Abstract
Atherosclerosis in the carotid arteries is a major cause of ischemic stroke, which accounts for 85% of all stroke cases. Genetic factors contributing to carotid atherosclerosis remain poorly understood. The aim of this study was to identify chromosomal regions harboring genes contributing to carotid atherosclerosis in mice. From an intercross between BALB/cJ (BALB) and SM/J (SM) apolipoprotein E-deficient (Apoe−/−) mice, 228 female F2 mice were generated and fed a “Western” diet for 12 wk. Atherosclerotic lesion sizes in the left carotid artery were quantified. Across the entire genome, 149 genetic markers were genotyped. Quantitative trait locus (QTL) analysis revealed eight loci for carotid lesion sizes, located on chromosomes 1, 5, 12, 13, 15, 16, and 18. Combined cross-linkage analysis using data from this cross, and two previous F2 crosses derived from BALB, C57BL/6J and C3H/HeJ strains, identified five significant QTL on chromosomes 5, 9, 12, and 13, and nine suggestive QTL for carotid atherosclerosis. Of them, the QTL on chromosome 12 had a high LOD score of 9.95. Bioinformatic analysis prioritized Arhgap5, Akap6, Mipol1, Clec14a, Fancm, Nin, Dact1, Rtn1, and Slc38a6 as probable candidate genes for this QTL. Atherosclerotic lesion sizes were significantly correlated with non-HDL cholesterol levels (r = 0.254; p = 0.00016) but inversely correlated with HDL cholesterol levels (r = −0.134; p = 0.049) in the current cross. Thus, we demonstrated the polygenic control of carotid atherosclerosis in mice. The correlations of carotid lesion sizes with non-HDL and HDL suggest that genetic factors exert effects on carotid atherosclerosis partially through modulation of lipoprotein homeostasis.
Keywords: plaque, vessels, linkage mapping, haplotype analysis, dyslipidemia
Stroke is the leading cause of extended disability and a major cause of mortality in the United States (Mozaffarian et al. 2015). 800,000 people are estimated to experience a new or recurrent stroke and 131,000 die of stroke annually in this country. Ischemic stroke accounts for ∼85% of all stroke cases and a large fraction of them are caused by atheromas in the carotid arteries (Donnan et al. 2008). Plaque in the carotid arteries directly or indirectly, though thrombus formation, blocks the blood flow to the brain (Markus and Cullinane. 2001; Matteis et al. 1999). Genetic studies of twins and families indicate that carotid arterial intima-media thickness and plaque, which reflect a thickening of the carotid artery wall and the presence of large irregular arterial wall deposits, respectively, is a genetically determined trait with heritability ranging from 30 to 65% (Sacco et al. 2009; Swan et al. 2003; Zhao et al. 2008). Recent genome-wide association studies (GWAS) have identified over a dozen common variants associated with carotid intima-media thickness and plaque, including LRIG1, EDNRA, SLC17A4, PIK3CG, PINX1, ZHX2, APOC1, LDLR, ANGPT1, ZBTB7C, HDAC9, the BCAR1-CFDP1-TMEM170A locus, EBF1, and PCDH15 (Bis et al. 2011; Du et al. 2015; Xie et al. 2015). However, these variants explain only a tiny fraction of the total heritability of the traits, suggesting that many more remain to be discovered. Furthermore, it is challenging to assess causality between a variant and disease in humans due to small gene effects, complex genetic structures, and environmental influences. Genetic studies of animal models have contributed greatly to the understanding of the genetic basis of human diseases, including atherosclerosis. Apoe−/− mice develop all phases of atherosclerotic lesions in large- and medium-sized arteries, including the carotid arteries. QTL analysis for carotid atherosclerosis has been performed on two F2 populations derived from C57BL/6 (B6), C3H/HeJ (C3H), and BALB/cJ (BALB) strains and identified several significant and suggestive loci for the trait (Li et al. 2008; Rowlan et al. 2013a). Nevertheless, more crosses are needed to identify new QTL and expedite the finding of underlying genes for carotid atherosclerosis. We have recently found that Apoe−/− mice with a SM/J (SM) genetic background developed significantly larger atherosclerotic lesions than those with a BALB background (Liu et al. 2015). In the present study, we generated a female F2 cohort from an intercross between the two Apoe−/− strains to search for loci contributing to carotid atherosclerosis. The combined cross analysis using data from multiple intercrosses has been shown to improve the resolution of shared QTL and increase the power of identify new QTL not found in an individual cross (Li et al. 2005). Thus, in this study we also performed a combined cross-linkage analysis using data from the current cross and two previously reported B6 × C3H and B6 × BALB intercrosses (Li et al. 2008; Rowlan et al. 2013a).
Materials and Methods
Animals and experimental design
BALB and SM Apoe−/− mice were generated in our laboratory using the classic congenic breeding strategy, as described by Liu et al. (2015) and Zhang et al. (2012). The two Apoe−/− strains were crossed to generate F1s, which were intercrossed to generate a F2 population. Mice were weaned at 3 wk of age onto a chow diet. At 6 wk of age, female F2 mice were switched onto a Western diet containing 21% fat, 34.1% sucrose, 0.15% cholesterol, and 19.5% casein (TD 88137; Envigo) and maintained on the diet for 12 wk.
Quantitation of carotid atherosclerosis
Atherosclerotic lesion sizes in the left common carotid artery and its main branches were measured as previously reported with minor modifications (Li et al. 2008). Briefly, the vasculature of mice was perfused through the heart with 4% paraformaldehyde, then the distal portion of the left common carotid artery and its adjacent branches were dissected en bloc and embedded in OCT compound (Tissue-Tek). Cryosections in 10 μm thickness were collected in every three sections, stained with oil red O and hematoxylin, and counterstained with fast green. Lesion areas were measured under a microscope using Zeiss AxioVision 4.8 software. Carotid lesion sizes on all sections were added up for each mouse and this sum was used for statistical analysis.
Measurements of plasma lipids and glucose
Plasma total cholesterol, HDL cholesterol, triglyceride, and glucose were measured using assay kits as reported (Tian et al. 2005; Wang et al. 2015). Non-HDL cholesterol was calculated as the difference between total and HDL cholesterol.
Genotyping
The Illumina mouse LD linkage panel consisting of 377 SNP loci was used to genotype F2 mice, as reported (Wang et al. 2015). Microsatellite markers were typed for chromosome 8 where only one SNP marker was informative. DNA from the two parental strains and F1s served as controls. After excluding uninformative and poorly typed SNPs, 149 markers were included in genome-wide QTL analysis.
Statistical analysis
QTL analysis was performed using J/qtl. Genome-wide LOD score thresholds for significant or suggestive linkage were determined through 1000 permutations, as reported (Wang et al. 2015; Su et al. 2006; Yuan et al. 2009).
Combined cross analysis
A combined cross analysis was performed using data from the current cross and two previously published B6 × C3H and B6 × BALB intercrosses (Li et al. 2008; Rowlan et al. 2013a). Genotype data for the chromosomal regions where a suggestive or significant QTL was found in an individual cross were recoded as “High” for F2s homozygous for the allele contributing to a larger lesion size, “Low” for F2s homozygous for the allele contributing to a smaller lesion size, and “H” for F2s with heterozygous alleles at each marker. For all other regions where no QTL was found, alleles at each marker were recoded based on the progenitor strain phenotype as reported (Wergedal et al. 2007). Phenotype data on carotid lesion sizes were switched from the total lesion area to the average of the top five lesion sizes for each F2 mouse in all crosses.
Prioritization of candidate genes
Bioinformatic tools were used to prioritize candidate genes for major QTL that were mapped in two or more crosses derived from different parental strains. Probable candidate genes were those that contained one or more nonsynonymous SNPs or a SNP in the upstream regulatory region, and that SNP was shared by the progenitor strains carrying the high allele but different from the one shared by the progenitor strains carrying the low allele at a QTL, as reported (Rowlan et al. 2013b; Grainger et al. 2016).
Data and reagent availability
BALB-Ape−/− mice are available upon request. Supplemental Material, File S1 contains original genotype and phenotype data used for the current study.
Results
Trait value frequency distribution
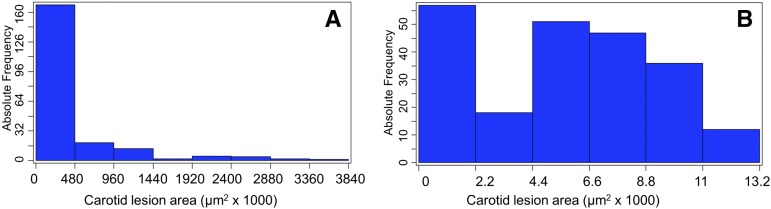
Values of atherosclerotic lesion sizes in the left carotid arteries of 228 F2 mice were distributed in the Pareto manner: the frequency of F2 mice with a total lesion size of ≤ 480 × 1000 μm2 was the highest and then decreased with increasing lesion sizes (Figure 1). After being log2-transformed, these values exhibited a bimodal distribution with 25% of the F2 mice (n = 57) falling under the no or small lesion peak on the left (Log2 value < 2.2) and the remaining 75% falling under the bell-shaped curve on the right.
Figure 1.
Frequency distributions of untransformed (A) and log2-transformed (B) total carotid lesion areas of 228 female F2 mice derived from BALB-Apoe−/− and SM-Apoe−/− mice. Each histogram indicates the number of individual F2 mice with a certain lesion area. Apoe−/−, apolipoprotein E-deficient.
QTL analysis of carotid lesion sizes
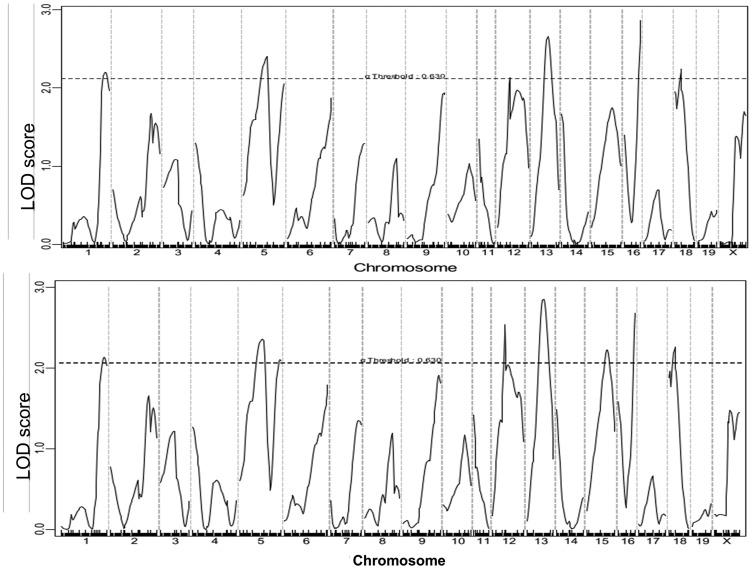
Genome-wide scans for carotid lesion sizes were performed using both a nonparametric algorithm to analyze nontransformed lesion data and a parametric algorithm to analyze Log2-transformed lesion data (Figure 2). Eight suggestive QTL, located on chromosomes 1, 5, 12, 13, 15, 16, and 18, were detected. With the exception of the QTL on distal chromosome 5 and the one on chromosome 15, which were only detected with the nonparametric algorithm, all QTL were detected on both scans (Table 1). The QTL on chromosome 12 peaked at 30.28 cM and had a LOD score of 2.48. This QTL replicated Cath1, a locus for carotid atherosclerosis originally mapped in the B6 × C3H Apoe−/− intercross and then replicated in the B6 × BALB Apoe−/− intercross (Li et al. 2008; Rowlan et al. 2013a). Two QTL on chromosome 5 were detected: the proximal one had a suggestive LOD score of 2.33 and peaked at 63.4 cM, and the distal one had a LOD score of 2.03 and peaked at 99.4 cM. The distal locus overlapped with Cath2, mapped initially in the B6 × C3H Apoe−/− intercross as a suggestive QTL for carotid atherosclerosis and then replicated in the B6 × BALB Apoe−/− intercross as a highly significant QTL (Li et al. 2008; Rowlan et al. 2013a). The locus on chromosome 13 peaked at 34.02 cM and had a suggestive LOD score of 2.8. This QTL replicated Cath3, mapped in the B6 × BALB Apoe−/− intercross (Rowlan et al. 2013a). The QTL on chromosome 15 peaked at 46.74 cM and had a suggestive LOD score of 2.24. This QTL overlapped with a suggestive locus for atherosclerosis in the innominate artery and mapped a B6 × C3H Apoe−/− intercross (Bennett et al. 2015). We named it Cath5 as this QTL was mapped in two separate crosses. The QTL on chromosome 18 had a suggestive LOD score of 2.22 and peaked at 16.27 cM. It replicated a suggestive QTL for carotid atherosclerosis mapped in the B6 × BALB Apoe−/− intercross (Rowlan et al. 2013a), and was named Cath6.
Figure 2.
Genome-wide QTL analysis for carotid lesion sizes in the F2 population. Chromosomes 1 through 20 are represented numerically on the x-axis. y-axis represents LOD score. The horizontal dashed line denotes the genome-wide threshold for suggestive linkage, which was determined by 1000 permutations. Top panel: a genome-wide scan using untransformed carotid lesion data performed with the nonparametric algorism; bottom panel: a genome-wide scan using log2-transformed carotid lesion data performed with the parametric mode. LOD, logarithm of the odds; QTL, quantitative trait locus.
Table 1. QTL identified for carotid lesion areas in female F2 mice derived from an intercross between BALB-Apoe−/− and SM-Apoe−/− mice.
| Locus | Chr | Analysis | LODa | p-Valueb | Peak (cM) | 95% C.I.c | High Allele | Mode of Inheritanced |
|---|---|---|---|---|---|---|---|---|
| 1 | Nonparametric | 2.17 | 0.535 | 91.52 | 75.52–97.02 | — | Heterosis | |
| 5 | Nonparametric | 2.33 | 0.422 | 63.4 | 34.19–101.24 | BALB | Additive | |
| Cath2 | 5 | Nonparametric | 2.03 | 0.630 | 99.4 | 79.4–101.2 | BALB | Dominant |
| Cath1 | 12 | Nonparametric | 2.48 | 0.324 | 30.28 | 19.41–63.41 | SM | Additive |
| Cath3 | 13 | Nonparametric | 2.8 | 0.163 | 34.02 | 22.02–46.02 | SM | Dominant |
| Cath5 | 15 | Nonparametric | 2.24 | 0.474 | 46.74 | 26.74–62.74 | SM | Recessive |
| 16 | Nonparametric | 2.58 | 0.274 | 44.66 | 13.43–46.66 | BALB | Dominant | |
| Cath6 | 18 | Nonparametric | 2.22 | 0.497 | 16.27 | 3.73–27.73 | SM | Additive |
| 1 | Parametric | 2.23 | 0.545 | 87.52 | 77.52–97.02 | — | Heterosis | |
| 5 | Parametric | 2.38 | 0.413 | 67.27 | 33.4–101.4 | BALB | Additive | |
| Cath2 | 5 | Parametric | 2.03 | 0.630 | 99.4 | 79.4–101.2 | BALB | Additive |
| Cath1 | 12 | Parametric | 2.1 | 0.644 | 30.28 | 23.41–65.41 | SM | Additive |
| Cath3 | 13 | Parametric | 2.64 | 0.267 | 32.02 | 22.02–47.99 | SM | Dominant |
| 16 | Parametric | 2.81 | 0.205 | 46.66 | 13.43–46.66 | BALB | Dominant | |
| Cath6 | 18 | Parametric | 2.21 | 0.552 | 16.27 | 3.73–25.73 | SM | Additive |
Chr, chromosome; LOD, logarithm of the odds; QTL, quantitative trait locus.
LOD scores were obtained from genome-wide scans using J/qtl. LOD score threshold for suggestive QTL > 2.054; for significance > 3.314 established by 1000 permutation tests.
p-values represent genome-wide significance at each locus.
95% C.I. was determined through whole-genome scans.
Inheritance was determined based on the effect of each parental allele at the nearest genomic marker.
The QTL on chromosome 1 peaked at 91.52 cM and had a LOD score of 2.17. It overlapped with Ath1, a QTL for aortic atherosclerosis mapped in a number of crosses (Wang et al. 2005; Grainger et al. 2016; Zhang et al. 2012). The QTL on chromosome 16 peaked at 46.66 cM and had a score of 2.58, and this QTL was novel. The SM allele was associated with increased lesion sizes for chromosome 12, 13, 15, and 18 QTL, while the BALB allele was associated with increased lesion sizes for the chromosome 5 and 16 QTL (Table 2). The chromosome 1 QTL affected lesion formation in a heterotic manner in that F2 mice with heterozygous alleles exhibited increased lesion size over those with homozygous alleles.
Table 2. Effects of BALB and SM alleles on carotid lesion area at identified QTL in female F2 mice derived from BALB-Apoe−/− and SM-Apoe−/− mice.
| Locus Name | Chr | Analysis | Peak Marker | Peak (cM) | BB | BS | SS | p-Value |
|---|---|---|---|---|---|---|---|---|
| 1 | Nonparametric | rs3685643 | 91.52 | 281.5 ± 662.4 | 522.8 ± 1049.5 | 260.9 ± 466.9 | 0.016 | |
| 5 | Nonparametric | rs3726547 | 63.4 | 604.1 ± 1250.9 | 341.5 ± 710.1 | 273.3 ± 504.0 | 0.006953 | |
| Cath2 | 5 | Nonparametric | rs13478578 | 99.4 | 454.6 ± 629.3 | 412.1 ± 1023.7 | 285.8 ± 635.7 | 0.008146 |
| Cath1 | 12 | Nonparametric | rs13481509 | 30.28 | 171.1 ± 389.1 | 374.9 ± 952.2 | 652.9 ± 917.3 | 0.002917 |
| Cath3 | 13 | Nonparametric | rs6259014 | 34.02 | 313.9 ± 650.0 | 412.7 ± 938.2 | 427.7 ± 793.2 | 0.143 |
| Cath5 | 15 | Nonparametric | rs13482641 | 46.74 | 244.8 ± 397.0 | 294.2 ± 664.1 | 711.2 ± 1281.3 | 0.03 |
| 16 | Nonparametric | rs3721202 | 44.66 | 426.5 ± 1249.9 | 485.8 ± 192.8 | 192.8 ± 405.0 | 0.002091 | |
| Cath6 | 18 | Nonparametric | rs3683699 | 16.27 | 256.1 ± 440.2 | 423.9 ± 1059.8 | 539.0 ± 759.6 | 0.005427 |
| 1 | Parametric | rs3685643 | 87.52 | 4.3 ± 3.8 | 6.1 ± 3.7 | 5.8 ± 3.2 | 0.01199282 | |
| 5 | Parametric | rs3726547 | 67.27 | 6.8 ± 3.3 | 5.2 ± 3.6 | 4.8 ± 3.8 | 0.00624255 | |
| Cath2 | 5 | Parametric | rs13478578 | 99.4 | 6.4 ± 3.6 | 5.6 ± 3.5 | 4.2 ± 3.8 | 0.00941731 |
| Cath1 | 12 | Parametric | rs13481509 | 30.28 | 4.7 ± 3.2 | 5.3 ± 3.8 | 6.8 ± 3.8 | 0.00787988 |
| Cath3 | 13 | Parametric | rs6259014 | 32.02 | 4.6 ± 3.8 | 5.7 ± 3.7 | 6.0 ± 3.6 | 0.14731571 |
| 16 | Parametric | rs3721202 | 46.66 | 5.8 ± 3.4 | 6.2 ± 3.7 | 4.0 ± 3.6 | 0.00150063 | |
| Cath6 | 18 | Parametric | rs3683699 | 16.27 | 5.3 ± 3.5 | 5.0 ± 3.9 | 7.1 ± 3.2 | 0.00613849 |
Measurements for carotid lesion areas are expressed as means ± SD. The unit for these measurements is: µm2 × 1000 for nonparametric analysis. For parametric analysis, the values are log2-transformed total carotid lesion areas. The Kruskal–Wallis test was used on the nonparametric data and ANOVA on the parametric data to determine the significance (p-value) of the differences among the BB, BS, and SS genotypes. Chr, chromosome; BB, homozygous for the BALB allele at the linked peak marker; BS, heterozygous for both BALB and SMJ; SS, homozygous for the SMJ allele.
Combined cross analysis for overlapping QTL
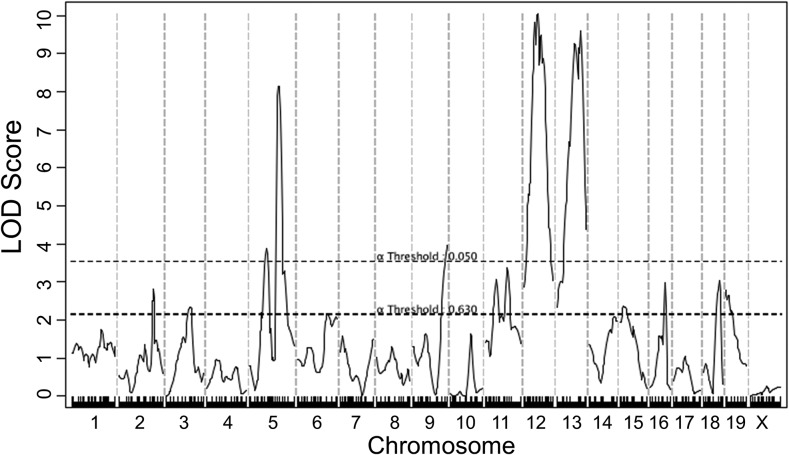
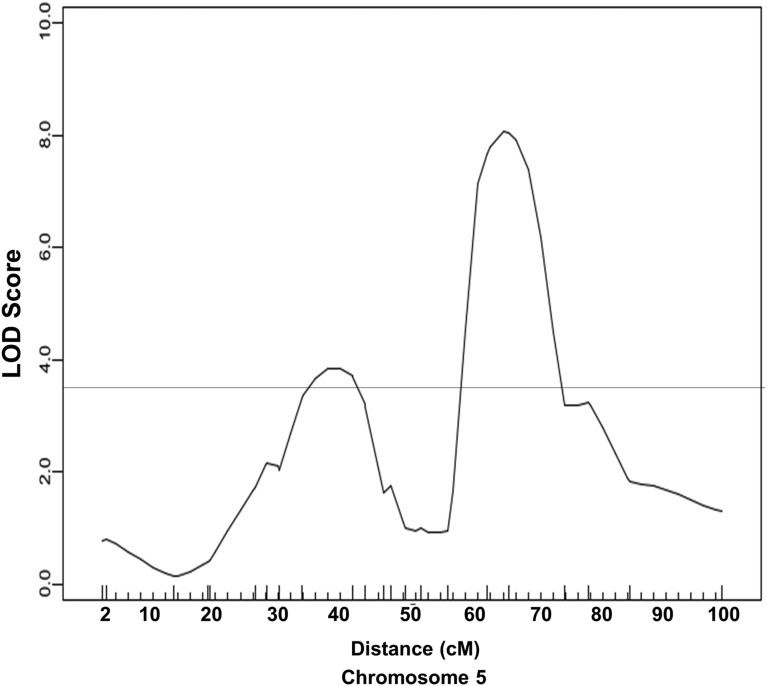
Combined cross analysis was performed for carotid atherosclerosis using data from the current cross and two previously reported B6 × C3H and B6 × BALB intercrosses (Li et al. 2008; Rowlan et al. 2013a). Five significant QTL, located on chromosomes 5, 9, 12, and 13, and nine suggestive QTL on chromosomes 2, 3, 6, 11, 15, 16, 18, and 19, were identified (Figure 3 and Table 3). The majority of these QTL had been identified as significant or suggestive QTL in one or more individual crosses, but the LOD scores for the significant QTL on chromosomes 5, 9, 12, and 13 were higher compared to those determined in an individual cross. The 95% C.I. was relatively smaller than that in an individual cross for most QTL. A LOD score plot for chromosome 5 revealed two disparate peaks, indicating the presence of two QTL for carotid atherosclerosis (Figure 4). The distal QTL replicated Cath2, mapped in all the three crosses (Li et al. 2008; Rowlan et al. 2013a). The proximal QTL was visible as a distinct peak in the current cross as well as the previously reported B6 × BALB intercross (Rowlan et al. 2013a), and was named Cath7 to represent a new locus for carotid atherosclerosis. The significant QTL on chromosome 9 was initially mapped as a suggestive QTL in the B6 × BALB intercross (Rowlan et al. 2013a), and was named Cath8. The suggestive QTL on chromosomes 6, 11, 15, 16, and 18 were each mapped in one or more individual crosses, while the suggestive QTL on chromosomes 2, 3, and 19 were only detected in the combined cross.
Figure 3.
Genome-wide QTL analysis for carotid lesion sizes using combined data from the current cross and two previously reported B6 × BALB and B6 × C3H Apoe−/− intercrosses. The horizontal dotted lines indicate the thresholds for genome-wide suggestive and significant linkage, as determined by 1000 permutations. Apoe−/−, apolipoprotein E-deficient; LOD, logarithm of the odds; QTL, quantitative trait locus.
Table 3. Significant and suggestive QTL for carotid atherosclerosis identified in combined cross analysis of data from the current cross and the two previously reported crosses.
| Locus | Chr | Trait | LOD | Peak (cM) | 95% C.I. | Peak (Mb) | 95% C.I. (Mb) |
|---|---|---|---|---|---|---|---|
| 2 | Carotid lesion | 2.77 | 80.22 | 44.22–98.22 | 159.59 | 71.96–170.59 | |
| 3 | Carotid lesion | 2.31 | 50.01 | 26.01–64.01 | 114.85 | 56.96–138.77 | |
| Cath7 | 5 | Carotid lesion | 3.84 | 39.05 | 34.19–44.28 | 65.31 | 61.51–69.16 |
| Cath2 | 5 | Carotid lesion | 8.06 | 66.35 | 63.84–70.35 | 127.32 | 124.83–131.29 |
| Cath4 | 6 | Carotid lesion | 2.15 | 66.21 | 1.53–88.79 | 120.60 | 6.44–145.75 |
| Cath8 | 9 | Carotid lesion | 3.92 | 75.33 | 66.37–75.33 | 114.09 | 103.61–114.09 |
| 11 | Carotid lesion | 3.02 | 26.1 | 18.2–32.2 | 45.28 | 30.91–54.19 | |
| 11 | Carotid lesion | 3.32 | 51 | 17.99–69.99 | 83.84 | 30.91–105.15 | |
| Cath1 | 12 | Carotid lesion | 9.95 | 32.59 | 23.47–44.59 | 70.23 | 48.06–88.56 |
| Cath3 | 13 | Carotid lesion | 9.49 | 53.35 | 36.02–56.02 | 100.5 | 68.40–103.48 |
| Cath5 | 15 | Carotid lesion | 2.35 | 11.26 | 3.8–37.8 | 30.76 | 7.87–71.92 |
| 16 | Carotid lesion | 2.94 | 41.66 | 28.95–43.66 | 64.11 | 37.35–72.76 | |
| Cath6 | 18 | Carotid lesion | 3.01 | 39.73 | 31.73–41.73 | 62.50 | 56.27–65.17 |
| 19 | Carotid lesion | 2.74 | 2.43 | 2.43–26.43 | 3.65 | 3.65–36.64 |
LOD score threshold for suggestive QTL was 2.128 and was 3.508 for significant QTL. Significant QTL are highlighted in bold. Chr, chromosome; LOD, logarithm of the odds; QTL, quantitative trait loci.
Figure 4.
Interval mapping graph for carotid lesion size on chromosome 5 using combined data from the current cross and previously reported B6 × BALB and B6 × C3H Apoe−/− intercrosses. The horizontal line denotes the threshold for significant linkage. Apoe−/−, apolipoprotein E-deficient; LOD, logarithm of the odds.
Candidate genes for Cath1
Cath1 on chromosome 12 was mapped in the current cross and two previously reported B6 × C3H and B6 × BALB Apoe−/− intercrosses (Li et al. 2008; Rowlan et al. 2013a). For this QTL, the B6 and SM alleles were associated with increased lesion sizes, while the C3H and BALB alleles were associated with smaller lesion sizes. We used the Sanger SNP database to search for positional candidate genes that contain nonsynonymous SNP(s) or SNP(s) in upstream regulatory regions that are shared by the low allele strains (BALB and C3H) but are different from ones carried by the high allele strain (B6) under the linkage peak. The SM strain was not included due to its incomplete genomic sequences for the region. Twenty-four candidate genes were identified (Table 4). Among them, Eapp, Foxa1, Fancm, Nin, Dact1, Rtn1, and Trmt5 contained one or more nonsynonymous SNPs with a low SIFT (Sorting Intolerant From Tolerant) score, predicting a high likelihood that an amino acid substitution has an adverse effect on protein function.
Table 4. Haplotype analysis for Cath1 on chromosome 12 (52–75 Mb).
| Chr | Position | Gene | dbSNP | High Allele | Low Allele | Consequence | Amino Acid Change | SIFT Score | Tolerated | |
|---|---|---|---|---|---|---|---|---|---|---|
| C57BL/6 | BALB_cJ | C3H_HeH | ||||||||
| 12 | 52006466 | Dtd2 | rs46701436 | A | G | G | Missense variant | Cn 7:V/A | 0.92 | Yes |
| 12 | 52023971 | Gpr33 | rs29173669 | A | G | G | Missense variant | Cn 95:V/A | 0.71 | Yes |
| 12 | 52027979 | Gpr33 | rs51561875 | T | G | G | 5ʹ-UTR variant | |||
| 12 | 52027989 | Gpr33 | rs49936313 | T | A | A | 5ʹ-UTR variant | |||
| 12 | 52027993 | Gpr33 | rs47019843 | C | T | T | 5ʹ-UTR variant | |||
| 12 | 52519522 | Arhgap5 | rs29198609 | T | C | C | Missense variant | Cn 1092:V/A | 1 | Yes |
| 12 | 52887261 | Akap6 | rs29183247 | G | A | A | Missense variant | Cn 512:R/Q | 0.2 | Yes |
| 12 | 52887389 | Akap6 | rs29223294 | A | G | G | Missense variant | Cn 555:T/A | 0.47 | Yes |
| 12 | 53140291 | Akap6 | rs48484112 | G | A | A | Missense variant | Cn 1496:R/H | 1 | Yes |
| 12 | 54203369 | Egln3 | rs29130898 | A | G | G | Missense variant | Cn 65:C/R | 0.89 | Yes |
| 12 | 54203615 | Egln3 | rs29122127 | T | G | G | 5ʹ-UTR variant | |||
| 12 | 54203690 | Egln3 | rs13473456 | G | A | A | 5ʹ-UTR variant | |||
| 12 | 54695720 | Eapp | rs29183105 | G | A | A | Missense variant | Cn 22:A/V | 0.01 | No |
| 12 | 54941453 | Baz1a | rs29195192 | G | A | A | Missense variant | Cn 88:L/F | 0.04 | Yes |
| 12 | 54999084 | Baz1a | rs29196908 | G | C | C | 5ʹ-UTR variant | |||
| 12 | 57303392 | Mipol1 | rs29163022 | G | A | A | 5ʹ-UTR variant | |||
| 12 | 57325598 | Mipol1 | rs46300008 | A | G | G | Missense variant | Cn 148:K/E | 1 | Yes |
| 12 | 57325623 | Mipol1 | rs13481473 | A | G | G | Missense variant | Cn 156:H/R | 1 | Yes |
| 12 | 57542267 | Foxa1 | rs13481474 | T | C | C | Missense variant | Cn 389:H/R | 0 | No |
| 12 | 57576142 | Ttc6 | rs50478178 | G | C | C | Missense variant | Cn 109:R/P | 1 | Yes |
| 12 | 57725789 | Ttc6 | rs48534883 | T | C | C | Splice region variant | |||
| 12 | 58267790 | Clec14a | rs31966428 | T | C | C | Missense variant | Cn 349:I/V | 0.23 | Yes |
| 12 | 58268339 | Clec14a | rs13465063 | T | C | C | Missense variant | Cn 166:T/A | 1 | Yes |
| 12 | 58268988 | Clec14a | rs29162388 | C | G | G | 5ʹ-UTR variant | |||
| 12 | 58268992 | Clec14a | rs29194398 | G | C | C | 5ʹ-UTR variant | |||
| 12 | 64471729 | Fscb | rs13481500 | G | A | A | Missense variant | Cn 988:P/S | 1 | Yes |
| 12 | 64472091 | Fscb | rs29131205 | G | C | C | Missense variant | Cn 867:A/G | 0.21 | Yes |
| 12 | 64472965 | Fscb | rs585463036 | C | A | A | Missense variant | |||
| 12 | 64473313 | Fscb | rs29220106 | G | A | A | Missense variant | Cn 460:P/S | 0.2 | Yes |
| 12 | 64950146 | Klhl28 | rs33846378 | C | T | T | Missense variant | Cn 474:V/I | 0.07 | Yes |
| 12 | 65113969 | Fancm | rs212043559 | A | T | T | Missense variant | Cn 1407:N/I | 0 | No |
| 12 | 65130342 | Fancm | rs29212900 | A | C | C | Missense variant | Cn 1987:I/L | 0.47 | Yes |
| 12 | 65130397 | Fancm | rs29213465 | A | T | T | Missense variant | Cn 2005:Q/L | 1 | Yes |
| 12 | 65130436 | Fancm | rs29184120 | A | C | C | Missense variant | Cn 2018:K/T | 0 | No (low confidence) |
| 12 | 65149007 | Mis18bp1 | rs50634267 | C | T | T | Missense variant | Cn 661: R/Q | 0.27 | Yes |
| 12 | 65152837 | Mis18bp1 | rs29200949 | T | C | C | Splice region variant | |||
| 12 | 65172467 | Mis18bp1 | rs3695606 | T | A | A | 5ʹ-UTR variant | |||
| 12 | 65172551 | Mis18bp1 | rs3696207 | A | G | G | 5ʹ-UTR variant | |||
| 12 | 69204274 | Pole2 | rs3704977 | T | C | C | Splice region variant | |||
| 12 | 69223117 | Pole2 | rs29135637 | T | C | C | Missense variant | Cn 78:M/V | 0.43 | Yes |
| 12 | 69741794 | Atp5s | rs29193315 | G | A | A | Missense variant | Cn 156: V/I | 1 | Yes |
| 12 | 70043177 | Nin | rs32225358 | C | T | T | Missense variant | Cn 1155:E/K | 0.06 | Yes |
| 12 | 70043386 | Nin | rs29192398 | C | T | T | Missense variant | Cn 1085:R/Q | 0.01 | No |
| 12 | 70043389 | Nin | rs29159683 | G | T | T | Missense variant | Cn 1084:S/Y | 0.02 | No |
| 12 | 70043915 | Nin | rs29149025 | T | C | C | Missense variant | Cn 909:K/E | 1 | Yes |
| 12 | 70180988 | Abhd12b | rs29173916 | G | T* | T* | Missense variant | Cn 258:M/I | 1 | Yes |
| 12 | 70183081 | Abhd12b | rs51691757 | A | G | G | Splice region variant | |||
| 12 | 70183205 | Abhd12b | rs32247424 | A | G* | G* | Stop retained variant, 3ʹ-UTR variant | |||
| 12 | 70193813 | Pygl | rs32246688 | G | T | T | Splice region variant | |||
| 12 | 70197551 | Pygl | rs29151561 | A | G | G | Splice region variant | |||
| 12 | 70201877 | Pygl | rs13467444 | T | C | C | Missense variant | Cn 323:M/V | 1 | Yes |
| 12 | 70231391 | Pygl | rs45983203 | C | T | T | 5ʹ-UTR variant | |||
| 12 | 70231392 | Pygl | rs48603304 | T | A | A | 5ʹ-UTR variant | |||
| 12 | 70231439 | Pygl | rs50231886 | A | T | T | 5ʹ-UTR variant | |||
| 12 | 70231450 | Pygl | rs32251907 | A | T | T | 5ʹ-UTR variant | |||
| 12 | 71318068 | Dact1 | rs29185339 | C | T | T | Missense variant | Cn 504:P/L | 0.02 | No |
| 12 | 71318500 | Dact1 | rs29222974 | G | C | C | Missense variant | Cn 648:R/P | 0.44 | Yes |
| 12 | 72408325 | Rtn1 | rs3695552 | T | C | C | Missense variant | Cn 76:E/G | 0 | No |
| 12 | 72408926 | Rtn1 | rs29209324 | T | C | C | 5ʹ-UTR variant | |||
| 12 | 72454073 | Lrrc9 | rs29198846 | G | A | A | Missense variant | Cn 191:R/H | 0.97 | Yes |
| 12 | 73281229 | Trmt5 | rs29130757 | G | T | T | Missense variant | Cn 400:P/H | 0 | No |
| 12 | 73285238 | Trmt5 | rs29166240 | A | T | T | Missense variant | Cn 15:L/M | 0.15 | No (low confidence) |
| 12 | 73285259 | Trmt5 | rs29162033 | A | T | T | Missense variant | Cn 8:F/I | 0.06 | Yes (low confidence) |
| 12 | 73285271 | Trmt5 | rs29141846 | C | A | A | Missense variant | Cn 4:V/L | 1 | Yes |
| 12 | 73287081 | Slc38a6 | rs48749977 | T | C | C | 5ʹ-UTR variant | |||
| 12 | 73350619 | Slc38a6 | rs13481528 | C | T | T | Missense variant | Cn 345:A/V | 1 | Yes |
Functional candidate genes are denoted in bold. A smaller SIFT score denotes a higher likelihood of protein function change. Chr, chromosome; dbSNP, Single Nucleotide Polymorphism Database identifier; SIFT, Sorting Intolerant From Tolerant; Cn, Coding non-synonymous polymorphism; UTR, untranslated region.
Relationships of carotid atherosclerosis with plasma lipids and glucose
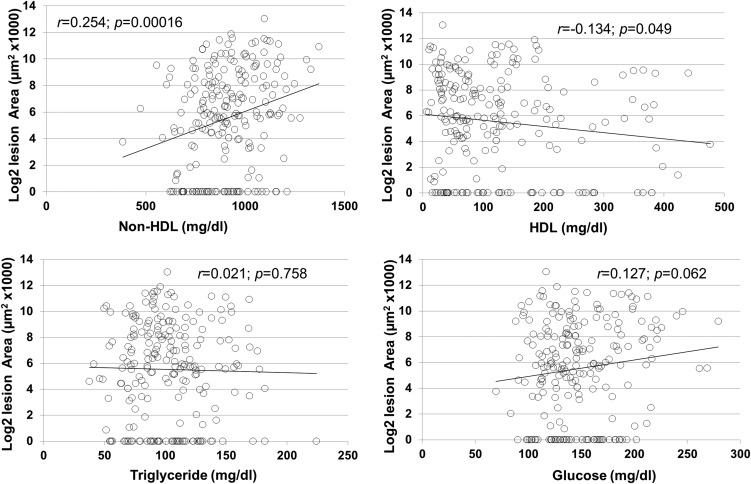
Associations of carotid lesion sizes with plasma HDL, non-HDL cholesterol, triglyceride, and glucose levels were evaluated using the F2 population (Figure 5). A significant correlation with non-HDL cholesterol levels was observed (r = 0.254; p = 0.00016). F2 mice with higher non-HDL cholesterol levels tended to develop larger carotid lesions. The value of the correlation coefficient r2 indicates that non-HDL accounted for 6.45% of the variance in carotid lesion sizes among the F2 population. A marginal, but statistically significant, inverse correlation with HDL cholesterol levels was observed (r = −0.134; p = 0.049). F2 mice with higher HDL cholesterol levels tended to develop smaller carotid lesions. HDL accounted for 1.8% of the variance in lesion sizes of the F2 mice. No correlation with triglyceride levels was found (r = 0.021; p = 0.758). There was a trend toward a significant correlation with plasma glucose levels (r = 0.127; p = 0.062).
Figure 5.
Scatterplots showing the correlations of carotid lesion sizes with plasma non-HDL (A), HDL cholesterol (B), triglyceride (C), and glucose (D) in the F2 population. Each point represents an individual value of a F2 mouse. The correlation coefficient (r) and significance (p) are shown. Log2-transformed carotid total areas were used for the analyses. HDL, high-density lipoprotein.
Discussion
Genetic factors contributing to carotid atherosclerosis, which is a major cause of ischemic stroke, are poorly understood. In this study, we performed QTL analysis using data from a newly generated intercross and combined data from three independent intercrosses to search for QTL contributing to carotid atherosclerosis. Five significant QTL and > 10 suggestive QTL were identified for the trait. Bioinformatic tools were successfully used to reduce the number of candidate genes for Cath1. Moreover, plasma non-HDL cholesterol was found to explain 6.5% of the variance in carotid lesion sizes of the F2 population.
Atherosclerotic lesions in the left carotid artery were measured after F2 mice were fed a Western diet for 12 wk. Under this condition, these mice, which were on the Apoe-null background, developed severe hyperlipidemia (Wang et al. 2015). Nevertheless, we found that a large fraction of F2 mice developed little or no atherosclerotic lesions in the carotid artery. The same phenomenon has also been observed in two other intercrosses previously constructed for QTL analysis of carotid atherosclerosis in the mouse (Li et al. 2008; Rowlan et al. 2013a). In contrast, all the F2 mice developed atherosclerotic lesions in the aortic root (Grainger et al. 2016). As the aortic root and the carotid arteries are exposed to the same level of lipoproteins and the same type of blood cells, the site-specific difference in the development of atherosclerosis should be attributable to local factors, such as vascular geometry, blood flow dynamics, and vessel wall properties. A genetic study of aortic arch curvature and atherosclerosis in a mouse cross has linked genetic factors regulating aortic arch geometry to aortic lesion formation (Tomita et al. 2010).
We and others have found that QTL identified for atherosclerotic lesions in the aortic root can be quite different from those mapped in another site of the vasculature, even in the same crosses (Li et al. 2008; Zhang et al. 2012; Rowlan et al. 2013a; Grainger et al. 2016; Bennett et al. 2015). Because the aortic root is easy to study in mice, genetic studies of atherosclerosis have largely focused on this site. However, this site has little clinical significance to humans. In contrast, the carotid arteries are the most extensively studied vessels in humans with ultrasonography because of their close association with the brain and ready accessibility.
Cath1 on chromosome 12, Cath2 on chromosome 5, Cath3 on chromosome 13, and Cath4 on chromosome 6 are four significant QTL for carotid atherosclerosis thus far mapped in two Apoe−/− mouse intercrosses (Li et al. 2008; Rowlan et al. 2013a). Three of the four QTL were replicated in the current BALB × SM Apoe−/− intercross, and all of them were replicated in the combined cross analysis. The QTL on chromosome 15 overlapped in the C.I. with a suggestive locus affecting both atherosclerotic lesion size and composition in the innominate artery of Apoe−/− mice (Bennett et al. 2009). We named it Cath5 to represent a locus for carotid atherosclerosis in the mouse. Naming a suggestive locus is considered appropriate if it is repeatedly observed (Abiola et al. 2003). The QTL on chromosome 18 overlapped in the C.I. with a suggestive locus for carotid atherosclerosis mapped in the B6 × BALB Apoe−/− intercross, and was named Cath6.
Five significant QTL and nine suggestive QTL for carotid atherosclerosis were identified in the combined cross analysis. Nearly all of these QTL were mapped in one or more individual crosses. However, the combined cross analysis had an increased power of detecting shared QTL by two or more crosses. Indeed, all five significant QTL had a higher LOD score than that achieved in an individual cross. The current and previous B6 × BALB F2 crosses suggested the presence of two QTL on chromosome 5 for carotid atherosclerosis (Rowlan et al. 2013a), while the combined cross analysis clearly demonstrated the presence of two disparate QTL on the chromosome. We named the proximal QTL Cath7 to represent a new locus for carotid atherosclerosis. The significant QTL on distal chromosome 9 identified by the combined cross analysis overlapped with a suggestive QTL previously mapped in the B6 × BALB F2 cross (Rowlan et al. 2013a), and was named Cath8. Consistent with the conclusion drawn by Li et al. (2005), we found that the C.I. defined by the combined cross analysis was smaller than that defined in an individual cross for most of the QTL.
Cath1 has been mapped in three intercrosses derived from mouse strains, including B6, C3H, and BALB, whose genome sequences are publicly available through the Sanger mouse genomes project. By examining genes containing variants that were shared among the low allele strains (BALB and C3H) but different from those carried by the low allele strain (B6), we reduced the number of candidate genes to 24. Because a QTL is yielded from changes in the function or the quantity of a gene product, we concentrated on genes carrying a nonsynonymous SNP or a SNP in the upstream regulatory region. Nin, Dact1, and Rtn1, which are located underneath the linkage peak and contain one or more nontolerated nonsynonymous SNPs, have shown suggestive associations with increased risk of ischemic stroke (Meschia et al. 2011) or lipoprotein particle size (Frazier-Wood et al. 2013).
A significant correlation was observed between non-HDL cholesterol levels and atherosclerotic lesion sizes in the present cross. Our previous study of a F2 population also showed a correlation between carotid lesion sizes and non-HDL cholesterol levels (Rowlan et al. 2013a). A marginal inverse correlation of HDL cholesterol levels with lesion sizes was observed in this cross, and also in two previous crosses (Li et al. 2008; Rowlan et al. 2013a). These findings are consistent with the observations made in humans (Mora et al. 2007; Sanossian et al. 2007). No correlation between carotid lesion sizes and plasma triglyceride levels was observed in this cross, nor in previous crosses (Li et al. 2008; Rowlan et al. 2013a). We have observed a trend toward a significant correlation of carotid lesion sizes with fasting plasma glucose levels in this cross. Blood glucose levels of the F2 mice were markedly elevated by feeding of a Western diet (Wang et al. 2015). In humans, impaired fasting glucose homeostasis has also been associated with preclinical carotid atherosclerosis (De Michele et al. 2002).
In summary, we have identified a number of QTL for carotid atherosclerosis, demonstrating the polygenic control of the disorder. The significant correlations of carotid lesion sizes with HDL and non-HDL cholesterol levels suggest that some loci exert effects on carotid atherosclerosis partially through action on lipoproteins. Using bioinformatics tools, we have reduced the list of candidate genes for a major atherosclerosis locus.
Supplementary Material
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.116.037879//DC1.
Acknowledgments
This work was supported by National Institutes of Health grants DK097120 and HL112281. Andrew Grainger is a recipient of the Robert R. Wagner Fellowship from the University of Virginia School of Medicine.
Footnotes
Communicating editor: D. W. Threadgill
Literature Cited
- Abiola O., Angel J. M., Avner P., Bachmanov A. A., Belknap J. K., et al. , 2003. The nature and identification of quantitative trait loci: a community’s view. Nat. Rev. Genet. 4: 911–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett B. J., Wang S. S., Wang X., Wu X., Lusis A. J., 2009. Genetic regulation of atherosclerotic plaque size and morphology in the innominate artery of hyperlipidemic mice. Arterioscler. Thromb. Vasc. Biol. 29: 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett B. J., Davis R. C., Civelek M., Orozco L., Wu J., et al. , 2015. Genetic architecture of atherosclerosis in mice: a systems genetics analysis of common inbred strains. PLoS Genet. 11: e1005711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bis J. C., Kavousi M., Franceschini N., Isaacs A., Abecasis G. R., et al. , 2011. Meta-analysis of genome-wide association studies from the CHARGE consortium identifies common variants associated with carotid intima media thickness and plaque. Nat. Genet. 43: 940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Michele M., Panico S., Celentano E., Covetti G., Intrieri M., et al. , 2002. Association of impaired glucose homeostasis with preclinical carotid atherosclerosis in women: impact of the new American Diabetes Association criteria. Metabolism 51: 52–56. [DOI] [PubMed] [Google Scholar]
- Donnan G. A., Fisher M., Macleod M., Davis S. M., 2008. Stroke. Lancet 371: 1612–1623. [DOI] [PubMed] [Google Scholar]
- Du R., Zhou J., Lorenzano S., Liu W., Charoenvimolphan N., et al. , 2015. Integrative mouse and human studies implicate ANGPT1 and ZBTB7C as susceptibility genes to ischemic injury. Stroke 46: 3514–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier-Wood A. C., Manichaikul A., Aslibekyan S., Borecki I. B., Goff D. C., et al. , 2013. Genetic variants associated with VLDL, LDL and HDL particle size differ with race/ethnicity. Hum. Genet. 132: 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger A. T., Jones M. B., Li J., Chen M. H., Manichaikul A., et al. , 2016. Genetic analysis of atherosclerosis identifies a major susceptibility locus in the major histocompatibility complex of mice. Atherosclerosis 254: 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Li Y., Zhang Z., Gilbert T. R., Matsumoto A. H., et al. , 2008. Quantitative trait locus analysis of carotid atherosclerosis in an intercross between C57BL/6 and C3H apolipoprotein E-deficient mice. Stroke 39: 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Lyons M. A., Wittenburg H., Paigen B., Churchill G. A., 2005. Combining data from multiple inbred line crosses improves the power and resolution of quantitative trait loci mapping. Genetics 169: 1699–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Li J., Chen M. H., Liu Z., Shi W., 2015. Variation in type 2 diabetes-related phenotypes among apolipoprotein E-deficient mouse strains. PLoS One 10: e0120935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus H., Cullinane M., 2001. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 124: 457–467. [DOI] [PubMed] [Google Scholar]
- Matteis M., Vernieri F., Caltagirone C., Troisi E., Rossini P. M., et al. , 1999. Patterns of cerebrovascular reactivity in patients with carotid artery occlusion and severe contralateral stenosis. J. Neurol. Sci. 168: 47–51. [DOI] [PubMed] [Google Scholar]
- Meschia J. F., Worrall B. B., Rich S. S., 2011. Genetic susceptibility to ischemic stroke. Nat. Rev. Neurol. 7: 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora S., Szklo M., Otvos J. D., Greenland P., Psaty B. M., et al. , 2007. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis 192: 211–217. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D., Benjamin E. J., Go A. S., Arnett D. K., Blaha M. J., et al. , 2015. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 131: e29–e322 (erratum: Circulation 131: e535; Circulation 133: e417). [DOI] [PubMed] [Google Scholar]
- Rowlan J. S., Zhang Z., Wang Q., Fang Y., Shi W., 2013a New quantitative trait loci for carotid atherosclerosis identified in an intercross derived from apolipoprotein E-deficient mouse strains. Physiol. Genomics 45: 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlan J. S., Li Q., Manichaikul A., Wang Q., Matsumoto A. H., et al. , 2013b Atherosclerosis susceptibility loci identified in an extremely atherosclerosis-resistant mouse strain. J. Am. Heart Assoc. 2: e000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco R. L., Blanton S. H., Slifer S., Beecham A., Glover K., et al. , 2009. Heritability and linkage analysis for carotid intima-media thickness: the family study of stroke risk and carotid atherosclerosis. Stroke 40: 2307–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanossian N., Saver J. L., Navab M., Ovbiagele B., 2007. High-density lipoprotein cholesterol: an emerging target for stroke treatment. Stroke 38: 1104–1109. [DOI] [PubMed] [Google Scholar]
- Su Z., Li Y., James J. C., Matsumoto A. H., Helm G. A., et al. , 2006. Genetic linkage of hyperglycemia, body weight and serum amyloid-P in an intercross between C57BL/6 and C3H apolipoprotein E-deficient mice. Hum. Mol. Genet. 15: 1650–1658. [DOI] [PubMed] [Google Scholar]
- Swan L., Birnie D. H., Inglis G., Connell J. M., Hillis W. S., 2003. The determination of carotid intima medial thickness in adults–a population-based twin study. Atherosclerosis 166: 137–141. [DOI] [PubMed] [Google Scholar]
- Tian J., Pei H., James J. C., Li Y., Matsumoto A. H., et al. , 2005. Circulating adhesion molecules in apoE-deficient mouse strains with different atherosclerosis susceptibility. Biochem. Biophys. Res. Commun. 329: 1102–1107. [DOI] [PubMed] [Google Scholar]
- Tomita H., Zhilicheva S., Kim S., Maeda N., 2010. Aortic arch curvature and atherosclerosis have overlapping quantitative trait loci in a cross between 129S6/SvEvTac and C57BL/6J apolipoprotein E-null mice. Circ. Res. 106: 1052–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Grainger A. T., Manichaikul A., Farber E., Onengut-Gumuscu S., et al. , 2015. Genetic linkage of hyperglycemia and dyslipidemia in an intercross between BALB/cJ and SM/J apoe-deficient mouse strains. BMC Genet. 16: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Ria M., Kelmenson P. M., Eriksson P., Higgins D. C., et al. , 2005. Positional identification of TNFSF4, encoding OX40 ligand, as a gene that influences atherosclerosis susceptibility. Nat. Genet. 37: 365–372. [DOI] [PubMed] [Google Scholar]
- Wergedal J. E., Ackert-Bicknell C. L., Beamer W. G., Mohan S., Baylink D. J., et al. , 2007. Mapping genetic loci that regulate lipid levels in a NZB/B1NJxRF/J intercross and a combined intercross involving NZB/B1NJ, RF/J, MRL/MpJ, and SJL/J mouse strains. J. Lipid Res. 48: 1724–1734. [DOI] [PubMed] [Google Scholar]
- Xie G., Myint P. K., Voora D., Laskowitz D. T., Shi P., et al. , 2015. Genome-wide association study on progression of carotid artery intima media thickness over 10 years in a chinese cohort. Atherosclerosis 243: 30–37. [DOI] [PubMed] [Google Scholar]
- Yuan Z., Pei H., Roberts D. J., Zhang Z., Rowlan J. S., et al. , 2009. Quantitative trait locus analysis of neointimal formation in an intercross between C57BL/6 and C3H/HeJ apolipoprotein E-deficient mice. Circ. Cardiovasc. Genet. 2: 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Rowlan J. S., Wang Q., Shi W., 2012. Genetic analysis of atherosclerosis and glucose homeostasis in an intercross between C57BL/6 and BALB/cJ apolipoprotein E-deficient mice. Circ. Cardiovasc. Genet. 5: 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Cheema F. A., Bremner J. D., Goldberg J., Su S., et al. , 2008. Heritability of carotid intima-media thickness: A twin study. Atherosclerosis 197: 814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.