Abstract
Scleraxis (Scx) is a known regulator of tendon development, and recent work has identified the role of Scx in bone modeling. However, the role of Scx in fracture healing has not yet been explored. This study was conducted to identify the role of Scx in cortical bone development and fracture healing. Scx green fluorescent protein–labeled (ScxGFP) reporter and Scx-knockout (Scx-mutant) mice were used to assess bone morphometry and the effects of fracture healing on Scx localization and gene expression, as well as callus healing response. Botulinum toxin (BTX) was used to investigate muscle unloading effects on callus shape. Scx-mutant long bones had structural and mechanical defects. Scx gene expression was elevated and bmp4 was decreased at 24 h after fracture. ScxGFP+ cells were localized throughout the healing callus after fracture. Scx-mutant mice demonstrated disrupted callus healing and asymmetry. Asymmetry of Scx-mutant callus was not due to muscle unloading. Wild-type littermates (age matched) served as controls. This is the first study to explore the role of Scx in cortical bone mechanics and fracture healing. Deletion of Scx during development led to altered long bone properties and callus healing. This study also demonstrated that Scx may play a role in the periosteal response during fracture healing.—McKenzie, J. A., Buettmann, E., Abraham, A. C., Gardner, M. J., Silva, M. J., Killian, M. L. Loss of scleraxis in mice leads to geometric and structural changes in cortical bone, as well as asymmetry in fracture healing.
Keywords: skeletal repair, callus, development, transcription factors, tendon
The fracture repair process requires a coordinated series of events for proper healing, and the process of fracture healing mimics the developmental process of endochondral bone formation in skeletal elements (1–5). Although fracture healing has been studied for decades, the processes involved in stem cell recruitment, proliferation, and differentiation, such as where the mesenchymal cells originate, is still poorly understood (1).
Members of the TGF-β superfamily, such as bone morphogenetic protein (BMP)-2 and TGF-β3, have been shown to act as promoters of mesenchymal stem cell differentiation toward osteogenic and chondrogenic lineages (6–10). These morphogens (1, 3), as well as BMP-4 (11), are also known to influence bone healing after fracture. Although dispensable in small animal models of long bone fracture healing (12), the role of BMP-4 in long bone development is evident. Recent work has shed light on the development of bone ridges in long bones, specifically at the interface between tendon and bone (13–20). The shape and formation of bone ridges on skeletal elements is regulated, first, by specification of progenitors by TGF-β, followed by differentiation mediated by BMP-4 and scleraxis (Scx), a tendon progenitor marker (15, 19). Mice that lack BMP-2 (21), BMP-4 (19), or Scx (22) during limb bud development do not develop bone ridges, and subsequent findings have shown that these structures are derived from a unique population of cells that express both Sox-9, a chondrocyte progenitor marker, and Scx (15, 23, 24).
Scx is a member of the basic helix-loop-helix family of transcription factors and is critical for the proper development and maturation of tendons (18, 22, 25–27) and their attachments to bone (13–15). Loss of Scx leads to impaired, but not complete absence of, tendon formation (22). In addition, Scx-knockout (Scx-mutant) mice fail to form tendon–bone eminences and ridges (19, 22), which is in part due to loss of BMP-4 signaling (19). Although the development of bone shape and structure is known to be mediated in part through Scx regulation of BMP-4, there has been little work investigating the role of Scx on long bone development and healing.
The mechanisms by which bone ridges form and contribute to the shape and loading of long bones may provide novel insights into the fracture-healing process, given the similarities between bone modeling during development and remodeling after injury. Muscle loading has been shown to maintain bone ridges during embryonic growth (19), and muscle contraction is required for bidirectional callus formation and ossification after fracture in the postnatal developing skeleton (20). Therefore, we hypothesized that a key regulator of bone ridge formation, Scx, also regulates callus formation after fracture in the adult long bone. Specifically, we posited that loss of Scx would lead to delayed and impaired callus formation, caused in part by reduced muscle loading of the fractured bone.
MATERIALS AND METHODS
Animal models
All studies were approved by the Washington University Institutional Animal Studies Committee. Mice in this study were raised on a C57BL/6 background, maintained in a 12-h light/dark cycle in standard housing, and given unrestricted access to food and water. A total of 102 mice were used in this study. All mice were housed in shared cages (no more than 5 mice per cage) in barrier facilities until time of fracture, after which all mice were housed in standard animal housing in compliance with the University’s Animal Welfare Guidelines for the Care and Use of Research Animals. Wild-type (WT) C57BL/6J mice (10 wk) were used for gene expression experiments (n = 5, female) and for unloading and fracture-healing experiments (n = 8 male) (The Jackson Laboratory, Bar Harbor, ME, USA). Scx green fluorescent protein–labeled (ScxGFP) transgenic mice (10 wk; n = 3 females) were bred in-house and used in immunohistochemistry experiments. In the remaining experiments, Scx-mutant mice (n = 43; 18 males and 25 females) and WT littermates (n = 47; 29 males and 18 females) were used (22). Scxflx/flx mice were mated with Prx1Cre mice to target Scx deletion in limb bud progenitor cells. Scxflx/flx and ScxGFP mice were generously provided by Dr. Ronen Schweitzer (Shriner’s Research Center, Portland, OR, USA) (26). Prx1Cre mice (strain 005584) were purchased from The Jackson Laboratory. The genotypes of mice were determined from toe and tail biopsies by using PCR-based analysis of DNA for Cre, Scx-null, Scx-WT, and Scx-flox alleles (13, 22). Scx-mutant mice were developed by using Scxflx/flx mice crossed with Prx1-Cre; Scxflx/WT mice. Targeted deletion of Scx occurred in homozygous Cre+ pups in Prx1-Cre (limb bud mesenchyme) lineage cells (n = 39 mice, used for fracture healing and phenotyping experiments, Prx1Cre+ male sires). Littermates that were Cre− and did not have a Scx-null allele were used as WT controls. Investigators were not blinded to mouse genotype at the time of experiments given the marked phenotype of Scx-mutants (i.e., hyperdorsiflexion of the forepaw).
Phenotypic bone characterization
WT and Scx-mutant mice (n = 8 WT; 8 mutant; 8 wk) were euthanized via carbon dioxide asphyxiation and cervical dislocation; carcasses were frozen until biomechanical and morphometric testing. At time of testing, the carcasses were thawed. Uninjured femurs, ulnae, and tibiae were dissected from WT and Scx-mutant mice, immersed in PBS, and stored at 4°C for <48 h before testing. Femurs and ulnae were scanned in air with microcomputed tomography (microCT40; 16 μm standard resolution, 55 kVp, 145 μA, and 300 ms integration; Scanco, Brüttisellen Switzerland) for geometric and structural measurements. At the long bone midpoint, 0.3 mm (20 slices) was analyzed for tissue mineral density (TMD), cortical area (Ct.Ar.), and polar moment of inertia (I). After the scans, bone lengths were measured with digital precision calipers, and bones were subjected to 3-point bending (span length = 7 mm) at a loading rate of 0.1 mm/s (Instron Electropuls, Norwood, MA, USA). Structural mechanical outcomes for both ulnae and femurs were rigidity, maximum load, postyield displacement (PYD), work-to-fracture, and yield load. Estimated material properties were Young’s modulus, yield stress, and maximum stress. Tibiae were used for microindentation of the cortical bone using reference point indentation (ActiveLife Sciences, Santa Barbara, CA, USA) to determine localized cortical bone material properties. Reference point indentation establishes a small fracture (<300 μm) and propagates it through cyclic penetration, thereby capturing a bone’s fracture resistance. This method has been correlated with traditional measures of whole-bone toughness (29), which is known to be an important material property for determining fragility (30). Measured parameters were first cycle indentation distance (ID 1st), indentation distance increase (IDI), total indentation distance (TID), first cycle unloading slope (US 1st), and average creep indentation distance (CID).
Dynamic histomorphometry
Mice allocated for dynamic histomorphometry were given calcein [10 mg/kg body weight (BW), i.p.; Sigma-Aldrich, St. Louis, MO, USA] and Alizarin red (25 mg/kg BW, i.p.; Sigma-Aldrich) fluorochrome injections spaced at 4 or 5 d, with the last label given 2 d before euthanasia. Mice were assayed between 3 wk of age and skeletal maturity (∼16–18 wk; n = 6 WT; n = 10 mutant). After euthanasia, femurs and ulnae were dissected from soft tissue and processed for plastic embedding. Bones were dehydrated in a series of graded ethanol solutions changed hourly (70%, 3× at 95%, 3× at 100%). Bones were then immediately transferred to infiltration solutions at 4°C (85% methyl-methacrylate, 15% dibutyl phthalate, with 0, 1, or 2% benzoyl peroxide), and solutions were changed every 24 h, with samples spending 48 h at each percentage of benzoyl peroxide. After infiltration, samples were embedded in the 2% benzoyl peroxide solution in glass vials at 37°C. After the solution cured into a solid, samples were removed from glass and sectioned transversely at the midpoint (100 μm, SP1600; Leica, Buffalo Grove, IL, USA). Samples were mounted to glass slides and sanded down to 30-µm thickness for imaging (IX51; Olympus, Center Valley, PA, USA) and analysis (Osteo 2010; Bioquant, Nashville, TN, USA).
Bone fracture model
Closed right femur fractures were produced according to a published procedure (28). In brief, the mice were anesthetized with isoflurane (2–3%). After shaving fur and disinfecting with Betadine, a small incision was made at the knee lateral and parallel to the patellar tendon to dislocate the patella. To provide access to the intramedullary canal, we made a hole in the distal end of the femur and placed thin tungsten guide wire (0.125 mm diameter) into the femur. The mouse was secured in a materials testing machine using a custom 3-point testing fixture (Dynamight; Instron, Norwood, MA, USA). The femur was broken at the mid-diaphysis with a rapid-displacement controlled ramp (30 mm/s). To prevent excessive angulation of the femur, displacement ramp was limited to 1 mm above the resting platform. Immediately after fracture, the broken femur was stabilized with a 24-gauge stainless steel hollow rod (guided over the tungsten wire). In case of nonfracture due to hip dislocation (observed in Scx-mutant mice), a second attempt was made to realign and fracture the femur. If, after 3 attempts, a fracture was not obtained, the mouse was euthanized immediately. The guide wire was removed, the stainless steel rod was trimmed to the end of the femur, and the wound was sutured closed. To manage pain after surgery, we gave all mice 1 dose of buprenorphine (0.05 mg/kg, subcutaneously).
Postfracture expression of scx-related genes
WT mice (C57BL/6J; age 10 wk, n = 5; The Jackson Laboratory) were subjected to a mid-diaphyseal fracture. At 24 h after injury, both right and left femora were harvested for analysis. The overlying soft tissues and muscle were stripped carefully to preserve the periosteum. The central 7 mm of the femur was isolated and the marrow removed by centrifugation (15 s at 13,000 rpm). Femora were weighed before pulverization in liquid nitrogen cooled in microdismembrator flasks (B. Braun, Melsungen, Germany). Samples were suspended in 1 ml Trizol (Thermo Fisher Scientific, Waltham, MA, USA), mixed with chloroform (200 µl; Sigma-Aldrich), and spun down in a phase-lock gel tube for 7 min (5Prime, Gaithersburg, MD, USA). The upper phase was transferred to a new tube, and RNA extraction continued according to the manufacturer’s instructions (RNeasy mini kit with DNAse away; Qiagen, Valencia, CA, USA). RNA quality (2100 Bioanalyzer, Agilent Technologies, Santa Clara, CA, USA) and quantity (Nanodrop; Thermo Fisher Scientific) were assessed for each sample. cDNA was obtained using VILO Superscript III (Thermo Fisher Scientific). Quantitative RT-PCR (qRT-PCR) was performed with murine-specific TaqMan probes (Thermo Fisher Scientific) for scx (Mm01205675_m1), bmp-4 (Mm00432087_m1), and tgf-b3 (Mm00436960_m1), with 18s (MM00432359_m1) as a reference gene. TaqMan qRT-PCR was performed (StepOnePlus; Thermo Fisher Scientific). Relative gene expression (fracture vs. nonfracture) was computed using the 2−ΔΔCt method.
Postfracture localization of scleraxis
ScxGFP mice were euthanized 14 d after fracture surgery (n = 3). Fractured hindlimbs were dissected with surrounding soft tissues removed and fixed overnight in 4% paraformaldehyde. Samples were decalcified for 14 d (14% EDTA) and processed for histological analysis (paraffin sections, 5 µm). Immunohistochemistry was performed for GFP localization in the fracture callus. In brief, sections were deparaffinized with xylenes and hydrated in graded alcohol solutions. Endogenous peroxidase activity was quenched with 3% H2O2 for 10 min at room temperature. Samples were blocked with 10% rabbit serum for 1 h at room temperature. Primary antibody was incubated overnight at 4°C in a humidified chamber (chicken anti-GFP, 1:100; ab13970; Abcam, Cambridge, MA, USA). The following day, sections were washed in PBS before secondary antibody application for 1 h at room temperature (biotinylated rabbit anti-chicken, 1:500, ab6752; Abcam). Sections were washed again in PBS before addition of streptavidin HRP (1:400). To visualize the stain, DAB substrate was used without any counterstaining. Sections were dehydrated, placed in xylene, coverslipped before imaging, and imaged at ×10 and 20 (BX51; Olympus, Center Valley, PA, USA).
Fracture-healing experiments
Adult WT and Scx-mutant mice (age, 10–100 wk) were subjected to midpoint femoral fractures. Mice that had displaced fractures, deemed irreparable by the orthopedic surgeon (M.J.G.), were euthanized immediately after fracture (n = 9, Scx-mutant mice; n = 0, WT mice). At d 14 or 28 after fracture, mice were euthanized for fracture callus analyses. After death, plain radiographs were obtained (MX-20; Faxitron, Tucson, AZ, USA) to image the fracture in the sagittal plane. Digitized radiograph images at d 14 after fracture were analyzed for ventral/dorsal callus area and asymmetry using OrthoRead (Boise State University, Boise, ID, USA) (31). Asymmetry was calculated using:
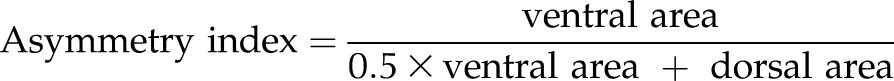 |
where ventral and dorsal areas are expressed in pixels. After radiography, both left (nonfractured, contralateral) and right (fractured) femurs were removed and processed for bone morphometry. Bones were carefully dissected to remove musculature and fixed for 24 h in 10% formalin. After fixation, bones were scanned in air using microCT (microCT40; Scanco, Switzerland; 16 μm standard resolution, 55 kVp, 145 μA, and 300 ms integration). Fracture callus volume, bone volume, and callus mineral density were calculated for a callus volume spanning 200 slices (3.2 mm), above and below the fracture, excluding the cortical bone. After scanning, bones were processed for histological staining. Femurs were decalcified in 14% EDTA for 2 wk at room temperature. Femurs were embedded in paraffin and sectioned at the middle of the callus. Serial sections were stained for safranin O and Alcian blue/picrosirius red by the Musculoskeletal Research Center histology core and imaged with a ×5 objective (AxioImager.A2 microscope, AxioVision SE64 software, release 4.9.1; Zeiss, Thornwood, NY, USA).
Unloading and fracture healing
Scx mutants have documented musculotendinous defects (13, 19, 22). To separate the effects of Scx deletion from the effects of muscle unloading, a set of unloading fracture experiments was performed (age, 15 wk). Immediately after fracture surgery and placement of the stainless steel rod, 0.15 U (10 μl) of botulinum toxin A (BTX; Allergan, Parsippany-Troy Hills, NJ, USA) in 0.1% saline was injected with a 27-gauge needle directly into the quadriceps. Hindlimb paralysis was observationally confirmed for several days after fracture. Mice were euthanized at 14 d and analyzed by microCT and histology by the same methods as were used in the Scx-mutant mice. This group is referred to as BTX-WT.
Statistical analysis
Data are expressed as means ± sd. All statistical comparisons were performed with Prism 6.0 (GraphPad, La Jolla, CA, USA). Unpaired, 1-tailed Student’s t tests were performed on WT and Scx-mutant mice to compare phenotypic differences using plain radiograph, microCT, and mechanical testing. Normality distribution was tested with the D’Agostino and Pearson omnibus normality test. For normally distributed data, correlations were used to compare the multiplicity of infection (MOI) with material properties of the femur and ulna for 8-wk-old WT and Scx-mutant mice. For nonnormally distributed data, Spearman correlations were used to compare MOI with material properties of the femur and ulna for 8-wk-old WT and Scx-mutant mice. The Wilcoxon matched-pairs signed rank test (1-tailed) was used for comparisons of log2-transformed −ΔΔCt gene expression data between nonfractured contralateral and fractured femurs. A 1-way ANOVA with Holm-Sidak’s multiple comparisons test were used to compare postfracture callus density and volume at d 14 for the WT, Scx-mutant, and BTX-WT groups and for comparison of callus density and volume at d 28 after fracture for WT and Scx-mutant groups. Statistical significance was set at P < 0.05.
RESULTS
Bone structure and strength are altered in the Scx-mutant mice
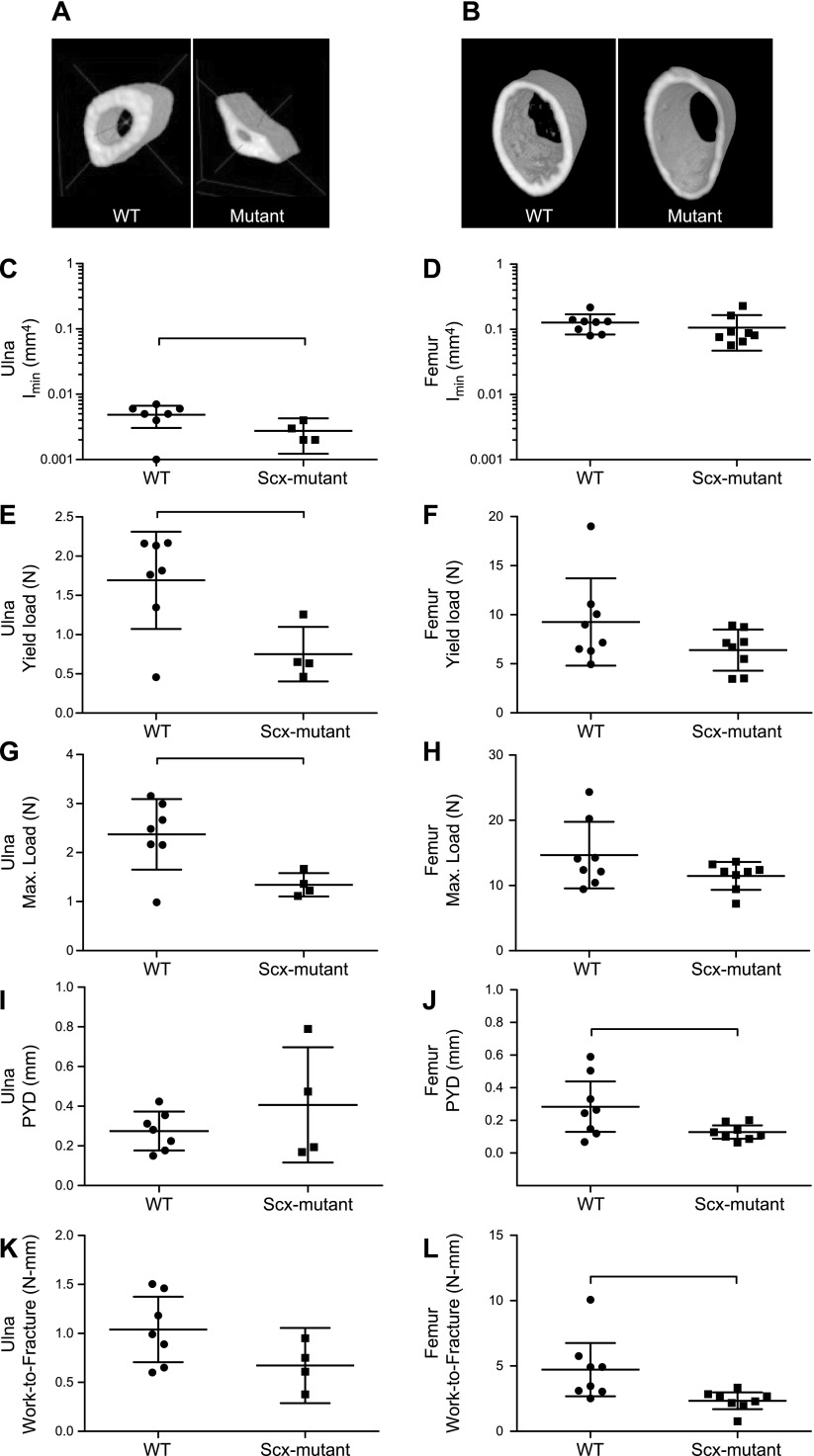
In 8-wk-old mice, bone structure and strength were evaluated with microCT, dynamic histomorphometry, 3-point bending and reference point indentation testing (Fig. 1, Table 1, and Supplemental Fig. S1). The BW of Scx-mutant mice decreased significantly compared with that of WT mice. In Scx-mutant femurs, there were some significant defects in femur mechanical properties, but bone size and density were not different from those of WT femurs. Specifically, Scx-mutant femurs had significantly lower postyield displacement and work-to-fracture than did the WT femurs, indicating impaired fracture resistance. However, rigidity, yield load, maximum load, Young’s modulus, yield stress, and maximum stress were not different for femurs between groups (Fig. 1 and Table 1). There was a significant correlation between the femur material properties and MOIs in both WT and Scx-mutant mice (Supplemental Fig. S1 and Table S1). In WT mice, increasing MOI led to significantly increased postyield displacement; however, this was not true of Scx-mutant mice (Supplemental Fig. S1A). Young’s modulus was negatively correlated with increasing MOI in both WT and Scx-mutant mice (Supplemental S1B). In Scx-mutant mice, increasing MOI was negatively correlated with Young’s modulus, ultimate stress, and modulus of toughness (Supplemental Fig. S1C–E). Scx-mutant ulnae had reduced area, MOI, yield load, and maximum load (Fig. 1). All other measured parameters were not significantly different between Scx-mutant and WT ulnae. Reference point indentation testing on tibiae from Scx-mutant and WT mice demonstrated differences in nearly all parameters measured (Table 1). ID 1st and CID were significantly elevated in Scx-mutant mice, whereas US 1st was lower. TID was 40% higher in Scx-mutant but the increase was not significant (P = 0.057). Dynamic histomorphometry labeling of ulnae and femurs demonstrated clearly labeled surfaces on Scx-mutant and WT groups.
Figure 1.
A, B) Reconstructions of the ulna (A) and femur (B) at the mid-diaphysis showed a distinct difference in the shape of the ulna, but not femur, for Scx-mutant compared to WT mice at 8 wk of age. C–L) After 3-point bending tests and measurement of Imin (C, D), yield load (E, F), maximum load (G, H), PYD (I, J), and work-to-fracture (K, L), some structural properties differed between WT and Scx-mutant in both the ulna (C, E, G, I, K) and femur (D, F, H, J, L). Bars indicate significant difference between groups. P < 0.05.
TABLE 1.
Mechanical testing of WT and Scx-mutant mice
| WT |
Scx-Mutant |
|
|---|---|---|
| Parameter | Average ± sd | Average ± sd |
| BW (g) | 25.44 ± 5.34 | 20.35 ± 3.17** |
| MicroCT and 3-point bending (femur) | ||
| TMD (mgHA/cm3) | 867.60 ± 34.40 | 841.60 ± 85.40 |
| Ct.Ar. (mm2) | 0.78 ± 0.13 | 0.72 ± 0.14 |
| I (mm) | 0.13 ± 0.04 | 0.11 ± 0.06 |
| Rigidity (N/mm2) | 579.20 ± 168.40 | 437.70 ± 121.50 |
| Yield load (N) | 9.26 ± 4.45 | 6.40 ± 2.10 |
| Maximum load (N) | 14.70 ± 5.09 | 11.50 ± 2.13 |
| PYD (mm) | 0.28 ± 0.19 | 0.13 ± 0.05* |
| Work-to-fracture (N/mm) | 4.72 ± 2.44 | 2.34 ± 0.77** |
| Young’s modulus (MPa) | 5061.00 ± 2603.00 | 4772.00 ± 1882.00 |
| Yield stress (MPa) | 95.20 ± 67.90 | 80.80 ± 44.40 |
| Maximum stress (MPa) | 145.40 ± 74.30 | 142.10 ± 51.30 |
| MicroCT and 3-point bending (ulna) | ||
| TMD (mgHA/cm3) | 839.50 ± 62.00 | 824.30 ± 29.50 |
| Ct.Ar. (mm2) | 0.31 ± 0.09 | 0.20 ± 0.03* |
| I (mm) (4) | 0.05 ± 0.01 | 0.03 ± 0.01* |
| Rigidity (N/mm2) | 57.56 ± 24.73 | 23.67 ± 3.58* |
| Yield load (N) | 1.69 ± 0.62 | 0.75 ± 0.35* |
| Maximum load (N) | 2.37 ± 0.72 | 1.34 ± 0.24** |
| PYD (mm) | 0.28 ± 0.10 | 0.41 ± 0.29 |
| Work-to-fracture (N/mm) | 1.04 ± 0.36 | 0.67 ± 0.24 |
| Young's modulus (MPa) | 12,814.00 ± 4413.00 | 9516.00 ± 3,854.00 |
| Yield stress (MPa) | 165.70 ± 43.70 | 123.50 ± 70.30 |
| Maximum stress (MPa) | 145.40 ± 74.30 | 142.10 ± 51.30 |
| Reference point indentation testing (tibia) | ||
| ID first (µm) | 86.50 ± 38.90 | 131.30 ± 22.80* |
| IDI (µm) | 23.30 ± 4.80 | 22.80 ± 2.90 |
| TID (µm) | 101.50 ± 38.10 | 141.50 ± 20.80 |
| US first (µm) | 0.17 ± 0.04 | 0.09 ± 0.01* |
| CID (µm) | 4.50 ± 1.35 | 6.88 ± 0.53** |
P < 0.05, **P < 0.01.
Scx is expressed in fracture healing
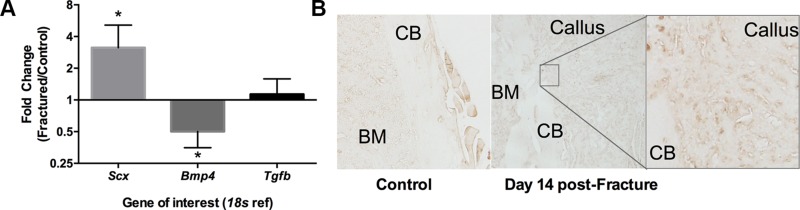
At 24 h after fracture, compared to contralateral nonfractured bones, expression of scx was elevated ∼3-fold (P = 0.031), and bmp-4 was reduced ∼2-fold (P = 0.031), with no difference between groups in tgf-b3 expression (P = 0.500) (Fig. 2A). ScxGFP expression in WT mice was absent in the contralateral nonfractured cortical bone and localized to the cartilage and woven bone of the callus at 14 d after fracture (Fig. 2B).
Figure 2.
A) Gene expression of scx, bmp4, and tgfb was evaluated in WT mice 24 h after fracture. Expression of scx was upregulated in the fractured limb compared with the contralateral nonfractured limb, whereas bmp4 was downregulated and tgfb unchanged. *P < 0.05, fractured vs. nonfractured limbs for respective gene. B) Immunohistochemistry for GFP on a subset of Scx:GFP mice at d 14 after fracture showed that ScxGFP was evident in the newly formed woven bone and cartilage of the callus, but not in the uninjured, contralateral cortical bone (CB) of the femur. BM, bone marrow.
Fracture healing was impaired in Scx-mutant mice, but not because of tendon imbalance
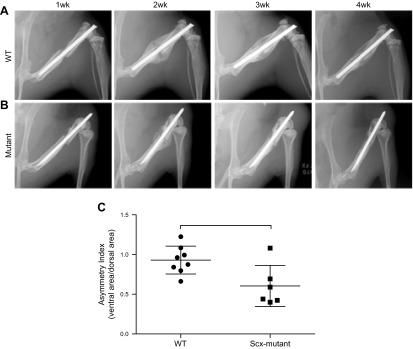
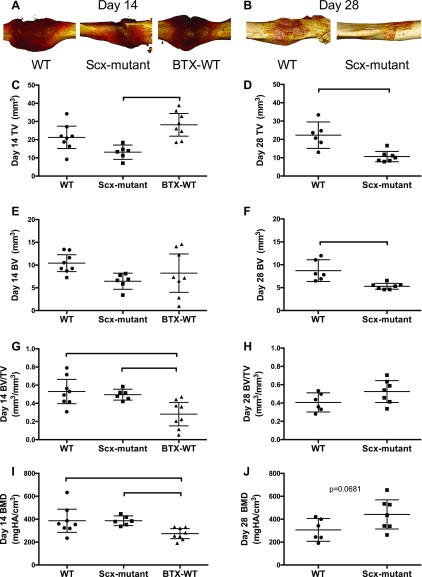
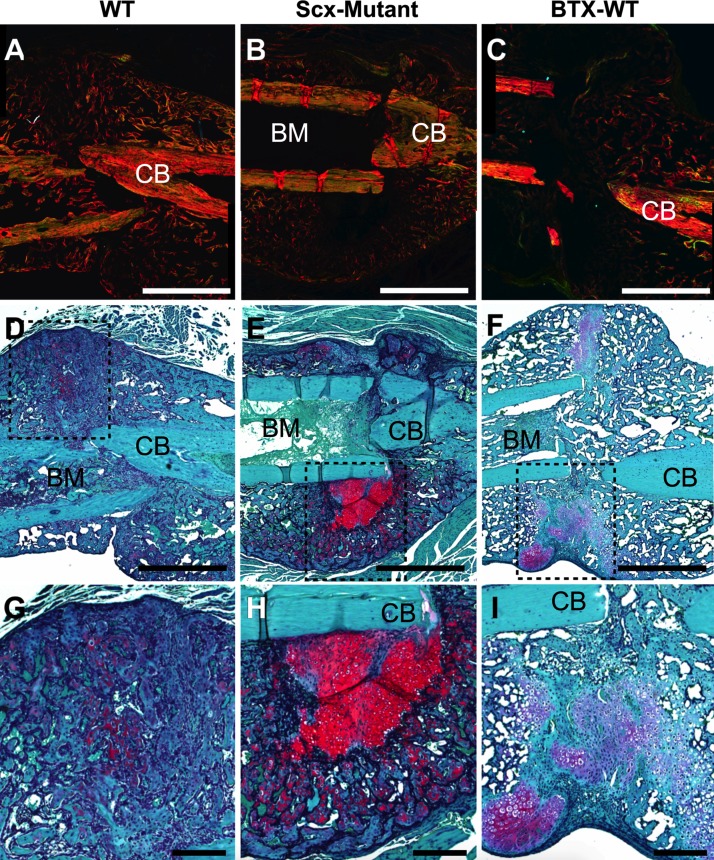
Scx-mutant mice had an unusually high percentage of fractures deemed irreparable (7/22 in Scx-mutant mice; 0/14 in WT mice) because of lack of repair stabilization, or poor fracture location (e.g., concomitant fracture in tibia) and were immediately euthanized. Although WT fractures healed well, with robust callus formation by 2 wk (Fig. 3A), Scx-mutant fractures healed nonuniformly around the fracture site (Fig. 3B). Callus asymmetry for Scx-mutant mice was significantly reduced compared to WT mice at d 14 after fracture (Fig. 3C). MicroCT reconstructions of the healing callus at d 14 showed some bone formation in all groups; although 1 Scx-mutant mouse did not form any callus, and the remaining formed calluses primarily on the dorsal surface of the femur (Fig. 4A). At d 14 postfracture, callus volume was decreased in Scx-mutant compared to WT, but increased in BTX-WT mice (Fig. 4C). Callus density was significantly decreased in BTX-WT compared to both WT and Scx-mutant mice (Fig. 4G, I) at 2 wk. At 28 d after fracture, the callus volume was still significantly lower in Scx-mutant compared to WT mice (Fig. 4B, D). The callus density was not different between groups at d 28 (Fig. 4H, J). In WT mice, the callus density significantly decreased from d 14 to 28 (P < 0.05), whereas density in the Scx-mutant mice did not change over time. Fracture callus organization was assessed qualitatively at d 14 (Fig. 5). Using polarized light imaging, the collagen organization was compared in WT, Scx-mutant, and BTX-WT samples (Fig. 5A–C). The Scx-mutant mice had decreased birefringence, a measure of collagen anisotropy, compared to the WT group. Staining with safranin O and alcian blue at d 14 showed larger areas of cartilage in Scx-mutant and BTX-WT groups (Fig. 5D–F). In addition, there was an absence of bone on the ventral side of the Scx-mutant mice (Fig. 5E), with robust bone formation on the dorsal side (Fig. 5E, H). Magnified images of WT, Scx-mutant and BTX-WT mice show bridging of callus at d 14 (Fig. 5G–I).
Figure 3.
X-ray images were taken at 7 d intervals after fracture. A) In WT mice, a healing bridged callus was visible at d 14, and the size diminished by d 28, consistent with remodeling. B) In the Scx-mutant mice an asymmetric, partially bridged, smaller callus was evident at d 14 and was less apparent by d 28.
Figure 4.
A, B) MicroCT reconstructions of WT, Scx-mutant, and BTX-WT mice at 14 d (A) and 28 d (B) (WT and Scx-mutant only) after fracture. Scx-mutant mice did not have robust callus formation on all surfaces of the femur. Administration of BTX to the quadriceps at the time of fracture did not alter the callus morphology. C) Fracture callus volume at d 14 was reduced in the Scx-mutant group and increased in the BTX-WT mice compared to WT. D) At d 28, the callus volume was reduced in the Scx-mutant group compared to WT. E, F) Callus bone volume (BV) at d 14 (E) and at d 28 (F) were lower in the Scx-mutant group vs. the WT. G–J) Callus BV/total volume (TV) (G, H) and bone mineral density (BMD) (I, J) were decreased in the BTX-WT mice compared to WT (G, I); callus density did not differ between WT and Scx-mutant mice at either healing time point (H, J). Bars indicate significant difference between groups; P < 0.05.
Figure 5.
Scx-mutant mice experienced delayed callus healing. A–C) Polarized light imaging of picrosirius red-/Alcian blue-stained callus sections at d 14 for WT (A), Scx-mutant (B), and BTX-WT (C) mice. Scx-mutant and BTX-WT calluses were less organized, indicated by reduced refringence, compared to WT. D–F) Serial sections stained with safranin O at d 14 after fracture showed increased cartilage (red) staining and hypertrophic chondrocytes on Scx-mutant (E) and BTX-WT (F) sections, compared to WT (D). G) Higher magnification of the WT callus shows clear bridging with little cartilage remaining. H, I) Scx-mutant (H) and BTX-WT (I) sections have both woven bone and low-mineralized, high-cartilage areas. BM, bone marrow; CB, cortical bone. Scale bars, 1 mm (A–F); 200 μm (G–I).
DISCUSSION
Although Scx is known to be important in tendons, this is the first study to explore the role of Scx in long bone structure and strength, as well as fracture healing. Our objective was to identify the role of Scx in cortical development and fracture healing. We found that loss of Scx led to morphological changes in the ulna and mechanical deficiencies in the ulna, femur, and tibia. In mutant mice, femur PYD was reduced and tibial ID 1st and CID were increased compared to WT mice. This finding suggests that mutant mice may have an altered collagen structure (32) compared to WT mice. In addition, mutant mice did not have altered TMD compared to WT mice, further suggesting that reductions in bulk scale mechanical properties (e.g., PYD, work-to-fracture, yield, and maximum load) observed in mutant mice may be related to collagen organization and geometric changes and not to altered mineral density. For this study, we did not systematically characterize the collagen fibril organization or fibril size of nonfractured long bone. In future work, advanced imaging techniques could be used, such as second-harmonic generation, quantitative polarized imaging, or transmission electron microscopy, to determine whether microscopic changes in collagen fibril size and organization of long bone exists in Scx-mutant limbs. The dramatic differences in bone morphology caused by loss of Scx may be caused by differences in limb patterning and/or mechanical loading during development. Two T-box family transcription factors, Tbx5 and Tbx4, are expressed in the forelimb and hindlimb, respectively, and the timing of expression for these genes during embryonic growth differ (33, 34). Proximal structures (e.g., stylopod) of the limb are formed earlier than distal (e.g., autopod) structures (35, 36). However, to our knowledge, the timing and role of Scx during patterning of these elements has not been thoroughly investigated. In regards to mechanical loading, the ulna and femur have quite different anatomic attachment of tendons and, thus, likely observe different bending environments induced by applied muscle forces. The ulna is surrounded by long tendon/muscle units of the forearm, particularly the flexor and extensor muscles that control hand movement. Primarily, the ulna acts as a hinge joint with limited out-of-plane movement. The ulna also has a corresponding element, the radius, which allows for supination and pronation. The femur, however, experiences a different loading environment, and it acts more as a ball-and-socket joint that allows for nearly 360° of rotation, flexion, extension, ab-/adduction, and internal/external rotation.
Our results indicate that there is an important role for Scx in bone formation and healing. Increased expression of Scx was evident in the callus at an early timepoint (24 h) as well as later (14 d). Scx-mutant mice had an asymmetric callus that healed in a similar timeframe to a WT callus. Contrary to our hypothesis that the Scx-mutant callus asymmetry was due to muscle unloading, BTX injections into the quadriceps of WT mice at the time of fracture did not model the scx-mutant phenotype.
The morphological deficits associated with the lack of Scx in the fracture callus, observed in this study, suggest the important role of this transcription factor in bone fracture callus formation. First, we showed that scx expression is up-regulated in the fractured cortical bone at 24 h postfracture. Other genes associated with scx, such as bmp-2 and bmp-4, have been studied extensively in the bone fracture and remodeling process (11, 12, 20, 28, 37–41). The temporal expression of bmps has been previously shown to synchronize with endochondral formation and periosteal response (3). In the present study, we excluded any hematoma, marrow, and periosteal tissue from the fractured cortex and found an increase in the transcriptional response of scx in cortical bone postinjury. This is the first study to explore the expression of scx postfracture and localization of ScxGFP in the fracture callus. In addition, we showed that absence of Scx throughout development had a profound influence on the size and shape of the fracture callus. It remains unclear what downstream targets, regulated by Scx, are responsible for the observed callus asymmetry and altered bone formation. However, upstream regulators of Scx, specifically TGF-β, have been previously shown to be instrumental in long bone fracture healing (42–46). A mechanistic, temporal investigation to identify the role of Scx in the formation and remodeling of the fracture callus could elucidate the specification and timing of Scx activity during various stages of healing.
Altered mechanical loading during fracture healing is a known regulator of callus formation (47–50). Although we made efforts to manipulate the loading environment by using localized BTX injections into the quadriceps, the reduced callus size and altered shape found in Scx-mutant mice could not be replicated by localized muscle denervation alone. It is possible that the contributions of Scx to fracture callus formation were mounted as an injury response, rather than as a bone remodeling response. Other studies involving models of bone remodeling that proceed via woven bone formation, such as mechanical loading-induced stress fractures, have shown that BMP-2 is dispensable in intramembranous ossification (32). Dynamic, compressive tibial loading has been shown to induce lamellar bone formation and upregulation of bone formation genes (51). However, unpublished gene expression data from our lab shows that such loading regimens did not induce differential expression of scx, bmp-4, or tgfb3 at 4, 24, or 72 h after a bout of single loading in adult C57Bl/6 mice. The present study provides insight into the role of Scx during bone remodeling, and suggests that Scx may be a contributing factor for endochondral bone formation.
There are some limitations to this work. Although we showed that constitutive deletion of Scx led to altered bone shape, structure, and mechanics in the adult skeleton, these impairments may amplify the differences in callus shape and size between Scx-mutant and WT mice. Inducible deletion of Scx at time of fracture would provide more information on the specific role of Scx during callus formation. In addition, targeted BTX-induced paralysis of the quadriceps muscle group may not have sufficiently unloaded the tendon/bone, as the tendon was still intact and able to apply passive tension to the knee. It would be interesting to explore patellar tendon laceration in addition to muscle denervation to fully capture the effect of localized muscle unloading on the healing bone. Last, this study focused only on deletion of Scx in fracture healing, and we did not explore the potential anabolic role that Scx may play during callus formation and remodeling. However, others have shown that adenoviral-mediated scx, transduced in stem cells and delivered to sites of tendon–bone fibrocartilage injury, demonstrate the potential for improving rotator cuff healing (52). Therefore, it is possible that intervention at time of fracture via scx gene therapy may enhance healing, promote bridging, and limit fracture nonunions.
Many of the key molecular processes involved in the development and growth of the skeleton are mirrored during the fracture-healing process of the injured adult skeleton (5). The identification of key factors involved in the development of the shape and structure of long bones can aid in elucidating novel molecules for therapeutic targeting. Although extensive research has investigated the contributions of BMPs in bone modeling and fracture callus formation (7, 12, 21), little work has focused on transcriptional regulation that bridges the developmental processes of bone shape and the adult healing process that drives fracture callus size and shape. This study is the first to explore the role of Scx in fracture healing, a transcription factor previously shown to influence tendon growth and healing (13–15, 19, 22, 25–27). In addition, it demonstrated a role for Scx in postnatal bone structure/function. In summary, the findings provide novel insight into the potential role of Scx in long bone modeling, healing, and remodeling.
ACKNOWLEDGMENTS
This work was funded by the U.S. National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) Grants F32 AR064652 (to M.L.K.), and R21 AR066798 (to M.J.S. and M.J.G.); Washington University Musculoskeletal Research Center Grant NIH-P30 AR057235; the Orthopaedic Research and Education Foundation, and the Children’s Discovery Institute (Postdoctoral Fellowship to M.L.K.). The authors thank Daniel Leib and Crystal Idleburg (Musculoskeletal Research Center) for microCT and histology assistance. The authors declare no conflicts of interest.
Glossary
- BMP
bone morphogenetic protein
- BTX
botulinum toxin A
- BW
body weight
- CID
creep indentation distance
- Ct.Ar.
cortical area
- GFP
green fluorescent protein
- I
polar moment of inertia
- ID 1st
first cycle indentation distance
- IDI
indentation distance increase
- microCT
microcomputed tomography
- MOI
moment of inertia
- PYD
postyield displacement
- qRT-PCR
quantitative RT-PCR
- Scx
scleraxis
- ScxGFP
scleraxis green fluorescent protein–labeled
- Scx-mutant
scleraxis knockout
- TID
total indentation distance
- TMD
tissue mineral density
- US 1st
first cycle unloading slope
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
M. L. Killian, J. A. McKenzie, and M. J. Silva designed the study; M. L. Killian, J. A. McKenzie, E. Buettmann, A. C. Abraham, and M. J. Gardner conducted the study; M. L. Killian, J. A. McKenzie, E. Buettmann, A. C. Abraham and M. J. Silva interpreted the study; M. L. Killian, J. A. McKenzie drafted the manuscript; M. L. Killian, J. A. McKenzie, E. Buettmann, A. C. Abraham, and M. J. Silva revised the manuscript; and M. L. Killian, J. A. McKenzie, A. C. Abraham, E. Buettmann, M. J. Silva, and M. J. Gardner approved the final manuscript.
REFERENCES
- 1.Marsell R., Einhorn T. A. (2011) The biology of fracture healing. Injury 42, 551–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Epari, D. R., Duda, G. N., Thompson, M. S. (2010) Mechanobiology of bone healing and regeneration: in vivo models. Proc. Inst. Mech. Eng. H224, 1543–1553 doi: 10.1243/09544119JEIM808. [DOI] [PubMed] [Google Scholar]
- 3.Cho T. J., Gerstenfeld L. C., Einhorn T. A. (2002) Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J. Bone Miner. Res. 17, 513–520 [DOI] [PubMed] [Google Scholar]
- 4.Scammell B. E., Roach H. I. (1996) A new role for the chondrocyte in fracture repair: endochondral ossification includes direct bone formation by former chondrocytes. J. Bone Miner. Res. 11, 737–745 [DOI] [PubMed] [Google Scholar]
- 5.Gerstenfeld L. C., Cullinane D. M., Barnes G. L., Graves D. T., Einhorn T. A. (2003) Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J. Cell. Biochem. 88, 873–884 [DOI] [PubMed] [Google Scholar]
- 6.Oka K., Oka S., Hosokawa R., Bringas P. Jr., Brockhoff H. C. II, Nonaka K., Chai Y. (2008) TGF-beta mediated Dlx5 signaling plays a crucial role in osteo-chondroprogenitor cell lineage determination during mandible development. Dev. Biol. 321, 303–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kempen D. H., Lu L., Hefferan T. E., Creemers L. B., Maran A., Classic K. L., Dhert W. J., Yaszemski M. J. (2008) Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials 29, 3245–3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang A. H., Motlekar N. A., Stein A., Diamond S. L., Shore E. M., Mauck R. L. (2008) High-throughput screening for modulators of mesenchymal stem cell chondrogenesis. Ann. Biomed. Eng. 36, 1909–1921 [DOI] [PubMed] [Google Scholar]
- 9.Hofbauer L. C., Dunstan C. R., Spelsberg T. C., Riggs B. L., Khosla S. (1998) Osteoprotegerin production by human osteoblast lineage cells is stimulated by vitamin D, bone morphogenetic protein-2, and cytokines. Biochem. Biophys. Res. Commun. 250, 776–781 [DOI] [PubMed] [Google Scholar]
- 10.Abe E., Yamamoto M., Taguchi Y., Lecka-Czernik B., O’Brien C. A., Economides A. N., Stahl N., Jilka R. L., Manolagas S. C. (2000) Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by noggin. J. Bone Miner. Res. 15, 663–673 [DOI] [PubMed] [Google Scholar]
- 11.Guimarães J. M., Guimarães I. C., Duarte M. E., Vieira T., Vianna V. F., Fernandes M. B., Vieira A. R., Casado P. L. (2013) Polymorphisms in BMP4 and FGFR1 genes are associated with fracture non-union. J. Orthop. Res. 31, 1971–1979 [DOI] [PubMed] [Google Scholar]
- 12.Tsuji K., Cox K., Bandyopadhyay A., Harfe B. D., Tabin C. J., Rosen V. (2008) BMP4 is dispensable for skeletogenesis and fracture-healing in the limb. J. Bone Joint Surg. Am. 90(Suppl 1), 14–18 [DOI] [PubMed] [Google Scholar]
- 13.Killian M. L., Thomopoulos S. (2016) Scleraxis is required for the development of a functional tendon enthesis. FASEB J. 30, 301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zelzer E., Blitz E., Killian M. L., Thomopoulos S. (2014) Tendon-to-bone attachment: from development to maturity. Birth Defects Res. C Embryo Today 102, 101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blitz E., Sharir A., Akiyama H., Zelzer E. (2013) Tendon-bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development 140, 2680–2690 [DOI] [PubMed] [Google Scholar]
- 16.Thomopoulos S., Das R., Birman V., Smith L., Ku K., Elson E. L., Pryse K. M., Marquez J. P., Genin G. M. (2011) Fibrocartilage tissue engineering: the role of the stress environment on cell morphology and matrix expression. Tissue Eng. Part A 17, 1039–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomopoulos S., Genin G. M., Galatz L. M. (2010) The development and morphogenesis of the tendon-to-bone insertion: what development can teach us about healing. J. Musculoskelet. Neuronal Interact. 10, 35–45 [PMC free article] [PubMed] [Google Scholar]
- 18.Pryce B. A., Watson S. S., Murchison N. D., Staverosky J. A., Dünker N., Schweitzer R. (2009) Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development 136, 1351–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blitz E., Viukov S., Sharir A., Shwartz Y., Galloway J. L., Pryce B. A., Johnson R. L., Tabin C. J., Schweitzer R., Zelzer E. (2009) Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Dev. Cell 17, 861–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rot C., Stern T., Blecher R., Friesem B., Zelzer E. (2014) A mechanical Jack-like mechanism drives spontaneous fracture healing in neonatal mice. Dev. Cell 31, 159–170 [DOI] [PubMed] [Google Scholar]
- 21.Tsuji K., Bandyopadhyay A., Harfe B. D., Cox K., Kakar S., Gerstenfeld L., Einhorn T., Tabin C. J., Rosen V. (2006) BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat. Genet. 38, 1424–1429 [DOI] [PubMed] [Google Scholar]
- 22.Murchison N. D., Price B. A., Conner D. A., Keene D. R., Olson E. N., Tabin C. J., Schweitzer R. (2007) Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 134, 2697–2708 [DOI] [PubMed] [Google Scholar]
- 23.Sugimoto Y., Takimoto A., Akiyama H., Kist R., Scherer G., Nakamura T., Hiraki Y., Shukunami C. (2013) Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development 140, 2280–2288 [DOI] [PubMed] [Google Scholar]
- 24.Eyal S., Blitz E., Shwartz Y., Akiyama H., Schweitzer R., Zelzer E. (2015) On the development of the patella. Development 142, 1831–1839 [DOI] [PubMed] [Google Scholar]
- 25.Schweitzer R., Zelzer E., Volk T. (2010) Connecting muscles to tendons: tendons and musculoskeletal development in flies and vertebrates. Development 137, 2807–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schweitzer R., Chyung J. H., Murtaugh L. C., Brent A. E., Rosen V., Olson E. N., Lassar A., Tabin C. J. (2001) Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 128, 3855–3866 [DOI] [PubMed] [Google Scholar]
- 27.Pryce B. A., Brent A. E., Murchison N. D., Tabin C. J., Schweitzer R. (2007) Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev. Dyn. 236, 1677–1682 [DOI] [PubMed] [Google Scholar]
- 28.Logan M., Martin J. F., Nagy A., Lobe C., Olson E. N., Tabin C. J. (2002) Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis 33, 77–80 [DOI] [PubMed] [Google Scholar]
- 29.Gallant M. A., Brown D. M., Organ J. M., Allen M. R., Burr D. B. (2013) Reference-point indentation correlates with bone toughness assessed using whole-bone traditional mechanical testing. Bone 53, 301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritchie R. O., Nalla R. K., Kruzic J. J., Ager J. W. III, Balooch G., Kinney J. H. (2006) Fracture and ageing in bone: toughness and structural characterization. Strain 42, 225–232 [Google Scholar]
- 31.Porter S. M., Dailey H. L., Hollar K. A., Klein K., Harty J. A., Lujan T. J. (2016) Automated measurement of fracture callus in radiographs using portable software. J. Orthop. Res. 34, 1224–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abraham A. C., Agarwalla A., Yadavalli A., Liu J. Y., Tang S. Y. (2016) Microstructural and compositional contributions towards the mechanical behavior of aging human bone measured by cyclic and impact reference point indentation. Bone 87, 37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Esteban C., Tsukui T., Yonei S., Magallon J., Tamura K., Izpisua Belmonte J. C. (1999) The T-box genes Tbx4 and Tbx5 regulate limb outgrowth and identity. Nature 398, 814–818 [DOI] [PubMed] [Google Scholar]
- 34.Ouimette J. F., Jolin M. L., L’honoré A., Gifuni A., Drouin J. (2010) Divergent transcriptional activities determine limb identity. Nat. Commun. 1, 35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dudley A. T., Ros M. A., Tabin C. J. (2002) A re-examination of proximodistal patterning during vertebrate limb development. Nature 418, 539–544 [DOI] [PubMed] [Google Scholar]
- 36.Niswander L. (2003) Pattern formation: old models out on a limb. Nat. Rev. Genet. 4, 133–143 [DOI] [PubMed] [Google Scholar]
- 37.McBride-Gagyi S. H., McKenzie J. A., Buettmann E. G., Gardner M. J., Silva M. J. (2015) Bmp2 conditional knockout in osteoblasts and endothelial cells does not impair bone formation after injury or mechanical loading in adult mice. Bone 81, 533–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seeherman H. J., Bouxsein M., Kim H., Li R., Li X. J., Aiolova M., Wozney J. M. (2004) Recombinant human bone morphogenetic protein-2 delivered in an injectable calcium phosphate paste accelerates osteotomy-site healing in a nonhuman primate model. J. Bone Joint Surg. Am. 86-A, 1961–1972 [DOI] [PubMed] [Google Scholar]
- 39.Seeherman H. J., Li X. J., Bouxsein M. L., Wozney J. M. (2010) rhBMP-2 induces transient bone resorption followed by bone formation in a nonhuman primate core-defect model. J. Bone Joint Surg. Am. 92, 411–426 [DOI] [PubMed] [Google Scholar]
- 40.Li R. H., Bouxsein M. L., Blake C. A., D’Augusta D., Kim H., Li X. J., Wozney J. M., Seeherman H. J. (2003) rhBMP-2 injected in a calcium phosphate paste (alpha-BSM) accelerates healing in the rabbit ulnar osteotomy model. J. Orthop. Res. 21, 997–1004 [DOI] [PubMed] [Google Scholar]
- 41.Edwards R. B. III, Seeherman H. J., Bogdanske J. J., Devitt J., Vanderby R. Jr., Markel M. D. (2004) Percutaneous injection of recombinant human bone morphogenetic protein-2 in a calcium phosphate paste accelerates healing of a canine tibial osteotomy. J. Bone Joint Surg. Am. 86-A, 1425–1438 [DOI] [PubMed] [Google Scholar]
- 42.Lind M., Schumacker B., Søballe K., Keller J., Melsen F., Bünger C. (1993) Transforming growth factor-beta enhances fracture healing in rabbit tibiae. Acta Orthop. Scand. 64, 553–556 [DOI] [PubMed] [Google Scholar]
- 43.Rosier R. N., O’Keefe R. J., Hicks D. G. (1998) The potential role of transforming growth factor beta in fracture healing. Clin. Orthop. Relat. Res. (355, Suppl)S294–S300 [DOI] [PubMed] [Google Scholar]
- 44.Tsiridis E., Upadhyay N., Giannoudis P. (2007) Molecular aspects of fracture healing: which are the important molecules? Injury 38(Suppl 1), S11–S25 [DOI] [PubMed] [Google Scholar]
- 45.Fischer C., Doll J., Tanner M., Bruckner T., Zimmermann G., Helbig L., Biglari B., Schmidmaier G., Moghaddam A. (2016) Quantification of TGF-ß1, PDGF and IGF-1 cytokine expression after fracture treatment vs. non-union therapy via masquelet. Injury 47, 342–349 [DOI] [PubMed] [Google Scholar]
- 46.Zimmermann G., Henle P., Küsswetter M., Moghaddam A., Wentzensen A., Richter W., Weiss S. (2005) TGF-beta1 as a marker of delayed fracture healing. Bone 36, 779–785 [DOI] [PubMed] [Google Scholar]
- 47.Gardner M. J., Putnam S. M., Wong A., Streubel P. N., Kotiya A., Silva M. J. (2011) Differential fracture healing resulting from fixation stiffness variability: a mouse model. J. Orthop. Sci. 16, 298–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lacroix D., Prendergast P. J. (2002) A mechano-regulation model for tissue differentiation during fracture healing: analysis of gap size and loading. J. Biomech. 35, 1163–1171 [DOI] [PubMed] [Google Scholar]
- 49.Claes L. E., Heigele C. A. (1999) Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J. Biomech. 32, 255–266 [DOI] [PubMed] [Google Scholar]
- 50.Carter D. R., Blenman P. R., Beaupré G. S. (1988) Correlations between mechanical stress history and tissue differentiation in initial fracture healing. J. Orthop. Res. 6, 736–748 [DOI] [PubMed] [Google Scholar]
- 51.Holguin N., Brodt M. D., Silva M. J. (2016) Activation of Wnt signaling by mechanical moading is impaired in the bone of old mice. [E-pub ahead of print] J. Bone Miner. Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gulotta L. V., Kovacevic D., Packer J. D., Deng X. H., Rodeo S. A. (2011) Bone marrow-derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am. J. Sports Med. 39, 1282–1289 [DOI] [PubMed] [Google Scholar]