Abstract
Neurotrophins, such as brain-derived neurotrophic factor (BDNF), are prominent regulators of neuronal survival, growth and differentiation during development. While trophic factors are viewed as well-understood but not innovative molecules, there are many lines of evidence indicating that BDNF plays an important role in the pathophysiology of many neurodegenerative disorders, depression, anxiety and other psychiatric disorders. In particular, lower levels of BDNF are associated with the aetiology of Alzheimer’s and Huntington’s diseases. A major challenge is to explain how neurotrophins are able to induce plasticity, improve learning and memory and prevent age-dependent cognitive decline through receptor signalling. This article will review the mechanism of action of neurotrophins and how BDNF/tropomyosin receptor kinase B (TrkB) receptor signaling can dictate trophic responses and change brain plasticity through activity-dependent stimulation. Alternative approaches for modulating BDNF/TrkB signalling to deliver relevant clinical outcomes in neurodegenerative and neuropsychiatric disorders will also be described.
Keywords: activity-dependent expression, deep brain stimulation, neuroprotection, neurotrophin, signalling, synaptic plasticity
INTRODUCTION
Neurotrophins are an essential family of secreted proteins for neuronal development. Nerve growth factor (NGF) was discovered more than 60 years ago, and it was found to be necessary for the survival and development of the peripheral nervous system [1–3]. Early studies determined that target sites in the periphery produce NGF [4], peripheral neurons express NGF receptors [5], and that NGF is retrogradely transported to the soma of these neurons to ensure survival during the period of naturally occurring cell death [6–8]. The discovery of NGF laid the groundwork for the identification of additional neurotrophic factors. Brain-derived neurotrophic factor (BDNF) was established as the main trophic factor in the central nervous system, where it is abundantly expressed and influences many aspects of neuronal function, such as neuronal growth, morphology, synaptic and structural plasticity [9]. The lack of BDNF in the central nervous system has widespread effects, such as aberrant neuronal morphology and synaptic function, reinforcing the notion that BDNF integrates different circuits and signalling pathways [10–13].
In this review, we will focus on tropomyosin receptor kinase B (TrkB) receptor signalling in the central nervous system and consider several mechanisms of action that might account for the diverse effects of BDNF on neuronal function and mammalian behaviour. We will summarize present evidence from studies describing the role of BDNF in the pathophysiology of neurodegenerative diseases and review recent clinical strategies for restoring BDNF signalling in disease.
BDNF AND TrkB RECEPTOR SIGNALLING
The neurotrophin family comprises of NGF, BDNF, neurotrophin-3 (NT-3) and neurotrophin-4 (NT-4) that have all evolved from a common neurotrophin ancestor gene. Their actions are dependent on binding to transmembrane receptor systems – the tropomyosin receptor tyrosine kinase family and the p75 neurotrophin receptor [14]. Neurotrophins have preferential binding for specific receptors: NGF binds to TrkA, BDNF and NT-4 to TrkB, and NT-3 to TrkC. However, there are a number of promiscuous interactions. All four neurotrophins can bind to the p75 receptor and the association of p75 with Trk receptors can regulate the affinity of Trk receptors for each respective neurotrophin, allowing for greater control of ligand–receptor interactions within this system [15–19]. NT-3’s cognate receptor is TrkC; however, NT-3 can also bind to TrkA and TrkB receptors. Still there is fidelity in neurotrophin; Trk interactions that are probably determined by many factors, such as local concentration, intracellular localization (axons compared with cell body), axonal transport, neuronal activity, half-lives, turnover and the form of the ligands [20]. Neurotrophins are synthesized as precursor proteins or pro-neurotrophins that undergo cleavage of the N-terminal portion (pro-domain) to produce the mature proteins. Pro-neurotrophins are released and have biological activity that involves regulating cell survival and growth cone dynamics [21–24]. Their pro-domains are important for protein folding and intracellular sorting of neurotrophins. Recent studies have implicated BDNF in the pathophysiology of psychiatric and neurodegenerative diseases with a potential mechanism likely due to a single nucleotide polymorphism caused by a valine (Val) to methionine (Met) base change at position 66 in the BDNF prodomain [25–28]. This modification results in a decrease in regulated BDNF secretion, leading to alterations in anxiety-related behaviour, learning and memory [29,30]. The endogenous BDNF prodomain is highly expressed and is secreted in an activity-dependent manner from hippocampal neurons similar to mature BDNF and proBDNF. Interestingly, treatment of hippocampal neurons with exogenous Met-66 prodomain (but not Val-66) leads to growth cone retraction through engagement of p75NTR and SorCS2 [31], which suggests a mechanism underlying the alterations in neural processing in humans with the V66M polymorphism. Trk receptor signalling is initiated by dimerization and autophosphorylation at specific tyrosine residues. After neurotrophin binding, activated Trk receptors recruit adaptor proteins such as Shc and FRS2 and other important tyrosine kinase substrates, including phosphoinositide 3-kinase (PI3K) and phospholipase C-γ (PLC-γ ). The key docking sites on Trk receptors are Tyr-490 (Tyr-496 in human TrkA) in the juxtamembrane region and Tyr-790 (Tyr-791 human TrkA) in the tail of the cytoplasmic domain. PLC-γ binds to Tyr-790 and this interaction has been proposed to facilitate interactions with ion channels, such as the VR1 capsaicin channel. Through residue Tyr-490, Shc or FRS2 become tyrosine phosphorylated and provide a scaffold for other signalling proteins that lead to the activation of the Ras/MAPK (mitogen-activated protein kinase) or the PI3K/Akt pathways. These phosphorylation events have many consequences. Analysis of deletions of the MAPK enzymes mimics a mouse model of autism [32] and prevents apoptosis during brain development [33]. The tyrosine phosphorylation of PLC-γ is closely associated with status epilepticus [34]. Further studies are required to explain how the activation of specific signalling cascades is related to circuit-level changes.
Trk RECEPTOR TRANSACTIVATION
Besides ligand-induced direct effects, activation of Trk receptors can also occur by other receptor systems. One particular mechanism studied in recent years is that of transactivation of Trk receptors by G-protein-coupled receptors (GPCRs). Adenosine is a neuromodulator that leads to TrkA receptor autophosphorylation in hippocampal cells and PC12-TrkA cells within 1–2 h of treatment [35]. This effect does not occur due to production of neurotrophins, but it is dependent on activation of the adenosine A2A receptors and sustained PI3K/Akt signalling downstream of TrkA. Pituitary adenylate cyclase-activating polypeptide (PACAP) can also transactivate Trk receptor with a time course and mechanism similar to adenosine [36]. Transactivation elicited by these GPCR ligands leads to neuroprotective effects, such as increased survival of PACAP-treated basal forebrain cholinergic neurons after axotomy [37], providing an alternative method for increasing neurotrophin signalling in neurodegenerative diseases.
Other ligands can transactivate Trk receptors such as epidermal growth factor (EGF) [38], glucocorticoids [39], dopamine [40] and zinc [41]. Remarkably, EGF signalling activates TrkB and TrkC in mouse embryonal precursor cells, providing an essential mechanism for regulating their migration into the developing cortex [38]. In addition, the interplay between glucocorticoid and BDNF signalling is important for eliciting neuroprotection [39], modulating target gene expression [42,43] and promoting neuronal plasticity in response to stress [44].
Depending upon the circumstances, GPCR ligands, such as adenosine and PACAP, may be neuroprotective against injury initiated by ischaemia, hypoxia or vascular damage. GPCR signalling through Trk neurotrophin receptors leads to selective activation of the PI3K/Akt pathway over a prolonged time course. Intracellular signalling interactions between adenosine and Trk receptors therefore provide a new avenue for developing new approaches to address neurological disorders. Small molecules like adenosine may be used to target populations of neurons that express both adenosine and Trk receptors and therefore be considered as potential treatments for a wide number of nervous system disorders, including cerebral ischaemia, amyotrophic lateral sclerosis (ALS), Parkinson’s disease (PD) and other neurodegenerative and neuropsychiatric conditions.
The strategies to apply neurotrophic factors in human neurological diseases are based on an assumption of symptomatic treatment of injured or deprived neurons. This treatment implies not only cell survival, but also restoration of proper synaptic functioning of vulnerable neurons. As the signal transduction pathways that are activated by BDNF have become better understood, novel strategies will be devised to manipulate these pathways through transactivation and the development of new drugs. In addition, further understanding of the core pathophysiological mechanism for neurodegenerative and psychiatric disorders will eventually assist in the development of rational therapies that engage the neurotrophin signalling.
BDNF AFFECTS ION CHANNELS AND PLASTICITY
Many interactions exist between TrkB receptors and ion channels. BDNF has an effect upon Kv1.3 currents and can block α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor currents. The catalytic activity of TrkB is required for the decrease in AMPA receptor activity, implying that there is an association between TrkB and AMPA receptors. In the hippocampus, TrkB receptors are expressed both pre- and post-synaptically to enhance long-term potentiation (LTP) [45]. The tyrosine kinase activity of TrkB is involved in activity-dependent changes in synaptic efficacy and may serve as a synaptic tag [46].
Application of BDNF to cultured neurons results in rapid increases in the frequency of spontaneous action potentials and excitatory synaptic activities [9]. Increased phosphorylation of N -methyl-D-aspartate (NMDA) and potassium channels occurs as a result of BDNF engagement of TrkB, resulting in downstream tyrosine kinase signalling [47] and interactions with postsynaptic density 95 (PSD95) [48]. The acute effects of BDNF frequently depend on developmental stage and recruitment of different signalling molecules to the TrkB receptor [49]. For example, signalling by BDNF increases clustering of postsynaptic ion channels, including γ -aminobutyric acid A (GABAA) and NMDA receptors in the hippocampus [50]. Many effects of BDNF on synaptic structure and connectivity are governed by neuronal activity [51].
In addition to phosphorylation of TrkB, there are several mechanisms that can account for BDNF’s effects upon synaptic plasticity. One important regulatory mechanism is the processing of neurotrophins. The unprocessed form of BDNF, proBDNF, can have opposite effects compared with the mature form. Cleavage of proBDNF to mature BDNF is crucial for the late form of LTP in the hippocampus [52]. Trafficking of ion channels and synaptic proteins also represents a prominent mechanism for changing the activity of synapses. For instance, a reduction in the number of surface NMDA receptors can occur by endocytosis of NR2B subunits through dephosphorylation and other posttranslational modifications [53]. Endocytosis of NMDA receptors may also involves internalization of GluR2-containing AMPA receptors. Indeed, expression of a mutant GluR2 that underwent increased endocytosis led to a reduction in synaptic responses mediated by AMPA and NMDA receptors. One hypothesis that might link the increase in endocytosis of NMDA and AMPA receptors is that AMPA receptors can stabilize synaptic spines and dendritic morphology. Thus, removal of AMPA receptors together with synaptic NMDA receptors can lead to spine elimination. The decrease in numbers of surface AMPA and NMDA receptors is critical. These key events integrate several signalling pathways that can be affected by BDNF signalling. For example, BDNF can rapidly induce phosphorylation of postsynaptic NR1 and NR2B subunits, providing an important mechanism for modulating synaptic plasticity [54,55].
BDNF IN PATHOPHYSIOLOGY OF DISEASES AND ITS THERAPEUTIC POTENTIAL
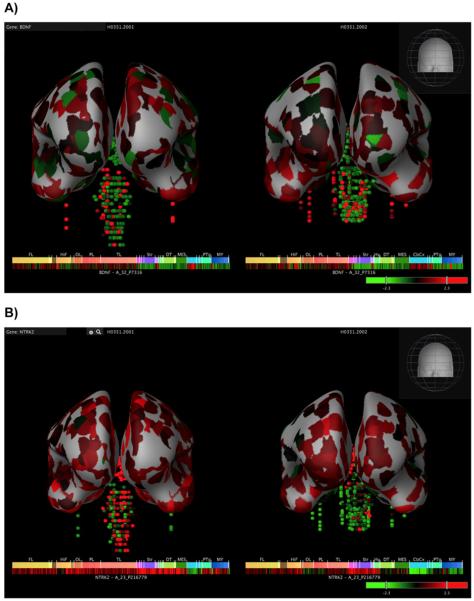
Neurotrophic factors have been implicated in the neuropathology of a wide variety of neurodegenerative and psychiatric disorders and have been considered as a therapeutic strategy for neuropsychiatric disorders. Human gene expression mapping has expanded the knowledge of the anatomical distribution of the BDNF and NTRK2 (TrkB) gene [56] (Figures 1A and 1B). The finding that neurotrophic factors modulate neuronal survival and axonal growth was a rationale for developing therapeutic approaches for neurodegenerative disorders such as ALS and spinal cord injury. Clinical trials 20 years ago were met with disappointing results, in part due to difficulties of delivery and unanticipated side effects. Indeed, neurotrophins are large, sticky proteins that do not diffuse well into tissues and do not cross the blood–brain barrier. The problems in managing the dose and pharmacokinetics of these proteins have hindered the application of neurotrophic factors as a therapeutic intervention for many neurodegenerative diseases. The hypothesis underlying clinical approaches, as well as development of therapeutic strategies using neurotrophic factors assumes that these disease states result in (i) decreased availability of neurotrophins; (ii) a decrease in the number of neurotrophin receptors on affected neurons; or (iii) decreased neuronal survival due to programmed cell death, injury, axotomy or inflammation. These deficits can be ameliorated by the addition of trophic factors. In all of these conditions, the assumption has been that exogenous neurotrophic factors would provide symptomatic treatment for the disease state, rather than a cure for these nervous system disorders.
Figure 1. Human BDNF expression.
(A) BDNF levels shown in human donors from Allen Institute for Brain Science, Allen Human Brain Atlas [Internet]. Available from: http://human.brain-map.org. BDNF gene assessed by DNA probe A_32_P7316. (B) Neurotrophic tyrosine kinase, receptor, type 2 (NTRK2) levels shown in human donors from Allen Institute for Brain Science, Allen Human Brain Atlas [Internet]. Available from: http://human.brain-map.org. Assessed by DNA probe A_23_P216 779 in the same two donors as (A). Heatmap color represents the z-score ranging from green (low expression) through red (high expression).
Before neurotrophic factors can be considered for further clinical application, a number of hurdles must be overcome, including their limited diffusion, short half-lives and the poor pharmacokinetics. The low penetrability of the blood–brain barrier towards proteins of the size of neurotrophic factors has hindered progress towards a therapeutic strategy. In this regard, it must be established that the trophic factors reach the target neurons in sufficient amounts. Another challenge is to regulate the amounts of trophic factor, as high concentrations of BDNF can cause the down-regulation of TrkB receptors, as has been reported in motor neuron clinical trials [57]. It is possible that a large number of diverse side effects accompany the use of BDNF and ciliary neurotrophic factor (CNTF) at high doses, such as fever, fatigue, weight loss, paraesthesias and diarrhoea. Besides direct delivery by minipump, a number of approaches, including cell grafts and viral delivery using adeno-associated or lentiviruses in non-toxic systems have been developed to allow for transduction in a cell-specific and inducible manner. Hence, numerous methodological issues must be addressed before trophic factors are applied in a safe and efficacious manner to provide an appropriate amount in the correct target.
ALZHEIMER’S DISEASE
A link to Alzheimer disease (AD) was made in the 1980s based on studies on aged animals in which lesioned cholinergic neurons in the basal forebrain could be rescued with intracerebroventricular NGF [58]. Treatment with NGF led to concomitant improvements in memory function. Neurotrophins modulates long-term potentiation, dendritic complexity and axon branching, processes that promote synaptic efficacy and thus learning and memory. Consequently, levels of neurotrophins have an effect on the progression neurodegenerative disorders. Low levels of neurotrophins have been observed in various neurological and neurodegenerative disorders [59–61]. BDNF is reduced in the cortex and the Meynert nucleus basalis, which are major sources of inputs that innervate cholinergic neurons, a neuronal cell type that is selectively vulnerable to degeneration in AD [62]. In patients with AD, low BDNF levels have also been reported in the dentate gyrus and in neurons that have neurofibrillary tangles, a hallmark of AD [63,64]. In a well-studied mouse model of AD (Tg2576), mature BDNF levels were decreased and proBDNF levels were increased [65]. Low levels of BDNF have also been linked to the pathological and behavioural deficits that are associated with neurodegeneration. More importantly, several lines of evidence suggest that BDNF can have therapeutic benefits in AD. Genetic delivery of BDNF in primate and rodent models of AD increased levels of BDNF in the entorhinal cortex and improved synaptic loss as well as learning and memory, thus highlighting the important roles of BDNF in neurodegenerative disorders [66]. The effect of BDNF on reversing symptomatic effects of neurodegeneration appear to be independent of Aβ clearance as levels of Aβ remain unchanged upon BDNF therapy [66,67]. Thus BDNF could be exerting its effects by modulating functional synapses through promoting synapse formation and repair.
A lack of BDNF in central nervous system (CNS) neurons also results in changes in expression of genes that have been implicated in AD pathogenesis. Withdrawal of BDNF in cultured hippocampal neurons results in a substantial decrease in genes involved in synaptic function, vesicular trafficking, endosomal function and MAPK signalling [68]. These changes correlate with previously reported decreases in gene expression in AD and aging where levels of BDNF are significantly reduced [69]. A comparison of the gene classes that change in AD and in BDNF-deprived hippocampal neurons highlight similarities in genes involved in vesicular trafficking and synaptic function [68,69]. Genes involved in synaptic vesicle trafficking and neurotransmission are also largely down-regulated in CA1 pyramidal neurons of post-mortem AD patients. Remarkably, TrkB mRNA in the mild cognitive impairment and AD cohorts decreases to less than half of control subjects with no cognitive impairment [70]. Indeed, lower levels of a major Trk scaffold protein, ARMS/Kidins220, results in age-dependent degeneration in the entorhinal cortex, an area that is selectively vulnerable in AD [71]. BDNF through TrkB is responsible for the tyrosine phosphorylation of ARMS/Kidins220. Thus, therapeutic interventions that target promoting BDNF signalling can have a significant impact on slowing disease progression. Raising the levels of BDNF in AD patients is likely to result in additional Trk receptor signalling, leading to increased survival or neurotransmission of cholinergic neurons.
An alternative approach that can increase BDNF signalling is through neural stem cell transplantation. Hippocampal neural stem cell transplantation can rescue the learning and memory deficits in AD mice by inducing a BDNF-dependent increase in synaptic density (without changes to tau pathology) [67]. Similarly, a recent study showed that striatal transplantation of neural stem cells into a mouse model of dementia with Lewy bodies improves motor and cognitive function by restoring BDNF levels. In this study, transplanting BDNF-depleted neural stem cells did not improve behaviour, whereas BDNF delivery via bilateral injection of adeno-associated virus mimicked the benefits of BDNF-expressing stem cells [72].
PARKINSON’S DISEASE
Various reports indicate that BDNF may also be important in the pathogenesis of PD. Reduced expression of BDNF mRNA has been reported in the substantia nigra pars compacta, a region that is selectively vulnerable to substantial neuronal loss in PD [62,73]. There are also isoform specific alterations in TrkB expression in PD. Levels of truncated TrkB are decreased in striatal axons, and increased in striatal soma and in substantia nigra pars compacta distal dendrites. In comparison, full-length TrkB is decreased in striatal neurites and substantia nigra pars compacta soma and dendrites, whereas it is increased in striatal somata and in substantia nigra pars compacta axons [74]. The localization of TrkB receptors is significant, since the signalling pathways can be enhanced through MAPK and PI3K/Akt enzymatic activities [75].
Experimental evidence supports the role of BDNF in promoting survival of dopaminergic neurons in the substantia nigra [76]. In addition, reduced BDNF production is closely associated with pathogenic mutations in α-synuclein in familial PD [77,78]. Targeted BDNF deletion studies leads to lower BDNF levels in PD mouse models, resulting in loss of dopaminergic neurons in the substantia nigra and reduction in striatal dopamine output. These symptomatic features parallel clinical manifestations of PD in humans [79–81]. Thus, BDNF can prevent loss of dopaminergic neurons and repair impaired synapses in PD.
HUNTINGTON’S DISEASE
BDNF also plays a crucial role in the pathogenesis of Huntington’s disease (HD). Striatal medium spiny neurons are prone to degeneration in HD and depend on BDNF for their survival through TrkB signalling [82]. BDNF produced in cortical neurons and anterogradely transported to the striatum supports survival of medium spiny neurons [83]. The huntingtin protein is thought to play a role in the transport and activity-dependent release of BDNF and mutant huntingtin can interfere with the transport and release of BDNF to the striatum [84,85]. Thus, mutations in the huntingtin protein greatly affect BDNF levels in striatal neurons. This is evident in studies that reported low expression of BDNF in mouse models of HD and in post-mortem human HD brains [85–87]. Furthermore, genetic manipulation of BDNF in cortical neurons leads to morphological and behavioural deficits that are similar to symptomatic features of HD in mice, which highlights the protective role of BDNF on striatal neurons [80,88]. Measurement of BDNF in a mouse model of HD indicated an increase in both BDNF and proBDNF protein [89]. Also, evidence from transcriptional profiling studies demonstrates a close association of human HD with molecular and phenotypic correlates of BDNF depletion in the mouse cortex [88]. Thus, these findings strongly link striatum-specific atrophy in HD to decreased cortical BDNF by mutant huntingtin.
AMYOTROPHIC LATERAL SCLEROSIS
The progressive loss of motor neuron function that manifests as muscle atrophy, weakness and spasticity in ALS can be prevented by neurotrophin-based therapeutic approaches. BDNF has been reported to slow progression of motor neuron atrophy in an animal model of ALS [90]. Moreover, the TrkB agonist 7,8-dihydroxyflavone (7,8-DHF) improved motor neuron deficits in the superoxide dismutase 1 (SOD1) (G93A) ALS mouse model [91], although efforts to replicate the positive effects of 7,8-DHF upon TrkB activity have not been successful [92]. An alternative approach is to use agonist monoclonal antibodies for TrkB [92]. The truncated form of TrkB, which is found on motor neurons and glia, as well as other non-neuronal cells [93], is involved in disease onset in an ALS mouse model [94]. Despite the therapeutic potential of BDNF, previous clinical trials in ALS patients have failed due to difficulties in administered BDNF to reach degenerating neurons [95]. Strategies to modulate BDNF signalling and thus motor neuron function can circumvent these challenges.
DEPRESSION
Recent clinical studies have also demonstrated an association between low levels of BDNF and depressive disorders. BDNF infusion in the mouse midbrain produces anti-depressive like effects in behavioural models of depression [96,97]. Furthermore, reduction in BDNF mRNA in the hippocampus in response to forced swim test in animal models of depression further emphasizes the importance of BDNF in the therapeutic response to antidepressant treatment [98]. BDNF signalling has been proposed to be a downstream target of many antidepressant treatments [99–101]. Consistent with this idea, heterozygous BDNF-knockout mice and TrkB mutant mice are resistant to antidepressant treatment while undergoing the forced swim test [102], suggesting that antidepressants employ their effects through modulating levels of BDNF, as well as signalling through TrkB.
SCHIZOPHRENIA
Recent human post-mortem studies have revealed altered BDNF and TrkB expression in cases of schizophrenia across several brain regions thus implicating neurotrophin signalling in the aetiology of schizophrenia. In particular, different studies have found that BDNF mRNA and protein levels were decreased in the hippocampus, prefrontal cortex, anterior cingulate cortex and superior temporal gyrus of schizophrenia patients compared with controls [103–108]. TrkB and TrkC mRNA was also reduced in the dorsolateral prefrontal cortex of schizophrenia cases [109]. In addition, the interaction of BDNF and TrkB gene single nucleotide polymorphisms could confer susceptibility to paranoid schizophrenia in the Chinese Han population [110]. To elucidate the impact of neurotrophins in the aetiology of schizophrenia, a recent whole exome sequencing study of 48 schizophrenia-related psychosis cases revealed several missense polymorphisms and novel mutations in neurotrophin genes [111]. Remarkably, the majority of the rare genetic variants discovered were in the NGF–NTRK1–ARMS/Kidins220–TRIO pathway, which can regulate neurotrophin signalling and synaptic transmission [112,113]. This mounting evidence implicates neurotrophin signalling in the pathophysiology of schizophrenia and requires further biochemical studies to identify the specific mechanisms and contributors.
CLINICAL APPROACHES FOR INCREASING BDNF LEVELS IN VIVO: ELECTROCONVULSIVE THERAPY
Considerable attention has been given to the use of electroconvulsive therapy (ECT) in major depression [114] and PD [115]. In a few cases of AD, ECT has been applied in treatment of AD-related severe agitation. ECT improved severe agitation without compromising cognitive function in an early-onset AD patient [116] highlighting the use of ECT as a treatment modality for behavioural symptoms of AD. Efforts to use deep brain stimulation have been extended to animal models of Rett syndrome, which resulted in a rescue of cognitive function [117]. ECT has been used as a therapy for several mood disorders, although the mechanism by which it relieves depressive symptoms is unknown.
One plausible mechanism for the positive effects of deep brain stimulation is increased secretion of trophic factors due to enhanced neuronal activity. During intense activity, such as seizure, there is a dramatic increase in mRNAs encoding NGF and BDNF in the dentate gyrus, CA1 and CA3 hippocampal regions, as well as immediate early genes. These results indicated that activity-dependent regulation of BDNF occurs and is in keeping with other physiological stimuli, such as depolarization, neurotransmitters, light, hormones and exercise that also influence the expression and levels of trophic factors. With regard to physical exercise, BDNF gene transcription can be enhanced through metabolites, such as β-hydroxybutyrate, which is produced in the liver and travels through the bloodstream into the brain where it inhibits histone deacetylases [118].
Studies have shown that ECT treatment in rodents increases the expression of hippocampal and amygdala BDNF mRNA [119], as well as BDNF protein levels. This response is consistent with the evidence that links neurotrophins to neuronal plasticity. Neurotrophins, particularly BDNF, increase neurotransmitter release from neurons during activity, which result in the reinforcement and stabilization of synaptic connections and networks [9]. An axiom of neurotrophin responsiveness is that activity-dependent changes in the nervous system occur frequently to change the levels of BDNF. The suggestion from ECT treatment studies is that other physiological and environmental stimuli, such as neuronal activity, novel stimuli and physical activity have an impact on the levels of trophic factors [120].
CONCLUDING REMARKS
The therapeutic application of neurotrophins has been beset with a number of obstacles, but in the future it is probable that these problems will be overcome. Higher-order functions, such as the circuits involved in pain, anxiety, depression, obesity and other maladaptive behaviours can be modulated by changing the levels of NGF and BDNF. BDNF regulates the formation and maintenance of neuronal networks associated with psychiatric disorders and TrkB has been genetically associated with gamma oscillations in the brain [121]. In addition, BDNF provides trophic support and increases synaptogenesis, dendritic and axonal branching through TrkB signalling. Decreased levels of BDNF are associated with depression and become enhanced following anti-depressant treatment. In addition, lower serum BDNF levels have been related to hippocampal volume decrease and memory decline in adulthood, whereas higher serum BDNF levels have been found to be protective against developing dementia [122,123]. New cell-based methods with BDNF gene delivery are successful in preventing cochlear spiral ganglion neuron degeneration and deafness [124]. Increased neuronal activity or physical exercise can lower the risk of these conditions through increases in trophic factors. Because neurotrophin signalling is germane for many neurodegenerative and psychiatric disorders, a promising approach will be to increase the levels of neurotrophins or signalling through Trk receptors via transactivation. These insights may provide new therapies to treat psychiatric disorders, such as depression, and neurodegenerative diseases, such as PD and AD.
Acknowledgments
FUNDING
This work was supported by the National Institutes of Health [grant numbers NS21072, AG025970 and MH025970].
Abbreviations
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- AMPA
α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
- BDNF
brain-derived neurotrophic factor
- 7,8-DHF
7,8-dihydroxyflavone
- ECT
electroconvulsive therapy
- EGF
epidermal growth factor
- GPCR
G-protein-coupled receptor
- HD
Huntington’s disease
- LTP
long-term potentiation
- MAPK
mitogen-activated protein kinase
- NGF
nerve growth factor
- NMDA
N-methyl-D-aspartate
- NT-3
neurotrophin-3
- NT-4
neurotrophin-4
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PD
Parkinson’s disease
- PI3K
phosphoinositide 3-kinase
- PLC-γ
phospholipase C-γ
- Trk
tropomyosin receptor kinase
REFERENCES
- 1.Levi-Montalcini R. Growth control of nerve cells by a protein factor and its antiserum. Science. 1964;143:105–110. doi: 10.1126/science.143.3602.105. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 2.Levi-Montalcini R, Booker B. Destruction of the sympathetic ganglia in mammals by an antiserum to a nerve-growth protein. Proc. Natl. Acad. Sci. U.S.A. 1960;46:384–391. doi: 10.1073/pnas.46.3.384. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levi-Montalcini R, Angeletti PU. Essential role of the nerve growth factor in the survival and maintenance of dissociated sensory and sympathetic embryonic nerve cells in vitro. Dev. Biol. 1963;6:653–659. doi: 10.1016/0012-1606(63)90149-0. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 4.Davies AM, Bandtlow C, Heumann R, Korsching S, Rohrer H, Thoenen H. Timing and site of nerve growth factor synthesis in developing skin in relation to innervation and expression of the receptor. Nature. 1987;326:353–358. doi: 10.1038/326353a0. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 5.Sutter A, Riopelle RJ, Harris-Warrick RM, Shooter EM. Nerve growth factor receptors. Characterization of two distinct classes of binding sites on chick embryo sensory ganglia cells. J. Biol. Chem. 1979;254:5972–5982. PubMed. [PubMed] [Google Scholar]
- 6.Dumas M, Schwab ME, Thoenen H. Retrograde axonal transport of specific macromolecules as a tool for characterizing nerve terminal membranes. J. Neurobiol. 1979;10:179–197. doi: 10.1002/neu.480100207. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 7.Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 8.Ginty DD, Segal RA. Retrograde neurotrophin signaling: Trk-ing along the axon. Curr. Opin. Neurobiol. 2002;12:268–274. doi: 10.1016/s0959-4388(02)00326-4. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 9.Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 10.Cohen-Cory S, Fraser SE. Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature. 1995;378:192–196. doi: 10.1038/378192a0. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 11.Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. U.S.A. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, et al. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J. Neurosci. 1999;19:4972–4983. doi: 10.1523/JNEUROSCI.19-12-04972.1999. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeanneteau F, Deinhardt K, Miyoshi G, Bennett AM, Chao MV. The MAP kinase phosphatase MKP-1 regulates BDNF-induced axon branching. Nat. Neurosci. 2010;13:1373–1379. doi: 10.1038/nn.2655. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chao MV, Hempstead BL. p75 and Trk: a two-receptor system. Trends Neurosci. 1995;18:321–326. CrossRefPubMed. [PubMed] [Google Scholar]
- 15.Hempstead BL, Martin-Zanca D, Kaplan DR, Parada LF, Chao MV. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991;350:678–683. doi: 10.1038/350678a0. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 16.Squinto SP, Stitt TN, Aldrich TH, Davis S, Bianco SM, Radziejewski C, et al. trkB encodes a functional receptor for brain-derived neurotrophic factor and neurotrophin-3 but not nerve growth factor. Cell. 1991;65:885–893. doi: 10.1016/0092-8674(91)90395-F. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 17.Benedetti M, Levi A, Chao MV. Differential expression of nerve growth factor receptors leads to altered binding affinity and neurotrophin responsiveness. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7859–7863. doi: 10.1073/pnas.90.16.7859. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bibel M, Hoppe E, Barde YA. Biochemical and functional interactions between the neurotrophin receptors trk and p75NTR. EMBO J. 1999;18:616–622. doi: 10.1093/emboj/18.3.616. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esposito D, Patel P, Stephens RM, Perez P, Chao MV, Kaplan DR, et al. The cytoplasmic and transmembrane domains of the p75 and Trk A receptors regulate high affinity binding to nerve growth factor. J. Biol. Chem. 2001;276:32687–32695. doi: 10.1074/jbc.M011674200. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 20.Yano H, Torkin R, Martin LA, Chao MV, Teng KK. Proneurotrophin-3 is a neuronal apoptotic ligand: evidence for retrograde-directed cell killing. J. Neurosci. 2009;29:14790–14802. doi: 10.1523/JNEUROSCI.2059-09.2009. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deinhardt K, Kim T, Spellman DS, Mains RE, Eipper BA, Neubert TA, et al. Neuronal growth cone retraction relies on proneurotrophin receptor signaling through Rac. Sci. Signal. 2011;4:ra82. doi: 10.1126/scisignal.2002060. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J. Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 24.Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 25.Ventriglia M, Bocchio Chiavetto L, Benussi L, Binetti G, Zanetti O, Riva MA, et al. Association between the BDNF 196 A/G polymorphism and sporadic Alzheimer’s disease. Mol. Psychiatry. 2002;7:136–7. doi: 10.1038/sj.mp.4000952. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 26.Sklar P, Gabriel SB, McInnis MG, Bennett P, Lim Y, Tsan G, et al. Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Mol. Psychiatry. 2002;7:579–93. doi: 10.1038/sj.mp.4001058. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 27.Neves-Pereira M, Mundo E, Muglia P, King N, Macciardi F, Kennedy JL. The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: evidence from a family-based association study. Am. J. Hum. Genet. 2002;71:651–655. doi: 10.1086/342288. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu E, Hashimoto K, Iyo M. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: the possibility to explain ethnic mental traits. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2004;126B:122–123. doi: 10.1002/ajmg.b.20118. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 29.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 30.Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anastasia A, Deinhardt K, Chao MV, Will NE, Irmady K, Lee FS, et al. Val66Met polymorphism of BDNF alters prodomain structure to induce neuronal growth cone retraction. Nat. Commun. 2013;4:2490. doi: 10.1038/ncomms3490. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pucilowska J, Vithayathil J, Tavares EJ, Kelly C, Karlo JC, Landreth GE. The 16p11.2 deletion mouse model of autism exhibits altered cortical progenitor proliferation and brain cytoarchitecture linked to the ERK MAPK pathway. J. Neurosci. 2015;35:3190–3200. doi: 10.1523/JNEUROSCI.4864-13.2015. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia Y, Wang CZ, Liu J, Anastasio NC, Johnson KM. Brain-derived neurotrophic factor prevents phencyclidine-induced apoptosis in developing brain by parallel activation of both the ERK and PI-3K/Akt pathways. Neuropharmacology. 2010;58:330–336. doi: 10.1016/j.neuropharm.2009.10.009. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu B, Huang YZ, He XP, Joshi RB, Jang W, McNamara JO. A peptide uncoupling BDNF receptor TrkB from phospholipase Cgamma1 prevents epilepsy induced by status Epilepticus. Neuron. 2015;88:484–491. doi: 10.1016/j.neuron.2015.09.032. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee FS, Chao MV. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3555–3560. doi: 10.1073/pnas.061020198. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee FS, Rajagopal R, Kim AH, Chang PC, Chao MV. Activation of Trk neurotrophin receptor signaling by pituitary adenylate cyclase-activating polypeptides. J. Biol. Chem. 2002;277:9096–9102. doi: 10.1074/jbc.M107421200. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 37.Takei N, Torres E, Yuhara A, Jongsma H, Otto C, Korhonen L, et al. Pituitary adenylate cyclase-activating polypeptide promotes the survival of basal forebrain cholinergic neurons in vitro and in vivo: comparison with effects of nerve growth factor. Eur. J. Neurosci. 2000;12:2273–2280. doi: 10.1046/j.1460-9568.2000.00118.x. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 38.Puehringer D, Orel N, Luningschror P, Subramanian N, Herrmann T, Chao MV, et al. EGF transactivation of Trk receptors regulates the migration of newborn cortical neurons. Nat. Neurosci. 2013;16:407–415. doi: 10.1038/nn.3333. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeanneteau F, Garabedian MJ, Chao MV. Activation of Trk neurotrophin receptors by glucocorticoids provides a neuroprotective effect. Proc. Natl. Acad. Sci. U.S.A. 2008;105:4862–4867. doi: 10.1073/pnas.0709102105. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwakura Y, Nawa H, Sora I, Chao MV. Dopamine D1 receptor-induced signaling through TrkB receptors in striatal neurons. J. Biol. Chem. 2008;283:15799–15806. doi: 10.1074/jbc.M801553200. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang YZ, Pan E, Xiong ZQ, McNamara JO. Zinc-mediated transactivation of TrkB potentiates the hippocampal mossy fiber-CA3 pyramid synapse. Neuron. 2008;57:546–558. doi: 10.1016/j.neuron.2007.11.026. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 42.Jeanneteau FD, Lambert WM, Ismaili N, Bath KG, Lee FS, Garabedian MJ, et al. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proc. Natl. Acad. Sci. U.S.A. 2012;109:1305–1310. doi: 10.1073/pnas.1114122109. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lambert WM, Xu CF, Neubert TA, Chao MV, Garabedian MJ, Jeanneteau FD. Brain-derived neurotrophic factor signaling rewrites the glucocorticoid transcriptome via glucocorticoid receptor phosphorylation. Mol. Cell. Biol. 2013;33:3700–3714. doi: 10.1128/MCB.00150-13. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arango-Lievano M, Lambert WM, Bath KG, Garabedian MJ, Chao MV, Jeanneteau F. Neurotrophic-priming of glucocorticoid receptor signaling is essential for neuronal plasticity to stress and antidepressant treatment. Proc. Natl. Acad. Sci. U.S.A. 2015;112:15737–15742. doi: 10.1073/pnas.1509045112. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gartner A, Polnau DG, Staiger V, Sciarretta C, Minichiello L, Thoenen H, et al. Hippocampal long-term potentiation is supported by presynaptic and postsynaptic tyrosine receptor kinase B-mediated phospholipase Cgamma signaling. J. Neurosci. 2006;26:3496–3504. doi: 10.1523/JNEUROSCI.3792-05.2006. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu Y, Ji Y, Ganesan S, Schloesser R, Martinowich K, Sun M, et al. TrkB as a potential synaptic and behavioral tag. J. Neurosci. 2011;31:11762–11771. doi: 10.1523/JNEUROSCI.2707-11.2011. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slack SE, Pezet S, McMahon SB, Thompson SW, Malcangio M. Brain-derived neurotrophic factor induces NMDA receptor subunit one phosphorylation via ERK and PKC in the rat spinal cord. Eur. J. Neurosci. 2004;20:1769–1778. doi: 10.1111/j.1460-9568.2004.03656.x. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 48.Cao C, Rioult-Pedotti MS, Migani P, Yu CJ, Tiwari R, Parang K, et al. Impairment of TrkB-PSD-95 signaling in Angelman syndrome. PLoS Biol. 2013;11:e1001478. doi: 10.1371/journal.pbio.1001478. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki K, Sato M, Morishima Y, Nakanishi S. Neuronal depolarization controls brain-derived neurotrophic factor-induced upregulation of NR2C NMDA receptor via calcineurin signaling. J. Neurosci. 2005;25:9535–9543. doi: 10.1523/JNEUROSCI.2191-05.2005. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elmariah SB, Crumling MA, Parsons TD, Balice-Gordon RJ. Postsynaptic TrkB-mediated signaling modulates excitatory and inhibitory neurotransmitter receptor clustering at hippocampal synapses. J. Neurosci. 2004;24:2380–2393. doi: 10.1523/JNEUROSCI.4112-03.2004. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mariga A, Glaser J, Mathias L, Xu D, Xiao M, Worley P, et al. Definition of a bidirectional activity-dependent pathway involving BDNF and Narp. Cell Rep. 2015;13:1–10. doi: 10.1016/j.celrep.2015.10.064. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 53.Lussier MP, Sanz-Clemente A, Roche KW. Dynamic regulation of N-methyl-D-aspartate (NMDA) and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by posttranslational modifications. J. Biol. Chem. 2015;290:28596–28603. doi: 10.1074/jbc.R115.652750. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin SY, Wu K, Levine ES, Mount HT, Suen PC, Black IB. BDNF acutely increases tyrosine phosphorylation of the NMDA receptor subunit 2B in cortical and hippocampal postsynaptic densities. Brain Res. Mol. Brain Res. 1998;55:20–27. doi: 10.1016/s0169-328x(97)00349-5. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 55.Suen PC, Wu K, Levine ES, Mount HT, Xu JL, Lin SY, et al. Brain-derived neurotrophic factor rapidly enhances phosphorylation of the postsynaptic N-methyl-D-aspartate receptor subunit 1. Proc. Natl. Acad. Sci. U.S.A. 1997;94:8191–8195. doi: 10.1073/pnas.94.15.8191. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399. doi: 10.1038/nature11405. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thoenen H, Sendtner M. Neurotrophins: from enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nat. Neurosci. 2002;5(Suppl.):1046–1050. doi: 10.1038/nn938. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 58.Williams LR, Varon S, Peterson GM, Wictorin K, Fischer W, Bjorklund A, et al. Continuous infusion of nerve growth factor prevents basal forebrain neuronal death after fimbria fornix transection. Proc. Natl. Acad. Sci. U.S.A. 1986;83:9231–9235. doi: 10.1073/pnas.83.23.9231. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Durany N, Michel T, Kurt J, Cruz-Sanchez FF, Cervas-Navarro J, Riederer P. Brain-derived neurotrophic factor and neurotrophin-3 levels in Alzheimer’s disease brains. Int. J. Dev. Neurosci. 2000;18:807–13. CrossRef. [PubMed] [Google Scholar]
- 60.Hock C, Heese K, Hulette C, Rosenberg C, Otten U. Region-specific neurotrophin imbalances in Alzheimer disease: decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch. Neurol. 2000;57:846–851. doi: 10.1001/archneur.57.6.846. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 61.Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 62.Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Prog. Neurobiol. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 63.Narisawa-Saito M, Wakabayashi K, Tsuji S, Takahashi H, Nawa H. Regional specificity of alterations in NGF, BDNF and NT-3 levels in Alzheimer’s disease. Neuroreport. 1996;7:2925–2928. doi: 10.1097/00001756-199611250-00024. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 64.Murer MG, Boissiere F, Yan Q, Hunot S, Villares J, Faucheux B, et al. An immunohistochemical study of the distribution of brain-derived neurotrophic factor in the adult human brain, with particular reference to Alzheimer’s disease. Neuroscience. 1999;88:1015–1032. doi: 10.1016/s0306-4522(98)00219-x. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 65.Benedetti E, D’Angelo B, Cristiano L, Di Giacomo E, Fanelli F, Moreno S, et al. Involvement of peroxisome proliferator-activated receptor beta/delta (PPAR beta/delta) in BDNF signaling during aging and in Alzheimer disease: possible role of 4-hydroxynonenal (4-HNE) Cell Cycle. 2014;13:1335–1344. doi: 10.4161/cc.28295. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat. Med. 2009;15:331–337. doi: 10.1038/nm.1912. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Muller FJ, Loring JF, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mariga A, Zavadil J, Ginsberg SD, Chao MV. Withdrawal of BDNF from hippocampal cultures leads to changes in genes involved in synaptic function. Dev. Neurobiol. 2015;75:173–192. doi: 10.1002/dneu.22216. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berchtold NC, Coleman PD, Cribbs DH, Rogers J, Gillen DL, Cotman CW. Synaptic genes are extensively downregulated across multiple brain regions in normal human aging and Alzheimer’s disease. Neurobiol. Aging. 2013;34:1653–1661. doi: 10.1016/j.neurobiolaging.2012.11.024. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ginsberg SD, Alldred MJ, Che S. Gene expression levels assessed by CA1 pyramidal neuron and regional hippocampal dissections in Alzheimer’s disease. Neurobiol. Dis. 2012;45:99–107. doi: 10.1016/j.nbd.2011.07.013. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duffy AM, Schaner MJ, Wu SH, Staniszewski A, Kumar A, Arevalo JC, et al. A selective role for ARMS/Kidins220 scaffold protein in spatial memory and trophic support of entorhinal and frontal cortical neurons. Exp. Neurol. 2011;229:409–420. doi: 10.1016/j.expneurol.2011.03.008. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goldberg NR, Caesar J, Park A, Sedgh S, Finogenov G, Masliah E, et al. Neural stem cells rescue cognitive and motor dysfunction in a transgenic model of dementia with lewy bodies through a BDNF-dependent mechanism. Stem Cell Reports. 2015;5:791–804. doi: 10.1016/j.stemcr.2015.09.008. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parain K, Murer MG, Yan Q, Faucheux B, Agid Y, Hirsch E, et al. Reduced expression of BDNF protein in Parkinson’s disease substantia nigra. Neuroreport. 1999;10:557–561. doi: 10.1097/00001756-199902250-00021. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 74.Fenner ME, Achim CL, Fenner BM. Expression of full-length and truncated trkB in human striatum and substantia nigra neurons: implications for Parkinson’s disease. J. Mol. Histol. 2014;45:349–361. doi: 10.1007/s10735-013-9562-z. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 75.Jha SK, Jha NK, Kar R, Ambasta RK, Kumar P. p38 MAPK and PI3K/AKT signalling cascades in Parkinson’s disease. Int. J. Mol. Cell. Med. 2015;4:67–86. PubMed. [PMC free article] [PubMed] [Google Scholar]
- 76.Mogi M, Togari A, Kondo T, Mizuno Y, Komure O, Kuno S, et al. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci. Lett. 1999;270:45–48. doi: 10.1016/s0304-3940(99)00463-2. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 77.Kohno R, Sawada H, Kawamoto Y, Uemura K, Shibasaki H, Shimohama S. BDNF is induced by wild-type alpha-synuclein but not by the two mutants, A30P or A53T, in glioma cell line. Biochem. Biophys. Res. Commun. 2004;318:113–118. doi: 10.1016/j.bbrc.2004.04.012. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 78.Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat. Rev. Neurol. 2009;5:311–322. doi: 10.1038/nrneurol.2009.54. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 79.Porritt MJ, Batchelor PE, Howells DW. Inhibiting BDNF expression by antisense oligonucleotide infusion causes loss of nigral dopaminergic neurons. Exp. Neurol. 2005;192:226–234. doi: 10.1016/j.expneurol.2004.11.030. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 80.Baquet ZC, Gorski JA, Jones KR. Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. J. Neurosci. 2004;24:4250–4258. doi: 10.1523/JNEUROSCI.3920-03.2004. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fumagalli F, Racagni G, Colombo E, Riva MA. BDNF gene expression is reduced in the frontal cortex of dopamine transporter knockout mice. Mol. Psychiatry. 2003;8:898–899. doi: 10.1038/sj.mp.4001370. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 82.Nguyen KQ, Rymar VV, Sadikot AF. Impaired TrkB signaling underlies reduced BDNF-mediated trophic support of striatal neurons in the R6/2 mouse model of Huntington’s disease. Front. Cell. Neurosci. 2016;10:37. doi: 10.3389/fncel.2016.00037. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 84.Gauthier LR, Charrin BC, Borrell-Pages M, Dompierre JP, Rangone H, Cordelieres FP, et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 85.Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L, et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science. 2001;293:493–498. doi: 10.1126/science.1059581. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 86.Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington’s disease. Prog. Neurobiol. 2007;81:294–330. doi: 10.1016/j.pneurobio.2007.01.003. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 87.Zuccato C, Marullo M, Conforti P, MacDonald ME, Tartari M, Cattaneo E. Systematic assessment of BDNF and its receptor levels in human cortices affected by Huntington’s disease. Brain Pathol. 2008;18:225–238. doi: 10.1111/j.1750-3639.2007.00111.x. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strand AD, Baquet ZC, Aragaki AK, Holmans P, Yang L, Cleren C, et al. Expression profiling of Huntington’s disease models suggests that brain-derived neurotrophic factor depletion plays a major role in striatal degeneration. J. Neurosci. 2007;27:11758–11768. doi: 10.1523/JNEUROSCI.2461-07.2007. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jeon J, Kim W, Jang J, Isacson O, Seo H. Gene therapy by proteasome activator, PA28gamma, improves motor coordination and proteasome function in Huntington’s disease YAC128 mice. Neuroscience. 2016;324:20–28. doi: 10.1016/j.neuroscience.2016.02.054. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 90.Mitsumoto H, Ikeda K, Klinkosz B, Cedarbaum JM, Wong V, Lindsay RM. Arrest of motor neuron disease in wobbler mice cotreated with CNTF and BDNF. Science. 1994;265:1107–1110. doi: 10.1126/science.8066451. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 91.Korkmaz OT, Aytan N, Carreras I, Choi JK, Kowall NW, Jenkins BG, et al. 7,8-Dihydroxyflavone improves motor performance and enhances lower motor neuronal survival in a mouse model of amyotrophic lateral sclerosis. Neurosci. Lett. 2014;566:286–291. doi: 10.1016/j.neulet.2014.02.058. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Todd D, Gowers I, Dowler SJ, Wall MD, McAllister G, Fischer DF, et al. A monoclonal antibody TrkB receptor agonist as a potential therapeutic for Huntington’s disease. PLoS One. 2014;9:e87923. doi: 10.1371/journal.pone.0087923. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fulgenzi G, Tomassoni-Ardori F, Babini L, Becker J, Barrick C, Puverel S, et al. BDNF modulates heart contraction force and long-term homeostasis through truncated TrkB.T1 receptor activation. J. Cell Biol. 2015;210:1003–1012. doi: 10.1083/jcb.201502100. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yanpallewar SU, Barrick CA, Buckley H, Becker J, Tessarollo L. Deletion of the BDNF truncated receptor TrkB.T1 delays disease onset in a mouse model of amyotrophic lateral sclerosis. PLoS One. 2012;7:e39946. doi: 10.1371/journal.pone.0039946. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.The BDNF Study Group (Phase III) A controlled trial of recombinant methionyl human BDNF in ALS. Neurology. 1999;52:1427–1433. doi: 10.1212/wnl.52.7.1427. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 96.Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacol. Biochem. Behav. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 97.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Russo-Neustadt A, Beard RC, Cotman CW. Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology. 1999;21:679–682. doi: 10.1016/S0893-133X(99)00059-7. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 99.Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int. J. Neuropsychopharmacol. 2015;18:pyu033. doi: 10.1093/ijnp/pyu033. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bjorkholm C, Monteggia LM. BDNF – a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J. Neurosci. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, et al. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J. Neurosci. 2005;25:372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Issa G, Wilson C, Terry AV, Jr, Pillai A. An inverse relationship between cortisol and BDNF levels in schizophrenia: data from human postmortem and animal studies. Neurobiol. Dis. 2010;39:327–333. doi: 10.1016/j.nbd.2010.04.017. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 105.Ray MT, Shannon Weickert C, Webster MJ. Decreased BDNF and TrkB mRNA expression in multiple cortical areas of patients with schizophrenia and mood disorders. Transl. Psychiatry. 2014;4:e389. doi: 10.1038/tp.2014.26. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thompson Ray M, Weickert CS, Wyatt E, Webster MJ. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J. Psychiatry Neurosci. 2011;36:195–203. doi: 10.1503/jpn.100048. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol. Psychiatry. 2003;8:592–610. doi: 10.1038/sj.mp.4001308. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 108.Wong J, Hyde TM, Cassano HL, Deep-Soboslay A, Kleinman JE, Weickert CS. Promoter specific alterations of brain-derived neurotrophic factor mRNA in schizophrenia. Neuroscience. 2010;169:1071–1084. doi: 10.1016/j.neuroscience.2010.05.037. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weickert CS, Ligons DL, Romanczyk T, Ungaro G, Hyde TM, Herman MM, et al. Reductions in neurotrophin receptor mRNAs in the prefrontal cortex of patients with schizophrenia. Mol. Psychiatry. 2005;10:637–650. doi: 10.1038/sj.mp.4001678. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 110.Lin Z, Su Y, Zhang C, Xing M, Ding W, Liao L, et al. The interaction of BDNF and NTRK2 gene increases the susceptibility of paranoid schizophrenia. PLoS One. 2013;8:e74264. doi: 10.1371/journal.pone.0074264. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kranz TM, Goetz RR, Walsh-Messinger J, Goetz D, Antonius D, Dolgalev I, et al. Rare variants in the neurotrophin signaling pathway implicated in schizophrenia risk. Schizophr. Res. 2015;168:421–428. doi: 10.1016/j.schres.2015.07.002. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Arevalo JC, Wu SH, Takahashi T, Zhang H, Yu T, Yano H, et al. The ARMS/Kidins220 scaffold protein modulates synaptic transmission. Mol. Cell Neurosci. 2010;45:92–100. doi: 10.1016/j.mcn.2010.06.002. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arevalo JC, Yano H, Teng KK, Chao MV. A unique pathway for sustained neurotrophin signaling through an ankyrin-rich membrane-spanning protein. EMBO J. 2004;23:2358–2368. doi: 10.1038/sj.emboj.7600253. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch. Gen. Psychiatry. 2012;69:150–158. doi: 10.1001/archgenpsychiatry.2011.1456. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moldovan AS, Groiss SJ, Elben S, Sudmeyer M, Schnitzler A, Wojtecki L. The treatment of Parkinson’s disease with deep brain stimulation: current issues. Neural Regen. Res. 2015;10:1018–1022. doi: 10.4103/1673-5374.160094. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aksay SS, Hausner L, Frolich L, Sartorius A. Severe agitation in severe early-onset Alzheimer’s disease resolves with ECT. Neuropsychiatr. Dis. Treat. 2014;10:2147–2151. doi: 10.2147/NDT.S71008. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hao S, Tang B, Wu Z, Ure K, Sun Y, Tao H, et al. Forniceal deep brain stimulation rescues hippocampal memory in Rett syndrome mice. Nature. 2015;526:430–434. doi: 10.1038/nature15694. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sleiman SF, Henry J, Al-Haddad R, El Hayek L, Abou Haidar E, Stringer T, et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body beta-hydroxybutyrate. eLife. 2016;5:e15092. doi: 10.7554/eLife.15092. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zetterstrom TS, Pei Q, Grahame-Smith DG. Repeated electroconvulsive shock extends the duration of enhanced gene expression for BDNF in rat brain compared with a single administration. Brain Res. Mol. Brain Res. 1998;57:106–110. doi: 10.1016/s0169-328x(98)00077-1. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 120.Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiol. Dis. 2013;57:47–55. doi: 10.1016/j.nbd.2012.06.011. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 121.Zheng K, An JJ, Yang F, Xu W, Xu ZQ, Wu J, et al. TrkB signaling in parvalbumin-positive interneurons is critical for gamma-band network synchronization in hippocampus. Proc. Natl. Acad. Sci. U.S.A. 2011;108:17201–17206. doi: 10.1073/pnas.1114241108. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Weinstein G, Beiser AS, Choi SH, Preis SR, Chen TC, Vorgas D, et al. Serum brain-derived neurotrophic factor and the risk for dementia: the Framingham Heart Study. JAMA Neurol. 2014;71:55–61. doi: 10.1001/jamaneurol.2013.4781. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J. Neurosci. 2010;30:5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pinyon JL, Tadros SF, Froud KE, Young Acy, Tompson IT, Crawford EN, et al. Close-field electroporation gene delivery using the cochlear implant electrode array enhances the bionic ear. Sci. Transl. Med. 2014;6:233ra54. doi: 10.1126/scitranslmed.3008177. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]