Abstract
Fused‐pentagons results in an increase of local steric strain according to the isolated pentagon rule (IPR), and for all reported non‐IPR clusterfullerenes multiple (two or three) metals are required to stabilize the strained fused‐pentagons, making it difficult to access the single‐atom properties. Herein, we report the syntheses and isolations of novel non‐IPR mononuclear clusterfullerenes MNC@C76 (M=Tb, Y), in which one pair of strained fused‐pentagon is stabilized by a mononuclear cluster. The molecular structures of MNC@C76 (M=Tb, Y) were determined unambiguously by single‐crystal X‐ray diffraction, featuring a non‐IPR C 2v(19138)‐C76 cage entrapping a nearly linear MNC cluster, which is remarkably different from the triangular MNC cluster within the reported analogous clusterfullerenes based on IPR‐obeying C82 cages. The TbNC@C76 molecule is found to be a field‐induced single‐molecule magnet (SMM).
Keywords: clusterfullerenes, cyanide compounds, endohedral fullerenes, non-IPR carbon cage, single-molecule magnets
Fullerenes are closed carbon cages with hollow interiors, and such unique structures bring about intriguing physical and chemical properties.1 Most fullerenes isolated during the past three decades are based on classical carbon cages composed of hexagons and pentagons only,1, 2 for which the stability is generally determined by the isolated pentagon rule (IPR) proposed by Kroto in the 1980s.3 According to IPR, fused‐pentagons result in an increase of local steric strain of a carbon cage, thus destabilizing the fullerene.3, 4 Stabilization of the strained fused‐pentagon within a non‐IPR fullerene cage has been fulfilled by either endohedral or exohedral derivatization.4 In particular, for endohedral fullerenes which are a special class of fullerene with an atom, ion, or cluster entrapped in the interior of carbon cage,5 the strong coordination of the entrapped metal ion(s) with the fused‐pentagon gives rise to an intramolecular electron transfer and consequently stabilization of the non‐IPR endohedral fullerene.4, 5, 6 Most of the non‐IPR endohedral fullerenes reported to date are based on clusterfullerenes7 owing to the feasibility of entrapping multiple metals in diverse forms of metal clusters, such as Sc3N@C68,6a,6b Gd3N@C2n (2n=78, 82, 84),6c–6e LaSc2N@C80,6f and Sc2S@C72.6g Noteworthy, for these reported non‐IPR clusterfullerenes, multiple (two or three) metal ions are required to stabilize simultaneously the charged metal clusters and the fused‐pentagons. Hence, it is desirable to synthesize novel non‐IPR endohedral fullerenes containing mononuclear metal clusters.
Clusterfullerenes have been recently recognized as single molecule magnets (SMMs) with potential applications in spintronics, quantum computing, and high‐density storage devices.8, 9 To date only a few endohedral fullerene SMMs have been reported, including LnxSc3−xN@C80 (Ln=Dy, Ho, x=1, 2)9a–9d and Dy2TiC@C80,9e which are all based on an Ih‐C80 cage entrapping multiple rare‐earth‐metal ions that are fixed as a triangle along with the central non‐magnetic ion (N or C). For such clusterfullerene SMMs based on multiple metal centers, their magnetic properties are generally determined jointly by the entrapped individual paramagnetic constituents, making it difficult to access the single‐atom properties. Very recently we reported new SMMs based on terbium cyanide clusterfullerenes TbNC@C82, which provide a model system for the study of endohedral fullerene SMM owing to its structural simplicity resulted from the mononuclear nature.10a Thus, it is highly desirable to synthesize new mononuclear clusterfullerene SMMs based on other carbon cages.
Herein we report novel non‐IPR mononuclear clusterfullerene SMM containing one pair of fused‐pentagons, which is stabilized by a mononuclear cyanide cluster. Two C76‐based mononuclear cyanide clusterfullerenes MNC@C76 (M=Tb, Y) are synthesized and isolated, and their molecular structures are determined unambiguously by single‐crystal X‐ray diffraction, revealing the non‐IPR feature of the C76 cage as well as the geometry of the entrapped MNC cluster. The electronic and magnetic properties of MNC@C76 are further characterized, and TbNC@C76 molecule is identified as a field‐induced SMM.
MNC@C76 (M=Tb, Y) were synthesized by a modified Krätschmer–Huffman DC arc discharge method using a mixture of Tb4O7 (or Y2O3) and graphite (molar ratio of M:C=1:15) as the raw material under 400 mbar He and 10 mbar N2 gas.10 Isolations of MNC@C76 (M=Tb, Y) were performed by multi‐step HPLC (see Supporting Information for experimental details). The high purities of MNC@C76 (M=Tb, Y) were confirmed by laser desorption time‐of‐flight (LD‐TOF) mass spectroscopic analyses (see Supporting Information Figure S4 and S6).
High quality cocrystals of MNC@C76 (M=Tb, Y) with NiII(OEP) (OEP=octaethylporphyrin), MNC@C76⋅Ni(OEP)⋅2 C6H6, were obtained by layering a benzene solution of NiII(OEP) over the solution of MNC@C76 in benzene (for TbNC@C76) or carbon disulfide (for YNC@C76),6b–6g, 10, 11 and were used for the X‐ray crystallographic study. Figure 1 a,d show the relative orientations of MNC@C76 and NiII(OEP) molecules in MNC@C76⋅Ni(OEP)⋅2 (C6H6) cocrystals. For both cases of TbNC@C76 and YNC@C76, the C76 cage is fully ordered, enabling the unambiguous determination of the carbon cage framework. However, the entrapped MNC cluster is disordered (see Supporting Information Figures S7–S8). For clarity, only the major site of the cluster was shown in Figure 1. The asymmetric unit of MNC@C 2v(19 138)‐C76⋅NiII(OEP)⋅2 (C6H6) has no crystallographic imposed symmetry and contains an intact fullerene molecule together with an intact NiII(OEP) molecule and two solvent benzene molecules (Figure 1 a,d). A remarkable structural feature of both cages of TbNC@C76 and YNC@C76 is that there is one pair of fused‐pentagon within the same C 2v(19138)‐C76 cage (see Figure 1 b,e), thus violating IPR.2, 3, 4 Hence, MNC@C 2v(19138)‐C76 (M=Tb, Y) represents novel non‐IPR mononuclear clusterfullerenes.
Figure 1.
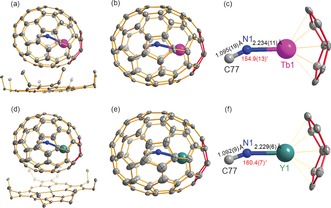
Single‐crystal X‐ray structures of TbNC@C 2v(19138)‐C76 (a,b) and YNC@C 2v(19138)‐C76 (d,e) shown with only the major Tb/Y (Tb1/Y1) positions.14 The fused‐pentagon pair is highlighted in red. The structures of the major TbNC (c) and YNC (f) clusters within C 2v(19138)‐C76 cage with X‐ray determined bond lengths, bond angles, and the interactions of the Tb/Y atom with the closest portions of the cage are also shown. Solvent molecules, hydrogen atoms and minor metal positions are omitted for clarity. Purple Tb; cyan Y; blue N; gray C; green Ni.
Quite similar to the cases of other reported clusterfullerenes including YNC@Cs(6)‐C82 and TbNC@C82 mononuclear cyanide clusterfullerenes,10 the entrapped TbNC/YNC clusters within TbNC@C 2v(19138)‐C76 and YNC@C 2v(19138)‐C76 both exhibit disorders. In fact, as many as 7 and 5 metal sites are refined for TbNC@C 2v(19138)‐C76 and YNC@C 2v(19138)‐C76, respectively (see Supporting Information Figures S7–S8). Among them, the major metal site has an occupancy of 0.689(3) and 0.871(2) for Tb and Y, respectively, which locates just under the junction of the fused‐pentagon (see Figure 1 c,f). This is quite similar to the reported non‐IPR clusterfullerenes such as Sc3N@D3(6140)‐C68 6b and Sc2S@Cs(10528)‐C72.6g Thus, it is the strong coordination interaction between Tb/Y metal and the cage that stabilizes the fused‐pentagon within the non‐IPR C 2v(19138)‐C76 cage.
For the reported YNC@Cs(6)‐C82 and TbNC@C2(5)‐C82 mononuclear cyanide clusterfullerenes, the entrapped MNC clusters both take a triangular geometry, and it is difficult to distinguish N and C atoms crystallographically because of their similarities on the atomic size and scattering power.10, 11d However, for the present case of MNC@C 2v(19138)‐C76, N and C atoms within MNC cluster can be distinguished by combining the crystallographic data with DFT computational results. Our DFT computations of MNC@C76 (M=Tb, Y) reveal that, for the non‐IPR cage isomers (C 2v, C1, Cs) of C76, nearly linear (slightly V‐shaped) M‐N‐C coordination is always preferred with the energy being 15–18 kJ mol−1 lower than that for linear M‐C‐N coordination. This agrees well with the M‐N‐C bond angle (154.9(13)° and 160.4(7)° for Tb and Y, respectively, see Figure 1 c, f) determined by X‐ray crystallography (see Supporting Information S4 for details). Hence, except for the non‐IPR feature of the C76 cage, the nearly linear M‐N‐C configuration of the entrapped MNC cluster within MNC@C76 highlights another remarkable difference with the triangular geometry of the MNC cluster for the analogous clusterfullerenes based on IPR‐obeying C82 cages, YNC@Cs(6)‐C82 and TbNC@C82.10 A plausible explanation is that for non‐IPR MNC@C76 a stronger M–cage interaction is required to stabilize the fused‐pentagon as confirmed by the smaller distance of the shortest M–cage contact (see Figure S9 and Table S4), thus the coordination bonding between the metal atom and [NC]− ligand is weakened via the change of the bidentate [NC]− ligand (for the triangular MNC cluster within MNC@C82) to a monodentate one (for the nearly linear MNC cluster within MNC@C76).
Such a dramatic geometric change of the entrapped TbNC cluster upon changing the carbon cage from IPR‐obeying C82 to non‐IPR C76 is further confirmed in terms of the N−C bond length. Interestingly, while the X‐ray determined N−C bond length for YNC@Cs(6)‐C82 and TbNC@C82 is in the range 0.935(11) to 1.05(4) Å,10 it elongates to 1.095(19) and 1.092(9) Å for TbNC@C 2v(19138)‐C76 and YNC@C 2v(19138)‐C76, respectively (see Figure 1 c, f). These values are approaching those of the reported N−C triple bonds in traditional cyanide/nitrile compounds and cyano coordination complexes (1.12–1.17 Å).12 Thus, it is reasonable to assign the N−C bond within MNC@C 2v(19138)‐C76 as a triple bond, which appears to be compressed within MNC@C82 despite of the larger cage size. This phenomenon is somewhat surprising if simply considering the cage‐size effect, and can be interpreted by the weakened M–[NC]− coordination bonding induced by the stronger M–cage interaction, which is required to stabilize the fused‐pentagon of the non‐IPR C76 cage as discussed above.
Figure 2 A shows the UV/Vis‐NIR absorption spectra of TbNC@C 2v(19138)‐C76 and YNC@C 2v(19138)‐C76 dissolved in carbon disulfide (CS2), and their characteristic absorption data are summarized in Table S6. Interestingly, their overall absorption spectra, the characteristic absorption peaks, the optical band‐gap (ΔE gap,optical) and color of CS2 solutions are almost identical, confirming their identity on the cage isomeric structure which predominantly determines the electronic absorption of endohedral fullerene with the same type of entrapped species.5, 6
Figure 2.
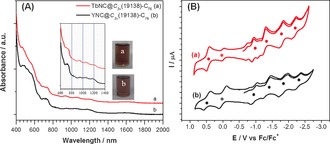
A) UV/Vis‐NIR spectra of TbNC@C 2v(19138)‐C76 (a) and YNC@C 2v(19138)‐C76 (b) dissolved in CS2. Insets: Enlarged spectral region (600–1400 nm) and the photographs of samples in CS2. B) Cyclic voltammograms of TbNC@C 2v(19138)‐C76 (a) and YNC@C 2v(19138)‐C76 (b) in o‐DCB solution. Ferrocene (Fc) was added as the internal standard and all potentials are referenced to the Fc/Fc+ couple, TBAPF6 as supporting electrolyte, scan rate: 100 mV s−1. The half‐wave potential (E 1/2) of each redox step is marked with a solid dot to aid comparison. The asterisk labels the oxidation peak of Fc.
The electronic properties of TbNC@C 2v(19138)‐C76 and YNC@C 2v(19138)‐C76 are further investigated by cyclic voltammetry. Figure 2 B shows their cyclic voltammograms measured in o‐dichlorobenzene (o‐DCB) with tetrabutylammonium hexafluorophosphate (TBAPF6) as supporting electrolyte (see also Figures S14–S15), and their characteristic redox potentials are summarized in Table 1, which includes also those of other analogous C82‐ and C76‐based endohedral fullerenes for comparison. Again, the characteristic redox potentials and the electrochemical gaps (ΔE gap,ec) of TbNC@C 2v(19138)‐C76 and YNC@C 2v(19138)‐C76 are almost identical (with the difference being less than 0.05 V, see Table 1), confirming further the decisive role of the carbon cage on the electronic properties of endohedral fullerenes with the same type of entrapped species.5, 6 MNC@C 2v(19138)‐C76 show a larger separation between the second and third reduction steps (0.52 and 0.50 V for TbNC@C76 and YNC@C76, respectively) than those between the first two reduction steps (first‐second, 0.35–0.38 V) and the last two reduction steps (third‐fourth, 0.41–0.42 V), and this phenomenon is similar to the cases of YNC@Cs(6)‐C82 and TbNC@C82 (Cs(6), C2(5), C 2v(9)).10 Such a resemblance on the electrochemical behavior between MNC@C 2v(19138)‐C76 and MNC@C82 suggests that they adopt the same electronic configuration, namely [M3+(NC)−]2+@[C2n]2−, resulting in a closed‐shell electronic configuration with non‐degenerate low‐lying LUMO and accessible LUMO+1 orbitals.5, 6, 10, 13a
Table 1.
Redox Potentials (V vs. Fc/Fc+), electrochemical gaps (ΔE gap,EC) of MNC@C 2v(19138)‐C76 and other reported C82‐ and C76‐based endohedral fullerenes.
| Sample | E 1/2 [V vs. Fc/Fc+] | ΔE gap,EC [V][a] | Ref. | ||||
|---|---|---|---|---|---|---|---|
| E red | E ox | ||||||
| 1st | 2nd | 3rd | 4th | 1st | |||
| TbNC@C 2v(19138)‐C76 | −0.91 | −1.26 | −1.78 | −2.19 | 0.45 | 1.36 | This work |
| YNC@C 2v(19138)‐C76 | −0.93 | −1.31 | −1.81 | −2.23 | 0.46 | 1.39 | This work |
| TbNC@C2(5)‐C82 | −0.88 | −0.97 | −1.55 | −1.91 | 0.50 | 1.38 | 10c |
| TbNC@Cs(6)‐C82 | −0.59 | −0.84 | −1.77 | −1.92 | 0.55 | 1.14 | 10a |
| TbNC@C 2v(9)‐C82 | −0.46 | −0.81 | −1.78 | −1.96 | 0.55 | 1.07 | 10a |
| YNC@Cs(6)‐C82 | −0.59 | −0.84 | −1.76 | −1.92 | 0.56 | 1.15 | 10b |
| Sm@C 2v(19138)‐C76 | −0.69 | −1.04 | −1.62 | −1.97 | 0.32 | 1.01 | 13b |
[a] ΔE gap,EC=E 1/2,ox(1)−E 1/2,red(1).
While YNC@C76 is diamagnetic since there is no unpaired electron for the Y3+ cation, Tb3+ has eight 4f electrons with a 7F6 Hund ground state, indicating that TbNC@C76 is paramagnetic. We then studied the magnetic properties of TbNC@C76 with a superconducting quantum interference device (SQUID). Figure 3 A shows the normalized magnetizations of TbNC@C76 versus the applied field‐temperature quotient x=μ0H/T measured at seven temperatures between 1.8 and 10 K. The good scaling in this temperature range indicates that the ligand field, which splits the Hund ground state, is so strong that the low temperature magnetization may be described with one Jz level. Based on a perfect fit between the experimental magnetization data and the non‐collinear magnetic moment model proposed previously for DyxSc3−xN@C80,9a–9c the magnetic moment |μ| of TbNC@C76 is determined to be 8.9 μB, which agrees well with the theoretical limit of 9 μB. Therefore, the Tb ground state is assigned to be Jz=±6 (see Supporting Information S7). Such a large Jz value is a prerequisite for SMM.10a
Figure 3.
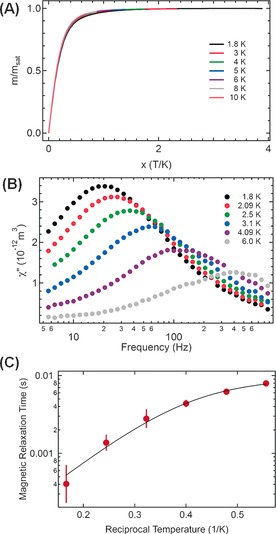
A) Magnetization of TbNC@C 2v(19138)‐C76 versus the applied field temperature quotient x. The color codes of the different temperatures are indicated. The magnetization curves scale with the applied field temperature quotient x=μ0H/T. B) Imaginary part of AC susceptibility measured at different temperatures for TbNC@C 2v(19138)‐C76. μ0H=B0+B1*sin(ωt), B0=200 mT, B1=0.25 mT. C) Magnetic relaxation times (τ) determined from the data in (B) as a function of reciprocal temperature. The solid line is a 3‐parameter fit using the similar function applied for DySc2N@C80 in Ref. 9a, resulting in the thermal barrier (Δeff/k B) of 12±2 K, a prefactor (τ0) of 80±40 μs and a temperature independent lifetime (τc) of 9±1 ms.
Similar to the case of HoSc2N@C80,9d the AC susceptibility shown in Figure 3 B qualifies TbNC@C76 as a field‐induced SMM or more specifically single‐ion magnet (SIM) which is a SMM containing only one single magnetic ion.8b, 10a In low fields (μ0H=0.2 T), the AC susceptibility shows significant temperature dependence of the magnetic relaxation times. Figure 3 C shows an Arrhenius plot of the magnetization lifetimes in an applied field μ0H=0.2 T with a fit9a extracting characteristic kinetic parameters for the demagnetization of the observed super‐paramagnetism. Above 4 K, a thermal de‐magnetization barrier (Δeff/k B) of 12±2 K with a prefactor (τ0) of 80±40 μs can be obtained. At lower temperatures, the magnetic relaxation time saturates where the fit indicates a maximum lifetime (τc) of 9±1 ms for the temperature independent decay of the magnetization (see Supporting Information S7).
In summary, two novel non‐IPR mononuclear clusterfullerenes MNC@C76 (M=Tb, Y) have been successfully synthesized and isolated, featuring the stabilization of one pair of fused‐pentagons by a mononuclear MNC cluster. The MNC cluster entrapped within the non‐IPR C 2v(19138)‐C76 cage is found to take a nearly linear configuration, which is remarkably different from the triangular geometry of the MNC cluster for the reported IPR‐obeying C82 cage‐based mononuclear cyanide clusterfullerenes. TbNC@C 2v(19138)‐C76 and YNC@C 2v(19138)‐C76 exhibit almost identical electronic properties as shown by UV/Vis‐NIR spectroscopic and cyclic voltammetric studies. TbNC@C76 is identified to be a field‐induced SMM with a maximum lifetime of 9±1 ms. Our study on the novel non‐IPR mononuclear clusterfullerenes provides new insights into the exceptional stabilities of strained fullerene molecules.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
We thank Profs. L.‐S. Zheng and J. Tao (Xiamen University, China) for valuable discussions. This work was partially supported by the National Natural Science Foundation of China (NNSFC, Nos. 21132007, 21371164, 2151101074, 51572254) [to S.F.Y.], the 973 project (2014CB845601) and the NNSFC (no. U1205111, 21390390, 51572231) [to S.Y.X.], DFG (grant PO 1602/1–2 and DU225/31‐1), and the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement No 648295 “GraM3”) [to A.A.P.], and the Swiss National Science Foundation (200021L_147201) within the DACH program [to T.G.]. Computational resources were provided by the Center for Information Services and High Performance Computing (ZIH) in TU Dresden. We thank Ulrike Nitzsche for technical assistance with computational resources in IFW Dresden.
F. Liu, S. Wang, C.-L. Gao, Q. Deng, X. Zhu, A. Kostanyan, R. Westerström, F. Jin, S.-Y. Xie, A. A. Popov, T. Greber, S. Yang, Angew. Chem. Int. Ed. 2017, 56, 1830.
Contributor Information
Prof. Dr. Su‐Yuan Xie, Email: syxie@xmu.edu.cn.
Dr. Alexey A. Popov, Email: a.popov@ifw-dresden.de.
Prof. Dr. Thomas Greber, Email: greber@physik.uzh.ch
Prof. Dr. Shangfeng Yang, Email: sfyang@ustc.edu.cn.
References
- 1. Hirsch A., Brettreich M., Fullerenes: Chemistry and Reactions, Wiley-VCH, Weinheim, 2005. [Google Scholar]
- 2.
- 2a. Fowler P. W., Manolopoulos D. E., An Atlas of Fullerenes, Oxford Press, Clarendon, 1995; [Google Scholar]
- 2b.“Carbon: Fullerenes”: Liu F. P., Yang S. F., in Encyclopedia of Inorganic and Bioinorganic Chemistry (Eds.: C. M. Lukehart, R. A. Scott), Wiley, Hoboken, 2014, DOI: 10.1002/9781119951438.eibc0033.pub2. [Google Scholar]
- 3. Kroto H. W., Nature 1987, 329, 529–531. [Google Scholar]
- 4. Tan Y. Z., Xie S. Y., Huang R. B., Zheng L. S., Nat. Chem. 2009, 1, 450–460. [DOI] [PubMed] [Google Scholar]
- 5.
- 5a. Popov A. A., Yang S. F., Dunsch L., Chem. Rev. 2013, 113, 5989–6113; [DOI] [PubMed] [Google Scholar]
- 5b. Wang T. S., Wang C. R., Acc. Chem. Res. 2014, 47, 450–458; [DOI] [PubMed] [Google Scholar]
- 5c. Lu X., Feng L., Akasaka T., Nagase S., Chem. Soc. Rev. 2012, 41, 7723–7760; [DOI] [PubMed] [Google Scholar]
- 5d. Rodríguez-Fortea A., Balch A. L., Poblet J. M., Chem. Soc. Rev. 2011, 40, 3551–3563; [DOI] [PubMed] [Google Scholar]
- 5e. Chaur M. N., Melin F., Ortiz A. L., Echegoyen L., Angew. Chem. Int. Ed. 2009, 48, 7514–7538; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 7650–7675. [Google Scholar]
- 6.
- 6a. Stevenson S., Fowler P. W., Heine T., Duchamp J. C., Rice G., Glass T., Harich K., Hajdu E., Bible R., Dorn H. C., Nature 2000, 408, 427–428; [DOI] [PubMed] [Google Scholar]
- 6b. Olmstead M. M., Lee H. M., Duchamp J. C., Stevenson S., Marciu D., Dorn H. C., Balch A. L., Angew. Chem. Int. Ed. 2003, 42, 900–903; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2003, 115, 928–931; [Google Scholar]
- 6c. Beavers C. M., Chaur M. N., Olmstead M. M., Echegoyen L., Balch A. L., J. Am. Chem. Soc. 2009, 131, 11519–11524; [DOI] [PubMed] [Google Scholar]
- 6d. Mercado B. Q., Beavers C. M., Olmstead M. M., Chaur M. N., Walker K., Holloway B. C., Echegoyen L., Balch A. L., J. Am. Chem. Soc. 2008, 130, 7854–7855; [DOI] [PubMed] [Google Scholar]
- 6e. Zuo T., Walker K., Olmstead M. M., Melin F., Holloway B. C., Echegoyen L., Dorn H. C., Chaur M. N., Chancellor C. J., Beavers C. M., Balch A. L., Athans A. J., Chem. Commun. 2008, 1067–1069; [DOI] [PubMed] [Google Scholar]
- 6f. Zhang Y., Ghiassi K. B., Deng Q., Samoylova N. A., Olmstead M. M., Balch A. L., Popov A. A., Angew. Chem. Int. Ed. 2015, 54, 495–499; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 505–509; [Google Scholar]
- 6g. Chen N., Beavers C. M., Mulet-Gas M., Rodríguez-Fortea A., Munoz E. J., Li Y.-Y., Olmstead M. M., Balch A. L., Poblet J. M., Echegoyen L., J. Am. Chem. Soc. 2012, 134, 7851–7860. [DOI] [PubMed] [Google Scholar]
- 7. Yang S. F., Liu F. P., Chen C. B., Jiao M. Z., Wei T., Chem. Commun. 2011, 47, 11822–11839. [DOI] [PubMed] [Google Scholar]
- 8.
- 8a. Woodruff D. N., Winpenny R. E. P., Layfield R. A., Chem. Rev. 2013, 113, 5110–5148; [DOI] [PubMed] [Google Scholar]
- 8b. Dreiser J., J. Phys. Condens. Matter 2015, 27, 183203. [DOI] [PubMed] [Google Scholar]
- 9.
- 9a. Westerström R., Dreiser J., Piamonteze C., Muntwiler M., Weyeneth S., Brune H., Rusponi S., Nolting F., Popov A. A., Yang S. F., Dunsch L., Greber T., J. Am. Chem. Soc. 2012, 134, 9840–9843; [DOI] [PubMed] [Google Scholar]
- 9b. Westerström R., Dreiser J., Piamonteze C., Muntwiler M., Weyeneth S., Kramer K., Liu S. X., Decurtins S., Popov A. A., Yang S. F., Dunsch L., Greber T., Phys. Rev. B 2014, 89, 060406; [Google Scholar]
- 9c. Westerström R., Uldry A. C., Stania R., Dreiser J., Piamonteze C., Muntwiler M., Matsui F., Rusponi S., Brune H., Yang S. F., Popov A. A., Buchner B., Delley B., Greber T., Phys. Rev. Lett. 2015, 114, 087201; [DOI] [PubMed] [Google Scholar]
- 9d. Dreiser J., Westerström R., Zhang Y., Popov A. A., Dunsch L., Kramer K., Liu S. X., Decurtins S., Greber T., Chem. Eur. J. 2014, 20, 13536–13540; [DOI] [PubMed] [Google Scholar]
- 9e. Junghans K., Schlesier C., Kostanyan A., Samoylova N. A., Deng Q. M., Rosenkranz M., Schiemenz S., Westerström R., Greber T., Büchner B., Popov A. A., Angew. Chem. Int. Ed. 2015, 54, 13411–13415; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 13609–13613. [Google Scholar]
- 10.
- 10a. Liu F. P., Gao C.-L., Deng Q. M., Zhu X. J., Kostanyan A., Westerström R., Wang S., Tan Y.-Z., Tao J., Xie S.-Y., Popov A. A., Greber T., Yang S. F., J. Am. Chem. Soc. 2016, 138, 14764–14771; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10b. Yang S. F., Chen C. B., Liu F. P., Xie Y. P., Li F. Y., Jiao M. Z., Suzuki M., Wei T., Wang S., Lu X., Chen Z. F., Akasaka T., Sci. Rep. 2013, 3, 1487; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10c. Liu F. P., Wang S., Guan J., Wei T., Zeng M. X., Yang S. F., Inorg. Chem. 2014, 53, 5201–5205. [DOI] [PubMed] [Google Scholar]
- 11.
- 11a. Olmstead M. M., Beavers C. M., Balch A. L., Wang G., Yee G. T., Shu C. Y., Xu L., Elliott B., Echegoyen L., Duchamp J. C., Dorn H. C., Inorg. Chem. 2008, 47, 5234–5244; [DOI] [PubMed] [Google Scholar]
- 11b. Wei T., Wang S., Liu F. P., Tan Y. Z., Zhu X. J., Xie S. Y., Yang S. F., J. Am. Chem. Soc. 2015, 137, 3119–3123; [DOI] [PubMed] [Google Scholar]
- 11c. Wei T., Wang S., Lu X., Huang J., Liu F. P., Li Q. X., Xie S. Y., Yang S. F., J. Am. Chem. Soc. 2016, 138, 207–214; [DOI] [PubMed] [Google Scholar]
- 11d. Zuo T. M., Xu L., Beavers C. M., Olmstead M. M., Fu W., Crawford T. D., Balch A. L., Dorn H. C., J. Am. Chem. Soc. 2008, 130, 12992–12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.
- 12a. Harris K. J., Wasylishen R. E., Inorg. Chem. 2009, 48, 2316–2332; [DOI] [PubMed] [Google Scholar]
- 12b. Stevens P. A., Madix R. J., Stohr J., J. Chem. Phys. 1989, 91, 4338–4345; [Google Scholar]
- 12c. Orpen A. G., Brammer L., Allen F. H., Kennard O., Watson D. G., Taylor R. J., J. Chem. Soc. Dalton Trans. 1989, S1–S83. [Google Scholar]
- 13.
- 13a. Lu X., Slanina Z., Akasaka T., Tsuchiya T., Mizorogi N., Nagase S., J. Am. Chem. Soc. 2010, 132, 5896–5905; [DOI] [PubMed] [Google Scholar]
- 13b. Hao Y. J., Feng L., Xu W., Gu Z. N., Hu Z., Shi Z. J., Slanina Z., Uhlik F., Inorg. Chem. 2015, 54, 4243–4248. [DOI] [PubMed] [Google Scholar]
- 14. CCDC 997467, 1509471, contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via The Cambridge Crystallographic Data Centre.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


