Abstract
Complex lesions of the thoracic aorta are traditionally treated in 2 surgical steps with the elephant trunk technique. A relatively new approach is the frozen elephant trunk (FET) technique, which potentially allows combined lesions of the thoracic aorta to be treated in a 1-stage procedure combining endovascular treatment with conventional surgery using a hybrid prosthesis. These are very complex and time-consuming operations, and good results can be obtained only if appropriate strategies for myocardial, cerebral, and visceral protection are adopted. However, the FET technique is associated with a non-negligible incidence of spinal cord injury, due to the extensive coverage of the descending aorta with the excessive sacrifice of intercostal arteries. The indications for the FET technique include chronic thoracic aortic dissection, acute or chronic type B dissection when endovascular treatment is contraindicated, chronic aneurysm of the thoracic aorta, and chronic aneurysm of the distal arch. The FET technique is also indicated in acute type A aortic dissection, especially when the tear is localized in the aortic arch; in cases of distal malperfusion; and in young patients. In light of the great interest in the FET technique, the Vascular Domain of the European Association for cardio-thoracic Surgery published a position paper reporting the current knowledge and the state of the art of the FET technique. Herein, we describe the surgical techniques involved in the FET technique and we report our experience with the FET technique for the treatment of complex aortic disease of the thoracic aorta.
Keywords: Aortic arch, Hybrid, Aortic surgery, Frozen elephant trunk
Introduction
The treatment of complex pathologies of the thoracic aorta has been and remains a challenge for cardiovascular surgeons. Until the early 2000s, the combined pathologies of the arch and of the descending thoracic aorta were mainly treated by a 2-stage approach, the elephant trunk (ET) technique described in 1983 by Borst et al. [1]. Over time, this technique evolved [2,3], but the most important change was the introduction of a hybrid prosthesis that consists of a distal endovascular stent graft and a proximal conventional surgical graft. The modified technique was named Frozen Elephant Trunk (FET) technique [4]. The FET procedure has gained popularity because it has simplified the treatment of complex thoracic 1–7 aortic pathologies.
In Europe, at the moment, 2 hybrid prosthesis are available for the FET procedure that have obtained the ‘Conformité Européenne’ mark: the E-Vita Open Plus (Jotec GmbH, Hechingen, Germany) and the Thoraflex hybrid device (Vascutek Terumo, Inchinnan, Scotland, UK). The E-Vita Open Plus was the first commercially available hybrid prosthesis, and it is composed of a proximal part consisting of a vascular prosthesis and a distal part consisting a self-expandable nitinol stent graft. In 2012, a hybrid prosthesis known as the Thoraflex hybrid device was introduced by Vascutek Terumo. The proximal part consists of a quadruple-branched vascular prosthesis, and the distal part is a self-expandable nitinol stent graft with a different stent shape. The multi-branched portion allows individual arch vessel reimplantation to be performed and perfusion of the lower part of the body to be started through the fourth branch once the distal anastomosis is completed.
Until December 2014, more than 28,000 hybrid prostheses were implanted worldwide [5]. Due to the great interest in the FET technique, the Vascular Domain of the European Association for Cardio-thoracic Surgery (EACTS) felt the need to write a position paper in which, with the help of other experienced aortic surgeons, the current knowledge and the state of the art regarding the FET technique were presented [6]. The study design included a PubMed search, and information was extracted from 97 relevant publications. The FET technique was found to be used to treat acute and chronic aortic dissection, atherosclerotic aneurysms involving the arch and the descending aorta, and even other aortic diseases, such as penetrating aortic ulcers. All hybrid devices available in the market were utilized in the studies. The authors reported in-hospital mortality rates ranging from 1.8% to 17.2%, and other complications were similar to those reported in series of classical arch surgery procedures, except for spinal cord injuries. The incidence of paraplegia or paraparesis was significantly higher when the FET technique was used. Various mechanisms seem to play a role in the occurrence of this devastating complication: the coverage of the descending aorta beyond T7–T8; the longer duration of spinal cord ischemia; air or corpusculate microehboli or throhboehbolish. The study had some limitations: being limited to single-center experiences, its retrospective design, the inclusion of different surgical methods and techniques, the small number of some series due to the rarity of this complex pathology, differences regarding the hybrid devices utilized, and the heterogeneity of the patient population.
However, despite the above limitations, the position paper of the EACTS Vascular Domain provided useful recommendations. In particular, in cases of type A dissection, it was suggested to perform the FET technique to close the primary entry tear in the distal portion of the aortic arch or in the proximal half of descending aorta, to treat or to prevent concomitant malperfusion syndrome, or to avoid the future dilatation of the false lumen in the distal aorta. Furthermore, in cases of type B dissection, the use of FET was advised when endovascular approach was not possible or when the risk of retrograde progression of the dissection was high. Moreover, the FET technique can be considered in patients affected by extensive thoracic or thoracoabdominal aortic disease when either repeated open surgery or endovascular treatment is expected.
In Bologna, we started our FET program in 2007, and since then we have operated on more than 200 patients. Our experience started with the E-Vita Open prosthesis, which was used in 157 patients, and since the branched Thoraflex device became available, we have implanted 44 of those devices.
The indications for the FET procedure were type A chronic dissection in 94 patients (with the large majority of these patients having residual dissection after treatment of acute type A dissection), chronic degenerative aneurysm in 60 patients, and chronic type B aortic dissection associated with an ascending arch aneurysm in 21 patients. Twenty-two patients were operated on for acute type A aortic dissection and 4 for acute type B dissection. Of the patients, 104 had undergone a previous aortic operation.
The main surgical procedures were total arch replacement and FET in 64 patients; only FET in 5 patients; and total arch, FET, and other procedures in 132 patients. In the majority of patients (132), associated procedures such as proximal aortic root surgery were performed. The key aspects of these operations are accurate assessment of the aortic anatomy, the implementation of reliable methods of organ protection, and the use of effective surgical techniques and strategies. The entire aorta must be carefully investigated before the operation; especially in cases of acute or chronic dissection, it is mandatory to know the origin of the visceral arteries (true or false lumen) and the presence and the localization of the distal re-entry sites. As is also the case for the use of TEVAR to treat acute dissection, the usual recommendation is to avoid any oversizing of the stent to reduce the risk of stent-induced new entry. In chronic aortic aneurysms, it is fundamental to know the exact diameters of the distal landing zone in the descending thoracic aorta to ensure the correct sizing of the stent graft. In such cases, stent oversizing is indicated to permit optimal distal stent-graft sealing.
Surgical technique
Our surgical approach included a full median sternotomy and antegrade selective cerebral perfusion (ASCP) according to the Kazui’s technique [7], with moderate hypothermia as method of brain protection as has been recently described [8,9]. Briefly, after systemic heparinization, a guide-wire was inserted through the femoral artery in the true lumen of the descending thoracic aorta under transesophageal echocardiographic control. For cardiopulmonary bypass institution, the arterial cannulation sites usually were the right axillary artery, directly or through 8-mm Dacron graft interposition; the innominate artery; or the right carotid artery through 8-mm Dacron graft interposition. Venous drainage was achieved by cannulation of the right atrium or femoral vein in cases of complex reoperations. A left ventricle drain was inserted through the right superior pulmonary vein. Our strategy for cerebral protection consisted of ASCP with moderate hypothermia, as described in detail elsewhere [8]. In all patients, near-infrared spectroscopy was utilized to monitor cerebral perfusion. Circulatory arrest was performed at a target nasopharyngeal temperature of 25°C. Myocardial protection was achieved with the infusion of cold crystalloid cardioplegia via the modified Bretschneider solution (Custodiol; Koehler Chemie, Alsbach-Haenlein, Germany), which guarantees 3 hours of myocardial protection. After the arch was completely resected, and cannulation of the left carotid and of the left subclavian arteries for antegrade cerebral perfusion was instituted, the proximal descending aorta was prepared using an external Teflon felt fixed with some (usually 4) internal pledgeted U-stitches. In patients with aortic dissection, the false lumen was surgically obliterated at the level of the distal stump. The stent-graft system (E-Vita Open or Thoraflex hybrid device) was introduced in an antegrade fashion in the descending aorta over the previously positioned stiff guide-wire and released. For Thoraflex hybrid device positioning, once the device was released, a circumferential anastomosis between the collar and the previously prepared native aorta was performed to ensure that the implant was correctly sealed. Systemic perfusion was then restored in an antegrade manner through the side branch of the graft. The supra-aortic vessels were then separately reimplanted starting from the left subclavian artery. In most patients, proximal repair was performed after the left subclavian artery reimplantation in order to reduce the cardiac ischemic time. In other cases, all the supra-aortic vessels were first reimplanted, and the proximal repair was subsequently performed. The distal anastomosis can be localized just beyond the left subclavian artery, between the left subclavian artery and the left carotid artery, or even more proximally. It is clear that more proximal distal anastomoses can be performed more easily, with less risk of left recurrent nerve damage (Fig. 1).
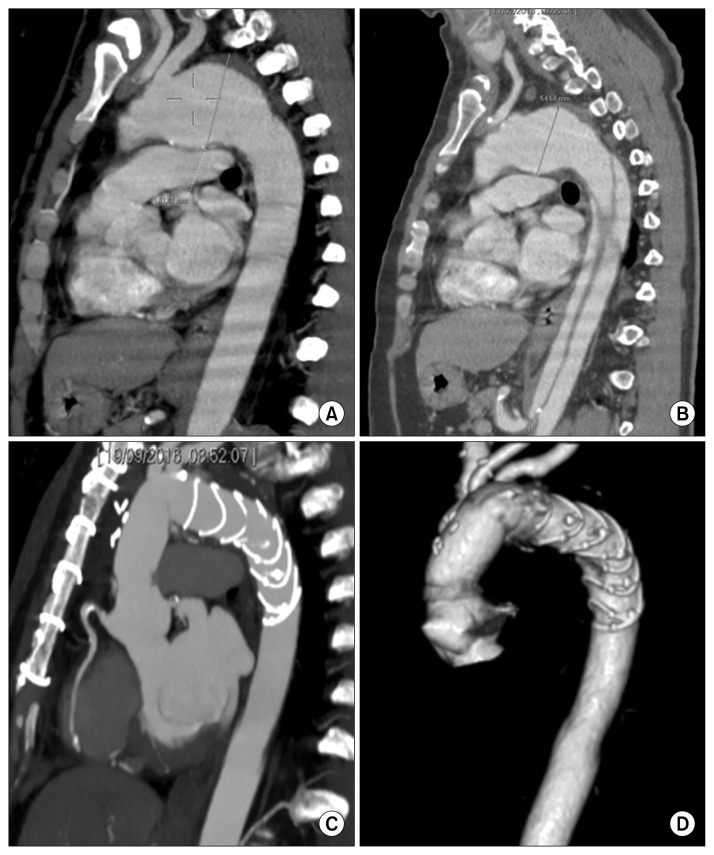
Fig. 1.
(A, B) Preoperative computed tomography scans with sagittal multi-planar reconstruction of a patient with chronic aneurysm of the aortic arch and type B acute dissection treated with a Thoraflex Hybrid device. (C, D) Postoperative results at follow-up.
For E-Vita Open Plus positioning, the stent-graft system was also introduced in an antegrade fashion in the descending aorta over the previously positioned stiff guide-wire and released. The incorporated Dacron graft was pulled back and the collar was sutured to the previously prepared descending aorta. Usually, 10 minutes of lower body reperfusion was then achieved through the graft while hemostasis at the distal anastomosis and preparation of the island containing the arch vessels was performed. The arch vessels island was then reimplanted and the distal flow definitively restored. Proximally, the Dacron graft was anastomosed to the native ascending aorta or to the previous aortic prosthesis in cases of aortic reoperation (Fig. 2).
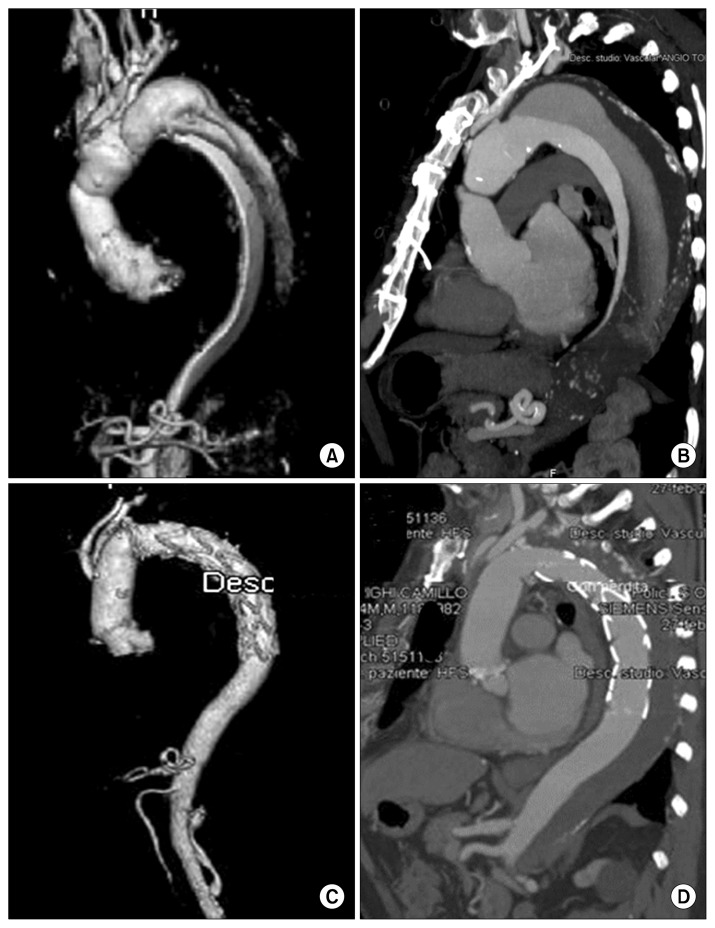
Fig. 2.
(A) Preoperative computed tomography scans with 3-dimensional volume rendering reconstruction and (B) sagittal multi-planar reconstruction of a patient with chronic dissection treated with the E-Vita Open Plus device. (C, D) Postoperative results at follow-up.
For spinal cord protection, we routinely used cerebrospinal fluid ( CSF) drainage, which was set up and positioned the day before the operation.
We preferred to use the Thoraflex hybrid device when the origins of the arch vessels were widely separated or when the arch vessels were severely involved with the dissecting process.
Following the experience of Tsagakis [10], we routinely used angioscopy during the procedure: before the deployment of the hybrid prosthesis in order to have a clear vision of the aortic anatomy, and after the deployment to assess the correct position and opening of the stent.
In our experience, 41 patients required endovascular extension at a mean time of 31 months after surgical intervention due to incomplete thrombosis of the false lumen in most the patients, and in a very few cases for inadequate distal sealing.
Conclusion
The treatment of complex lesions of the thoracic aorta still represents a challenge; however, it has been simplified by the introduction of the FET technique.
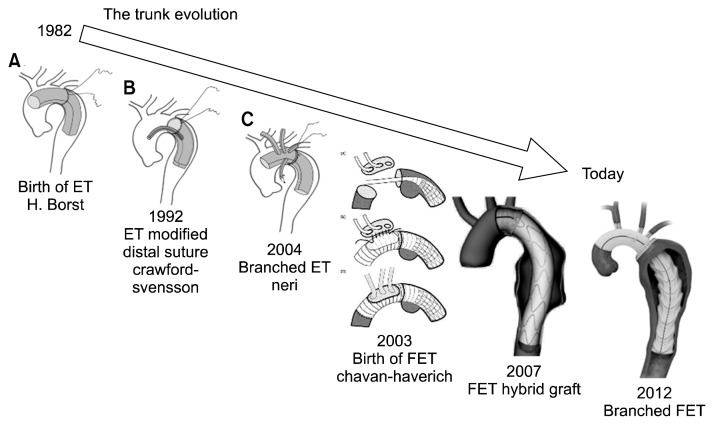
At the end of the 1990s, this technique was named the open stent-grafting technique by some Japanese surgeons [2,3], combining antegrade endovascular stenting simultaneously with arch repair. Some years later, in 2003, this procedure was modified by Karck et al. [ 4] with the introduction of a custom-made hybrid prosthesis, and it was referred to as the FET technique. Over the last years, the hybrid prosthesis has undergone several changes, most recently with the introduction of the branched FET in 2012 (Fig. 3).
Fig. 3.
Evolution of the ET technique over time. ET, elephant trunk; FET, frozen elephant trunk.
The use of this technique to treat complex lesions of the thoracic aorta is becoming increasingly common due to encouraging short-term and medium-term results [11–14]. Until December 2015, more than 28,000 prostheses were implanted worldwide, and interestingly, more than half were used in China. In a recent review, Ma et al. [5] reported an early mortality rate ranging from 6.4% to 15.8%. Similar results were reported in the recent position paper of the Vascular Domain of EACTS [6]. Data from the E-Vita registry demonstrated that the early results were comparable (without significant differences) between aortic dissection and chronic degenerative aneurysm, with in-hospital mortality rates of 17.1% and 13.2%, respectively [15]. One of the most important complications associated with FET is spinal cord injury (SCI), which has a non-negligible incidence [16].
SCI during FET surgery is multifactorial, and spinal cord ischemia and occlusion of the thoracic inter-costal arteries seem to be the most important risk factors. Its incidence could probably be reduced with shorter coverage of the descending aorta or with a shorter period of spinal cord ischemia.
The drainage of CSF has been demonstrated to be a useful means to prevent SCI, so we strongly recommend its use during FET surgery. In the consensus paper of EACTS, it was reported that SCI tends to occur more frequently in patients undergoing operations for chronic dissection [6]. The FET procedure has been demonstrated to be a very useful technique in both chronic and acute dissection because by restoring the flow in the true lumen and covering the proximal entry tears, thrombosis of the false lumen is promoted. In a recent meta-analysis we showed that partial or complete thrombosis of a persistent false lumen occurred in more than 90% of cases of acute type 1 aortic dissection treated using the FET technique [16]. Visceral ischemia due to the complete thrombosis of the false lumen can occur if the visceral arteries originate from the false lumen itself, and no re-entries are present in the distal portion of the aorta. In order to avoid this complication, computed tomography angiography of the entire aorta should be performed before the operation. We believe that the FET procedure should be contraindicated if reentries are not present in the distal descending thoracic and thoracoabdominal aorta or when the origin of the visceral arteries is from the false lumen.
In conclusion, the FET technique represents a feasible and efficient option in the treatment of complex thoracic aortic pathologies. This technique allows 1- stage repair and, if necessary, it offers a secure landing zone for additional endovascular procedures or second-stage open thoracoabdominal aortic aneurysm repair. Moreover, refinements in surgical technique have contributed to improved early and late outcomes.
Footnotes
This article was presented at the Bundang Aortic Surgery Symposium (BASS) in July, 2016 at Bundang, Korea.
Conflict of interest
No potential conflicts of interest relevant to this article are reported.
References
- 1.Borst HG, Walterbusch G, Schaps D. Extensive aortic replacement using “elephant trunk” prosthesis. Thorac Cardiovasc Surg. 1983;31:37–40. doi: 10.1055/s-2007-1020290. [DOI] [PubMed] [Google Scholar]
- 2.Suto Y, Yasuda K, Shiiya N, et al. Stented elephant trunk procedure for an extensive aneurysm involving distal aortic arch and descending aorta. J Thorac Cardiovasc Surg. 1996;112:1389–90. doi: 10.1016/S0022-5223(96)70157-5. [DOI] [PubMed] [Google Scholar]
- 3.Kato M, Ohnishi K, Kaneko M, et al. New graft-implanting method for thoracic aortic aneurysm or dissection with a stented graft. Circulation. 1996;94(9 Suppl):II188–93. [PubMed] [Google Scholar]
- 4.Karck M, Chavan A, Hagl C, Friedrich H, Galanski M, Haverich A. The frozen elephant trunk technique: a new treatment for thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 2003;125:1550–3. doi: 10.1016/S0022-5223(03)00045-X. [DOI] [PubMed] [Google Scholar]
- 5.Ma WG, Zheng J, Sun LZ, Elefteriades JA. Open stented grafts for frozen elephant trunk technique: technical aspects and current outcomes. Aorta (Stamford) 2015;3:122–35. doi: 10.12945/j.aorta.2015.14.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shrestha M, Bachet J, Bavaria J, et al. Current status and recommendations for use of the frozen elephant trunk technique: a position paper by the Vascular Domain of EACTS. Eur J Cardiothorac Surg. 2015;47:759–69. doi: 10.1093/ejcts/ezv085. [DOI] [PubMed] [Google Scholar]
- 7.Kazui T, Inoue N, Yamada O, Komatsu S. Selective cerebral perfusion during operation for aneurysms of the aortic arch: a reassessment. Ann Thorac Surg. 1992;53:109–14. doi: 10.1016/0003-4975(92)90767-X. [DOI] [PubMed] [Google Scholar]
- 8.Pacini D, Leone A, Di Marco L, et al. Antegrade selective cerebral perfusion in thoracic aorta surgery: safety of moderate hypothermia. Eur J Cardiothorac Surg. 2007;31:618–22. doi: 10.1016/j.ejcts.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 9.Di Bartolomeo R, Di Marco L, Armaro A, et al. Treatment of complex disease of the thoracic aorta: the frozen elephant trunk technique with the E-vita open prosthesis. Eur J Cardiothorac Surg. 2009;35:671–5. doi: 10.1016/j.ejcts.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Tsagakis K. Angioscopy as a supplement to frozen elephant trunk treatment. Ann Cardiothorac Surg. 2013;2:653–5. doi: 10.3978/j.issn.2225-319X.2013.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss G, Tsagakis K, Jakob H, et al. The frozen elephant trunk technique for the treatment of complicated type B aortic dissection with involvement of the aortic arch: multicentre early experience. Eur J Cardiothorac Surg. 2015;47:106–14. doi: 10.1093/ejcts/ezu067. [DOI] [PubMed] [Google Scholar]
- 12.Pacini D, Tsagakis K, Jakob H, et al. The frozen elephant trunk for the treatment of chronic dissection of the thoracic aorta: a multicenter experience. Ann Thorac Surg. 2011;92:1663–70. doi: 10.1016/j.athoracsur.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Di Eusanio M, Armaro A, Di Marco L, et al. Short- and midterm results after hybrid treatment of chronic aortic dissection with the frozen elephant trunk technique. Eur J Cardiothorac Surg. 2011;40:875–80. doi: 10.1016/j.ejcts.2011.01.068. [DOI] [PubMed] [Google Scholar]
- 14.Di Bartolomeo R, Pacini D, Savini C, et al. Complex thoracic aortic disease: single-stage procedure with the frozen elephant trunk technique. J Thorac Cardiovasc Surg. 2010;140(6 Suppl):S81–5. doi: 10.1016/j.jtcvs.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 15.Leontyev S, Tsagakis K, Pacini D, et al. Impact of clinical factors and surgical techniques on early outcome of patients treated with frozen elephant trunk technique by using EVITA open stent-graft: results of a multicentre study. Eur J Cardiothorac Surg. 2016;49:660–6. doi: 10.1093/ejcts/ezv150. [DOI] [PubMed] [Google Scholar]
- 16.Di Eusanio M, Castrovinci S, Tian DH, et al. Antegrade stenting of the descending thoracic aorta during DeBakey type 1 acute aortic dissection repair. Eur J Cardiothorac Surg. 2014;45:967–75. doi: 10.1093/ejcts/ezt493. [DOI] [PubMed] [Google Scholar]