Abstract
Pulmonary benign metastasizing leiomyoma (PBML) is defined as metastasis of a leiomyoma to lung tissue. It was first reported in 1937. P BML is known as a benign disease, but can undergo malignant transformation. Only 1 case of the malignant transformation of PBML to leiomyosarcoma has been reported previously. In this report, we present a case of malignant transformation of PBML.
Keywords: Leiomyoma, Leiomyosarcoma, Lung neoplasms
Case report
In June 2014, a 61-year-old female was admitted to Keimyung University Dongsan Medical Center because of a severe dry cough that had lasted for over 20 days. According to her medical history, she had undergone a hysterectomy in 2005, and the pathologic diagnosis was leiomyoma of the uterus. In 2006, chest computed tomography (CT) findings demonstrated multiple nodules in both lungs (Fig. 1A, B) and a CT-guided needle biopsy was performed. Microscopic examination revealed a proliferating spindle cell tumor showing relatively bland nuclei, no pleomorphism, and no mitoses. In addition, immunohistochemical staining analysis was positive for smooth muscle actin, desmin, vimentin, estrogen receptor (ER), and progesterone receptor (PR), and negative for CD34, PAN-CK, and S100 proteins. The result of the pathologic study was benign leiomyoma, and the nodules were diagnosed as pulmonary benign metastasizing leiomyoma (PBML). Since the patient was asymptomatic, we did not resect the mass. After that, the patient did not return to our outpatient clinic for follow-up.
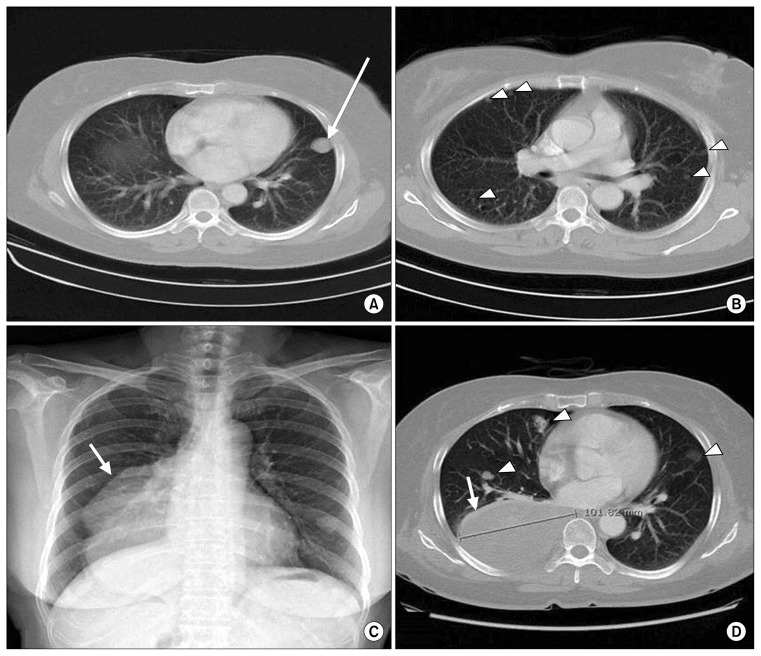
Fig. 1.
(A, B) Chest CT in 2006: The patient was diagnosed with BML by CT-guided biopsy of the left lower lung mass (arrow). Multiple nodules were present in both lung fields (arrow heads). (C) Preoperative posteroanterior chest X-ray, with a very large lobulated mass (arrow) found in the right lower lung field. (D) Chest CT in 2014, showing a pleural-based heterogeneous mass measuring 10 cm×12 cm (arrow) compressing the right lower lung and posterobasal segmental bronchus. Multiple nodules were seen in both lung fields (arrow heads). CT, computed tomography; BML, benign metastasizing leiomyoma.
Upon the patient’s return, a posteroanterior chest X-ray showed a very large mass on the right cardiac border (Fig. 1C). Chest CT demonstrated a 101-mm mass on the right lower lobe that compressed the posterobasal segmental bronchus (Fig. 1D). The other masses had not increased in size. A CT-guided needle biopsy of the huge mass revealed it to be leiomyoma. Initially, right lower lobectomy and mass excision were planned.
After right thoracotomy, a very large mass, 100 mm in size, originating from the visceral pleura of the right lower lobe was observed (Fig. 2A). The mass was in contact with the parietal and diaphragmatic pleura, but no invasion was noted. Near the mass, multiple fine, scattered, whitish nodules measuring less than 1 cm were found on the diaphragmatic, parietal, and visceral pleura. We performed an excision of the mass in the right lower lobe and excised fine nodules from the parietal and diaphragmatic pleura for biopsy. Intrapulmonary nodules found in the middle lobe and upper lobe were not excised, because they had not increased in size.
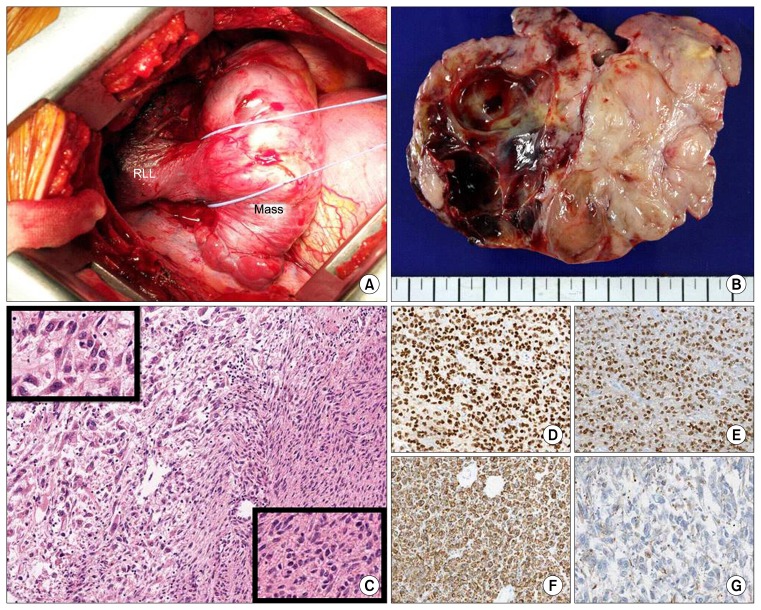
Fig. 2.
(A) Right thoracotomy, operator view. A huge mass originating in the visceral pleura of the RLL and a totally collapsed lower lobe were found via thoracotomy. (B) Cross-sectional view of the mass. A lobulated mass containing well-demarcated solid and cystic portions was found (13.0 cm×10.5 cm×4.5 cm). The mass showed cystic changes and had replaced the lung parenchyma. The visceral pleura was invaded. (C) Histologic features of the tumor (×100). The tumor consisted of a benign leiomyomatous lesion (left) and a malignant leiomyosarcoma (right). The benign leiomyomatous lesion had bland spindle cells (upper left inset, ×400) and the leiomyosarcoma had pleomorphic cells (lower right inset, ×400). (D–G) Immunohistochemical staining of the tumor (×100). (D) The tumor cells were positive for the estrogen receptor, (E) the progesterone receptor, and (F) smooth muscle actin. (G) The leiomyosarcoma was weakly positive for smooth muscle actin. RLL, right lower lobe.
Gross findings showed multiple lobulation and partial cystic change (Fig. 2B). On microscopic examination, the tumor was composed of leiomyoma and a broad necrotic lesion (Fig. 2C). The necrotic lesion showed severe cellular atypia, frequent mitoses (more than 15 per 10 high power fields [HPFs]), and continuity of the leiomyoma and leiomyosarcoma (Fig. 2C). Immunohistochemical staining revealed that the tumor was positive for smooth muscle actin (SMA), ER, and PR, but negative for CD34, pan-CK, S100, and HMB45 (Fig. 2D–G). The final diagnosis of the mass was leiomyosarcoma that had transformed from leiomyoma. The nodular lesions taken from the parietal and diaphragmatic pleura showed no malignant cells. The patient was discharged 8 days after the operation without complications.
We used positron emission tomography–computed tomography (PET-CT) to rule out metastasis of the leiomyosarcoma. Large heterogeneous hypermetabolic masses were found from the left para-aortic area to the left pelvic cavity (Fig. 3), and we diagnosed them as metastatic leiomyosarcoma. Additionally, increased fluorodeoxyglucose (FDG) uptake was observed in the peribronchial lymph nodes, the mediastinum, and the supraclavicular lymph nodes. Two months later, en-bloc resection of the retroperitoneal masses with left nephrectomy was performed, and the pathologic diagnosis was metastatic leiomyosarcoma. We decided to continue observing FDG uptake in the mediastinal and peribronchial lymph nodes rather than prescribing chemotherapy, because metastasis of leiomyosarcoma to the lymph nodes is rare. A chest CT scan done 1 year after surgery demonstrated no changes in the pulmonary nodules and no recurrence of the mass.
Fig. 3.
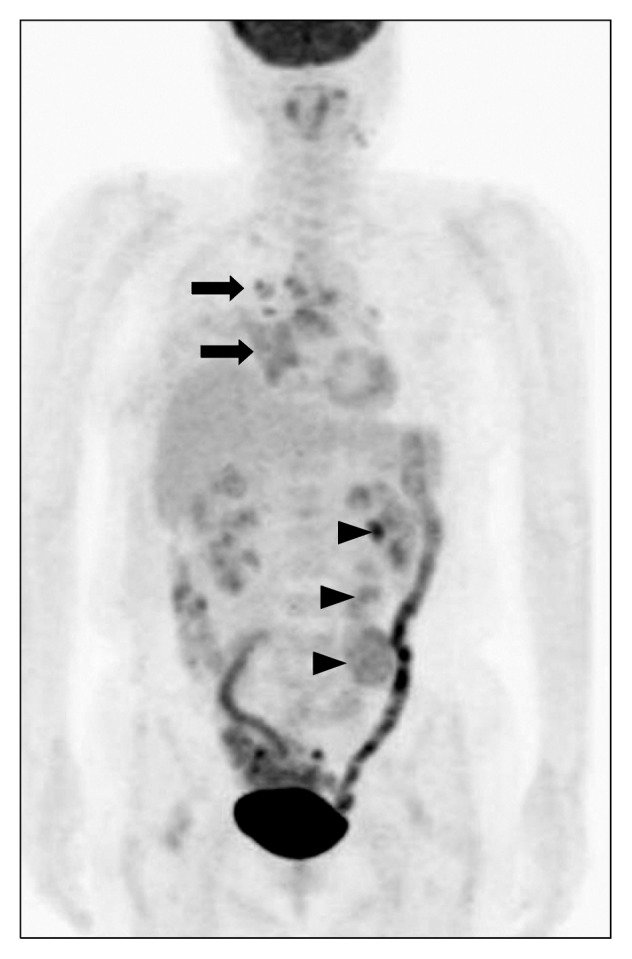
Positron emission tomography-computed tomography. Large heterogeneous hypermetabolic lobulated masses were seen from the left para-aortic area to the left pelvic cavity (arrow heads; maximum standardized uptake value, 4.7). Multiple hypermetabolic lymph nodes were observed at 2R, 4R/L, 7, and 10R (arrows).
Discussion
Uterine leiomyoma is the most common pelvic tumor in women. Leiomyomas are found in 12%–25% of women of reproductive age [1]. Benign metastasizing leiomyoma (BML) results from metastasis of a uterine leiomyoma; thus, BML patients have a history of uterine leiomyoma. The condition may occur at any age, but occurs most often in women from 30 to 74 years old. BML can occur in a number of different areas of the body, including but not limited to the lungs, para-aortic lymph nodes, abdominal lymph nodes, heart, breast, liver, and esophagus [2]. The most common site of occurrence is in the lungs, in which case it is known as PBML. PBML is a rare disease; only around 100 cases have been reported. The pathogenesis of PBML is unknown, but the most common theory is hematogenous spreading of leiomyoma during uterine surgery. Excisional biopsy is the standard diagnostic tool used to identify PBML. Immunohistochemical staining of affected tissue is positive for desmin, SMA, PR, and ER.
Most cases of PBML do not require treatment, but PBML that metastasizes or increases in size does need to be treated, as it can cause respiratory failure, chest pain, or hemoptysis. Surgical excision is the treatment of choice, but this is only possible in cases of resectable PBML. In some cases of PBML that a repositive for ER or PR, reducing hormone release via hysterectomy, bilateral adnexectomy, and/or hormone therapy may decrease the tumor’s size [3].
Leiomyoma can undergo various degenerative changes, including malignant transformation. The incidence of the sarcomatous transformation of benign uterine leiomyomas is only 0.1%–0.8%, and is most often seen in women in their 50s [4]. PBML can also undergo malignant transformation; excluding our case, just 1 case of the malignant transformation of PBML has been reported [5].
Radiologically, it is difficult to differentiate between normal PBML and PBML that has become malignant. The only effective diagnostic imaging tool is PET-CT [5]. On FDG PET-CT, heterogeneous FDG uptake of the tumor may reflect necrosis within the leiomyosarcoma. In our case, the retroperitoneal leiomyosarcoma showed this pattern. The standard diagnostic procedure for PBML is excisional lung biopsy. Pathologically, the current criteria used to differentiate a malignant smooth muscle tumor from a benign mass are the presence of necrosis, a high mitotic index (>5 mitoses seen in 50 HPFs), nuclear atypia, cellularity, and an ill-defined tumor border [6].
The malignant transformation of PBML is very rare. However, in primary pulmonary leiomyosarcoma (PPL), progress is slow, and metastasis of PPL occurs late in the disease process. If the condition is diagnosed early, wide excision with negative margins (R0) is the standard treatment. The 5-year survival rate in PPL patients who undergo complete resection is over 50%, and recurrence is rare. Lymph node resections are generally not necessary because PPL rarely shows lymph node involvement. For advanced lesions, depending on the size and grade of the tumor, radiation therapy, chemotherapy, or both may be indicated as an adjuvant therapy. However, the median survival of patients with an unresectable sarcoma or metastatic disease is about 12 months [7,8].
Overall, early diagnosis and complete mass resection are vital in cases of the malignant transformation of PBML. However, preventive resection or diagnostic excisional biopsy is not recommended for all PBML patients, since the occurrence of a malignant change is extremely rare. Instead, regular observation and follow-up chest CT are recommended. If there is a very large, newly developed mass that is increasing in size rapidly, or if a malignant mass is found on PET-CT, diagnostic mass resection should be considered.
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol. 1990;94:435–8. doi: 10.1093/ajcp/94.4.435. [DOI] [PubMed] [Google Scholar]
- 2.Chen S, Liu RM, Li T. Pulmonary benign metastasizing leiomyoma: a case report and literature review. J Thorac Dis. 2014;6:E92–8. doi: 10.3978/j.issn.2072-1439.2014.04.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esteban JM, Allen WM, Schaerf RH. Benign metastasizing leiomyoma of the uterus: histologic and immunohistochemical characterization of primary and metastatic lesions. Arch Pathol Lab Med. 1999;123:960–2. doi: 10.5858/1999-123-0960-BMLOTU. [DOI] [PubMed] [Google Scholar]
- 4.Bharambe BM, Deshpande KA, Surase SG, Ajmera AP. Malignant transformation of leiomyoma of uterus to leiomyosarcoma with metastasis to ovary. J Obstet Gynaecol India. 2014;64:68–9. doi: 10.1007/s13224-012-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogawa M, Hara M, Ozawa Y, et al. Benign metastasizing leiomyoma of the lung with malignant transformation mimicking mediastinal tumor. Clin Imaging. 2011;35:401–4. doi: 10.1016/j.clinimag.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Mahadevia PS, Tanaka K, Fineberg S. Rosai and Ackerman’s surgical pathology, 9th edition author: Juan Rosai Mosby, Edinburgh, 2004. Diagn Cytopathol. 2006;34:382–3. doi: 10.1002/dc.20292. [DOI] [Google Scholar]
- 7.Arnold LM, 3rd, Burman SD, O-Yurvati AH. Diagnosis and management of primary pulmonary leiomyosarcoma. J Am Osteopath Assoc. 2010;110:244–6. [PubMed] [Google Scholar]
- 8.Ramanathan T. Primary leiomyosarcoma of the lung. Thorax. 1974;29:482–9. doi: 10.1136/thx.29.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]