Abstract
Signaling by insulin and target of rapamycin are both required for cell growth, but their interrelationships remain poorly defined. It was reported that Akt, an essential component of the insulin pathway, stimulates growth by phosphorylating and inhibiting tuberous sclerosis complex 2 (TSC2). Here we evaluate this model genetically in Drosophila by engineering Tsc2 mutants in which the Akt phosphorylation sites are changed to nonphosphorylatable or phospho-mimicking residues. Strikingly, such mutants completely rescue the lethality and cell growth defects of Tsc2-null mutants. Taken together, our data suggest that Tsc2 is not a critical substrate of Akt in normal Drosophila development.
Keywords: Cell growth, tumor suppressor, insulin signaling, target of rapamycin (TOR)
The growth of higher eukaryotic cells is regulated not only by intercellular growth factors such as insulin or insulin-like growth factors (IGFs), but also more directly by the availability of nutrients (Gingras et al. 2001; Shamji et al. 2003; Fingar and Blenis 2004; Hafen 2004). Insulin and IGFs act through a canonical signaling pathway composed of insulin receptor (InR), insulin receptor substrate (IRS), phosphoinositide-3-kinase (PI3K), phosphoinositide-dependent kinase 1 (PDK1), and Akt (also called PKB). The nutrient-sensitive growth pathway is less well defined, but appears to involve the tuberous sclerosis tumor suppressor proteins TSC1 and TSC2, the small GTPase Rheb, and target of rapamycin (TOR), a Ser/Thr kinase specifically inhibited by the immunosuppressant rapamycin (Garami et al. 2003; Inoki et al. 2003; Tee et al. 2003; Zhang et al. 2003). Both pathways converge on a common set of effectors involved in protein translation, including ribosomal S6 protein kinase (S6K) and eukaryotic initiation factor 4E-binding protein (4E-BP). Recent studies in both mammalian cells and Drosophila have demonstrated the importance of the insulin/TOR signaling network in regulating cell growth (Fingar and Blenis 2004; Hafen 2004).
A long-standing question concerning insulin and TOR signaling is how the TOR-mediated nutrient signals intersect with the canonical insulin signaling pathway (Gingras et al. 2001; Shamji et al. 2003). The simplest model places TOR downstream from Akt in a linear fashion. This linear model was initially proposed based on the identification of Ser 2448 of mammalian TOR as an Akt phosphorylation site (Nave et al. 1999). The physiological relevance of this phosphorylation event was unclear, because substitution of Ser 2448 to alanine does not perturb mammalian TOR activity (Sekulic et al. 2000), and this site is not conserved in Drosophila TOR (Oldham et al. 2000; Zhang et al. 2000). An alternative model suggests that insulin and TOR act in parallel pathways. This parallel model was proposed based on the differential sensitivity of certain S6K mutants to rapamycin and the PI3K inhibitor wortmannin (Dennis et al. 1996; Hara et al. 1998). Such behavior can be best explained by the existence of parallel inputs into S6K from TOR and insulin signaling. For example, it is possible that TOR provides a permissive signal that primes S6K activation when nutrients, in particular amino acids, are at sufficient levels (Hara et al. 1998; Gingras et al. 2001; Shamji et al. 2003).
Several recent studies have identified the TSC2 protein as an Akt substrate, thus providing another plausible way to place TOR downstream from Akt (Dan et al. 2002; Inoki et al. 2002; Manning et al. 2002; Potter et al. 2002). Collectively, these studies suggest that phosphorylation by Akt inactivates TSC2 by several mechanisms, including changes in subcellular localization, dissociation of the TSC1–TSC2 complex, or degradation of the TSC1–TSC2 complex (Dan et al. 2002; Inoki et al. 2002; Manning et al. 2002; Potter et al. 2002). Although these studies provided strong evidence for phosphorylation of TSC2 by Akt (mostly in cultured cells), this observation alone is not sufficient to infer a physiological role for this phosphorylation event. It remains to be determined to what extent phosphorylation of TSC2 by Akt contributes to Akt-mediated growth control in the context of normal development. Are all Akt-mediated growth signals transduced through TSC2 as suggested (Potter et al. 2002)? Could it be possible that phosphorylation by Akt only serves a minor role in regulating TSC2 activity in normal development?
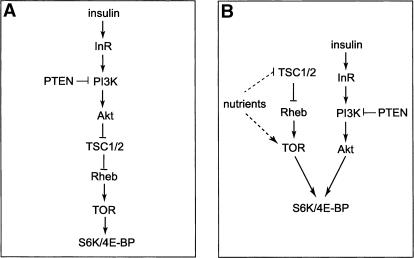
The fruit fly Drosophila provides a powerful system in which to investigate the insulin/TOR signaling network in an intact organism (Hafen 2004; Neufeld 2004). The insulin/TOR signaling network is highly conserved between Drosophila and mammalian cells, and its perturbation similarly affects cell size in both systems. Despite the large body of genetic studies in Drosophila, the relationship between insulin signaling and TOR remains controversial. Potter and coworkers have proposed a simple and linear model (Fig. 1A) that places Drosophila TSC2 (hereafter referred to as Tsc2) directly downstream from Akt (Potter et al. 2002). Specifically, they suggested that Akt directly phosphorylates Tsc2 on Ser 924 and Thr 1518, and that phosphorylation by Akt disrupts the Tsc1–Tsc2 complex and disturbs the subcellular localization of Tsc1 and Tsc2. It is worth noting that the only in vivo data provided by this study that support the functional relevance of these Akt phosphorylation sites involved overexpressing Tsc2 mutants in which the Akt phosphorylation sites are changed to alanine (Potter et al. 2002). Based on their observation that such alanine mutants showed a stronger growth-suppressing activity than the wild-type Tsc2 when overexpressed, the investigators concluded that Akt mediates growth through phosphorylating Tsc2 in vivo (Potter et al. 2002). However, this simple linear model is inconsistent with several observations in Drosophila (Oldham et al. 2000; Gao et al. 2002; Radimerski et al. 2002). For example, although perturbation of insulin and of TOR signaling both result in decreased cell size in Drosophila, the respective cell size phenotypes are distinct in that loss of TOR or nutrient starvation preferentially reduces the cytoplasmic volume of endoreplicating cells (Oldham et al. 2000). Such distinct mutant phenotypes are more consistent with TOR acting in a parallel pathway involved in amino acid sensing, instead of acting in a linear pathway downstream from insulin signaling. This parallel model (Fig. 1B) is further supported by the observation that cells doubly mutant for Tsc1 and PTEN display an additive increase in cell size as compared with either single mutation (Gao et al. 2002). An important distinction between these studies and that by Potter et al. (2002) is that the former were based on loss-of-function genetics whereas the latter was based on overexpression studies. Given the caveat often associated with protein overexpression, we attempted to re-examine the relationship between Akt and Tsc2 using an experimental approach that does not involve overexpression. Using a gene-replacement strategy, we show that mutations of the Akt phosphorylation sites had little effect on the biological activity of Tsc2. Our results suggest that Tsc2 is not a critical target of Akt during normal Drosophila development.
Figure 1.
Two models relating Akt and Tsc2 in cell growth control in Drosophila. (A) In the linear model, insulin signaling activates Akt, which phosphorylates and inactivates the Tsc1–Tsc2 complex. Inactivation of the Tsc1–Tsc2 complex by Akt in turn promotes cell growth through Rheb, TOR, and S6K/4E-BP. This model suggests that signals from Akt to S6K/4E-BP are all transduced through Tsc1–Tsc2 and TOR. (B) In the parallel model, the nutrient-sensitive TOR pathway converges with the canonical insulin pathway to regulate translation regulators in cell growth such as S6K and 4E-BP. This model suggests that TOR and the insulin/Akt pathways provide parallel inputs in cell growth control.
Results and Discussion
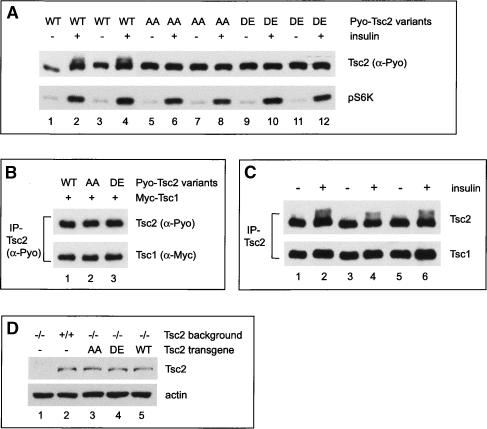
To investigate the relative contribution of Akt-mediated Tsc2 phosphorylation to overall growth control, we engineered Drosophila Tsc2 variants in which the previously identified Akt phosphorylation sites, S924 and T1518, were mutated to nonphosphorylatable alanine (Tsc2S924A/T1518A, abbreviated as Tsc2AA) or phospho-mimicking residues (Tsc2S924D/T1518E, abbreviated as Tsc2DE). We first characterized these mutants in the Drosophila S2 cells. As reported in mammalian cells (Dan et al. 2002; Inoki et al. 2002; Manning et al. 2002), insulin stimulation resulted in phosphorylation of wild-type Tsc2, and this phosphorylation was abolished when S924 and T1518 were changed to nonphosphorylatable (Tsc2AA) or phospho-mimicking residues (Tsc2DE) (Fig. 2A). This result is consistent with the previous report that identified S924 and T1518 of Tsc2 as the Akt phosphorylation sites (Potter et al. 2002). However, in contrast to reports that these phosphorylation sites regulate Tsc1–Tsc2 complex formation (Potter et al. 2002), Tsc2AA and Tsc2DE associate with Tsc1 with similar affinity as the wild-type Tsc2 (Fig. 2B). Thus, these results are consistent with observations made by Dan et al. (2002) and Manning et al. (2002) concerning the mammalian TSC1–TSC2 complex. We also examined the interactions between the endogenous Tsc1 and Tsc2 proteins, and found that insulin-induced phosphorylation of Tsc2 did not significantly affect its ability to associate with the endogenous Tsc1 in S2 cells (Fig. 2C). Taken together, we conclude that insulin signaling, at least in Drosophila S2 cells, leads to the phosphorylation of Tsc2 at Ser 924 and Thr 1518 as shown previously (Potter et al. 2002), although such phosphorylation does not appear to disrupt Tsc1–Tsc2 complex formation. We noted that even under our most optimized conditions in S2 cells, only a fraction of endogenous (Fig. 2C) or transfected (Fig. 2A) Tsc2 protein showed mobility shift upon insulin stimulation, which contrasts mammalian cells wherein insulin stimulation results in a much greater mobility shift of TSC2 (Dan et al. 2002; Inoki et al. 2002). The significance of this difference is unclear at present.
Figure 2.
Characterization of Tsc2 mutants with phosphorylation site mutations. (A) S2 cells expressing Polyoma (Pyo)-tagged Tsc2WT, Tsc2AA, or Tsc2DE were extracted directly (lanes 1,3,5,7,9,11) or after treatment with insulin for 20 min (lanes 2,4,6,8,10,12). The cell lysate was probed with α-Pyo antibody (top gel) and a phospho-specific antibody that detects activated S6K (bottom gel). Two sets of independent experiments were shown for each Tsc2 construct. Note the mobility shift of Tsc2WT resulting from insulin treatment (lanes 1–4), and the absence of such a shift for Tsc2AA (lanes 5–8) or Tsc2DE (lanes 9–12). (B) S2 cells coexpressing Myc-tagged Tsc1 and Pyo-tagged Tsc2WT, Tsc2AA, or Tsc2DE were lysed and the total cell lysate was immunoprecipitated with α-Pyo antibody. The α-Pyo immunoprecipitates were probed with antibodies against Pyo (upper gel) and Myc (lower gel). Note that all the Pyo-Tsc2 variants bind to Myc-Tsc1 with similar affinity. (C) S2 cells were extracted directly (lanes 1,3,5) or after treatment with insulin for 20 min (lanes 2,4,6). Endogenous Tsc2 protein was immunoprecipitated with α-Tsc2 antibody. The α-Tsc2 immunoprecipitates were probed with antibodies against Tsc2 (top gel) and Tsc1 (bottom gel). Three sets of independent experiments were shown. Despite the mobility shift of endogenous Tsc2 upon insulin treatment, a similar amount of Tsc1 was present in the α-Tsc2 immunoprecipitates (cf. lanes 1,3,5 and 2,4,6). (D) Embryo extract from Tsc2-null embryos (lane 1), wild-type flies (lane 2), Tsc2-null embryos rescued with Tsc2AA (lane 3), Tsc2-null embryos rescued with Tsc2DE (lane 4), and Tsc2-null embryos rescued with Tsc2WT (lane 5) was probed with antibodies against Tsc2 (top gel) and actin (bottom gel). The Tsc2-null embryos were identified using a balancer chromosome that carries GFP. (Lanes 3–5) All the rescued flies were homozygous for the respective transgene insertion. Note the absence of signal in Tsc2-null animals, and the similar levels of Tsc2 transgenes (lanes 3–5) as compared with the endogenous Tsc2 (lane 2).
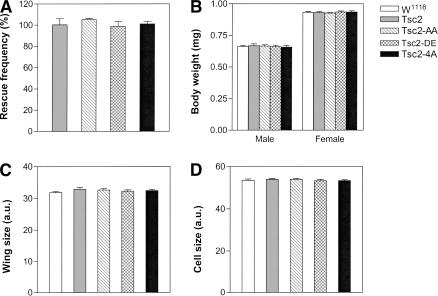
To investigate if Akt-mediated Tsc2 phosphorylation plays a critical physiological role in Drosophila development, we designed a strategy to compare the activity of nonphosphorylatable and phospho-mimicking mutants with their wild-type counterpart in intact organisms without protein overexpression. Specifically, we introduced the nonphosphorylatable Tsc2AA and the phospho-mimicking Tsc2DE, as well as a wild-type Tsc2 (Tsc2WT) control, into a Tsc2-null mutant background using a minigene cassette driven by the ubiquitous α-tubulin promoter. This expression system provided levels of Tsc2 transgenes similar to those of the endogenous protein (Fig. 2D). We reasoned that if Akt-mediated growth signals are normally transduced via phosphorylation of Tsc2, neither the nonphosphorylatable nor the phospho-mimicking mutant should rescue the lethality of the Tsc2-null mutant. Flies in which the endogenous Tsc2 is replaced with Tsc2AA or Tsc2DE should resemble loss-of-function mutants of Akt or Tsc2, respectively, which are lethal at the third or the second larval stage. Contrary to this prediction, however, we found that Tsc2AA and Tsc2DE rescued Tsc2-null animals to viable adults as efficiently as the Tsc2WT construct, with a rescue frequency near 100% for all the transgenes (Fig. 3A). Furthermore, the adult flies rescued by these constructs showed similar body weight (Fig. 3B), wing size (Fig. 3C), and cell size (Fig. 3D) as wild-type flies. Similar results were obtained using multiple independent transgenic lines for each construct (Table 1). These observations suggest that Akt-mediated Tsc2 phosphorylation does not contribute significantly to overall growth control in the context of normal Drosophila development. Indeed, fly strains in which the endogenous Tsc2 is replaced with these phosphorylation-site mutants are maintained as stable stocks. So far, we have yet to observe any noticeable difference among these strains.
Figure 3.
Mutations of the Akt phosphorylation sites do not perturb Tsc2 function in normal Drosophila development. Tsc2WT, Tsc2AA, Tsc2DE, and Tsc24A were tested for their ability to complement Tsc2-null mutant flies (see Materials and Methods for details). Note the similar rescue efficiency of each construct as well as the similar body weight (B), wing size (C), and cell size (D) of the rescued animals as compared with wild-type flies. (A) The percentage of Tsc2-null flies rescued to adulthood by each construct. (B) Body weight of the rescued adult flies as compared to wild-type flies (w1118). Both sexes are shown. (C) Wing size of the rescued adult flies compared with wild-type flies (w1118), measured by arbitrary units (a.u.). Male flies were analyzed. (D) Cell size of the rescued adult flies compared with wild-type flies (w1118), measured by arbitrary units (a.u.). Cell size was assessed by counting the number of wing hairs on the dorsal wing surface in a fixed-size area posterior to the anterior cross vein. Male flies were analyzed.
Table 1.
Summary of Tsc2 variants tested for their ability to rescue Tsc2-null mutant flies
| Construct | Description | No. of lines tested | No. of lines that rescue Tsc2-/- |
|---|---|---|---|
| Tsc2N1698K | GAP dead, disease-causing | 6 | 0 |
| Tsc2K1698A | GAP dead | 7 | 0 |
| Tsc2R884Q | Affecting Tsc1–Tsc2 complex, disease-causing | 5 | 0 |
| Tsc2R622W | Affecting Tsc1–Tsc2 complex, disease-causing | 5 | 0 |
| Tsc2AA | S924A/T1518A | 5 | 5 |
| Tsc2DE | S924D/T1518E | 12 | 12 |
| Tsc24A | T437A/S924A/T1054A/T1518A | 5 | 5 |
| Tsc2WT | Wild type Tsc2 | 3 | 3 |
All the constructs were tested for rescuing the null allele Tsc2192, as well as Tsc2193, another allele of Tsc2 that has an in-frame deletion (amino acids 472–527). Identical results were obtained using Tsc2192 and Tsc2193.
Besides S924 and T1518, Tsc2 contains two additional sites that match the Akt phosphorylation consensus motif, RXRXXS/T, at T437 and T1054. These sites were shown previously to play negligible role in relaying the growth signal from Akt to Tsc2 (Potter et al. 2002). To investigate the possibility that T437 and T1054 might serve as compensatory Akt phosphorylation sites when S924 and T1518 are changed to nonphosphorylatable alanine, we engineered a Tsc2 variant in which all four possible Akt phosphorylation sites are changed to alanine (Tsc2T437A/S924A/T1054A/T1518A, abbreviated as Tsc24A). When the activity of this Tsc2 mutant was examined in vivo using the rescue assay described above, we could not detect any significant difference between Tsc24A and Tsc2WT with respect to rescue frequency, body weight, wing size and cell size (Fig. 3A–D). These observations strengthen our conclusion that Tsc2 is not a critical target of Akt during normal Drosophila development.
Besides the Akt phosphorylation site mutants, we have tested several other Tsc2 mutants using the same rescue assay described above. These included two mutations in the GAP domain, Tsc2N1698K and Tsc2K1693A, as well as two mutations in the N-terminal noncatalytic region of Tsc2, Tsc2R884Q and Tsc2R622W (Table 1). Tsc2N1698K and Tsc2K1693A have been shown to abolish the GAP activity of Tsc2 toward Rheb (Zhang et al. 2003), and among them, Tsc2N1698K mimics a disease-causing mutation in TSC patients (N1643K of human TSC2) (Maheshwar et al. 1997). Tsc2R622W and Tsc2R884Q are also analogous to disease-causing mutations in TSC patients (R611W and R905Q of human TSC2, respectively) (Nellist et al. 2001). In humans, both R611W and R905Q have been shown to affect TSC1–TSC2 complex formation (Nellist et al. 2001; Inoki et al. 2002). In contrast to mutations of the Akt phosphorylation sites, which completely rescued the Tsc2-null, Tsc2N1698K, Tsc2K1693A, Tsc2R884Q, and Tsc2R622W all failed to rescue the lethality of Tsc2 mutant flies (0% rescue; see Table 1). These results not only provide controls for our rescue assay, but also confirm the disease-causing nature of the respective mutations in TSC patients.
In summary, we have developed an in vivo assay in which Tsc2 variants, expressed at levels similar to those of the endogenous Tsc2, are tested for their ability to complement Tsc2-null mutant flies. Using this gene replacement strategy, we show that mutations of the reported Akt phosphorylation sites had little effect on the biological activity of Tsc2 in normal Drosophila development. Because we assayed Tsc2 function by rescue frequency and adult cell size rather than the biochemical activity of Tsc2 per se, it is formally possible that the rescue activity of the phosphorylation-site mutants might reflect aberrant Tsc2 activity in conjunction with compensatory changes of other growth-regulatory pathways. We think this is highly unlikely given that multiple disease-causing mutations displayed no rescue activity in the same assay (Table 1), and it would be difficult to imagine how only one class of mutants invoked compensatory changes. Taken together, we suggest that Tsc2 is not a critical target of Akt during normal Drosophila development, a conclusion that differs from a previous report that assayed a nonphosphorylatable Tsc2 mutant by overexpression (Potter et al. 2002). Our data are more consistent with a model that places TOR and insulin signaling in parallel pathways (Fig. 1B) (Shamji et al. 2003; Hafen 2004). This model is supported not only by studies of certain S6K mutants in mammalian cells that differentially respond to rapamycin (or amino acid withdrawal) and PI3K inhibitor (Dennis et al. 1996; Hara et al. 1998), but also by the majority of genetic studies in Drosophila including data presented here (Oldham et al. 2000; Gao et al. 2002; Radimerski et al. 2002). We emphasize that our conclusion regarding the negligible contribution of Tsc2 phosphorylation by Akt concerns normal development in Drosophila, and it does not preclude a possible involvement of this phosphorylation event in regulating Tsc2 activity under abnormal conditions. For example, activation of oncogenes or inactivation of tumor suppressor genes might lead to abnormally high level of Akt activity that targets TSC2 in a “gain-of-function” manner, and inactivation of TSC2 in such context could still contribute significantly to Akt-mediated tumorigenesis. We have attempted to test this possibility in Drosophila by comparing the overgrowth phenotype resulting from a myristoylated constitutively active form of Akt (myr-Akt; Stocker et al. 2002) in flies in which the endogenous Tsc2 was replaced with rescue constructs containing Tsc2WT, Tsc2AA, or Tsc24A. In this experiment, we could not detect any significant difference in Akt-driven overgrowth in the various genetic backgrounds (data not shown; see Materials and Methods for details of fly genetics). Thus, at least with expression levels achieved in this experiment, the constitutively active Akt did not appear to signal to Tsc2. Whether even higher level of Akt activity could allow signaling to Tsc2 awaits further investigation. Irrespective of the results in Drosophila, it will be important to carry out similar experiments in mammals to determine whether inactivation of TSC2 contributes to tumorigenesis driven by abnormal Akt activity.
Our studies highlight the importance of studying signaling networks in the specific contexts in which the pathways operate. This is especially true when one investigates the cross-talk among different signaling pathways, which may vary between cultured cells and intact organisms or even among different cultured cells. For example, activation of the insulin pathway leads to Tsc2 phosphorylation in cultured Drosophila cells (Fig. 2), yet this phosphorylation does not appear to play a critical role during normal Drosophila development (Fig. 3). Similarly, although oncogenic Ras activates both MAPK and PI3K pathways in mammalian cells, loss of Ras only attenuates MAPK signaling without affecting PI3K in Drosophila (Prober and Edgar 2002). One possible explanation for such differences might be that as compared with intact animals, cross-talk among signaling pathways could be more extensive and flexible in cultured cells, especially under conditions in which signaling components are expressed or activated beyond physiological levels. Thus, caution should be exercised when extrapolating from one system to another. Along the same line, although our studies suggest that Tsc2 is not a critical target of Akt during normal Drosophila development, it remains to be determined whether the same is true for mammals. With the existence of TSC2 knockout mice, it should be possible to directly test the physiological significance of the Akt phosphorylation sites in mice using a rescue assay similar to that described here. Furthermore, such rescue assays should be generally applicable to assigning kinase–substrate relationships in vivo for other kinases and substrates.
Materials and methods
Molecular biology
Full-length wild-type Tsc2 and Tsc1 cDNAs were cloned into the pAc5.1/V5-HisB vector (Invitrogen). An N-terminal Polyoma (Pyo) or Myc epitope was added by PCR in place of the first Met codon of Tsc2 or Tsc1, respectively. All the missense mutations were introduced using the QuikChange site-directed mutagenesis kit (Stratagene), and the mutations were verified by sequencing. Wild-type Tsc2 and its variants were excised from the pAc5.1/V5-His vector and cloned downstream from the tubulin promoter (a gift from Jin Jiang, University of Texas Southwestern Medical Center, Dallas, TX) to generate the rescue constructs.
Cell transfection and immunoprecipitation
S2 cells were transfected using the Effectene transfection reagent (QIAGEN). Cells were washed by PBS and lysed with RIPA buffer (150 mM sodium chloride, 50 mM Tris-HCl at pH 8.0, 1% NP-40, 0.5% sodium-deoxycholate, 0.1% SDS, 1 mM PMSF) plus phosphatase and protease inhibitors cocktail. Tsc2 was immunoprecipitated with anti-Pyo or anti-Tsc2 antibody and protein G-Sepharose beads. The immunoprecipitate was washed four times with lysis buffer and resolved on 5% SDS-PAGE gels. For insulin treatment, S2 cells grown in serum-free media (SFM) were incubated with 10 μg/mL bovine insulin (Sigma) in SFM for 20 min. In our hands, this regime represented the most optimized condition to observe the mobility shift of Tsc2 upon phosphorylation in S2 cells. The phospho-S6K antibody against Thr 398 (corresponding to Thr 389 of human S6K) was from Cell Signaling Technology.
Drosophila genetics
All crosses were done at 25°C. Tsc2192 is a null allele that truncates Tsc2 at amino acid 919, thus deleting the C-terminal half of Tsc2 including the essential GAP domain (Ito and Rubin 1999). Transgenic fly strains containing the various Tsc2 rescue constructs on the second chromosome were first mated with Tsc2192 flies to generate flies of the genotype P/+; Tsc2192/TM6B, where P stands for the rescue construct insertion. These flies were then mated with Tsc2192/TM6B flies. Rescue frequency was derived by dividing the number of non-TM6B progeny from the cross by the number of expected progeny of this class, which equals half of the number of P/+; Tsc2192/TM6B siblings. Besides Tsc2192, we have also tested all the constructs described in this study (Table 1) using Tsc2193, another allele of Tsc2 that has an in-frame deletion (amino acids 472–527) (Ito and Rubin 1999). For each construct, identical results were obtained for both Tsc2 alleles.
To test the ability of constitutively active Akt to promote growth in the various Tsc2 mutant background, we used the eye-specific GMR-Gal4 to drive the expression of UAS-myr-Akt in flies of the following genotype: UAS-myr-Akt, P/GMR-Gal4; Tsc2192/Tsc2192, where P stands for the rescue construct insertions (Tsc2WT, Tsc2AA, or Tsc24A). The size of the compound eyes of these genotypes were compared under scanning electron microscope (SEM).
Data analysis
Wing size and cell size were measured on a Zeiss Axioplan microscope equipped with an AxioCam digital camera and analyzed using the Axiovision software. At least five wings from three independent experiments were analyzed for each genotype. At least 30 flies from four independent crosses were determined for body weight. Results were analyzed using GraphPad Prism software, and statistical significance was performed using a two-tailed, unpaired Student's t-test.
Acknowledgments
We thank Keith Wharton for critical reading of the manuscript. We thank Hugo Stocker and Ernst Hafen for the UAS-myr-Akt flies. D.J.P. is Virginia Murchison Linthicum Endowed Scholar in Medical Science. This work was supported by grants from the NIH, the American Heart Association, and the American Cancer Society.
Article published online ahead of print. Article and publication date are at http://www.genesdev.org/cgi/doi/10.1101/gad.1240504.
References
- Dan H.C., Sun, M., Yang, L., Feldman, R.I., Sui, X.M., Ou, C.C., Nellist, M., Yeung, R.S., Halley, D.J., Nicosia, S.V., et al. 2002. Phosphatidylinositol 3-kinase/Akt pathway regulates tuberous sclerosis tumor suppressor complex by phosphorylation of tuberin. J. Biol. Chem. 277: 35364-35370. [DOI] [PubMed] [Google Scholar]
- Dennis P.B., Pullen, N., Kozma, S.C., and Thomas, G. 1996. The principal rapamycin-sensitive p70(s6k) phosphorylation sites, T-229 and T-389, are differentially regulated by rapamycin-insensitive kinase kinases. Mol. Cell. Biol. 16: 6242-6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingar D.C. and Blenis, J. 2004. Target of rapamycin (TOR): An integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 23: 3151-3171. [DOI] [PubMed] [Google Scholar]
- Gao X., Zhang, Y., Arrazola, P., Hino, O., Kobayashi, T., Yeung, R.S., Ru, B., and Pan, D. 2002. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat. Cell Biol. 4: 699-704. [DOI] [PubMed] [Google Scholar]
- Garami A., Zwartkruis, F.J., Nobukuni, T., Joaquin, M., Roccio, M., Stocker, H., Kozma, S.C., Hafen, E., Bos, J.L., and Thomas, G. 2003. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol. Cell 11: 1457-1466. [DOI] [PubMed] [Google Scholar]
- Gingras A.C., Raught, B., and Sonenberg, N. 2001. Regulation of translation initiation by FRAP/mTOR. Genes & Dev. 15: 807-826. [DOI] [PubMed] [Google Scholar]
- Hafen E. 2004. Interplay between growth factor and nutrient signaling: Lessons from Drosophila TOR. Curr. Top. Microbiol. Immunol. 279: 153-167. [DOI] [PubMed] [Google Scholar]
- Hara K., Yonezawa, K., Weng, Q.P., Kozlowski, M.T., Belham, C., and Avruch, J. 1998. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J. Biol. Chem. 273: 14484-14494. [DOI] [PubMed] [Google Scholar]
- Inoki K., Li, Y., Zhu, T., Wu, J., and Guan, K.L. 2002. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4: 648-657. [DOI] [PubMed] [Google Scholar]
- Inoki K., Li, Y., Xu, T., and Guan, K.L. 2003. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes & Dev. 17: 1829-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito N. and Rubin, G.M. 1999. gigas, a Drosophila homolog of tuberous sclerosis gene product-2, regulates the cell cycle. Cell 96: 529-539. [DOI] [PubMed] [Google Scholar]
- Maheshwar M.M., Cheadle, J.P., Jones, A.C., Myring, J., Fryer, A.E., Harris, P.C., and Sampson, J.R. 1997. The GAP-related domain of tuberin, the product of the TSC2 gene, is a target for missense mutations in tuberous sclerosis. Hum. Mol. Genet. 6: 1991-1996. [DOI] [PubMed] [Google Scholar]
- Manning B.D., Tee, A.R., Logsdon, M.N., Blenis, J., and Cantley, L.C. 2002. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol. Cell 10: 151-162. [DOI] [PubMed] [Google Scholar]
- Nave B.T., Ouwens, M., Withers, D.J., Alessi, D.R., and Shepherd, P.R. 1999. Mammalian target of rapamycin is a direct target for protein kinase B: Identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem. J. 344 Pt 2: 427-431. [PMC free article] [PubMed] [Google Scholar]
- Nellist M., Verhaaf, B., Goedbloed, M.A., Reuser, A.J., Van Den Ouweland, A.M., and Halley, D.J. 2001. TSC2 missense mutations inhibit tuberin phosphorylation and prevent formation of the tuberin–hamartin complex. Hum. Mol. Genet. 10: 2889-2898. [DOI] [PubMed] [Google Scholar]
- Neufeld T.P. 2004. Genetic analysis of TOR signaling in Drosophila. Curr. Top. Microbiol. Immunol. 279: 139-152. [DOI] [PubMed] [Google Scholar]
- Oldham S., Montagne, J., Radimerski, T., Thomas, G., and Hafen, E. 2000. Genetic and biochemical characterization of dTOR, the Drosophila homolog of the target of rapamycin. Genes & Dev. 14: 2689-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter C.J., Pedraza, L.G., and Xu, T. 2002. Akt regulates growth by directly phosphorylating Tsc2. Nat. Cell Biol. 4: 658-665. [DOI] [PubMed] [Google Scholar]
- Prober D.A. and Edgar, B.A. 2002. Interactions between Ras1, dMyc, and dPI3K signaling in the developing Drosophila wing. Genes & Dev. 16: 2286-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radimerski T., Montagne, J., Hemmings-Mieszczak, M., and Thomas, G. 2002. Lethality of Drosophila lacking TSC tumor suppressor function rescued by reducing dS6K signaling. Genes & Dev. 16: 2627-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekulic A., Hudson, C.C., Homme, J.L., Yin, P., Otterness, D.M., Karnitz, L.M., and Abraham, R.T. 2000. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 60: 3504-3513. [PubMed] [Google Scholar]
- Shamji A.F., Nghiem, P., and Schreiber, S.L. 2003. Integration of growth factor and nutrient signaling: Implications for cancer biology. Mol. Cell 12: 271-280. [DOI] [PubMed] [Google Scholar]
- Stocker H., Andjelkovic, M., Oldham, S., Laffargue, M., Wymann, M.P., Hemmings, B.A., and Hafen, E. 2002. Living with lethal PIP3 levels: Viability of flies lacking PTEN restored by a PH domain mutation in Akt/PKB. Science 295: 2088-2091. [DOI] [PubMed] [Google Scholar]
- Tee A.R., Manning, B.D., Roux, P.P., Cantley, L.C., and Blenis, J. 2003. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr. Biol. 13: 1259-1268. [DOI] [PubMed] [Google Scholar]
- Zhang H., Stallock, J.P., Ng, J.C., Reinhard, C., and Neufeld, T.P. 2000. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes & Dev. 14: 2712-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Gao, X., Saucedo, L.J., Ru, B., Edgar, B.A., and Pan, D. 2003. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat. Cell Biol. 5: 578-581. [DOI] [PubMed] [Google Scholar]