, 1877) (Mollusca: Gastropoda) from Lake Malawi. Afr. Zool. 2010;45:315–320. doi: 10.3377/004.045.0210. [DOI] [Google Scholar]
- 41.McCreesh N., Arinaitwe M., Arineitwe W., Tukahebwa E., Booth M. Effect of water temperature and population density on the population dynamics of Schistosoma mansoni intermediate host snails. Parasites Vectors. 2014;7:1–9. doi: 10.1186/s13071-014-0503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pflüger W., Roushdy M., El-Emam M. Prepatency of Schistosoma haematobium in snails at different constant temperatures. J. Egypt. Soc. Parasitol. 1983;13:513–519. [PubMed] [Google Scholar]
- 43.Pflüger W. Experimental epidemiology of schistosomiasis. Z. Parasitenkd. 1980;63:159–169. doi: 10.1007/BF00927532. [DOI] [PubMed] [Google Scholar]
- 44.Mofolusho O.F., Benson O. Survival potential, fecundity and fertility of Biomphalaria pfeifferi (Krauss, 1848) during acclimatization in the laboratory. Zool. Ecol. 2013;23:157–161. [Google Scholar]
- 45.Joubert P., Pretorius S., De Kock K., Van Eeden J. The effect of constant low temperatures on the survival of Bulinus africanus (Krauss), Bulinus globosus (Morelet) and Biomphalaria pfeifferi (Krauss) S. Afr. J. Zool. 1984;19:314–316. doi: 10.1080/02541858.1984.11447901. [DOI] [Google Scholar]
- 46.Barbosa N., Pimentel-Souza F., Sampaio I. The effect of seasonal temperature and experimental illumination on reproductive rate in the snail Biomphalaria glabrata. Braz. J. Med. Biol. Res. 1986;20:685–696. [PubMed] [Google Scholar]
- 47.O’keeffe J. Population biology of the freshwater snail Bulinus globosus on the Kenya coast. I. Population fluctuations in relation to climate. J. Appl. Ecol. 1985;22:73–84. doi: 10.2307/2403328. [DOI] [Google Scholar]
- 48.Woolhouse M., Chandiwana S. Population biology of the freshwater snail Bulinus globosus in the Zimbabwe highveld. J. Appl. Ecol. 1990;27:41–59. doi: 10.2307/2403567. [DOI] [Google Scholar]
- 49.Mangal T.D., Paterson S., Fenton A. Predicting the impact of long-term temperature changes on the epidemiology and control of schistosomiasis: A mechanistic model. PLoS ONE. 2008;3:e1438. doi: 10.1371/journal.pone.0001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCreesh N., Booth M. The effect of increasing water temperatures on Schistosoma mansoni transmission and Biomphalaria pfeifferi population dynamics: An agent-based modelling study. PLoS ONE. 2014;9:e105917. doi: 10.1371/journal.pone.0101462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ngarakana-Gwasira E., Bhunu C., Masocha M., Mashonjowa E. Transmission dynamics of schistosomiasis in Zimbabwe: A mathematical and GIS approach. Commun. Nonlinear Sci. Num. Sim. 2016;35:137–147. doi: 10.1016/j.cnsns.2015.11.005. [DOI] [Google Scholar]
- 52.Pedersen U.B., Stendel M., Midzi N., Mduluza T., Soko W., Stensgaard A.-S., Vennervald B.J., Mukaratirwa S., Kristensen T.K. Modelling climate change impact on the spatial distribution of fresh water snails hosting trematodes in Zimbabwe. Parasites Vectors. 2014;7:536. doi: 10.1186/s13071-014-0536-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stensgaard A.S., Utzinger J., Vounatsou P., Hurlimann E., Schur N., Saarnak C.F.L., Simoonga C., Mubita P., Kabatereine N.B., Tchuente L.A.T., et al. Large-scale determinants of intestinal schistosomiasis and intermediate host snail distribution across Africa: Does climate matter? Acta Trop. 2013;128:378–390. doi: 10.1016/j.actatropica.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 54.Sorensen R.E., Minchella D.J. Parasite influences on host life history: Echinostoma revolutum parasitism of Lymnaea elodes snails. Oecologia. 1998;115:188–195. doi: 10.1007/s004420050507. [DOI] [PubMed] [Google Scholar]
- 55.Sterarns S. The Evolution of Life Histories. Oxford University Press; Oxford, UK: 1992. [Google Scholar]
- 56.Hurd H. Host fecundity reduction: A strategy for damage limitation? Trends Parasitol. 2001;17:363–368. doi: 10.1016/S1471-4922(01)01927-4. [DOI] [PubMed] [Google Scholar]
- 57.Lafferty K.D. The ecology of climate change and infectious diseases. Ecology. 2009;90:888–900. doi: 10.1890/08-0079.1. [DOI] [PubMed] [Google Scholar]
- 58.Brackenbury T., Appleton C. Effect of controlled temperatures on gametogenesis in the gastropods Physa acuta (Physidae) and Bulinus tropicus (Planorbidae) J. Mollus. Stud. 1991;57:461–469. doi: 10.1093/mollus/57.4.461. [DOI] [Google Scholar]
- 59.Manyangadze T., Chimbari M.J., Gebreslasie M., Pietro C., Mukaratirwa S. Modelling the spatial and seasonal distribution of suitable habitats of schistosomiasis intermediate host snails using maxent in Ndumo Area, Kwazulu-Natal province, South Africa. Parasites Vectors. 2016;9:572. doi: 10.1186/s13071-016-1834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Appleton C. The influence of above-optimal constant temperatures on South African Biomphalaria pfeifferi (Krauss) (Mollusca: Planorbidae) Trans. R. Soc. Trop. Med. Hyg. 1977;71:140–143. doi: 10.1016/0035-9203(77)90082-7. [DOI] [PubMed] [Google Scholar]
- 61.Kabatereine N.B., Brooker S., Tukahebwa E.M., Kazibwe F., Onapa A.W. Epidemiology and geography of Schistosoma mansoni in Uganda: Implications for planning control. Trop. Med. Int. Health. 2004;9:372–380. doi: 10.1046/j.1365-3156.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 62.Seppälä O., Jokela J. Immune defence under extreme ambient temperature. Biol. Lett. 2011;7:119–122. doi: 10.1098/rsbl.2010.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paull S.H., Johnson P.T.J. High temperature enhances host pathology in a snail-trematode system: Possible consequences of climate change for the emergence of disease. Freshwater Biol. 2011;56:767–778. doi: 10.1111/j.1365-2427.2010.02547.x. [DOI] [Google Scholar]
- 64.Studer A., Thieltges D., Poulin R. Parasites and global warming: Net effects of temperature on an intertidal host-parasite system. Mar. Ecol. Progr. Ser. 2010;415:11–22. doi: 10.3354/meps08742. [DOI] [Google Scholar]
- 65.Harrison A., Shiff C. Factors influencing the distribution of some species of aquatic snails. S. Afr. J. Sci. 1966;62:3–258. [Google Scholar]
- 66.Shiff C. Studies on Bulinus (Physopsis) globosus in Rhodesia. I. The influence of temperature on the intrinsic rate of natural increase. Ann. Trop. Med. Parasitol. 1964;58:94–105. doi: 10.1080/00034983.1964.11686219. [DOI] [PubMed] [Google Scholar]
- 67.Sturrock R., Sturrock B. The influence of temperature on the biology of Biomphalaria glabrata (say), intermediate host of Schistosoma mansoni on St. Lucia, West Indies. Ann. Trop. Med. Parasitol. 1972;66:385–390. doi: 10.1080/00034983.1972.11686839. [DOI] [PubMed] [Google Scholar]
- 68.Graham A. Effects of snail size and age on the prevalence and intensity of avian schistosome infection: Relating laboratory to field studies. J. Parasitol. 2003;89:458–463. doi: 10.1645/0022-3395(2003)089[0458:EOSSAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 69.Labaude S., Rigaud T., Cézilly F. Host manipulation in the face of environmental changes: Ecological consequences. Int. J. Parasitol. 2015;4:442–451. doi: 10.1016/j.ijppaw.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Studer A., Poulin R., Tompkins D. Local effects of a global problem: Modelling the risk of parasite-induced mortality in an intertidal trematode-amphipod system. Oecologia. 2013;172:1213–1222. doi: 10.1007/s00442-012-2569-4. [DOI] [PubMed] [Google Scholar]
- 71.Esch G.W., Gibbons J.W., Bourque J.E. An analysis of the relationship between stress and parasitism. Am. Midl. Nat. 1975;93:339–353. doi: 10.2307/2424167. [DOI] [Google Scholar]
- 72.Yang G.-J., Utzinger J., Sun L.-P., Hong Q.-B., Vounatsou P., Tanner M., Zhou X.-N. Effect of temperature on the development of Schistosoma japonicum within Oncomelania hupensis, and hibernation of O. hupensis. Parasitol. Res. 2007;100:695–700. doi: 10.1007/s00436-006-0315-8. [DOI] [PubMed] [Google Scholar]
- 73.Zhou X.N., Yang G.-J., Yang K., Wang X.-H., Hong Q.-B., Sun L.-P., Malone J.B., Kristensen T.K., Bergquist N.R., Utzinger J. Potential impact of climate change on schistosomiasis transmission in China. Am. J. Trop. Med. Hyg. 2008;78:188–194. [PubMed] [Google Scholar]
- 74.Morley N., Lewis J. Thermodynamics of cercarial development and emergence in trematodes. Parasitology. 2013;140:1211–1224. doi: 10.1017/S0031182012001783. [DOI] [PubMed] [Google Scholar]
- 75.Poulin R. Global warming and temperature-mediated increases in cercarial emergence in trematode parasites. Parasitology. 2006;132:143–151. doi: 10.1017/S0031182005008693. [DOI] [PubMed] [Google Scholar]
- 76.Martens W., Jetten T.H., Focks D.A. Sensitivity of malaria, schistosomiasis and dengue to global warming. Clim. Chang. 1997;35:145–156. doi: 10.1023/A:1005365413932. [DOI] [Google Scholar]
- 77.Macdonald G. The dynamics of helminth infections, with special reference to schistosomes. Transact. R. Soc. Trop. Med. Hyg. 1965;59:489–506. doi: 10.1016/0035-9203(65)90152-5. [DOI] [PubMed] [Google Scholar]
- 78.Anderson R.M., May R.M. Population dynamics of human helminth infections: Control by chemotherapy. Nature. 1982;297:557–563. doi: 10.1038/297557a0. [DOI] [PubMed] [Google Scholar]
- 79.Clements A.C., Lwambo N.J., Blair L., Nyandindi U., Kaatano G., Kinung’hi S., Webster J.P., Fenwick A., Brooker S. Bayesian spatial analysis and disease mapping: Tools to enhance planning and implementation of a schistosomiasis control programme in Tanzania. Trop. Med. Int. Health. 2006;11:490–503. doi: 10.1111/j.1365-3156.2006.01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Woolhouse M. On the application of mathematical models of schistosome transmission dynamics. I. Natural transmission. Acta Trop. 1991;49:241–270. doi: 10.1016/0001-706X(91)90077-W. [DOI] [PubMed] [Google Scholar]
- 81.Xu J.-F., Lv S., Wang Q.-Y., Qian M.-B., Liu Q., Bergquist R., Zhou X.-N. Schistosomiasis japonica: Modelling as a tool to explore transmission patterns. Acta Trop. 2015;141:213–222. doi: 10.1016/j.actatropica.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 82.Keeling M.J., Rohani P. Modeling Infectious Diseases in Humans and Animals. Princeton University Press; Princeton, NJ, USA: 2008. [Google Scholar]
- 83.Liang S., Maszle D., Spear R.C. A quantitative framework for a multi-group model of schistosomiasis japonicum transmission dynamics and control in Sichuan, China. Acta Trop. 2002;82:263–277. doi: 10.1016/S0001-706X(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 84.Zakaria H. Further study on the ecology of the intermediate host of schistosoma haematobium, bulinus truncatus baylis. Bull. Endem. Dis. 1955;1:123–155. [PubMed] [Google Scholar]
- 85.Mouritsen K.N., Jensen K.T. Parasite transmission between soft-bottom invertebrates: Temperature mediated infection rates and mortality in Corophium volutator. Mar. Ecol. Progr. Ser. 1997;151:123–134. doi: 10.3354/meps151123. [DOI] [Google Scholar]