Abstract
Background
Heart failure (HF) is divided into heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF). Mortality from HF is inversely related to left ventricular function. Additional studies are required to distinguish between these two types of HF. A previous study showed that HFrEF is less likely when electrocardiogram (ECG) findings are normal. This study aims to create a scoring system based on ECG findings that will predict the type of HF.
Methods
We performed a cross-sectional study analyzing ECG and echocardiographic data from 110 subjects with chronic HF. HFrEF was defined as an ejection fraction ≤ 40%.
Results
Fifty people were diagnosed with HFpEF and 60 people suffered from HFrEF. Multiple logistic regression analysis revealed certain ECG variables that were independent predictors of HFrEF, i.e., left atrial hypertrophy (LAH), QRS duration > 100 ms, right bundle branch block (RBBB), ST-T segment changes and prolongation of the QT interval. Based on receiver operating characteristic (ROC) curve analysis, we obtained a score for HFpEF of -1 to +3, while HFrEF had a score of +4 to +6 with 76% sensitivity, 96% specificity, a 95% positive predictive value, an 80% negative predictive value and an accuracy of 86%.
Conclusions
The scoring system derived from this study, including the presence or absence of LAH, QRS duration > 100 ms, RBBB, ST-T segment changes and prolongation of the QT interval can be used to predict the type of HF with satisfactory sensitivity and specificity.
Keywords: Chronic heart failure, Scoring system, Electrocardiogram features, Type of heart failure
Introduction
Heart failure (HF) has a high incidence, is one of the major causes of mortality from cardiovascular diseases in the world and is a major health problem in society [1]. HF is divided into heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF) [2]. In the literature, HFrEF and HFpEF are also referred to as systolic and diastolic HF, respectively. Patients with systolic dysfunction can also exhibit diastolic dysfunction, particularly in late stage HF [3]. The mortality from HF is inversely related to left ventricular systolic function. Ejection fraction is considered one of the strongest prognosistic factors that influences a poor outcome for HF patients [4].
Echocardiography is considered the gold standard for assessing diastolic or systolic dysfunction in patients with HF. However, an expert is required to conduct the examination and not all health facilities provide an echocardiography machine. Thus, a simple device that can measure both systolic and diastolic function can help a physician determine the diagnosis [5]. Electrocardiogram (ECG) and X-ray examinations are inexpensive tools that are accessible in almost all primary health care settings. A normal or minor change in the ECG is consistent with a low likelihood of left ventricle dysfunction. Conversely, left ventricle systolic dysfunction is usually accompanied by major ECG changes [6]. The ECG is useful because it can serve as an initial investigative tool that physicians can use to determine the presence of systolic and diastolic dysfunction in patients with chronic HF, though it cannot replace echocardiography.
A scoring system is a simple method for diagnosing disease [7]. Several scoring systems based on ECG findings have been studied to estimate left ventricular function. However, additional studies have suggested that scoring systems have limitations or are less accurate in estimating left ventricular function in patients with coronary heart disease [8, 9]. To our knowledge, no previous study has evaluated a scoring system based on ECG results to predict HFpEF or HFrEF in patients with HF.
Methods
We used a cross-sectional research design to determine a scoring system based on ECG findings to predict the type of HF (HFpEF or HFrEF). The population consisted of patients with chronic HF that were hospitalized or seen as outpatients in Dr. Sardjito General Hospital between April and July 2015 and in whom both echocardiography and ECG had been conducted.
The inclusion criteria were patients with HF diagnosed based on ESC (2012) or AHA (2013) guidelines, the presence of sinus rhythm, age > 18 years and agreement to participate in the study. The exclusion criteria were patients with congenital heart disease, primary valve disease, massive pericardial effusion, patients with acute coronary syndrome, severe pulmonary disease (cor pulmonale, pneumothorax) or who had pacemakers.
The independent variable in this study was the ECG findings in patients with chronic HF. The dependent variable was chronic HF (HFrEF or HFpEF). The confounding variable was patient medications.
Subjects were enrolled in the cardiovascular clinic, the hospital ward, and the echocardiography clinic at Dr. Sardjito general hospital. Subjects who met the inclusion criteria were included in the study and were enrolled consecutively. The collected data included demographic information, clinical examination, ECG and echocardiography results. Demographic data included age, sex and medication history. Clinical data included the NYHA class determination.
Twelve lead ECGs were obtained by a nurse or a cardiology resident with patients in the supine position at a speed of 25 mm/s. The ECGs were read by three expert physicians (who were blinded to the results of the echocardiogram). The ECG report included an evaluation of heart rhythm and rate, heart axis, presence or absence of chamber enlargement, intraventricular block, and ST-T segment changes, and the duration of the QRS complex, QT and QTc intervals. A wide QRS complex was defined as QRS duration > 100 ms. The echocardiogram was supervised by a cardiologist and two expert examiners (who were blinded to the ECG result). Echocardiography data included a determination of the ejection fraction (calculated by Simpson’s method) and the presence or absence of diastolic dysfunction.
HF was divided into HFrEF and HFpEF. The criterion for a diagnosis of HFrEF and HFpEF was an ejection fraction ≤ 40% and > 40%, respectively. Subjects were divided into two groups: HFrEF group and HfpEF group.
Bivariate analysis was used to analyze the relationship between the ECG results and HFrEF or HFpEF. Inter-variable bivariate analysis was analyzed with the Chi-square test followed by Fisher’s test. Further, variables in the bivariate analysis with a P < 0.25 were tested using a multivariate analysis, specifically logistic regression with a backward stepwise method. Each variable from the multivariate analysis was scored by using B and SE values (from SPSS analysis program). There were two scoring systems based on the probability and cut-off point from the ROC curve. Next, the scoring system was validated in several samples to obtain the diagnostic value. The entire data analysis was conducted in SPSS version 16 program.
Additional information about the research subjects that was deemed necessary for the study was collected from the medical record or via a direct interview. Sample data collection was non-probability sampling.
Ethics
The research was conducted after receiving permission from the Faculty of Medicine at UGM/RSUP Dr. Sardjito Ethical Committee. Informed consent was obtained from the subjects after they received complete information about the trial.
Results
Data were collected between April and July 2015 from patients seen in the cardiovascular clinic, hospital ward and echocardiography clinic at Dr. Sardjito General Hospital. A total of 110 HF patients met the inclusion criteria.
Sixty patient (54.5%) had systolic heart failure (HFrEF) and 50 patients had diastolic heart failure (HFpEF). There was no significant difference in the average age of patients in the HFpEF and HFrEF group (59.7 ± 9.2 and 57.9 ± 10.1 years, respectively). All of the subjects met NYHA class II or III criteria (Table 1).
Table 1. Basic Subject Characteristics Based on Heart Failure Type.
| Variable | HFpEF (n = 50) | HFrEF (n = 60) | Total (n = 110) | P |
|---|---|---|---|---|
| Age, years (± SD) | 59.7 ± 9.2 | 57.9 ± 10.1 | 58.6 ± 9.9 | 0.29 |
| Sex | 0.003 | |||
| Male, n (%) | 28 (56) | 49 (81.7) | 77 (70) | |
| Female, n (%) | 22 (44) | 11 (18.3) | 33 (30) | |
| Risk factor | ||||
| Hypertension, n (%) | 46 (92) | 39 (65) | 85 (77.2) | 0.001 |
| DM, n (%) | 19 (38) | 13 (21.7) | 32 (29.1) | 0.06 |
| Smoker, n (%) | 11 (22) | 29 (48.3) | 40 (36.3) | 0.004 |
| Therapy | ||||
| ACE-I/ARB, n (%) | 50 (100) | 60 (100) | 110 (100) | 1.000 |
| Diuretic, n (%) | 37 (74) | 57 (95) | 94 (85.4) | 0.002 |
| Beta blocker, n (%) | 21 (42) | 30 (50) | 51 (46.3) | 0.042 |
| CCB, n (%) | 22 (44) | 6 (10) | 28 (25.4) | < 0.0001 |
HFpEF: heart failure preserved ejection fraction; HFrEF: heart failure reduced ejection fraction; DM: diabetes mellitus; ACE-I: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker; CCB: calcium channel blocker.
Patients with HFrEF had a larger left atrial diameter, left atrial volume index (LAVI), left ventricular mass index (LVMI), left ventricular internal diameter end diastole (LVIDd) and E/A ratio compared to patients with HFpEF, while interventricular septal end diastole (IVSs), deceleration time (DT) and tricuspid annular plane systolic excursion (TAPSE) were lower in patients with HFrEF. Kinetic disturbance in the myocardium was found in all patients with HFrEF patients and in 30% of patients with HFpEF. Characteristics of the echocardiograms are shown in Table 2.
Table 2. Echocardiography Basic Characteristics Based on Heart Failure Type.
| Variables | HFpEF (n = 50) | HFrEF (n = 60) | P |
|---|---|---|---|
| LA diameter, mm (± SD) | 34.3 ± 5.2 | 41.5 ± 6.2 | < 0.0001 |
| LVIDd, mm (± SD) | 49.2 ± 7.1 | 64.9 ± 7.9 | < 0.0001 |
| IVSd, mm (± SD) | 12.5 ± 2.1 | 10.3 ± 2.6 | < 0.0001 |
| EF Simpson, % (± SD) | 59.2 ± 8.5 | 29.4 ± 6.8 | < 0.0001 |
| LVMI, g/m2 (± SD) | 133.3 ± 40.2 | 170.3 ± 46.7 | < 0.0001 |
| Septal e’ | 5.09 ± 1.1 | 4.3 ± 1.2 | < 0.0001 |
| Lateral e’ | 6.21 ± 1.6 | 5.6 ± 2.5 | 0.146 |
| LAVI, mL/m2 (± SD) | 34.5 ± 4.3 | 47.1 ± 14.4 | < 0.0001 |
| E/A ratio | 0.77 ± 0.2 | 1.8 ± 1.2 | < 0.0001 |
| DT, ms (± SD) | 234 ± 53.3 | 158.4 ± 52.8 | < 0.0001 |
| Diastolic dysfunction | 1.000 | ||
| Relaxation, n | 39 | 16 | |
| Pseudonormal, n | 11 | 15 | |
| Restrictive, n | 0 | 29 | |
| TAPSE, mm (± SD) | 23.2 ± 3.1 | 18.1 ± 4.3 | < 0.0001 |
| RVSP, mm Hg (± SD) | 6.64 ± 9.1 | 26.8 ± 22.6 | < 0.0001 |
| Myocard kinetic disturbance | < 0.0001 | ||
| Yes | 15 | 60 | |
| No | 35 | 0 | |
| Heart rate, /min (± SD) | 71.8 ± 12.8 | 81.7 ± 15 | 0.135 |
LA: left atrium; LVIDd: left ventricular internal diameter end diastole; IVSd: Interventricular septal end diastole; EF: ejection fraction; LVMI: left ventricular mass index; LAVI: left atrial volume index; DT: deceleration time; TAPSE: tricuspid annular plane systolic excursion; RVSP: right ventricle systolic pressure.
The major ECG abnormalities were a prolonged QT interval (62.7%), ST-T segment changes (55.4%) and prolonged QRS duration (48.2%). Thirty-eight percent of patients with HFpEF had ST-T segment changes and a prolonged QT interval, while a prolonged QT interval, prolonged QRS duration and ST-T segment changes were found in 83.3%, 71.7% and 70% of patients with HFrEF, respectively. ECG characteristics are shown in Table 3.
Table 3. ECG Basic Characteristics Based on Heart Failure Type.
| ECG parameter | HFpEF (n = 50) | HFrEF (n = 60) | Total (n = 110) | P |
|---|---|---|---|---|
| LAH, n (%) | 6 (12) | 22 (36.7) | 28 (25.5) | 0.003 |
| LVH, n (%) | 15 (30) | 33 (55) | 48 (43.6) | 0.008 |
| Poor R wave, n (%) | 5 (10) | 30 (50) | 35 (31.8) | < 0.0001 |
| LAD, n (%) | 15 (30) | 27 (45) | 42 (38.2) | 0.107 |
| RAD, n (%) | - | 3 (5) | 3 (2.7) | 0.249 |
| Q wave, n (%) | 9 (18) | 17 (28.3) | 26 (23.6) | 0.204 |
| Wide QRS, n (%) | 10 (20) | 43 (71.7) | 53 (48.2) | < 0.0001 |
| QRS duration, ms (± SD) | 97.3 ± 20.7 | 124 ± 30.4 | ||
| LBBB, n (%) | - | 12 (20) | 12 (10.9) | 0.001 |
| RBBB, n (%) | 7 (14) | 3 (5) | 10 (9.1) | 0.181 |
| ST-T changes, n (%) | 19 (38) | 42 (70) | 61 (55.4) | 0.001 |
| Prolong QT, n (%) | 19 (38) | 50 (83.3) | 69 (62.7) | < 0.0001 |
| Interval QTc, ms (± SD) | 453.2 ± 42.8 | 499 ± 50.9 |
LAH: left atrial hypertrophy; LVH: left ventricular hypertrophy; LAD: left axis deviation; RAD: right axis deviation; LBBB: left bundle branch block; RBBB: right bundle branch block.
Multivariate analysis (Table 4) demonstrated that the ECG variables influencing whether a patient had HFpEF or HFrEF were left atrial hypertrophy (LAH), a wide QRS complex, right bundle branch block (RBBB), ST-T changes and a prolonged QT interval. The power of the association from largest to smallest was a wide QRS (OR 12.657), prolonged QT interval (OR 7.401), LAH (OR 4.449), ST-T segment changes (OR 4.35) and RBBB (OR 0.109). Thus, the scores from these variables were used to calculate the scoring system (Table 5).
Table 4. ECG Variable Multivariate Analysis Variable ECG With Logistic Regression to Test the Possibility of Systolic Heart Failure or HFrEF (n = 110) .
| ECG parameter | OR | IK 95% | P |
|---|---|---|---|
| LVH | 1.24 | 0.284 - 5.407 | 0.774 |
| Poor R wave | 2.004 | 0.423 - 9.494 | 0.381 |
| LAD | 0.661 | 0.161 - 2.708 | 0.565 |
| RAD | # | # | # |
| Q wave | 3.756 | 0.947 - 14.891 | 0.060 |
| LBBB | # | # | # |
| LAH | 4.449 | 1.109 - 17.848 | 0.035* |
| Wide QRS | 12.657 | 3.277 - 48.895 | < 0.0001* |
| RBBB | 0.109 | 0.012 - 0.986 | 0.049* |
| ST-T changes | 4.35 | 1.277 - 14.817 | 0.019* |
| Prolong QT | 7.401 | 2.134 - 25.672 | 0.002* |
*Statistically significant. #n.a. LAH: left atrial hypertrophy; LVH: left ventricular hypertrophy; LAD: left axis deviation; RAD: right axis deviation; LBBB: left bundle branch block; RBBB: right bundle branch block.
Table 5. Calculated Score for Each ECG Variable Resulting From the Multivariate Analysis.
| ECG parameter | B | SE | B/SE | (B/SE)/x | Score |
|---|---|---|---|---|---|
| LAH | 1.493 | 0.709 | 2.105 | 1.068 | 1 |
| Wide QRS | 2.538 | 0.69 | 3.678 | 1.865 | 2 |
| RBBB | -2.214 | 1.123 | -1.971 | -1 | -1 |
| ST-T changes | 1.47 | 0.625 | 2.352 | 1.192 | 1 |
| Prolong QT | 2.002 | 0.635 | 3.152 | 1.599 | 2 |
LAH: left atrial hypertrophy; RBBB: right bundle branch block.
The first scoring model was based on a subject’s probability of having HFrEF. The minimum score was -1, and the maximum score was +6 (Table 5). A regression tool was used to calculate the probability of each subject having systolic HF (Table 6).
Table 6. Scoring System Based on the Probability of Systolic Heart Failure (HFrEF).
| Score | Probability (%) |
|---|---|
| -1 | 0.9 |
| 0 | 3.16 |
| 1 | 10.4 |
| 2 | 29.3 |
| 3 | 59.6 |
| 4 | 84 |
| 5 | 94.9 |
| 6 | 98.5 |
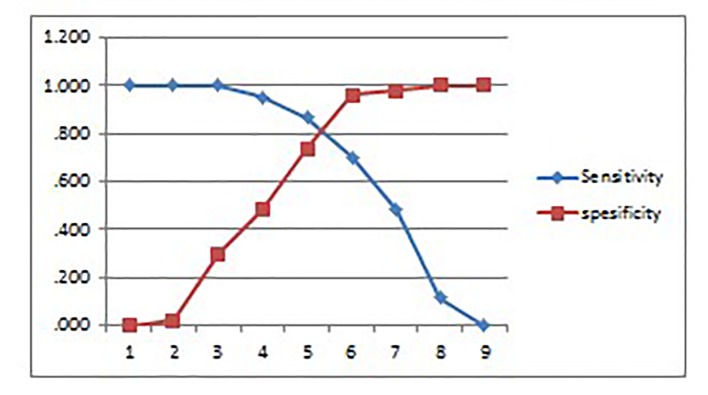
The second scoring model was based on a cut-off point derived from the receiver operating characteristic (ROC) curve analysis. The ROC method is based on a calculation between sensitivity and specificity values surrounding various cut-off points represented in the graph. Results of the ROC curve analysis are shown in Figure 1 and Table 7.
Figure 1.
The sensitivity and specificity based on a cut-off point derived from the ROC curve analysis.
Table 7. Sensitivity and Specificity for Each Point From Graph (Figure 1) Subjected to the Possibility of the Patient to Having Either HFpEF or HFrEF.
| No. | Positive if greater than or equal to | Sensitivity | 1 - specificity | Sensitivity | Specificity |
|---|---|---|---|---|---|
| 1 | -2.0000 | 1.000 | 1.000 | 1.000 | 0.000 |
| 2 | -0.5000 | 1.000 | 0.980 | 1.000 | 0.020 |
| 3 | 0.5000 | 1.000 | 0.700 | 1.000 | 0.300 |
| 4 | 1.5000 | 0.950 | 0.520 | 0.950 | 0.480 |
| 5 | 2.5000 | 0.867 | 0.260 | 0.867 | 0.740 |
| 6 | 3.5000 | 0.700 | 0.040 | 0.700 | 0.960 |
| 7 | 4.5000 | 0.483 | 0.020 | 0.483 | 0.980 |
| 8 | 5.5000 | 0.117 | 0.000 | 0.117 | 1.000 |
| 9 | 7.0000 | 0.000 | 0.000 | 0.000 | 1.000 |
From the cut-off analysis in Figure 1 and Table 7, the cut-off value was 4. A score of +4 to +6 indicated the possibility of systolic heart failure (HFrEF), and a score of -1 to +3 indicated the possibility diastolic heart failure (HFpEF).
The scoring system was validated with additional samples to better determine the diagnostic value of the result and demonstrated 76% sensitivity, 96% specificity, 95% positive predictive value, 80% negative predictive value and an accuracy of 86% (Table 8).
Table 8. Validity Test Based on the Scoring System of Heart Failure Patients (n = 50).
| Scoring system | ECHO (gold standard) |
|
|---|---|---|
| HFrEF (EF ≤ 40%) | HFpEF (EF > 40%) | |
| HFrEF (+4 SD +6) | 19 | 1 |
| HFpEF (-1 SD +3) | 6 | 24 |
HFpEF: heart failure preserved ejection fraction; HFrEF: heart failure reduced ejection fraction.
Discussion
In this study, hypertension was the major risk factor for patients with HFpEF (92%), while HFrEF was associated with multiple risk factors. This result is in agreement with Paulus and Tschope (2013) and Tsutsui et al (2010) who found that hypertension is comorbid in HFpEF, while HFrEF is mostly associated with ischemic heart disease with multiple risk factors such as hypertension, DM or smoking [10, 11].
Patients with HFrEF had evidence of morphological changes such as an increase in the size of the left ventricle accompanied by an increase in end-systolic and end-diastolic volume, reduced wall thickness and increased left ventricular mass. The morphology in patients with HFrEF was opposite to that observed in patients with HFpEF (Table 2) [12]. Most of the differences in echocardiography parameters between HFrEF and HFpEF were significant (P < 0.05), i.e., LVID size (64.9 ± 7.9 vs. 49.2 ± 7.1 mm) and IVSd (12.5 ± 2.1 vs. 10.3 ± 2.6 mm).
ECG changes are usually found in patients with HF. This finding is supported by Karaye and Sani (2008) who reported ECG abnormalities in 98.2% of patients with HF. Further, the majority of these patients (65.5%) had at least three types of ECG abnormalities [13]. If the ECG was normal, the probability that a patient had systolic dysfunction (HFrEF) was small [5, 6].
Multivariate analysis showed that LAH, a wide QRS complex, RBBB, ST-T segment changes and a prolonged QT interval were independent predictors of systolic heart failure (HFrEF) (Table 4).
LAH on ECG was found in 25.5% of patients (28 out of the total number of patients). Of these, 78.5% (22 cases; P = 0.003) were from patients with HFrEF. This result is similar to that reported in a study by Karaye and Sani (2008) in which LAH was more commonly found in patients with an ejection fraction < 50% compared to those with an ejection fraction ≥ 50% (77.5% vs. 22.4%; P = 0.001) [13].
In this study, a prolonged QRS duration > 100 ms was predominantly found in patients with HFrEF compared to those with HFpEF (81.1% vs. 18.9%), with values of 124 ± 30.4 vs. 97.3 ± 20.7 ms, respectively. Murkofsky et al (1998) showed that a prolonged QRS duration (> 0.10 s) is a very specific, though not a very sensitive, indicator of left ventricular systolic function. A QRS duration of > 0.10 s has a high likelihood of being associated with an ejection fraction < 45% [14].
RBBB was mostly found in patients with HFpEF compared to those with HFrEF (70% vs. 30%), while left bundle branch block (LBBB) was found in 20% of patients with HFrEF and in no patients with HFpEF. This result is similar to the findings by Lee et al (2009) [15]. RBBB is caused by myocardial ischemia, infarction, inflammation (myocarditis), chronic increase in right ventricular pressure (cor pulmonale) and a sudden dilatation of the right ventricle (observed in acute pulmonale secondary to emboli). Less common causes of RBBB include hypertension, cardiomyopathy and congenital heart disease [16]. RBBB is also found in subjects without any underlying disease (isolated RBBB) [17]. In our study, the presence of RBBB in patients with HFpEF might have been related to age, coronary artery disease, hypertension or due to isolated RBBB.
ST-T segment changes were evident in 61 patients (55.4%). Of these, 68.8% (42 patients) were found in patients with HFrEF and 19 in patients with HFpEF. This finding supports similar findings by Basnet et al (2009) who reported that ST-T segment changes are a common ECG finding that is present in 48.57% of patients with left ventricular systolic dysfunction [18]. Nielsen et al (2000) also found that ECGs with significant Q wave abnormalities, LBBB and ST-T segment changes (P < 0.012) are associated with left ventricle systolic dysfunction [19]. Strain pattern ST-T segment changes have a strong association with an LVIDd > 55 mm compared to a posterior wall thickness > 12 mm. This observation explains the strong association between strain pattern and ventricular hypertrophy with eccentric vs. concentric remodeling [20].
A prolonged QTc interval was found in 69 patients (62.7%). Of these, 72.4% (50 patients) were patients with HFrEF. The average QTc interval in patients with HFrEF was also longer than that of patients with HFpEF (499 ± 50.9 vs. 453.2 ± 42.8 ms; P = 0.000). Wilcox et al (2011) showed that patients with grade II or III diastolic dysfunction have longer QTc intervals compared to patients with non-diastolic or grade I diastolic dysfunction (QTc 461 ± 34 vs. 432 ± 32 ms; P = 0.0003) [21]. In our study, most of the patients with HFrEF had grade III diastolic dysfunction (restrictive type) (48.3%), while 78% of patients with HFpEF had grade I diastolic dysfunction (relaxation type).
Limitation
A limitation of our study is variable duration of HF therapy for each patient, though it is difficult to determine the impact this would have on our results.
Conclusion
Our study suggests that a scoring system based on ECG findings that include the presence or absence of LAH, a wide QRS duration, RBBB, ST-T segment changes and a prolonged extended QTc interval can be used to predict the type of HF (HFpEF and HFrEF) in patients with chronic HF. This scoring system has a sensitivity of 76%, a specificity of 96%, a positive predictive value of 95%, a negative predictive value of 80% and an accuracy of 86%. A score of -1 to +3 suggests the possibility of HFpEF, while a score of +4 to +6 suggests the possibility of HFrEF.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Vasan RS, Levy D. In: Heart Failure Updates. McMurray JJV, Pfeffer MA, editors. United Kongdom: Martin Dunitz; 2003. Heart Failure Due to Diastolic Dysfunction: Definition, Diagnosis and Treatment; pp. 1–13. [DOI] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC. et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 3.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V. et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 4.Ponikowski P, Jankowska EA, Banasiak W. In: Diastolic Heart Failure. Smiseth OA, Tendera M, editors. Springer-Verlag; London: 2008. Prognosis in Diastolic Heart Failure; pp. 213–220. [DOI] [Google Scholar]
- 5.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Stromberg A. et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;29(19):2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 6.Davie AP, Francis CM, Love MP, Caruana L, Starkey IR, Shaw TR, Sutherland GR. et al. Value of the electrocardiogram in identifying heart failure due to left ventricular systolic dysfunction. BMJ. 1996;312(7025):222. doi: 10.1136/bmj.312.7025.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahlan MS. Penelitian Prognostik dan Sistem Skoring. Jatinangor: Penerbit Alqa Prisma Interdelta; 2011. pp. 49–125. [Google Scholar]
- 8.Young SG, Abouantoun S, Savvides M, Madsen EB, Froelicher V. Limitations of electrocardiographic scoring systems for estimation of left ventricular function. J Am Coll Cardiol. 1983;1(6):1479–1488. doi: 10.1016/S0735-1097(83)80052-7. [DOI] [PubMed] [Google Scholar]
- 9.Fioretti P, Brower RW, Lazzeroni E, Simoons ML, Wijns W, Reiber JH, Bos RJ. et al. Limitations of a QRS scoring system to assess left ventricular function and prognosis at hospital discharge after myocardial infarction. Br Heart J. 1985;53(3):248–252. doi: 10.1136/hrt.53.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 11.Tsutsui H, Tsuchihashi-Makaya M, Kinugawa S. Clinical characteristics and outcomes of heart failure with preserved ejection fraction: lessons from epidemiological studies. J Cardiol. 2010;55(1):13–22. doi: 10.1016/j.jjcc.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee K, Massie B. Systolic and diastolic heart failure: differences and similarities. J Card Fail. 2007;13(7):569–576. doi: 10.1016/j.cardfail.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Karaye KM, Sani MU. Electrocardiographic abnormalities in patients with heart failure. Cardiovasc J Afr. 2008;19(1):22–25. [PMC free article] [PubMed] [Google Scholar]
- 14.Murkofsky RL, Dangas G, Diamond JA, Mehta D, Schaffer A, Ambrose JA. A prolonged QRS duration on surface electrocardiogram is a specific indicator of left ventricular dysfunction [see comment] J Am Coll Cardiol. 1998;32(2):476–482. doi: 10.1016/S0735-1097(98)00242-3. [DOI] [PubMed] [Google Scholar]
- 15.Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV. et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119(24):3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sauer WH. Overview of Right Bundle Branch Block. Up To Date 19.3.
- 17.Surawicz B, Tavel ME, Knilans TK, Gering LE. Philadelphia: Saunders Elsevier; 2008. Chou's Electrocardiography in Clinical Practice; pp. 75–92.pp. 29–43. [Google Scholar]
- 18.Basnet BK, Manandhar K, Shrestha R, Shrestha S, Thapa M. Electrocardiograph and chest X-ray in prediction of left ventricular systolic dysfunction. JNMA J Nepal Med Assoc. 2009;48(176):310–313. [PubMed] [Google Scholar]
- 19.Nielsen OW, Hansen JF, Hilden J, Larsen CT, Svanegaard J. Risk assessment of left ventricular systolic dysfunction in primary care: cross sectional study evaluating a range of diagnostic tests. BMJ. 2000;320(7229):220–224. doi: 10.1136/bmj.320.7229.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okin PM, Devereux RB, Nieminen MS, Jern S, Oikarinen L, Viitasalo M, Toivonen L. et al. Relationship of the electrocardiographic strain pattern to left ventricular structure and function in hypertensive patients: the LIFE study. Losartan Intervention For End point. J Am Coll Cardiol. 2001;38(2):514–520. doi: 10.1016/S0735-1097(01)01378-X. [DOI] [PubMed] [Google Scholar]
- 21.Wilcox JE, Rosenberg J, Vallakati A, Gheorghiade M, Shah SJ. Usefulness of electrocardiographic QT interval to predict left ventricular diastolic dysfunction. Am J Cardiol. 2011;108(12):1760–1766. doi: 10.1016/j.amjcard.2011.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]