Abstract
Background
Environmental exposures to indoor allergens are major contributors to asthma symptoms, particularly in inner cities. The effectiveness of household allergen reduction as an adjunct to National Asthma Education Prevention Program (NAEPP) guideline-based pharmacologic therapy in asthma has not been prospectively studied.
Objective
We studied the effect of individualized allergen reduction on ability to reduce asthma pharmacologic therapy over 40 weeks.
Methods
We performed a randomized, controlled trial to determine the effect of multi-faceted indoor allergen avoidance measures on ability to reduce asthma controller therapy in adults and children residing in New York City who were both sensitized and exposed to at least one indoor allergen. Asthma treatment and control were optimized in all subjects prior to randomization.
Results
125 subjects were randomized to receive individualized household allergen reduction and 122 received a sham intervention. Subjects in the intervention group significantly reduced all measured allergen levels (cat, dog, dust mite in the bedroom, roach and mouse in the kitchen and bedroom); those in the control group reduced only dust mite and mouse in the bedroom and roach in the kitchen. Participants in the intervention arm reduced NAEPP based therapy from step 4.4 at randomization to 3.50 post intervention (range 0–6); participants in the control arm reduced medication from step 4.4 to 3.4 (p = 0.76). There were no differences in other measured asthma outcomes.
Conclusion
Targeted allergen avoidance measures do not allow for reduction in asthma pharmacologic therapy compared to usual care in patients already receiving optimal controller therapy.
Keywords: asthma, allergens, cockroach, mouse, asthma controller
INTRODUCTION
Environmental exposures to indoor allergens are major contributors to asthma morbidity among individuals in all geographic regions. Household exposure to allergens is of particular concern among inner-city individuals, where time spent indoors and high incidence of sensitization to indoor allergens exists and correlates with asthma severity in a dose dependent fashion (1–8). Despite effectiveness of existing pharmacologic treatments for most patients with asthma, there is heightened concern over adverse effects of these agents over the short and long term, contributing to poor adherence to medication regimens (9–13). Asthma management guidelines emphasize the need for individualized environmental control measures for the treatment of asthma, but there is conflicting evidence of the efficacy of such measures and widely variable adherence to these recommendations by patients and providers (14, 15).
Previously conducted studies demonstrate variable results regarding benefits of household allergen reduction on asthma morbidity (2, 5, 16, 17). Limitations of single household allergen avoidance trials have directed attention to multi-faceted allergen reduction (16–18). Limited numbers of clinical trials have evaluated multiple allergen avoidance, especially in adults. In 2004, the Inner City Asthma Study reported on a multifaceted home based environmental intervention for children with asthma, tailored to each patient’s sensitization and environmental risk profile (19). Individuals randomized to environmental intervention demonstrated significantly fewer symptoms days (0.8 fewer symptom days per 2 week period) compared to individuals in the control group (5). However, in another multi-faceted allergen avoidance study, Carter, et al studied the effect of avoidance of dust and cockroach in a group of inner-city children with asthma and demonstrated no improvement (20). Mouse allergen in the inner city has also received considerable attention given the prevalence of mouse allergen in 95% of inner city households tested in the National Cooperative Inner city asthma study and the dose-dependent correlation of mouse allergen with asthma morbidity (3, 21–24). However, intervention trials based on household mouse allergen have not be reported.
Further complicating interpretation of study results is the fact that the effectiveness of multi-faceted environmental intervention as an adjunct to guideline-based pharmacologic therapy has not been prospectively studied (2, 5, 17, 25). We hypothesized that household allergen reduction among patients with asthma living in New York City may improve asthma control and allow for significant reduction in need for pharmacologic therapy. We performed a randomized, controlled trial in subjects receiving optimized asthma controller therapy to assess the effect of individualized, comprehensive, multi-faceted indoor allergen avoidance measures on the ability to step down asthma controller therapy in adults and children with mild to severe persistent asthma who were both sensitized and exposed to specific indoor allergens. To minimize the effect of poor medication adherence on asthma outcomes, medications were provided free of charge.
METHODS
Participants
Non-smoking adults and children with mild to severe persistent asthma, ≥6 years, were invited to participate at either Columbia University Medical Center in New York, New York or the Jacobi Medical Center in the Bronx, New York between March 2011 and July 2012. The study was approved by the Institutional Review Board of each institution and was posted on clinicaltrials.gov (NCT0159311). Subjects were recruited from pediatric and adult asthma and primary care clinics at the institutions as well as through printed advertisements. Written informed consent was obtained from each subject or guardian. Adolescents aged 12–17 provided assent. Enrolled subjects were either receiving controller therapy or had symptoms consistent with persistent asthma (26) if not receiving therapy. Additional inclusion criteria at screening included: Forced Expiratory Volume in 1 second (FEV1) ≥ 40% predicted and asthma confirmed by bronchodilator reversibility defined as having a 12% or greater increase in FEV1 15 minutes after administration of 2 puffs of albuterol or PC20 methacholine ≤ 8mg/ml if not using inhaled corticosteroids (ICS) or ≤16mg/ml if using ICS. Subjects had to sleep overnight at the same address at least 5 times per week, have a positive skin test (or ImmunoCAP if FEV1 < 60% precluded skin testing) to protein extracts of at least one common indoor allergen including dust mite German cockroach , mouse, Aspergillus mix, cat and dog. Skin testing was performed utilizing the percutaneous MultiTest method (MultiTest II, Lincoln Diagnostics). ImmunoCAP testing (ThermoFisher, Uppsala, Sweden) was performed as previously described (27). Following screening, subjects continued their usual asthma therapy or its equivalent for 21 days to allow for characterization of asthma severity and control. For subjects not previously receiving controller therapy, the study physician determined appropriate therapy based on National Asthma Education and Prevention Program (NAEPP) guidelines (26). To standardize therapy, medications were transitioned based on equivalency tables (Table 1) (28, 29). All medications were provided free of charge to the subjects; adherence was measured by built-in dose counter and pill count.
Table I.
Asthma treatment steps and associated controller therapy.
| Treatment Step | Medication |
|---|---|
| 0 | Albuterol MDI as needed |
| 1 | Montelukast 5mg daily for ages 6–11, 10mg daily for ages ≥12 years |
| 2 | Fluticasone DPI 100 mcg bid |
| 3 | Fluticasone DPI 200mcg bid |
| 4 | Fluticasone/salmeterol diskus 250mcg/50mcg bid |
| 5 | Fluticasone/salmeterol diskus 500mcg/50mcg bid |
| 6 | Fluticasone/salmeterol diskus 500mcg/50mcg plus montelukast one daily dosed by age |
Home evaluation/dust collection
One to ten days following screening, two trained home evaluators conducted a home visit. Visual assessment of the subject’s home, recording evidence of exposure to second hand environmental tobacco smoke (ETS), presence and number of pets, and condition of the living space, kitchen, bedroom and bathroom was performed. Two vacuumed settled dust samples were collected from the bed, bedroom floor and kitchen as previously described (24) (see Supplement for additional methods). Protein was extracted and Musm1 (mouse), Bla g1 (cockroach), Der f1 (dust mite) , Can f1 (dog) and Fel d1 (cat) allergen concentration was quantified by means of ELISA (30). Evidence of exposure above a pre-specified cutoff (31) (Table S1 on-line supplement) to at least one allergen to which the subject was sensitized, was required for study continuation. Subjects sensitized to only cat or dog were eligible only if they had a cat or dog in the home.
Run-in period (4 weeks)
At least 70% medication adherence was required to proceed to the run-in period. Asthma control was assessed based on measured FEV1 as well as subject recall of number of days with asthma symptoms, number of days with rescue medication use and number of nights with symptoms over the previous two weeks (Table 2) (28, 29). The most severe metric was used to determine control level, with level 1 denoting good control. A standardized NAEPP-guideline based algorithm (Table S2 on line-supplement) was used by the study physician to determine the appropriate treatment step (Range 1–6) required to achieve or maintain asthma control at the mild intermittent level (control level 1) during the subsequent run-in period (28, 29). The purpose of the four week run-in period was to allow transition to protocol driven asthma management, ensure asthma control and adherence with therapy, and determine baseline symptoms and physiologic and inflammatory parameters.
Table II.
Control level based on subject two-week recall
| Level of control | # days with symptoms | # days with rescue albuterol use | # nights with asthma symptoms | FEV1 (%predicted)* |
|---|---|---|---|---|
| 1 | 0–3 | 0–3 | 0–1 | ≥85 |
| 2 | 4–9 | 4–9 | 2 | 80–84 |
| 3 | 10–13 | 10–13 | 3–4 | 70–79 |
| 4 | 14 | 14 | 5–14 | <70 |
Modified to reflect FEV1 relative to FEV1 at run-in visit for all visits following randomization.
Randomization
Subjects with optimal asthma control (control level 1 with FEV1 modified to be ≥ 85% of FEV1 at run-in visit) and 70% adherence were randomized and maintained on the same asthma treatment step level. If asthma control was > 1, controller therapy was increased based on Table S2 and the run-in period was extended for two weeks. Subjects receiving Step 6 with control level >1 were maintained on step 6 and randomized.
Treatment Period
Subjects who were randomized to the Intervention arm received an individualized home-based program by two intervention counselors utilizing standardized modules targeting furry pets, cockroach, dust mites, rodents (i.e. mice, rats) and mold as described by Morgan, et al (5). All subjects in the intervention group received all intervention modules including the Safe Sleeping Zone module, organized around reducing all allergen levels in the bedroom(5). Subjects in the Intervention arm received targeted education about how indoor allergens can affect asthma and education about strategies for reduction of allergens in the home. Intervention Counselors provided materials needed for allergen reduction (eg mattress covers, cleaning products, Electrolux® vacuums, Swiffer® WetJet mops, and Orek® HEPA-air purifiers placed in the bedroom) and implemented the measures in the home while teaching the subjects how to maintain them. Any report or observation of ETS in the home prompted inclusion of the ETS remediation plan which included strategies for avoiding ETS in the home and public places, a HEPA air purifier, and encouraging smokers to smoke outside the home. Follow up visits by the Intervention Counselor with replacement of supplies occurred at Weeks 18 and 32. The group assigned to the control arm also received a total of three visits by the Intervention Counselor where they received educational materials unrelated to asthma (eg window guards), with no discussion of allergen avoidance. Subjects in the control arm were provided no information related to their own or their child’s allergen sensitization or exposure. Two follow-up home evaluation visits for exposure assessment and dust collection was conducted for all subjects at weeks 20 and 36 following randomization. Home evaluators were distinct from intervention counselors.
Clinical assessments were performed at the study site at baseline and every 8 weeks thereafter (Figure S1). Questionnaires based on two-week recall of symptoms, rescue bronchodilator use and nocturnal awakenings were administered at each visit and mean for the two week period was calculated. Information regarding asthma exacerbations was collected at every visit. Spirometry was performed with a KoKo spirometer (nSpire Health, Longmont, Colo) and percent predicted values were determined using Hankinson equations. Measurement of fraction of exhaled nitric oxide (FeNO) levels was measured with the NIOX Mino (Aerocrine, Solana, Sweden) following American Thoracic Society guidelines (32). All subjects completed an Asthma Control Test (ACT) (33) or childhood ACT if < 12 years(34), and Juniper mini Asthma Quality of Life (mini-AQLQ) (35) at baseline, week 24 and week 40. Total and allergen specific IgE was measured at randomization visit and at week 24 and 40. Composite asthma severity index (CASI) was calculated as a composite measure of clinical characterization of asthma (36).
Treatment reduction phase
During the 40 week treatment period, pharmacologic therapy was stepped up or down based on control level at each visit by investigators blinded to the treatment arm, using Table S3, with the goal of achieving or maintaining asthma control at the mild intermittent range (control level 1). The following protocol was utilized (29): if asthma control was at level 1, maintenance therapy was reduced one step. If asthma control was >1, maintenance therapy was increased by an appropriate number of steps. The option to use a prednisone burst at any time during the study was at the discretion of the study physician or the treating physician. Once asthma control was achieved, reduction of one step at a time was repeated at each visit until the subject was using only albuterol as needed (“Step 0”) or until asthma became uncontrolled at which point treatment was increased incrementally, one step at a time, until control was again achieved.
The primary outcome variable was reduction in asthma step therapy between randomization and 40 weeks of study treatment. Secondary outcomes included change in FEV1, rescue albuterol use, asthma symptoms, FeNO, score on theACT and miniAQLQ, and total and allergen specific IgE. Prespecified subgroup analysis based on factors known to contribute to asthma outcomes, including baseline age (< or ≥ 18 years), BMI (< or ≥ 30 kg/m2), asthma control level (1 or > 1), asthma therapy (Step 1–5 or Step 6), ETS and race were conducted, as was post-hoc analysis comparing subjects who, regardless of treatment group, did and did not have reduction in mouse and cockroach, allergens most associated with inner city asthma morbidity(2, 21).
It was estimated that 99 participants per group would be needed to achieve 80 percent power (two-sided type I error of 0.05) to detect a clinically meaningful difference between groups of 20% in therapy reduction. To allow sufficient power for subgroup analysis by age, a sample size of 150 subjects per group was estimated.
STATISTICAL ANALYSIS
Summary statistics were calculated to describe sample characteristics. For baseline demographics, the chi-square test was used to compare categorical variables, and the Wilcoxon rank-sum test was used to compare continuous variables. The mean post randomization outcome variables for each group and group differences were analyzed with linear mixed-effects models with visit and group as fixed effects. For variables with skewed distribution, log transformation was performed and ratio was reported. The random effects included a random intercept to account for the within-subject correlation between repeated measures over time. The primary outcome: step therapy, was analyzed as a continuous outcome. Pre-specified subgroup analyses were conducted to assess heterogeneity of treatment effects across nine characteristics, with a statistical test for interaction following recommended guidelines for subgroup analyses. (37) Statistical analyses were performed with SAS software (version 9.3, SAS Institute). The latent class mixed model analysis, to determine trajectory clusters for exposure to mouse and cockroach was implemented using the lcmm software package (38) in R 3.2.1.(39) No adjustments for multiple comparisons were made given the a priori nature of the hypotheses tested. Analyses were performed according to intention to treat with a two-sided alpha level of 0.05. Missing data was examined using linear mixed models. Mixed effect models were used for repeated measures over time (40)
RESULTS
Baseline characteristics of participants
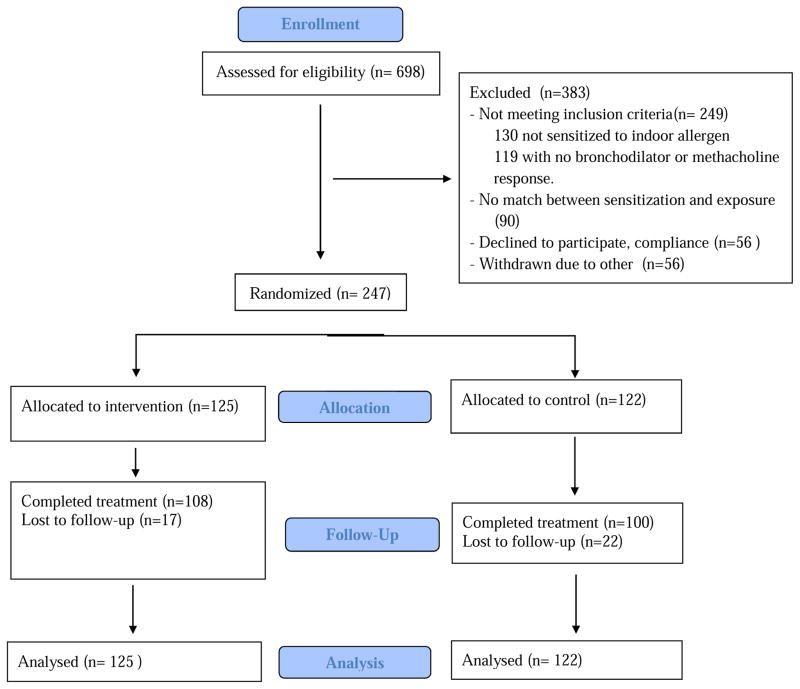
Six hundred and ninety-eight subjects were screened; 130 subjects were excluded based on negative allergen skin testing or Immunocap, 119 subjects were excluded based on lack of bronchodilator reversibility or methacholine hyperresponsiveness; 90 were excluded due to absence of home allergen exposure corresponding to specific sensitization (Figure 1). Two hundred and forty-seven subjects were randomized; 125 (56 children and 69 adults) into the intervention arm and 122 (54 children and 68 adults) into the control arm. There were no significant baseline differences between the control and intervention groups in all listed variables with the exception of the mini AQLQ which was higher in the control group (Table 3). At the time of randomization, ACT score in both groups revealed well-controlled asthma, defined as ACT > 19. Mean FEV1 was 85.4% predicted (SD=18.6) in the intervention group and 84.9% (18.1) predicted in the control group. The mean number of days in the 2 week interval on which subjects had symptoms was 2.34 (SD=3.00) in the intervention group compared with 1.87 (2.59) days in the control group (p=0.19). At baseline, slightly over half of subjects had asthma control level of 1 . Mean NAEPP treatment step required for optimal asthma control at baseline was similar in the two groups, 4.42 (SD=1.56) in the Intervention group, 4.41 (1.57) in the control group, (p = 0.96) with similar numbers of subjects requiring Step 6, or maximal therapy. 23% of children were on Step 5 or 6 and 69% of adults were on Step 5 or 6. 105 subjects in the intervention group (84%) and 97 subjects in the control group (80%) completed the study, with only 10% of completed subjects missing a clinic assessment.
Figure 1.
CONSORT diagram
Table III.
Characteristics of the Intention-to-Treat Participants at Randomization*
| Intervention N=125 | Usual Care N=122 | p-value | |
|---|---|---|---|
| Age (yrs.) | 0.99 | ||
| 6 to 17 | 56 (44.8%) | 54 (44.3%) | |
| 18 to 69 | 69 (55.2%) | 68 (55.7%) | |
| Sex: | 0.42 | ||
| Female | 73 (58.4%) | 64 (52.5%) | |
| Male | 52 (41.6%) | 58 (47.5%) | |
| Race/ethnicity: | 0.11 | ||
| Hispanic | 67 (55.4%) | 72 (61.0%) | |
| Black (non-Hispanic) | 47 (38.8%) | 45 (38.1%) | |
| White (non-Hispanic) | 7 (5.79%) | 1 (0.85%) | |
| Borough: | 0.60 | ||
| Bronx | 89 (71.8%) | 81 (66.4%) | |
| Manhattan | 29 (23.4%) | 30 (24.6%) | |
| Brooklyn | 4 (3.23%) | 7 (5.74%) | |
| Queens | 2 (1.61%) | 4 (3.28%) | |
| Body Mass Index (kg/m2) | 28.9 (8.75) | 27.8 (8.72) | 0.36 |
| Asthma symptoms (days past 2 weeks) | 2.34 (3.00) | 1.87 (2.59) | 0.19 |
| Rescue (albuterol) inhaler (days past 2 weeks) | 2.10 (2.77) | 1.72 (2.74) | 0.28 |
| Awakened with asthma symptoms (days past 2 weeks) | 1.17 (2.98) | 0.79 (2.19) | 0.25 |
| ACT (n=175) | 19.5 (4.14) | 20.2 (3.94) | 0.23 |
| Childhood ACT (n=69) | 21.9 (4.74) | 22.7 (3.60) | 0.45 |
| eNO (ppb) | 28.8 (25.5) | 27.0 (23.0) | 0.61 |
| Total IgE (kU/L) | 416 (556) | 517 (632) | 0.37 |
| FEV1/FVC ratio ‡ | 0.76 (0.09) | 0.78 (0.11) | 0.30 |
| FEV1 (% predicted) Pre ‡ | 85.4 (18.6) | 84.9 (18.1) | 0.84 |
| FEV1 (% predicted) Post ‡ | 90.4 (22.0) | 90.4 (17.3) | 0.99 |
| FEV1 (% predicted) Change ‡ | 6.79 (9.97) | 7.53 (10.2) | 0.57 |
| Reversibility 10 percent: Yes | 36 (29.0%) | 32 (26.4%) | 0.65 |
| Control Level: | |||
| 1 | 69 (55.6%) | 62 (51.2%) | 0.73 |
| 2 | 11 (8.87%) | 11 (9.09%) | |
| 3 | 17 (13.7%) | 23 (19.0%) | |
| 4 | 27 (21.8%) | 25 (20.7%) | |
| Control level mean | 2.02 (1.26) | 2.09 (1.24) | 0.64 |
| Treatment step at Randomization (categorical): | 0.10 | ||
| 1 | 2 (1.61%) | 3 (2.50%) | |
| 2 | 17 (13.7%) | 14 (11.7%) | |
| 3 | 22 (17.7%) | 21 (17.5%) | |
| 4 | 19 (15.3%) | 27 (22.5%) | |
| 5 | 14 (11.3%) | 3 (2.50%) | |
| 6 | 50 (40.3%) | 52 (43.3%) | |
| Treatment step at randomization (Continuous): | 4.42 (1.56) | 4.41 (1.57) | 0.96 |
| Juniper Mini Asthma Quality of Life Score + | 5.03 (1.32) | 5.57 (1.26) | 0.02 |
| Reported smokers in home: Yes | 43 (34.4%) | 33 (27.0%) | 0.27 |
| “Spend a lot of time with someone who smokes” | 41 (33.3%) | 30 (25.6%) | 0.24 |
| Cat/dog/pet rodent in your home now or 6 months prior: Yes | 24 (35.3%) | 21 (34.4%) | 0.99 |
Values are count (percent) or mean (standard deviation).
FEV1 Denotes forced expiratory volume in one second, and FVC denotes forced vital capacity.
Higher score denotes better quality of life.
Change in asthma outcomes in response to treatment group
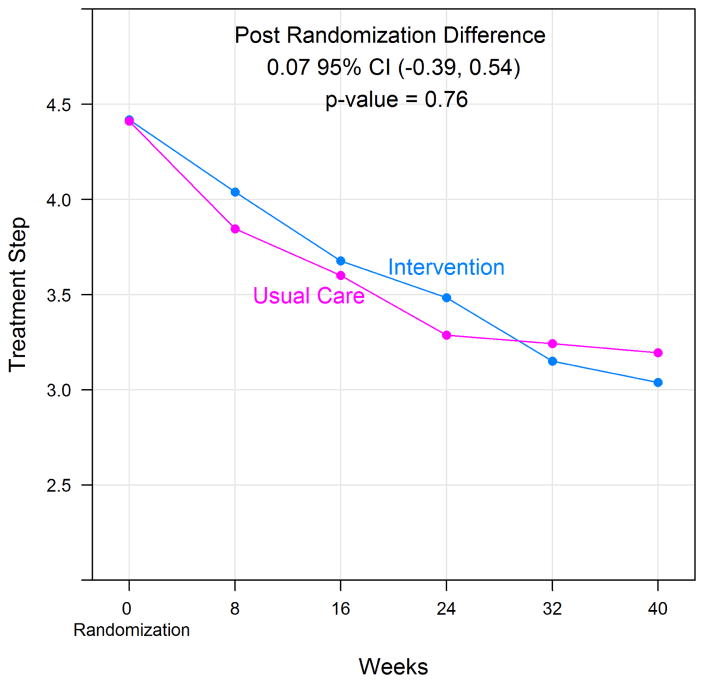
There was a significant decline in all mean measured allergen levels in the intervention group (cat, dog, dust mite, cockroach, mouse), however the control group only demonstrated a significant decline in allergen levels for dust mite and mouse in the bedroom and cockroach in the kitchen (Table 4). For the primary outcome, NAEPP treatment step, both groups reduced asthma step therapy, but there was no significant difference between treatment groups (Figure 2). Participants in the treatment group reduced NAEPP based therapy from treatment step 4.4 to treatment step 3.50 over the study period; participants in the control group reduced medications from step 4.4 to step 3.43 (p=0.76, 95%CI (−0.39, 0.54)). Table 5 presents the group specific mean and mean difference in the primary and secondary outcomes over the 40 weeks post-randomization. There was no significant difference in mean number of days with asthma symptoms, nocturnal awakenings or need for rescue bronchodilator therapy between groups. There was no significant difference in mean pre or post bronchodilator FEV1, childhood or adult ACT score, or the mini AQLQ between groups. Total serum IgE and allergen specific IgE levels were similar between the two treatment groups and did not significantly change over the course of the study period (Table S4). Eight subjects (6.4%) in the intervention group and 8 subjects (6.6%) in the control group experienced an exacerbation during the study period, (p =0.96). As a sensitivity analysis, we also calculated the effect of the intervention as a change from baseline (Table S5). Similarly, no intervention effect was observed as reflected by the p-values for the differences between groups.
Table IV.
Allergen reduction for each treatment group over the study period *
| Usual Care | Intervention | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Exposure (ug/g) | Location | Week: -2 | 16 | 32 | P-value | Week: -2 | 16 | 32 | P-value |
| Bla g 2 | Bedroom | 0.05 (0.04–0.06) | 0.04 (0.03–0.05) | 0.04 (0.03–0.05) | 0.63 | 0.05 (0.04–0.06) | 0.04 (0.03–0.04) | 0.03 (0.02–0.04) | <0.01 |
| Can f 1 | 0.27 (0.18–0.40) | 0.26 (0.17–0.38) | 0.22 (0.15–0.32) | 0.36 | 0.28 (0.18–0.41) | 0.19 (0.13–0.28) | 0.17 (0.12–0.25) | 0.03 | |
| Der f 1 | 0.06 (0.05–0.08) | 0.05 (0.04–0.06) | 0.05 (0.04–0.05) | 0.04 | 0.07 (0.06–0.09) | 0.05 (0.04–0.06) | 0.05 (0.04–0.05) | <0.01 | |
| Fel d 1 | 0.11 (0.08–0.16) | 0.10 (0.07–0.15) | 0.10 (0.07–0.14) | 0.87 | 0.11 (0.08–0.17) | 0.08 (0.06–0.12) | 0.07 (0.05–0.10) | 0.01 | |
| Mus m 1 | 0.48 (0.35–0.65) | 0.24 (0.17–0.33) | 0.33 (0.24–0.45) | 0.03 | 0.38 (0.28–0.51) | 0.20 (0.15–0.28) | 0.17 (0.13–0.23) | <0.01 | |
|
| |||||||||
| Bla g 2 | Kitchen | 0.64 (0.42–0.97) | 0.38 (0.26–0.55) | 0.32 (0.21–0.47) | <0.01 | 0.73 (0.48–1.11) | 0.49 (0.34–0.70) | 0.30 (0.21–0.44) | <0.01 |
| Mus m 1 | 2.51 (1.68–3.74) | 1.94 (1.24–3.04) | 1.76 (1.17–2.65) | 0.10 | 1.66 (1.12–2.46) | 1.47 (0.95–2.27) | 1.02 (0.69–1.51) | 0.02 | |
Numbers are geometric means (95% confidence intervals), the p-value for trend is computed using linear mixed models.
Figure 2.
Change in asthma treatment step over the study period.
Table V.
Treatment effect averaged over 40 Weeks of Follow-up.*
| Intervention* | Usual Care* | Effect † (95% CI) | p-value | |
|---|---|---|---|---|
| Treatment Step Final | 3.50 ± 0.16 | 3.43 ± 0.17 | 0.07 (−0.39, 0.54) | 0.76 |
| Asthma symptoms (days/2 weeks) | 2.44 ± 0.22 | 2.38 ± 0.23 | 0.06 (−0.57, 0.70) | 0.85 |
| Rescue (albuterol) inhaler (days/2wk) | 2.32 ± 0.23 | 2.15 ± 0.24 | 0.17 (−0.48, 0.82) | 0.61 |
| Awakened with asthma symptoms | 1.08 ± 0.16 | 0.81 ± 0.17 | 0.27 (−0.20, 0.73) | 0.26 |
| Asthma Control Test (ACT) (n=175) | 20.1 ± 0.38 | 20.9 ± 0.40 | −0.85 (−1.93, 0.24) | 0.12 |
| Childhood ACT (n=69) | 22.6 ± 0.58 | 22.9 ± 0.62 | −0.31 (−2.01, 1.39) | 0.71 |
| eNO ‡ | 23.6 ± 0.65 | 26.1 ± 0.75 | 0.90 (0.75, 1.08) + | 0.26 |
| FEV1 (% predicted) Pre | 83.8 ± 1.45 | 82.8 ± 1.51 | 1.03 (−3.09, 5.15) | 0.62 |
| FEV1 (% predicted) Post | 89.8 ± 1.58 | 89.2 ± 1.64 | 0.60 (−3.87, 5.06) | 0.79 |
| Control Level | 1.57 ± 0.06 | 1.56 ± 0.06 | 0.01 (−0.16, 0.18) | 0.92 |
| Composite Asthma Score | 5.64 ± 0.25 | 5.66 ± 0.27 | −0.01 (−0.74, 0.71) | 0.97 |
| Exacerbations (n, %) | 8 (6.4%) | 8 (6.6%) | 0 | 0.96 |
| Juniper mini QOL | 5.41 ± 0.13 | 5.63 ± 0.14 | −0.22 (−0.61, 0.16) | 0.26 |
| Mite IgE (kU/L) ‡ | 1.22 ± 0.09 | 1.09 ± 0.08 | 1.12 (0.69, 1.84) + | 0.64 |
| Cat IgE (kU/L) ‡ | 1.77 ± 0.15 | 1.78 ± 0.15 | 0.99 (0.57, 1.72) + | 0.98 |
| Cockroach IgE (kU/L) ‡ | 2.08 ± 0.17 | 1.75 ± 0.15 | 1.19 (0.69, 2.06) + | 0.53 |
| Mouse IgE (kU/L) ‡ | 1.16 ± 0.11 | 1.17 ± 0.10 | 0.99 (0.53, 1.85) + | 0.97 |
| Dog IgE (kU/L) ‡ | 1.47 ± 0.12 | 2.28 ± 0.19 | 0.64 (0.38, 1.10) + | 0.10 |
| Total IgE (kU/L) ‡ | 231.4 ± 13.6 | 250.3 ± 15.2 | 0.92 (0.63, 1.36) + | 0.69 |
Plus–minus values are means ±SE over the 40-week treatment period.
Unrounded values were used to determine the difference between groups for the 40 week treatment
Due to skewed distribution, log transformation was performed and the geometric mean and standard errors are reported. For these variables the ratio rather than difference is reported.
Prespecified subgroup analysis based on baseline age , BMI, asthma control level, asthma step therapy, ETS and race revealed no difference in ability to reduce step therapy between the intervention and control groups (Table S6). Sensitization and exposure for each allergen was similar between groups and is reported in Table S7. When analyzing only those subjects exposed and sensitized to roach and mouse, there was also no significant difference in ability to reduce step therapy between the intervention and control groups or any of the other measured outcomes.
Post-hoc comparison of subjects for whom mouse and cockroach allergen reduction occurred during the study period with subjects for whom specific allergen reduction did not occur, regardless of intervention assignment, was performed using a longitudinal cluster analysis. For both mouse and cockroach exposure, a two-group solution was found as optimal; subjects were clustered in with/without an allergen reduction throughout the study. Subjects who experienced significant reduction of mouse in the kitchen (n= 122) were able to reduce asthma therapy from step 4.25 at the time of randomization to step 2.74 at study completion; those subjects without significant decline in kitchen mouse (n=125) reduced asthma therapy from step 4.57 to 3.47 (effect size 0.53 95% CI (0.07, 0.99), p =0.02). Similar analysis comparing reduction of cockroach allergen in the kitchen or bedroom and reduction of mouse allergen in the bedroom did not lead to a significant difference in ability to reduce asthma treatment burden.
DISCUSSION
Exposure to indoor allergens among sensitized asthmatic patients has been associated with worse asthma severity and increased healthcare utilization (41–43). In inner city communities particularly, exposure and sensitization to indoor allergens has been identified as an independent risk factor for poor asthma-related outcomes. (1–3), (43, 44).Efforts to reduce indoor allergens has been recommended in published guidelines as a treatment for asthma (26). However, previously conducted environmental intervention studies have demonstrated conflicting results regarding effectiveness of household allergen reduction. The effect of household allergen avoidance on real-world asthma management is not clear, partly because of the lack of control for concurrent asthma therapy as interventions are implemented. In one study, Halken and colleagues demonstrated that reduction in dust mite exposure led to significant reduction in inhaled corticosteroid (ICS) dose among dust mite allergic children, however, the study was not designed to test prospectively this outcome and asthma therapy at baseline was not standardized prior to randomization(25).
Our study demonstrates that in a population of inner city adults and children exposed and sensitized to common indoor allergens and receiving optimal guideline-based asthma therapy, environmental control measures effectively reduced levels of all measured household allergens (roach, mouse, dust mite, cat and dog), but did not lead to further reduction in need for asthma controller therapy compared with a control group receiving a home visit which did not target allergies and asthma. However, in our study, the control group also experienced a significant reduction in concentration of several allergens (dust mite, roach and mouse in bedroom), thus making it difficult to assess the direct effect of the allergen intervention measures on reduction in asthma therapy through reduced allergen exposure. Allergen reduction in the control group of our study may have been due to preparation by subjects for expected visits to the home. Some of the treatment reduction in both groups, beyond the effect of allergen reduction, may have been due to enrollment in a study with regular follow up and dispensing of medications free of charge. The difference in our results compared with previously published studies showing an effect of environmental remediation may be due to the initial optimization of pharmacologic asthma therapy in our study. Additionally enrollment in our study required both sensitization and exposure to at least one indoor allergen, a criterion not used in many previous studies.
While it is recognized that environments outside of the home may differ considerably between children and adults, exposure to household allergens is expected to be similar and thus both age groups were included in this trial. To our knowledge, our study is the first prospective allergen reduction study controlling for concurrent asthma medication use. Post-hoc analysis showed that participants with a significant decline in mouse allergen in the kitchen, regardless of treatment arm, had a significant reduction in NAEPP step therapy compared with those participants not experiencing a reduction in mouse allergen. This finding was not seen in those participants with reduction in cockroach allergen, another allergen often cited as a major contributor to asthma morbidity in the inner city. Ahluwalia, et al also recently reported on the strong association of mouse allergen, but not cockroach allergen, with poor asthma outcomes in inner city children in Baltimore (3). Given the high prevalence of mouse allergen in inner city homes (23), these findings highlight the need to perform prospective studies targeting mouse allergen in the inner city. Some potential weaknesses of our study include the fact that our study was underpowered to detect differences in the effect of the intervention in children versus adults and did not include effects of air pollution and outdoor exposures.
Our study population required relatively high doses of controller therapy to achieve asthma control, consistent with advanced severity of asthma often noted in inner city residents (4, 45). However, even among those patients requiring the highest asthma step therapy, household allergen reduction did not allow for significant reduction in therapy or improvement in asthma control compared with the control group.
Supplementary Material
Highlights Box.
What is already known about this topic: Roach and mouse allergen appear to be the most important allergens associated with asthma morbidity in inner city residents. Intervention trials for reducing household allergens report mixed results in terms of improving asthma morbidity.
What does this article add to our knowledge? Individualized household allergen intervention does not lead to incremental reduction in asthma step-level care.
How does this study impact current management guidelines? This study highlights the need for further studies to inform current guidelines for allergen avoidance in asthmatic individuals.
Abbreviations
- NAEPP
National Asthma Education and Prevention Program
- FEV1
Forced Expiratory Volume in one second
- eNO
exhaled nitric oxide
- Mch
methacholine
- AQLQ
Asthma Quality of Life Questionnaire
- ACT
Asthma Control Test
Footnotes
ClinicalTrials.gov Identifier: NCT01593111
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chew GL, Perzanowski MS, Canfield SM, Goldstein IF, Mellins RB, Hoepner LA, et al. Cockroach allergen levels and associations with cockroach-specific IgE. The Journal of allergy and clinical immunology. 2008 Jan;121(1):240–5. doi: 10.1016/j.jaci.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 2.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. The New England journal of medicine. 1997 May 8;336(19):1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 3.Ahluwalia SK, Peng RD, Breysse PN, Diette GB, Curtin-Brosnan J, Aloe C, et al. Mouse allergen is the major allergen of public health relevance in Baltimore City. The Journal of allergy and clinical immunology. 2013 Oct;132(4):830–5. e1–2. doi: 10.1016/j.jaci.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gruchalla RS, Pongracic J, Plaut M, Evans R, 3rd, Visness CM, Walter M, et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. The Journal of allergy and clinical immunology. 2005 Mar;115(3):478–85. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Morgan WJ, Crain EF, Gruchalla RS, O'Connor GT, Kattan M, Evans R, 3rd, et al. Results of a home-based environmental intervention among urban children with asthma. The New England journal of medicine. 2004 Sep 9;351(11):1068–80. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 6.Arbes SJ, Jr, Gergen PJ, Vaughn B, Zeldin DC. Asthma cases attributable to atopy: results from the Third National Health and Nutrition Examination Survey. The Journal of allergy and clinical immunology. 2007 Nov;120(5):1139–45. doi: 10.1016/j.jaci.2007.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perzanowski MS, Sporik R, Squillace SP, Gelber LE, Call R, Carter M, et al. Association of sensitization to Alternaria allergens with asthma among school-age children. The Journal of allergy and clinical immunology. 1998 May;101(5):626–32. doi: 10.1016/S0091-6749(98)70170-8. [DOI] [PubMed] [Google Scholar]
- 8.Perzanowski MS, Ronmark E, Platts-Mills TA, Lundback B. Effect of cat and dog ownership on sensitization and development of asthma among preteenage children. American journal of respiratory and critical care medicine. 2002 Sep 1;166(5):696–702. doi: 10.1164/rccm.2201035. [DOI] [PubMed] [Google Scholar]
- 9.Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM, Group SS. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006 Jan;129(1):15–26. doi: 10.1378/chest.129.1.15. [DOI] [PubMed] [Google Scholar]
- 10.Wechsler ME, Lehman E, Lazarus SC, Lemanske RF, Jr, Boushey HA, Deykin A, et al. beta-Adrenergic receptor polymorphisms and response to salmeterol. American journal of respiratory and critical care medicine. 2006 Mar 1;173(5):519–26. doi: 10.1164/rccm.200509-1519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu AC, Butler MG, Li L, Fung V, Kharbanda EO, Larkin EK, et al. Primary Adherence to Controller Medications for Asthma is Poor. Annals of the American Thoracic Society. 2015 Jan 8; doi: 10.1513/AnnalsATS.201410-459OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelkes M, Janssens HM, de Jongste JC, Sturkenboom MC, Verhamme KM. Medication adherence and the risk of severe asthma exacerbations: a systematic review. The European respiratory journal. 2014 Oct 16; doi: 10.1183/09031936.00075614. [DOI] [PubMed] [Google Scholar]
- 13.McNally KA, Rohan J, Schluchter M, Riekert KA, Vavrek P, Schmidt A, et al. Adherence to combined montelukast and fluticasone treatment in economically disadvantaged african american youth with asthma. The Journal of asthma : official journal of the Association for the Care of Asthma. 2009 Nov;46(9):921–7. doi: 10.3109/02770900903229651. [DOI] [PubMed] [Google Scholar]
- 14.Carlton BG, Lucas DO, Ellis EF, Conboy-Ellis K, Shoheiber O, Stempel DA. The status of asthma control and asthma prescribing practices in the United States: results of a large prospective asthma control survey of primary care practices. The Journal of asthma : official journal of the Association for the Care of Asthma. 2005 Sep;42(7):529–35. doi: 10.1081/JAS-67000. [DOI] [PubMed] [Google Scholar]
- 15.Finkelstein JA, Lozano P, Shulruff R, Inui TS, Soumerai SB, Ng M, et al. Self-reported physician practices for children with asthma: are national guidelines followed? Pediatrics. 2000 Oct;106(4 Suppl):886–96. [PubMed] [Google Scholar]
- 16.Gotzsche PC, Johansen HK. House dust mite control measures for asthma: systematic review. Allergy. 2008 Jun;63(6):646–59. doi: 10.1111/j.1398-9995.2008.01690.x. [DOI] [PubMed] [Google Scholar]
- 17.Woodcock A, Forster L, Matthews E, Martin J, Letley L, Vickers M, et al. Control of exposure to mite allergen and allergen-impermeable bed covers for adults with asthma. The New England journal of medicine. 2003 Jul 17;349(3):225–36. doi: 10.1056/NEJMoa023175. [DOI] [PubMed] [Google Scholar]
- 18.Platts-Mills TA, Tovey ER, Mitchell EB, Mozarro H. Long-term effects of living in a dust-free room on patients with allergic asthma - reversal of bronchial hyper-reactivity. Monographs in allergy. 1983;18:153–5. [PubMed] [Google Scholar]
- 19.Crain EF, Walter M, O'Connor GT, Mitchell H, Gruchalla RS, Kattan M, et al. Home and allergic characteristics of children with asthma in seven U.S. urban communities and design of an environmental intervention: the Inner-City Asthma Study. Environmental health perspectives. 2002 Sep;110(9):939–45. doi: 10.1289/ehp.02110939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carter MC, Perzanowski MS, Raymond A, Platts-Mills TA. Home intervention in the treatment of asthma among inner-city children. The Journal of allergy and clinical immunology. 2001 Nov;108(5):732–7. doi: 10.1067/mai.2001.119155. [DOI] [PubMed] [Google Scholar]
- 21.Torjusen EN, Diette GB, Breysse PN, Curtin-Brosnan J, Aloe C, Matsui EC. Dose-response relationships between mouse allergen exposure and asthma morbidity among urban children and adolescents. Indoor air. 2013 Aug;23(4):268–74. doi: 10.1111/ina.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phipatanakul W, Eggleston PA, Wright EC, Wood RA. Mouse allergen. I. The prevalence of mouse allergen in inner-city homes. The National Cooperative Inner-City Asthma Study. The Journal of allergy and clinical immunology. 2000 Dec;106(6):1070–4. doi: 10.1067/mai.2000.110796. [DOI] [PubMed] [Google Scholar]
- 23.Phipatanakul W, Eggleston PA, Wright EC, Wood RA National Coooperative Inner-City Asthma S. Mouse allergen. II. The relationship of mouse allergen exposure to mouse sensitization and asthma morbidity in inner-city children with asthma. The Journal of allergy and clinical immunology. 2000 Dec;106(6):1075–80. doi: 10.1067/mai.2000.110795. [DOI] [PubMed] [Google Scholar]
- 24.Chew GL, Perzanowski MS, Miller RL, Correa JC, Hoepner LA, Jusino CM, et al. Distribution and determinants of mouse allergen exposure in low-income New York City apartments. Environmental health perspectives. 2003 Aug;111(10):1348–51. doi: 10.1289/ehp.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halken S, Host A, Niklassen U, Hansen LG, Nielsen F, Pedersen S, et al. Effect of mattress and pillow encasings on children with asthma and house dust mite allergy. The Journal of allergy and clinical immunology. 2003 Jan;111(1):169–76. doi: 10.1067/mai.2003.5. [DOI] [PubMed] [Google Scholar]
- 26.Program. NAEaP. Expert Panel Report 3, Guidelines for the Diagnosis and Management of Asthma. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute; Bethesda, MD: 2007. [Google Scholar]
- 27.Donohue KM, Al-alem U, Perzanowski MS, Chew GL, Johnson A, Divjan A, et al. Anti-cockroach and anti-mouse IgE are associated with early wheeze and atopy in an inner-city birth cohort. The Journal of allergy and clinical immunology. 2008 Nov;122(5):914–20. doi: 10.1016/j.jaci.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. The New England journal of medicine. 2011 Mar 17;364(11):1005–15. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szefler SJ, Mitchell H, Sorkness CA, Gergen PJ, O'Connor GT, Morgan WJ, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008 Sep 20;372(9643):1065–72. doi: 10.1016/S0140-6736(08)61448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Filep S, Tsay A, Vailes L, Gadermaier G, Ferreira F, Matsui E, et al. A multi-allergen standard for the calibration of immunoassays: CREATE principles applied to eight purified allergens. Allergy. 2012 Feb;67(2):235–41. doi: 10.1111/j.1398-9995.2011.02750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olmedo O, Goldstein IF, Acosta L, Divjan A, Rundle AG, Chew GL, et al. Neighborhood differences in exposure and sensitization to cockroach, mouse, dust mite, cat, and dog allergens in New York City. The Journal of allergy and clinical immunology. 2011 Aug;128(2):284–92. e7. doi: 10.1016/j.jaci.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Thoracic S, European Respiratory S. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. American journal of respiratory and critical care medicine. 2005 Apr 15;171(8):912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 33.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. The Journal of allergy and clinical immunology. 2004 Jan;113(1):59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Liu AH, Zeiger RS, Sorkness CA, Ostrom NK, Chipps BE, Rosa K, et al. The Childhood Asthma Control Test: retrospective determination and clinical validation of a cut point to identify children with very poorly controlled asthma. The Journal of allergy and clinical immunology. 2010 Aug;126(2):267–73. 73 e1. doi: 10.1016/j.jaci.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 35.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. The European respiratory journal. 1999 Jul;14(1):32–8. doi: 10.1034/j.1399-3003.1999.14a08.x. [DOI] [PubMed] [Google Scholar]
- 36.Wildfire JJ, Gergen PJ, Sorkness CA, Mitchell HE, Calatroni A, Kattan M, et al. Development and validation of the Composite Asthma Severity Index--an outcome measure for use in children and adolescents. The Journal of allergy and clinical immunology. 2012 Mar;129(3):694–701. doi: 10.1016/j.jaci.2011.12.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine--reporting of subgroup analyses in clinical trials. N Engl J Med. 2007 Nov 22;357(21):2189–94. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 38.Wood SN. Stable and efficient multiple smoothing parameter estimation for generalized additive models. Journal of the American Statistical Association. 2004;99:673–86. [Google Scholar]
- 39.Team RDC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. [Google Scholar]
- 40.Little RJ, Rubin DB. Causal effects in clinical and epidemiological studies via potential outcomes: concepts and analytical approaches. Annual review of public health. 2000;21:121–45. doi: 10.1146/annurev.publhealth.21.1.121. [DOI] [PubMed] [Google Scholar]
- 41.Kivity S, Solomon A, Soferman R, Schwarz Y, Mumcuoglu KY, Topilsky M. Mite asthma in childhood: a study of the relationship between exposure to house dust mites and disease activity. The Journal of allergy and clinical immunology. 1993 Apr;91(4):844–9. doi: 10.1016/0091-6749(93)90341-c. [DOI] [PubMed] [Google Scholar]
- 42.van der Heide S, de Monchy JG, de Vries K, Bruggink TM, Kauffman HF. Seasonal variation in airway hyperresponsiveness and natural exposure to house dust mite allergens in patients with asthma. The Journal of allergy and clinical immunology. 1994 Feb;93(2):470–5. doi: 10.1016/0091-6749(94)90356-5. [DOI] [PubMed] [Google Scholar]
- 43.van Strien RT, Verhoeff AP, van Wijnen JH, Doekes G, de Meer G, Brunekreef B. Infant respiratory symptoms in relation to mite allergen exposure. The European respiratory journal. 1996 May;9(5):926–31. doi: 10.1183/09031936.96.09050926. [DOI] [PubMed] [Google Scholar]
- 44.Huss K, Adkinson NF, Jr, Eggleston PA, Dawson C, Van Natta ML, Hamilton RG. House dust mite and cockroach exposure are strong risk factors for positive allergy skin test responses in the Childhood Asthma Management Program. The Journal of allergy and clinical immunology. 2001 Jan;107(1):48–54. doi: 10.1067/mai.2001.111146. [DOI] [PubMed] [Google Scholar]
- 45.Gergen PJ, Togias A. Inner city asthma. Immunology and allergy clinics of North America. 2015 Feb;35(1):101–14. doi: 10.1016/j.iac.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.