Abstract
Articular cartilage has little regenerative capacity. Recently, genetic lineage tracing experiments have revealed chondrocyte progenitors at the articular surface. We further characterized these progenitors by using in vivo genetic approaches. Histone H2B–green fluorescent protein retention revealed that superficial cells divide more slowly than underlying articular chondrocytes. Clonal genetic tracing combined with immunohistochemistry revealed that superficial cells renew their number by symmetric division, express mesenchymal stem cell markers, and generate chondrocytes via both asymmetric and symmetric differentiation. Quantitative analysis of cellular kinetics, in combination with phosphotungstic acid–enhanced micro–computed tomography, showed that superficial cells generate chondrocytes and contribute to the growth and reshaping of articular cartilage. Furthermore, we found that cartilage renewal occurs as the progeny of superficial cells fully replace fetal chondrocytes during early postnatal life. Thus, superficial cells are self-renewing progenitors that are capable of maintaining their own population and fulfilling criteria of unipotent adult stem cells. Furthermore, the progeny of these cells reconstitute adult articular cartilage de novo, entirely substituting fetal chondrocytes.—Li, L., Newton, P. T., Bouderlique, T., Sejnohova, M., Zikmund, T., Kozhemyakina, E., Xie, M., Krivanek, J., Kaiser, J., Qian, H., Dyachuk, V., Lassar, A. B., Warman, M. L., Barenius, B., Adameyko, I., Chagin, A. S. Superficial cells are self-renewing chondrocyte progenitors, which form the articular cartilage in juvenile mice.
Keywords: superficial zone, bone, adult stem cells, regeneration, osteoarthritis
Extensively hydrated articular cartilage that covers all joints is neither innervated nor vascularized and consists of superficial (in contact with synovial fluid), middle, and deep zones, beneath which lies the calcified matrix and subchondral bone. Parallel collagen fibers on the surface of the superficial zone along with its expression of the lubricant lubricin [encoded by proteoglycan 4 (PRG4 )] reduce friction. Cells in this zone are small, flat, and arranged along the surface of the cartilage. The middle zone has a high content of proteoglycans, which retain a large amount of water and absorb impact; collagen fibers are organized both vertically and in a meshwork to help distribute stress evenly throughout the cartilage. Finally, the deep zone transmits stress to underlying bone via the calcified matrix. The only type of cell that is present in the middle and deep zones is the articular chondrocyte (1, 2).
Osteoarthritis is the most common musculoskeletal disorder and affects 10–12% of the global population (3). The earliest changes associated with this condition include tissue fibrillation and attenuated mechanical integrity in the superficial zone of the articular cartilage (4), whereas later stages are characterized by pathological alterations in all tissues around joints (5). Superficial cells are also the first to be lost in connection with aging, with as much as 50% lost between ages 30 and 85 yr (4, 6).
Articular cartilage cannot completely repair itself after injury, which presents a serious challenge to engineering approaches that are designed to repair damaged tissue. In 2004, cells in the superficial zone were shown to form colonies in vitro and were therefore proposed to be chondrocyte progenitors (7), after which numerous descriptions of the presence of stem cell–related markers and in vitro clonogenicity and multilineage differentiation of superficial cells have appeared [reviewed in Jiang and Tuan (8)]. Finally, Kozhemyakina and colleagues (9) recently showed that genetically labeled superficial cells can, indeed, differentiate into mature articular chondrocytes in vivo.
Here, we further characterized these superficial zone cells, focusing on their cellular kinetics and contribution to cartilage growth and renewal in the physiological setting. We report that these cells behave as adult stem cells, with both symmetric and asymmetric divisions, and thereby maintain their population. Furthermore, we show that these cells generate chondrocytes that are used for the postnatal growth of articular cartilage as well as for its reshaping. Finally, we demonstrate that articular chondrocytes undergo apoptosis at the chondro-osseous junction, which facilitates endochondral growth of underlying epiphyseal bone, and that together with new chondrocytes generated from superficial cells, this process facilitates cartilage renewal in juvenile mice.
MATERIALS AND METHODS
Mouse strains
All animal work was approved and permitted by the Ethical Committee on Animal Experiments (Stockholm North Committee/Norra Djurförsöksetiska Nämd) and conducted according to the Swedish Animal Agency’s Provisions and Guidelines for Animal Experimentation recommendations.
Prg4-green fluorescent protein (GFP)-Causes Recombination (Cre)–estrogen receptor (ER) conjugate (CreER) (T2) [Prg4-GFP-CreER(T2)] mouse strain, recently described by Kozhemyakina et al. (9), expresses CreER(T2)-GFP under the transcriptional control of PRG4 (lubricin) promoter. This allows targeting of superficial cells at the surface of articular cartilage as these cells have high levels of PRG4 expression. Cre-mediated DNA recombination is tamoxifen dependent in this strain. It is important to emphasize that Prg4-CreER(T2) triggers DNA recombination in superficial cells only at neonatal and early postnatal age, whereas if tamoxifen is injected at age ≥1 mo, it causes recombination in superficial cells and 2–3 layers of underlying chondrocytes (9) (data in this study and unpublished observations). GFP is expressed at a low level and was not detected by confocal microscopy with settings that we used to detect fluorescent confetti proteins (see below); we shortened the name to Prg4-CreER(T2) to avoid confusion for the reader.
Col2-CreER binding tamoxifin (Col2-CreERt) strain was developed by Susan Mackem (10) and expresses CreERt protein under control of collagen type 2α1 promoter (Col2α1). Col2a1 gene is active in chondrocytes, facilitating high Col2-CreERt levels, specifically in chondrocytes. Cre-mediated DNA recombination is tamoxifen dependent in this strain, which allows genetic labeling and tracing of these cells and their progeny, as labeling occurs only during tamoxifen administration and a few hours later when tamoxifen is still in the tissue. Accordingly, all labeled cells observed after the chasing period where labeled either during the time of tamoxifen administrations or descendants of those cells.
Rosa26-Confetti is a reporter mouse strain that contains the Brainbow 2.1 construct (11). Upon Cre-mediated DNA recombination, 1 of 4 different fluorescent proteins (nucleus green, cytoplasmic red, cytoplasmic yellow, and membranous cyan) is expressed in a stochastic manner, which allows clonal separation (12).
For clonal genetic tracing, R26-Confetti strain was crossed with either Prg4-CreER(T2) or Col2-CreERt strains, which resulted in Prg4-CreER(T2):Confetti or Col2-CreERt:Confetti strains. For genetic tracing of superficial cells, Prg4-CreER(T2):Confetti stain received a single intraperitoneal injection of 125 µg tamoxifen per animal at d 0 to achieve a low recombination efficiency that was compatible with analysis of single recombinant clones. For clonal genetic tracing of chondrocytes, pregnant Col2-CreERt:Confetti females were injected with a single dose of 5.0 mg tamoxifen, intraperitoneal tamoxifen (T5648; Sigma-Aldrich, St. Louis, MO, USA) was dissolved in corn oil (C8267; Sigma-Aldrich). Subsequently, samples were placed into freshly prepared 4% formaldehyde in PBS and fixed for 6–48 h at 4°C on a roller. After, samples were either decalcified (EDTA) for 1–3 d or were cryopreserved directly. Cryopreservation was conducted by rolling in 30% sucrose (C27480; VWR International, Radnor, PA, USA) overnight at 4°C, embedding in optimum cutting temperature compound (102094-104; Sakura, Torrance, CA, USA), and cut into sections of between 10 and 150 µm on a cryostat (CM3050; Leica, Wetzlar, Germany) according to the specific applications.
The histone H2B (H2B)–GFP Tet-On mice strain was created by crossing Tg(tetO-HIST1H2BJ/GFP)47Efu/J mice (005104; The Jackson Laboratory, Bar Harbor, ME, USA) with the B6.Cg-Gt(ROSA)26Sortm1(rtTA*M2)Jae/J (006965) strain. The resulting strain accumulated H2B histone conjugated with GFP upon exposure to doxycycline. After cessation of doxycycline treatment, H2B-GFP was diluted with every cell cycle, which allowed visualization of slowly dividing cells on the basis of their GFP retention.
Confocal microscopy and image analysis
Confocal images and clonal analysis were performed by using a Zeiss LSM710 fluorescence microscope (Zeiss, Jena, Germany) and Imaris (Bitplane, Zurich, Switzerland; http://www.bitplane.com/) and ImageJ software (National Institutes of Health, Bethesda, MD, USA; http://imagej.net/Welcome) as previously described (13). In brief, for all confocal images 30- to 150-μm-thick sections of frozen samples were scanned throughout the entire thickness and 3-dimensional (3D) z stacks were generated. Analysis of the angle of cell division was performed by using ImageJ software with the Angle tool. 3D confocal images were analyzed in Surpass and Slice modes of Imaris software. The Measurement Points function was used to measure the distance between the nucleus and the distance from cells to the surface. The Surfaces function was used to measure individual cell volume and that of articular cartilage volume. The Spots function was used to count the number of cells of different colors.
Phosphotungstic acid–enhanced micro–computed tomography
Tissue contrast was achieved by exposure of tissue samples to 0.7% phosphotungstic acid solution in methanol for 2–4 wk as previously described (14). Thereafter, samples were embedded in agarose gel within Eppendorf tubes. For the tomographic measurement, a GE Phoenix VTome XL 240 system (GE Healthcare, Pittsburgh, PA, USA) that was equipped with a 180-kV/15-W nanofocus X-ray tube and high-contrast flat panel detector DXR250 with 2048 × 2048 pixel2 (200- × 200-µm2 pixel size) was used. The Eppendorf tube that contained each sample was mounted into the machine on the plastic rod by using hot-melt adhesive. In all measurements, the accelerating voltage was set to 70 kV and the tube current to 150 mA. The X-ray spectrum was filtered by a 0.2-mm-thick aluminum filter. Exposure time was 500 ms in 2300 positions around the 360° rotation. Obtained voxel resolution was 5 µm. Tomographic measurements were performed at 21°C. The micro–computed tomography (CT) system was calibrated by using the certificated ruby balls phantom Ø4 × 6 to guarantee the accuracy of dimensional measurement. Tomographic reconstruction was realized by using GE Phoenix Datos x 2.0 software (GE Healthcare) with median filter application, correction of a sample drift, and beam-hardening correction (6° in different material mode). CT data processing and visualization were implemented in VG Studio Max 2.2 (Volume Graphics, Heidelberg, Germany). Segmentation of bone cartilage in CT data was performed manually as described (14). The surface and volume of segmented cartilage 3D model was measured in GOM Inspect 8.0 Professional (GOM, Braunschweig, Germany; https://www.gemeasurement.com/inspection-ndt/radiography-and-computed-tomography/phoenix-datosx-ct-software).
Analysis of cell density and cell volume
Frozen sections from 3-, 30-, and 60-d-old mice were stained using DAPI (D1306; Thermo Fisher Scientific, Waltham, MA, USA) and Alexa Fluor 488 Phalloidin (A12379; Thermo Fisher Scientific) for nuclear and cytoskeleton staining, respectively. Analysis of images was performed by using Imaris 7.6.2 software as previously described.
TUNEL
For detection of apoptotic cells, TUNEL staining (In Situ Cell Death Detection Kit; 11684795910; Roche, Indianapolis, IN, USA) was applied according to manufacturer instructions. At least 3 discontinuous 100-μm–thick sections were analyzed by confocal microscopy for each leg.
To detect apoptotic cells in the calcified region of articular cartilage, we modified the retrieval procedure: instead of the standard proteinase K treatment (67 μg/ml concentration for 40 min at 37°C), tissue was exposed to 0.1% trypsin/PBS/0.1% Triton X-100 (Sigma-Aldrich) for 1 h at room temperature.
5-Ethynyl-2′-deoxyuridine, thymidine analog, labeling and detection
EdU (5-ethynyl-2′-deoxyuridine, thymidine analog; E10187; Thermo Fisher Scientific) was injected into mice from postnatal d 0 (P0) to P2 for 4 total injections. Mice were harvested at P2 or age 1 mo. After sample preparation, frozen sections were used for EdU detection with a click reaction in a mixture of 0.1 M Tris, pH 7.5, 2 mM CuSO4, 0.1 M ascorbic acid, and 2 µM Alexa Fluor Azide 647 for 30 min at room temperature, protected from light.
Histology and immunohistochemistry protocol
Frozen samples were sectioned into 25-µm-thick sections for immunohistochemistry or 10-µm-thick for Safranin O and Fast Green staining (477736, 2353459; Sigma-Aldrich). The following Abs were used: anti–phospho-histone H3 (04-817; Millipore, Billerica, MA, USA), anti–aPKC ζ (sc-17781; Santa Cruz Biotechnology, Santa Cruz, CA, US), anti-survivin (sc-17779; Santa Cruz Biotechnology), anti–caspase-3 rabbit IgG (9662; Cell Signaling Technology, Danvers, MA, USA), anti-Sox9 (HPA001758; Sigma-Aldrich), anti-Notch 1 (sc-6014; Santa Cruz Biotechnology), PE-conjugated anti-CD73 (127206; BioLegend, San Diego, CA, USA), and Alexa Fluor 647–conjugated donkey anti-mouse/rabbit IgG (715 605 150 and 711 605 152; The Jackson Laboratory). Anti-Col2 Ab was provided by Rikard Holmdahl (Karolinska Institute, Stockholm, Sweden).
For primary Abs of mouse origin, an additional blocking step with Vectorshield mouse-on-mouse kit (MKB-2213; Vector Laboratories, Burlingame, CA, USA) was performed. After blocking with 3% horse serum in PBS + 0.1% Triton X-100 for 30 min, slides were incubated with primary Ab overnight at 4°C, washed with PBS + 0.1% Tween 20, and incubated with corresponding secondary Ab for 1 h at room temperature protected from light. After counterstaining with DAPI, detection and imaging was performed by using a confocal Zeiss LSM710 fluorescence microscope.
Calcein–xylenol double labeling
Mice were injected with xylenol (0.09 mg/g) at either age 1 or 1.5 mo and calcein (10 mg/kg) 2 wk later, 24 h before tissue collection. Collected samples were placed into 4% formaldehyde for 12–24 h at 4°C on a roller and were cryopreserved and sectioned as described above. Analysis of the distance between mineralization fronts (labeled lines) was done on 3D confocal scans using Imaris software.
Statistical analysis
Statistical data are presented as means ± sem unless otherwise indicated. Unpaired Student’s t test or 1-way ANOVA was applied to calculate P values. All results were replicated in at least 3 different mice.
RESULTS
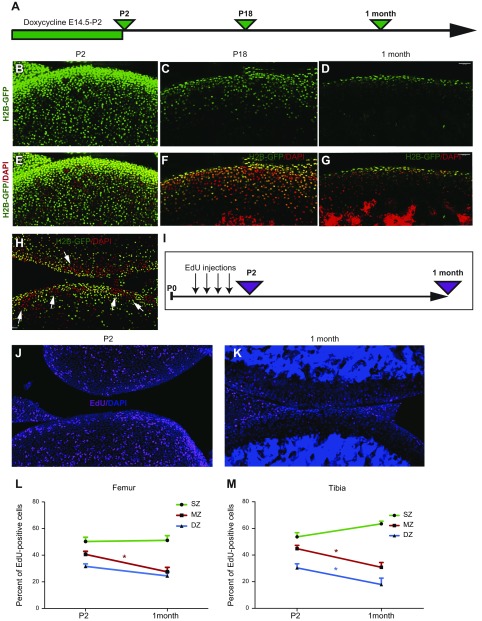
First, we analyzed the proliferative activity in the different zones of articular cartilage in young mice by labeling cells with H2B-GFP from embryonic day 14.5 (E14.5) to P2, then chasing these H2B-GFP–labeled cells for up to 1 mo (Fig. 1A). At the end of the labeling period, 90 ± 4% of chondrocytes were labeled (Fig. 1B, E; 96 ± 1% in the most superficial 150 μm of the tissue, that is, the estimated thickness of articular cartilage at age 1 mo), a value that fell to 76 ± 9 and 48 ± 6% at P18 and age 1 mo, respectively (Fig. 1C, F and D, G, respectively), a clear indication that cells in this region have proliferative activity. Of interest, the proliferative activity was not uniform and areas of high proliferation were observed (Fig. 1H, arrows).
Figure 1.
Superficial cells are slow-dividing cells. A) H2B-GFP mice were exposed to doxycycline from E14.5 to P2 and were harvested at P2, P18, and age 1 mo. B–G) The majority of cells were GFP-labeled after exposure to doxycycline (green cells; B, E). The number of labeled cells was reduced markedly after 16 d of chasing (C, F) and only superficial cells still expressed GFP after 1 mo of chasing (D, G). Images in panels E–G correspond to those in panels B–D, but with DAPI (red) channel added. Sample preparation, DAPI staining, laser power, and settings used for confocal scans were similar in B–G. H) To visualize residual GFP, laser power was increased twice compared with that in panel F. Clusters of high (red, arrows) and low (green) proliferative activity could be observed in P18 H2B-GFP mice. I) Between P0 and P2, mice were injected 4 times with EdU, and their bones were collected either on P2 or at age 1 mo. J, K) On P2, many cells throughout the entire articular cartilage were labeled with EdU (J; pink; nuclei were stained with DAPI, blue), whereas only a few cells retained EdU 1 mo later (K). L, M) Quantitative analysis revealed that superficial cells in both the femur (L) and tibia (M) retain EdU, whereas underlying chondrocytes lose this signal. DZ, deep zone; MZ, middle zone; SZ, superficial zone. Values are presented as means ± sem; n = 9. *P < 0.05.
Surprisingly, superficial cells were less proliferative than cells immediately below them, retaining a higher level of GFP (Fig. 1D, G). To independently confirm this observation, we performed pulse-chase labeling experiments with EdU (Fig. 1I) and also observed that surface cells divided less often and thereby retained the highest levels of EdU (Fig. 1J–M).
Next, we characterized the capacity of superficial cells to form chondrocytes in a clonal manner by crossing Prg4-CreER(T2) mice with R26-Confetti multicolor reporter mice. Upon injection of tamoxifen in these animals, Cre-mediated recombination activated expression of a green, yellow, red, or cyan fluorescent protein (Confetti-labeled cells), which allowed identification of the clonal progeny of individual cells.
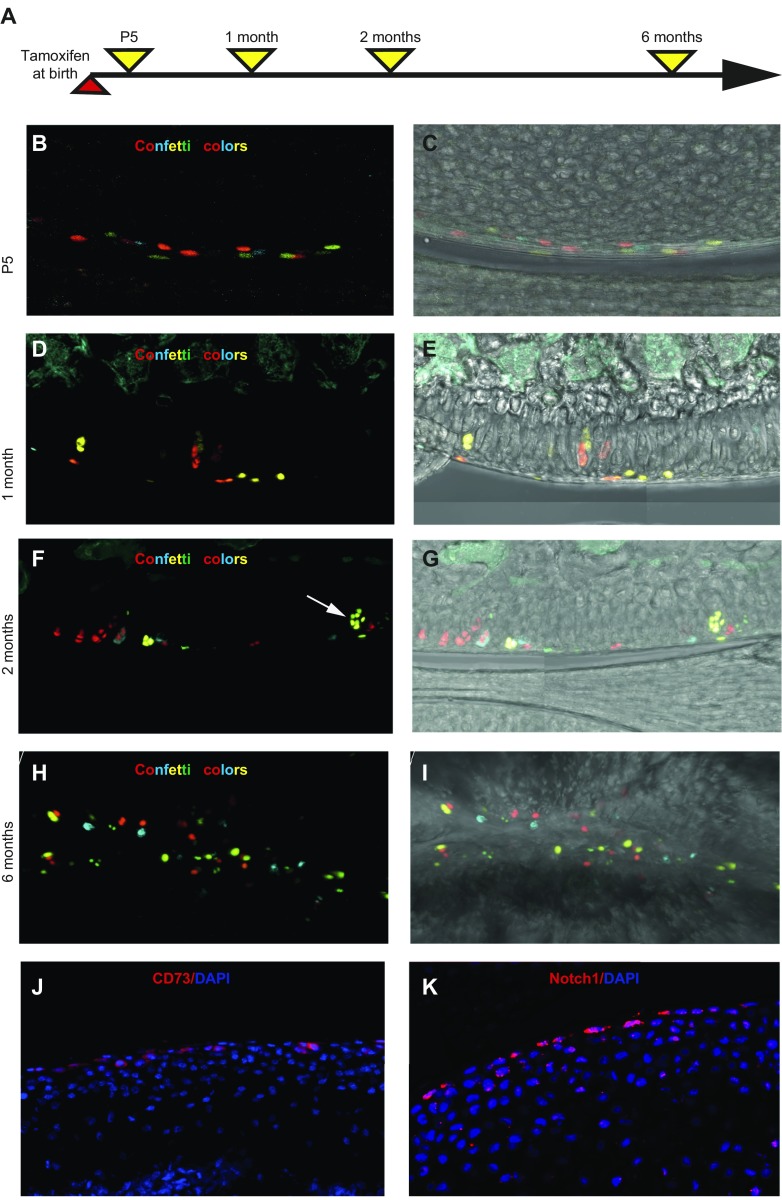
Mice were injected with tamoxifen at birth and analyzed 5 d and 1, 2, and 6 mo later (Fig. 2A). At P5, only superficial cells expressed fluorescent proteins (Fig. 2B, C), whereas after chasing for 1, 2, or 6 mo (Fig. 2D–I and Supplemental Fig. S1A), we observed chondrocytes that expressed fluorescent proteins in the middle and deep zones, which indicated that they were derived from superficial cells. Leakiness of the Cre-recombinase is an unlikely explanation for the observation as there were no fluorescent chondrocytes in mice that did not receive tamoxifen (Supplemental Fig. S1B). It has to be emphasized that a low level of tamoxifen (0.125 mg tamoxifen per newborn pup) was used throughout the study to achieve Cre-mediated recombination only in a fraction of cells to enable clonal separation (all figures except Supplemental Fig. S1C, D) as the standard tamoxifen dose (0.5 mg tamoxifen per newborn pup) caused a high recombination level (Supplemental Fig. S1D).
Figure 2.
Superficial cells are chondrocyte progenitors. A) PrgCreER(T2):Confetti mice were injected with tamoxifen at birth and analyzed at different timepoints. B–I) At P5, labeled confetti cells (red, yellow, cyan, and, occasionally, green) were observed in the superficial zone of articular cartilage (B, C), whereas labeled chondrocytes were seen clearly 1 (D, E), 2 (F, G), and 6 mo (H, I) after labeling. Large clonal clusters of labeled chondrocytes (arrow in panel F) could occasionally be observed. Images in panels C, E, G, I correspond to those in panels B, D, F, H but with the white light channel added. J, K) Expression of mesenchymal stem cell marker CD73 (J) and Notch1 (K) was observed in superficial chondrocytes. Samples are from 1-mo-old (J) and 5-d-old (K) mice, respectively.
Rather than forming single columns of cells, as has been proposed previously (9), we observed that the progeny of superficial cells seemed to form clusters, with as many as 10 chondrocytes/cluster (arrows in Figs. 2F and 3E, G, L and Supplemental Video 1). When all Prg4-Confetti–labeled clones were pseudocolored to be the same color, then it seemed that superficial cells formed columns (Supplemental Fig. S1C); however, those columns have a mixed clonal origin (Supplemental Fig. S1D). Thus, whereas deep articular chondrocytes in adult mice are derived from cells that were initially at the cartilage surface in newborn mice, which is consistent with appositional growth, beneath the surface cartilage, growth is also interstitial. Of interest, areas of high proliferative activity that were observed in H2B-GFP mice (Fig. 1H, arrows) may contain clonal clusters observed after Prg4-CreErt:Confetti tracing.
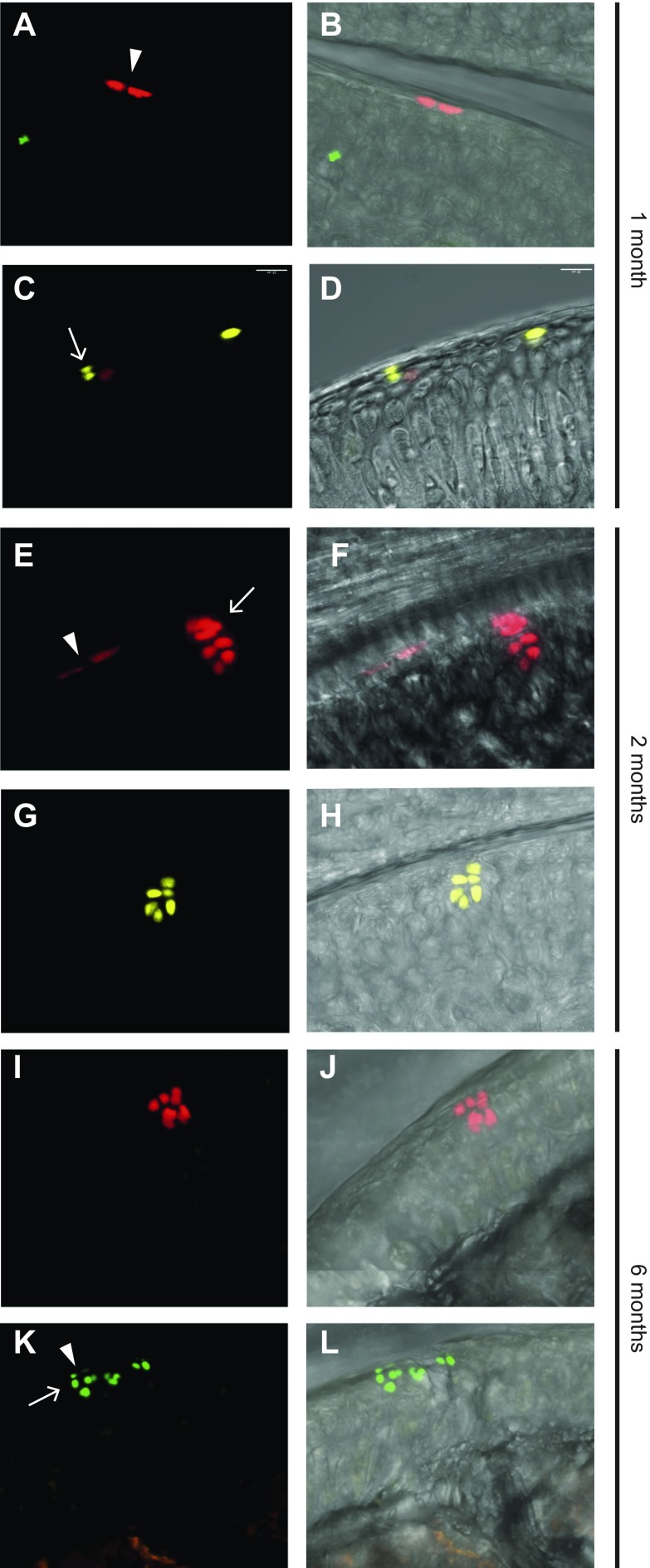
Figure 3.
Cell division in the superficial zone has different orientation. Prg4-traced cells either divided parallel to and then remained at the superficial surface (A, E, K, arrowheads) or divided perpendicularly to this surface (C, E, K, arrows). Clusters of chondrocytes generated from superficial cells can be seen at the cartilage surface together with (E, K) or separated from (G, I) progenitor cells. 3D confocal scans of 150-μm–thick sections were performed to examine the spatial relationship between clusters and the superficial cells above. Images in panels B, D, F, H, J, and L correspond to those in panels A, C, E, G, I, and K but with the white light channel added. Samples for panels A–D were collected at age 1 mo, those for panels E–H were collected at age 2 mo, and those for panels I–L were collected at age 6 mo.
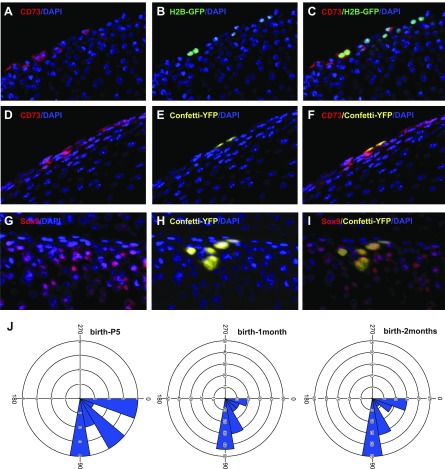
The ability of superficial cells to contribute to all layers of adult articular cartilage as well as their lower proliferative activity compared with underlying chondrocytes led us to investigate whether they express stem cell markers. We found that superficial cells at age 1 mo exclusively expressed the mesenchymal stem cell marker CD73 (Fig. 2J). We also found that they express Notch 1, as has been previously reported (7); however, we found that Notch1 was localized to superficial cells of the forming joint at P5 (Fig. 2K) and expressed by all articular cartilage cells at age 1 mo (Supplemental Fig. S1E). In contrast, we observed that CD73 expression is specifically maintained in superficial cells of the mature joint (Supplemental Fig. S1F). At age 2 mo, the number of CD73+ cells constituted 31.5 ± 6.1% of superficial cells (defined by flat morphology) and 11.9 ± 1.8% of all cells in articular cartilage (mean ± sd, n = 4).
The ability of Prg4-expressing cells in the superficial-most layer of the nascent joint to give rise to deeper zone cells in the articular cartilage (see above and ref. 9) suggests that these cells may resemble stem cells that both self-renew and give rise to differentiated progeny. Indeed, some superficial cells divided along the joint surface and then remained there, thus reflecting symmetric cell division (Fig. 3A, B, E, K, arrowheads), whereas others divided perpendicular to the joint surface, with one daughter cell remaining at the surface and another becoming a deeper zone chondrocyte, which reflects asymmetric cell division (Fig. 3C, D, E, K, arrows). Of interest, parent stem cells sometimes remained at the surface (Fig. 3C, K), but in other clones, the cluster did not contain any cells still within the superficial zone (Fig. 3G, I and Supplemental Video 1), which suggested that in some cases both daughter cells were recruited to differentiation—a process known as symmetric cell differentiation. To further investigate symmetric and asymmetric cell division, we combined immunohistochemistry with clonal tracing or H2B-GFP retention. Slowly dividing H2B-GFP–retaining superficial cells were positive for CD73, a stem cell marker (Fig. 4A–C). Colocalization of confetti clones with CD73 expression revealed that whereas cells that divided parallel to the surface retained the stem cell marker (Fig. 4D–F), those that divided perpendicular to the surface acquired expression of chondrocyte marker Sox9 (Fig. 4G–I).
Figure 4.
Superficial cells divide symmetrically and asymmetrically. A–C) H2B-GFP–retained cells (labeled as depicted in Fig. 1A and analyzed at age 1 mo) divided parallel to the cartilage surface and expressed CD73 stem cells marker. D–F) Confetti clone divided parallel to the cartilage surface and expressed CD73 stem cell marker. G–I) Confetti clone divided perpendicular to the cartilage surface and acquired expression of chondrogenic marker Sox9. Double-colored arrowheads in panels C, F, and I showed double-labeled cells and yellow arrowheads in panels G, H, and I show confetti-labeled cell without Sox9 expression. Ab-detected signal, red; signal from a single confetti channel, yellow; H2B-GFP signal, green; DAPI, blue. J) Spatially separated doublets of the same color revealed the orientation of cell division was predominantly perpendicular to the cartilage surface. A straight line was drawn through every separated doublet of one color, and angle of cell division parallel to the surface was designated as 0° and perpendicular as 90°, whereas angles between 90° and 180° were equalized to the corresponding angles between 0° and 90°. A low dose of tamoxifen was administrated to achieve rare recombination events that were spatially separated, and 3D confocal scans of 150-μm–thick sections were analyzed with Imaris software; 253, 133, and 29 doublets were analyzed for mice age 2 and 1 mo and 5 d, respectively; 10 mice were analyzed for every timepoint.
The combination of both symmetric and asymmetric cell division is a classic behavior of adult stem cells—referred to as population asymmetry—and implies that a pool of stem cells behaves as a population (15). We assessed the proportions of surface cells that exhibited symmetric and asymmetric cell divisions by examining the orientations of fluorescently labeled cell dyads. Indeed, 46.7 ± 3.6% of dyads were oriented at an angle between 70° and 90° to the surface of the cartilage, and 21.4 ± 1.8% were parallel to this surface; that is, at a 0–10° angle (Fig. 4J). To further support renewal of superficial cells, we made a time-lapse of their retention at the surface: the number of these cells when labeled at P0 remained stable between age 1 and 2 mo and slowly declined with aging (6.9 ± 0.8, 6.5 ± 1.0, and 3.9 ± 0.8 × 104 labeled cells/mm3 at age 1, 2, and 6 mo, respectively; P = 0.04 (1 vs. 6 mo), = 0.08 (2 vs. 6 mo); n = 9, only flat superficial cells within the top 15 μm of the cartilage surface were analyzed; low-recombination efficiency employed). This analysis suggests that whereas some superficial cells were lost as a result of symmetric differentiation into chondrocytes (Fig. 3G, I), symmetric cell division allowed them to maintain their population on the articular surface up to age 6 mo.
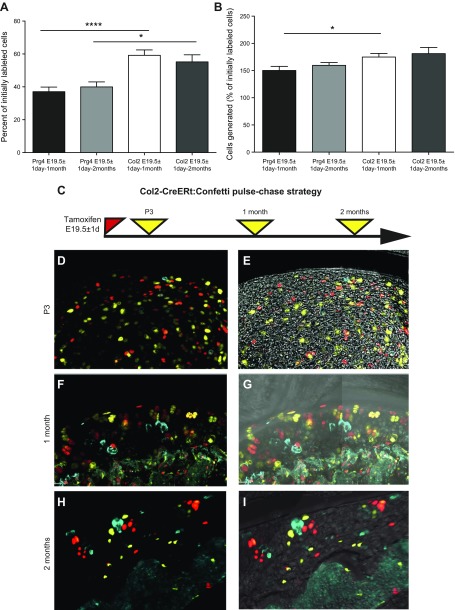
To assess the contribution of superficial stem cells to cartilage population by generating chondrocytes de novo, we again used the power of clonal genetic tracing, which allows one to extract this information from the frequency and size of labeled clones. The proportion of superficial cells that have undergone division was calculated as follows: if x is the number of clones containing >1 cell and y the number of single-labeled cells, the percentage will be x/(x + y) multiplied by 100. In this manner, we calculated that 36 ± 4% of superficial cells proliferated during the first month and 38 ± 5% during the first 2 mo of postnatal life (Fig. 5A). By also counting the number of cells in each clone, we found that superficial cells generated 151 ± 12 or 157 ± 7% of the initially labeled cells during either the first month or the first 2 mo of life, respectively (Fig. 5B). The number of clusters (defined as ≥4 cells) did grow substantially between 1 and 2 mo: from 23 clusters (constituted 93 cells) to 34 clusters (constituted 158 cells; the sum for 3 animals in each group).
Figure 5.
Assessment of proliferative activity of chondrocytes and superficial cells by clonal tracing. Every single-labeled cell after tracing period indicated was considered as quiescent, and every spatially separated clone that contained cells of the same color counted as initially labeled single progenitor. Thus, the sum of these two gives the number of cells labeled initially. A) The proportion of cells that had proliferated in Prg4-CreER(T2):Confetti mice and Col2-CreERt:Confetti animals traced for 1 or 2 mo was calculated by dividing the number of clones by the number of cells labeled initially. B) Overall increase in cell number was calculated as the total number of labeled cells divided by the number of cells labeled initially. A low degree of recombination (to avoid clonal overlap) was achieved by injecting a smaller amount of tamoxifen. 3D confocal scans of 150-μm–thick sections were analyzed. C) Strategy for labeling Col2-CreER(T):Confetti mice. D–I) Col2-CreER(T)–labeled chondrocytes throughout the articular cartilage, with occasional labeling of superficial cells (D, E). Representative images showing chondrocytes labeled around birth and analyzed 3 d (D, E), 1 mo (F, G), or 2 mo later (H, I; maximum intensity projection shown). On P3, single-labeled cells and clonal doublets were only observed, whereas at 1- and 2-mo timepoints, triplets and quadruplets could be observed. Values are presented as means ± sem; n = 9 mice for every point (A, B). *P < 0.05; ***P < 0.001.
As soon as superficial cells divide or differentiate asymmetrically, they become chondrocytes (the only type of cell under the superficial layer), which then continue to proliferate. To examine their proliferation in the same manner, we bred confetti reporter mice with the Col2-CreERt strain and labeled chondrocytes at birth (Fig. 5D–I). By using the calculations described above, clonal genetic analysis revealed that 62 ± 6% of Col2-CreERt–labeled chondrocytes proliferated within the first month of age (Fig. 5A), which is almost double the proliferation observed in Prg4-CreERt2–labeled superficial cells and is consistent with the findings from both the EdU- and H2B-GFP–retention experiments (Fig. 1). Overall, Col2-CreERt–labeled cells generated 181 ± 7% new chondrocytes during the 2-mo tracing period (Fig. 5B). Of interest, most of these cells formed doublets (38 ± 5%), triplets (9 ± 3%), or quadruplets (8 ± 4%), with no single clone containing >4 cells, in contrast to Prg4-CreERt–traced cells.
To estimate the relative contribution of superficial cells to the growth of articular cartilage, we analyzed cell (nuclei) density, which was 1.77-fold higher in the superficial zone than in the underlying cartilage (103.9 ± 6.0 and 58.8 ± 4.9 × 104 cells/mm3, respectively; P = 0.0002; n = 4). Thus, despite the slower proliferation in the superficial layer compared with the underlying chondrocytes (157 ± 7% vs. 181 ± 7%; Fig. 5B), this layer could increase the tissue volume 2.77-fold (1.57 × 1.77; if the same cell density was maintained). Together with the 1.81-fold increase provided by the initial underlying chondrocytes, cell proliferation could increase the volume of articular cartilage 4.58-fold (2.77+1.81) in 2 mo, provided that cell density does not change.
We observed that cell (nuclei) density was reduced 50% with age (Fig. 6A), with a corresponding increase in the average distance between adjacent nuclei (Fig. 6B). These parameters reflect increases in both cell size and matrix production, and together with changes in cell proliferation, should lead to a 9.16-fold increase in tissue volume [increase in cell number (4.58-fold) multiplied by a decrease in cell density (2-fold)].
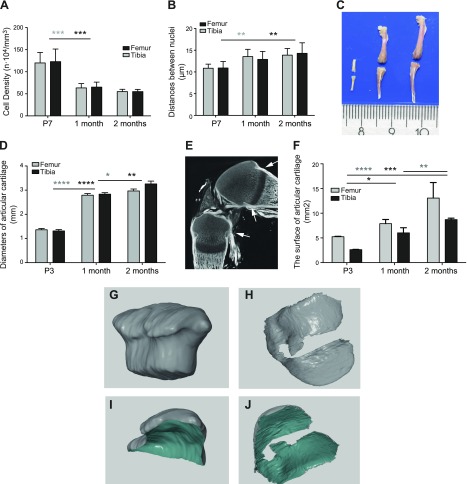
Figure 6.
Growth and reshaping of mouse articular cartilage. A, B) 3D confocal analysis of nuclear density (A) and of the distance between adjusted nuclei (B) was performed on 150-μm sections stained with DAPI by using Imaris software. C, D) Representative images of the femur and tibia at age 3 d and 1 and 2 mo (C) and the corresponding epiphyseal diameters measured (D). E) Micro-CT with phosphotungstic acid contrasting allowed visualization of soft tissues, such as cartilage, tendon, and all connective tissues, as shown for the 3-d-old knee joint. Arrows show the Ranvier grove delineating the cartilage surface for determination of the surface area at age 3 d. F) Manual segmentation of these scans allowed quantitative analysis of the area of the cartilage surface. G–J) Segmented cartilage was measured employing GOM Inspect 8.0 software as exemplified for 3-d-old knee cartilage (G, I) and 1-mo-old (H, J) tibial cartilage. The gray color depicts the outer and green the inner surface of articular cartilage. Values are presented as means ± sd; n = 9 (A, B, D, F) *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, as analyzed by one-way ANOVA.
Such tissue production seems to exceed the demands of the growing articular tissue. Indeed, simple estimations on the basis of increased epiphyseal diameter suggested 4.7- and 6.2-fold increases in cartilage volume for the femur and tibia, respectively, between birth and age 2 mo (Fig. 6C, D). However, quantitative analysis that employed micro-CT combined with phosphotungstic acid contrasting, which allowed visualization of all tissues, including cartilage and tendons (Fig. 6E), revealed that the surface of articular cartilage increased relatively modestly by 2.7- and 2.3-fold from P3 to age 2 mo for tibia and femur, respectively (Fig. 6F; notice that no statistical difference was found between femur and tibia at age 2 mo; P = 0.099; n = 6; and at P3 the articular surface was considered to be delineated by the groove of Ranvier; Fig. 6E, arrows). Instead, substantial tissue reshaping occurred: initially, articular cartilage was hemispherical in shape, but flattened as the bone enlarged [see the different projections in Fig. 6G–J and cross-sections in Fig. 7, as well as 3D reconstructions in Supplemental Videos 2 (cartilage at P3) and 3 (femoral cartilage at age 1 mo]. Of interest, the largest clusters in Prg4-CreER(T2):Confetti mice were located primarily on the lateral sides of the cartilage, which suggested that differences in clonal dynamics might contribute to reshaping.
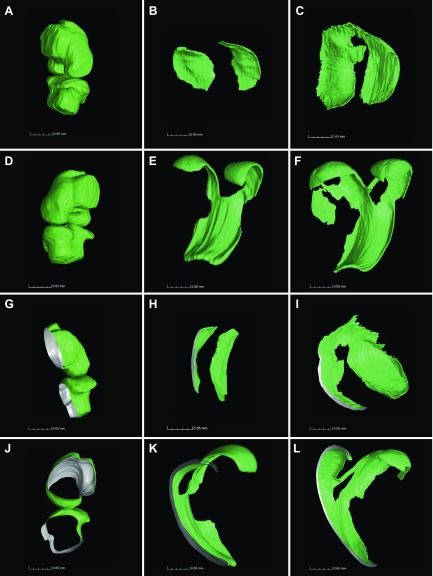
Figure 7.
Visualization of cartilage shape revealed substantial flattening and reshaping in ontogenesis. Cartilage was reconstructed employing VG Studio Max 2.2 software for 3-d-old mouse knee cartilage (A, D, G, J), 1-mo-old tibia (B, H), and 1-mo-old femur (E, K), as well as 2-mo-old tibia (C, I) and femur (F, L). Panels A and D represent different projections of the same joint, G–L cross-sections of CT scans (gray color, section plan), J shows middle sagittal slice of 3-d-old knee, and A–F are the same magnitudes, as well as G–L.
Thus, the above analysis revealed that superficial cells generate a clear excess of chondrocytes.
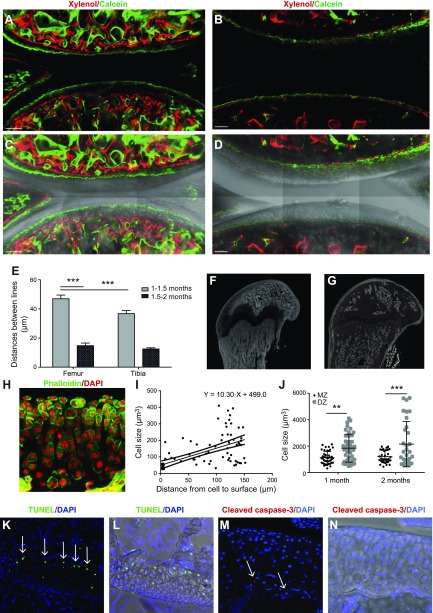
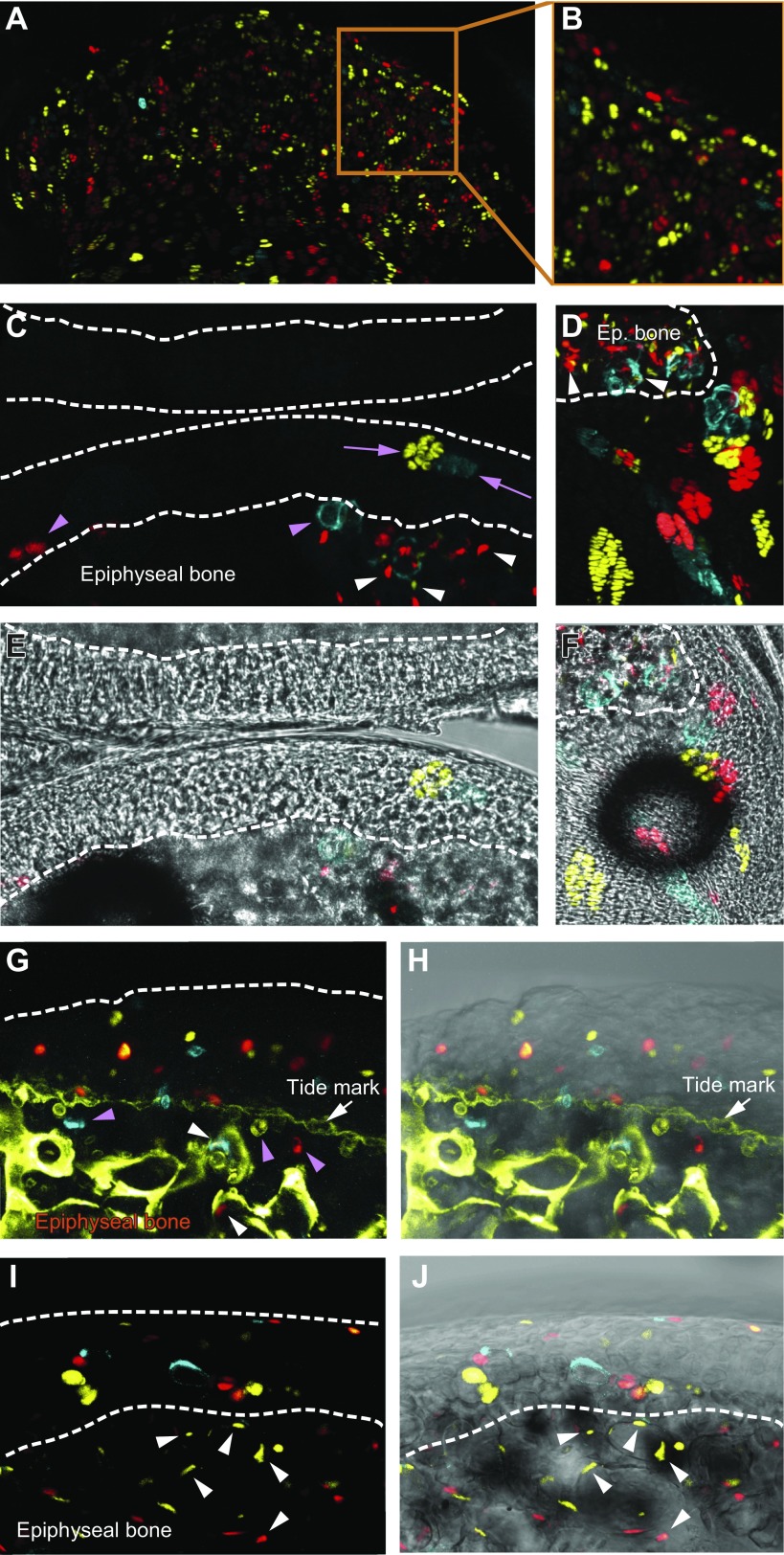
To understand what this excess is used for, we explored whether chondrocytes are consumed for growth of the underlying epiphyseal bone. Indeed, this bone continued to grow even after the secondary ossification center was formed: combined calcein-xylenol labeling revealed expansion of this bone (together with calcified cartilage) by 42 ± 8 μm and 14 ± 4 μm during the periods of age 1–1.5 and 1.5–2 mo, respectively (Fig. 8A–E), and an enlargement was also seen in micro-CT scans (Fig. 8F, G). To determine if this growth occurs via the mechanism of endochondral bone formation in which hypertrophic chondrocytes die and become substituted by newly formed bone, we assayed the hypertrophy and death of articular chondrocytes.
Figure 8.
The contribution of articular cartilage to the growth of underlying bone. A–D) Growth of epiphyseal bone, which underlies articular cartilage, was measured by double calcein–xylenol labeling at time periods of age 1–1.5 mo (A, C) and 1.5–2 mo (B, D). Images in panels C and D correspond to those in A and B, but with the white light channel added. E) Quantification of the average distance between green (calcein)- and red (xylenol)-labeled lines depicted in A and B. F, G) Micro-CT scans revealed expansion of epiphyseal bones from age 1–2 mo. H–J) 150-μm-thick sections were stained with phalloidin (green) and DAPI (red), and chondrocyte volume (I, J) was analyzed in 3D confocal scans using Imaris software. Volume of chondrocytes increased with their distance from the superficial zone (I), with extensive variation/clustering in the volume of cells in the deep zone (J). K, M) Cells stained positively for TUNEL (K, arrows) and cleaved caspase-3 (M, arrows) were observed at the chondro-osseous junction between articular cartilage and the underlying epiphyseal bone. Images in panels L and N correspond to those of K and M, respectively, but with the white light channel added. Values are presented as means ± sem; n = 9 (E, I, J). **P < 0.01; ***P < 0.001.
To determine chondrocyte hypertrophy, cartilage was stained with phalloidin (Fig. 8H) and cell volume was measured in 3D confocal scans. Indeed, a clear hypertrophy was observed the further that chondrocytes were located from the surface of articular cartilage (Fig. 8I). Surprisingly, cell size did not fulfill Gaussian distribution and 2 populations can be separated by size in the deep zone: the small cells of 738 ± 49 μm3 and true hypertrophic cells of 3130 ± 370 μm3 (P < 0.001; Fig. 8J). Apoptotic chondrocytes were detected at the chondro-osseous junction between articular cartilage and the underlying epiphyseal bone by both TUNEL detection (Fig. 8K, L) and by positive staining for cleaved caspase-3 (Fig. 8M). Thus, this experiment revealed that an excess of articular chondrocytes is used for endochondral growth of the underlying epiphyseal bone.
The program of cellular kinetics with chondrocytes generated at the surface and consumed at the chondro-osseous junction suggests tissue renewal. Indeed, chondrocytes that were labeled genetically with Col2-CreERt disappeared from cartilage (when labeled at birth, they constituted 6.5 ± 2.0 × 104 labeled cells/mm3 at age 1 mo, but 3.4 ± 0.6 × 104 labeled cells/mm3 at age 2 mo; P = 0.004; n = 9). In contrast, if superficial cells are labeled, the number of their progeny increased (Prg4-CreERt:Confetti mice labeled at birth constituted 7.9 ± 3.0 and 17.9 ± 7.5 × 104 labeled cells/mm3 at age 1 and 2 mo, respectively; P = 0.077; n = 10). The above data show that chondrocytes that were labeled with Col2-CreERt at birth slowly vanished from articular cartilage. To check whether all fetal chondrocytes were substituted by progeny of superficial cells, we performed Col2-CreERt tracing from E14.5 (the timepoint right after joint cavitation assuming a 24-h delay in maximum Cre activity; see Supplemental Fig. S1I, J for Col2 expression). At P4, this tracing revealed abundant cell labeling throughout epiphyseal cartilage, including areas right beneath the superficial zone as well as the growth plate (Fig. 9A, B). However, as early as age 19 d (P19), almost no labeled articular chondrocytes remained (only 2 clones were found within 2 full joints analyzed; Fig. 9C, D). At the same time, labeled cells remained within the growth plate (Fig. 9E, F). Furthermore, labeled chondrocytes were found to be buried within the bone just beneath articular cartilage (Fig. 9G, H), and extensive labeling was observed within epiphyseal bone (Fig. 9I, J), which suggested trans-differentiation (16). In contrast, the progeny of superficial cells have been observed at the chondro-osseous junction (Supplemental Fig. S1I) but never below, and no labeling within epiphyseal bone was observed even after 6 mo of tracing (not shown).
Figure 9.
Fetal chondrocytes did not remain in postnatal articular cartilage. Fetal chondrocytes of Col2-CreERt:Confetti mice were labeled by tamoxifen injection at E14.5. A, B) Tracing to P4 revealed abundant labeled cells distributed throughout the epiphyseal cartilage (A), including cartilage surface areas (B). C, E) Almost no labeled cells remained in articular cartilage when tracing was prolonged until P19 (images represent the only 2 clones found; pink arrows). D, F) Labeled chondrocytes persisted within the growth plate. G–J) Col2-CreERt:Confetti–labeled cells within epiphyseal bone. Bone and mineralized matrix were visualized by calcein labeling 24 h before sacrifice (G, H). Tracing was done from P6 to P40 (G, H) or from P0 to P30 (I, J). Pink arrowheads point toward chondrocytes at chondro-osseous junction. White arrowheads point toward cells lining bone surface or inside bone. Notice labeled cells in bone area in panels C and D where tracing was from E14.5 to P19. Maximum intensity projections are shown in panels A–H and single-plan scans in I and J. Panels E, F, H, and J correspond to C, D, G, and I, but with the white light channel added.
Thus, we concluded that fetal chondrocytes do not remain within adult mouse articular cartilage. Together, with the amount of cells that originated from superficial cells (see standard dose of tamoxifen; Supplemental Fig. S1D) and their capacity to fill all zones of articular cartilage (Supplemental Fig. S1I), these observations strongly suggest that the progeny of superficial cells fully substitute chondrocytes of fetal origin and adult joints are entirely formed by progeny of superficial cells.
DISCUSSSION
Superficial cells are slowly dividing chondrocyte progenitors
Here, we show that superficial cells are slow-dividing progenitors of middle- and deep-zone chondrocytes. In general, articular chondrocytes are thought to proliferate little during the juvenile period (17, 18) and, in this context, it is especially interesting that superficial cells divide even more slowly. Reports regarding superficial cell division on the basis of retention of [3H]-thymidine or EdU/BrdU are not in agreement: some indicate that superficial cells divide more rapidly than underlying chondrocytes (9, 18, 19) whereas others conclude the opposite (20, 21). It is known that results obtained with [3H]-thymidine or BrdU labeling can differ substantially (22), that EdU/BrdU can inhibit cell division (23, 24), and that prolonged incorporation of thymidine might reflect other cellular activities, such as DNA repair, mitochondrial renewal, and even RNA synthesis (25–27).
The 3 independent approaches we employed here—retention of H2B-GFP, analysis of clonal activity in confetti mice, and retention of EdU—all showed clearly that superficial cells divided less frequently than underlying chondrocytes. We also demonstrated the ability of superficial cells to generate chondrocytes de novo in a physiological setting, which was in agreement with the initial report (9). In addition, we observed that superficial cells give rise to clonal clusters, which indicates that articular cartilage growth is both appositional and interstitial. This interstitial growth might facilitate lateral cartilage expansion during juvenile growth.
Superficial cells of the nascent joint undergo both symmetric and asymmetric cell division
It was initially proposed that superficial cells of the nascent joint are chondrocyte progenitors (9). Our analysis revealed that these cells can renew themselves by symmetrical divisions, with both daughter cells becoming superficial cells that remain at the cartilage surface. We also observed symmetric and asymmetric differentiation of these cells. Thus, the behavior of superficial cells can be described as population asymmetry, where some stem cells can renew themselves symmetrically and others are lost via symmetric differentiation (15). Of interest, such behavior is typical for adult stem cells in their respective niches, as has been described for mouse germline stem cells (28), stem cells in intestinal crypts (12), and hair follicles (29, 30). Whether a special niche facilitates the renewal of superficial cells remains to be explored.
It has previously been reported that bovine superficial zone cells can be expanded in culture and be induced to different mesenchymal cell types as well as proliferate indefinitely (7). We showed that in vivo murine superficial cells generate only chondrocytes, which suggests unipotency; however, the observation that they proliferate slowly and express markers that are commonly seen in stem cells suggest that they may retain pluripotent stem cell properties in vivo, similar to cells with unipotent stem cell features in other tissues, such as muscle satellite stem cells, corneal limbus epithelial stem cells, and the recently described liver stem cells (31).
Currently, there are no satisfactory in vivo assays to test whether superficial cells have stem cell properties that can lead to repair or regeneration of damaged cartilage. Zhang et al. (32) induced superficial cell death by causing cell autonomous expression of diphtheria toxin. Although this resulted in high rates of cell death, there was evidence that surviving superficial cells could divide at a higher rate and partially repopulate cell-depleted areas. However, because diphtheria-induced cell death does not physically damage articular cartilage, this approach may not have been sufficient to promote a proper regenerative response. Indeed, ablation of muscle satellite stem cells does not cause a phenotype until tissue is physically damaged (33). Additional examples in which ablation of stem cells without physical damage to the microenvironment of the niche does not lead to a detectable phenotype include the ablation of hematopoietic stem cells (34), Lgr5-stem cells in the intestine (35), and bulge/hair germ stem cells in the hair follicle (36, 37). In all of these cases, the stem cell population is promptly restored. Multiple mechanisms are used for this process, from compensation by remaining stem cells (34, 38), to migration of cells from neighboring niches [that is, intestine or hair follicle (36)] and dedifferentiation of the progeny (39, 40). Thus, superficial cells might tend to restore their number upon partial ablation similar to the above-described stem cell populations (provided their niche is preserved). Further studies are required to understand whether this mechanism involves the direct compensation by remaining stem cells, dedifferentiation of chondrocytes, or migration of stem cells from a neighboring niche [which, in this case, is the synovial membrane (41)].
Superficial cells reconstitute adult articular cartilage
It was shown that early in embryogenesis all joint structures originate from growth differentiation factor 5 (GDF5)–expressing cells of interzone (42, 43). Recent elegant spatiotemporal characterization of the process of joint formation proposed an influx model in which there is a constant influx of cells into the GDF5+ domain of interzone and a simultaneous efflux of cells from this domain toward round epiphyseal chondrocytes, forming articular joints (44). Thus, new chondrocytes continue to be added appositionally and to contribute to enlargement of fetal epiphysis. Our data are in line with this general model of organogenesis of the joint and extend the observation from fetal to postnatal life. Indeed, we observed a constant influx of newly differentiated chondrocytes from Prg4+ superficial cells and the simultaneous loss of mature chondrocytes; however, in contrast to the fetal model, it seems that there is no influx of new progenitor cells. Instead, chondrocyte progenitors at the surface acquire a possibility to self-renew, as discussed above, and thereby maintain their population.
Of interest, if GDF5 marks the domain of chondrocyte progenitors in fetal development, this changes to Prg4 when joints are fully formed. Indeed, GDF5 expression first becomes restricted to the joint surface at E14.5–E15.5 (44) and vanishes from joints between E15.5 and P0 [varies between joints (43–45)]. In parallel, surface cells acquire Prg4 expression (43, 45). It is very likely that these are the same cells that turn off GFD5 expression and switch on Prg4. The process occurs 1–2 d after joint cavitation and likely coincides with acquired motility. Indeed, Prg4 expression was shown to be directly activated by mechanical stimuli (46). GDF5 expression is acquired movement-autonomously; however, maintenance of the GDF5+ progenitors requires mechanical stimulation and, without it, GDF5+ cells directly differentiate into chondrocytes (47). Thus, it is plausible that mechanical stimulation is responsible for the maintenance of progenitors and for their acquisition of the self-renewal capacity.
Finally, our cellular kinetics findings strongly suggest that when the mouse is fully grown, all articular chondrocytes in the knee joint have originated from superficial cells. This is again in line with the proposed influx model (44), which we would suggest be renamed as the influx-efflux model of cartilage formation, where new chondrocytes are constantly generated from progenitors. In the case of self-renewing Prg4-labeled progenitors, both our data (tracing from birth) and those previously reported [(9), tracing from E17.5], show that progeny of Prg4+ cells repopulate all zones of the articular cartilage. Prg4+ cells—when labeled at birth with a high level of recombination (Supplemental Fig. S1D)—result in labeling of virtually all underlying chondrocytes. Together with the fact that Col2+ chondrocytes labeled at fetal life are absent from articular cartilage after age 1 mo (Fig. 9), these observations strongly suggest complete substitution of fetal chondrocytes by progeny of Prg4+ cells during the juvenile period in mice.
Recently, mainly on the basis of EdU/BrdU pulse-chase experiments, Ray et al. (48) proposed an alternative model in which articular cartilage is formed by descendants of highly proliferative epiphyseal chondrocytes; however, such a model contradicts several previous reports in which genetic tracing was used in fetal development (42–44) as well as in postnatal life (present work and ref. 9). A plausible explanation for these differences can be related to methodological approach and requires further clarification. In addition, we cannot fully exclude that synovial cells, which can be labeled in our Prg4-tracing experiments, give rise to superficial cells, which, in turn, make chondrocytes. Stem cells were described for synovial membrane (49).
In summary, our present findings reveal that superficial cells are self-renewing chondrocyte progenitors and likely can be called unipotent adult stem cells. These cells are responsible for the formation of adult murine cartilage.
ACKNOWLEDGMENTS
The authors thank Susan Mackem (U.S. National Institutes of Health) for Col2-CreERt mouse strain, Bára Szarowská (Karolinska Institutet) for phosphotungstic acid contrasting, and Eugeniy Ivashkin (Karolinska Institutet) for assistance with Imaris software. This work was supported by The Swedish Research Council [2016-02835 (to A.S.C.), 2015-03387 (to V.D.)], Karolinska Institutet (including a FoAss extension grant, KID grant, Ulla och Gustaf af Ugglas Foundation and Karolinska Institutet Foundation for Rheumatism Research), King Gustaf V’s 80-year Jubileum Foundation, and the Swedish Foundation for Rheumatism. L.L. was supported by the Chinese Scholarship Council; P.T.N. was supported by Stiftelsen Frimurare Barnhuset i Stockholm and Sällskapet Barnavård; M.X. was supported by EMBO long-term postdoctoral fellowship; V.D. was supported by StratNeuro; and I.A. was supported by Bertil Hallsten Research Foundation and Åke Wiberg Foundation. Tomographic analysis (M.S., T.Z., and Jo.K.) was carried out in connection with the Central European Institute of Technology 2020 project (LQ1601), with financial support from the Ministry of Education, Youth, and Sports of the Czech Republic under the National Sustainability Programme II. The authors declare no conflicts of interest.
Glossary
- 3D
3-dimensional
- Cre
Causes Recombination
- CreERt
Cre-ER conjugate binding tamoxifen
- Col2a1
collagen type 2α1
- CT
computed tomography
- E1
embryonic day 1
- EdU
5-ethynyl-2′-deoxyuridine, thymidine analog
- ER
estrogen receptor
- GDF
growth differentiation factor
- GFP
green fluorescent protein
- H2B
histone H2B
- P0
postnatal day 0
- Prg4
proteoglycan 4
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
L. Li, P. T. Newton, and A. S. Chagin conceived, designed and performed experiments, analyzed data, and wrote the manuscript; T. Bouderlique, M. Sejnohova, and T. Zikmund performed experiments and analyzed data; E. Kozhemyakina, A. B. Lassar, and M. L. Warman conceived and designed experiments, provided material, and shared unpublished data; M. Xie, J. Krivanek, and V. Dyachuk performed experiments; J. Kaiser provided resources and supervision; H. Qian and B. Barenius conceived and designed experiments; and I. Adameyko conceived and designed experiments and analyzed data.
REFERENCES
- 1.Madry H., Luyten F. P., Facchini A. (2012) Biological aspects of early osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 20, 407–422 [DOI] [PubMed] [Google Scholar]
- 2.Hall B. K. (2015) Bones and Cartilage: Developmental and Evolutionary Skeletal Biology, 2nd ed., Elsevier, New York [Google Scholar]
- 3.Murray C. J., Vos T., Lozano R., Naghavi M., Flaxman A. D., Michaud C., Ezzati M., Shibuya K., Salomon J. A., Abdalla S., Aboyans V., Abraham J., Ackerman I., Aggarwal R., Ahn S. Y., Ali M. K., Alvarado M., Anderson H. R., Anderson L. M., Andrews K. G., Atkinson C., Baddour L. M., Bahalim A. N., Barker-Collo S., Barrero L. H., Bartels D. H., Basáñez M. G., Baxter A., Bell M. L., Benjamin E. J., Bennett D., Bernabé E., Bhalla K., Bhandari B., Bikbov B., Bin Abdulhak A., Birbeck G., Black J. A., Blencowe H., Blore J. D., Blyth F., Bolliger I., Bonaventure A., Boufous S., Bourne R., Boussinesq M., Braithwaite T., Brayne C., Bridgett L., Brooker S., Brooks P., Brugha T. S., Bryan-Hancock C., Bucello C., Buchbinder R., Buckle G., Budke C. M., Burch M., Burney P., Burstein R., Calabria B., Campbell B., Canter C. E., Carabin H., Carapetis J., Carmona L., Cella C., Charlson F., Chen H., Cheng A. T., Chou D., Chugh S. S., Coffeng L. E., Colan S. D., Colquhoun S., Colson K. E., Condon J., Connor M. D., Cooper L. T., Corriere M., Cortinovis M., de Vaccaro K. C., Couser W., Cowie B. C., Criqui M. H., Cross M., Dabhadkar K. C., Dahiya M., Dahodwala N., Damsere-Derry J., Danaei G., Davis A., De Leo D., Degenhardt L., Dellavalle R., Delossantos A., Denenberg J., Derrett S., Des Jarlais D. C., Dharmaratne S. D., Dherani M., Diaz-Torne C., Dolk H., Dorsey E. R., Driscoll T., Duber H., Ebel B., Edmond K., Elbaz A., Ali S. E., Erskine H., Erwin P. J., Espindola P., Ewoigbokhan S. E., Farzadfar F., Feigin V., Felson D. T., Ferrari A., Ferri C. P., Fèvre E. M., Finucane M. M., Flaxman S., Flood L., Foreman K., Forouzanfar M. H., Fowkes F. G., Fransen M., Freeman M. K., Gabbe B. J., Gabriel S. E., Gakidou E., Ganatra H. A., Garcia B., Gaspari F., Gillum R. F., Gmel G., Gonzalez-Medina D., Gosselin R., Grainger R., Grant B., Groeger J., Guillemin F., Gunnell D., Gupta R., Haagsma J., Hagan H., Halasa Y. A., Hall W., Haring D., Haro J. M., Harrison J. E., Havmoeller R., Hay R. J., Higashi H., Hill C., Hoen B., Hoffman H., Hotez P. J., Hoy D., Huang J. J., Ibeanusi S. E., Jacobsen K. H., James S. L., Jarvis D., Jasrasaria R., Jayaraman S., Johns N., Jonas J. B., Karthikeyan G., Kassebaum N., Kawakami N., Keren A., Khoo J. P., King C. H., Knowlton L. M., Kobusingye O., Koranteng A., Krishnamurthi R., Laden F., Lalloo R., Laslett L. L., Lathlean T., Leasher J. L., Lee Y. Y., Leigh J., Levinson D., Lim S. S., Limb E., Lin J. K., Lipnick M., Lipshultz S. E., Liu W., Loane M., Ohno S. L., Lyons R., Mabweijano J., MacIntyre M. F., Malekzadeh R., Mallinger L., Manivannan S., Marcenes W., March L., Margolis D. J., Marks G. B., Marks R., Matsumori A., Matzopoulos R., Mayosi B. M., McAnulty J. H., McDermott M. M., McGill N., McGrath J., Medina-Mora M. E., Meltzer M., Mensah G. A., Merriman T. R., Meyer A. C., Miglioli V., Miller M., Miller T. R., Mitchell P. B., Mock C., Mocumbi A. O., Moffitt T. E., Mokdad A. A., Monasta L., Montico M., Moradi-Lakeh M., Moran A., Morawska L., Mori R., Murdoch M. E., Mwaniki M. K., Naidoo K., Nair M. N., Naldi L., Narayan K. M., Nelson P. K., Nelson R. G., Nevitt M. C., Newton C. R., Nolte S., Norman P., Norman R., O’Donnell M., O’Hanlon S., Olives C., Omer S. B., Ortblad K., Osborne R., Ozgediz D., Page A., Pahari B., Pandian J. D., Rivero A. P., Patten S. B., Pearce N., Padilla R. P., Perez-Ruiz F., Perico N., Pesudovs K., Phillips D., Phillips M. R., Pierce K., Pion S., Polanczyk G. V., Polinder S., Pope C. A. III, Popova S., Porrini E., Pourmalek F., Prince M., Pullan R. L., Ramaiah K. D., Ranganathan D., Razavi H., Regan M., Rehm J. T., Rein D. B., Remuzzi G., Richardson K., Rivara F. P., Roberts T., Robinson C., De Leòn F. R., Ronfani L., Room R., Rosenfeld L. C., Rushton L., Sacco R. L., Saha S., Sampson U., Sanchez-Riera L., Sanman E., Schwebel D. C., Scott J. G., Segui-Gomez M., Shahraz S., Shepard D. S., Shin H., Shivakoti R., Singh D., Singh G. M., Singh J. A., Singleton J., Sleet D. A., Sliwa K., Smith E., Smith J. L., Stapelberg N. J., Steer A., Steiner T., Stolk W. A., Stovner L. J., Sudfeld C., Syed S., Tamburlini G., Tavakkoli M., Taylor H. R., Taylor J. A., Taylor W. J., Thomas B., Thomson W. M., Thurston G. D., Tleyjeh I. M., Tonelli M., Towbin J. A., Truelsen T., Tsilimbaris M. K., Ubeda C., Undurraga E. A., van der Werf M. J., van Os J., Vavilala M. S., Venketasubramanian N., Wang M., Wang W., Watt K., Weatherall D. J., Weinstock M. A., Weintraub R., Weisskopf M. G., Weissman M. M., White R. A., Whiteford H., Wiebe N., Wiersma S. T., Wilkinson J. D., Williams H. C., Williams S. R., Witt E., Wolfe F., Woolf A. D., Wulf S., Yeh P. H., Zaidi A. K., Zheng Z. J., Zonies D., Lopez A. D., AlMazroa M. A., Memish Z. A. (2012) Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2197–2223 [DOI] [PubMed] [Google Scholar]
- 4.Lotz M., Loeser R. F. (2012) Effects of aging on articular cartilage homeostasis. Bone 51, 241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldring M. B. (2012) Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther. Adv. Musculoskelet. Dis. 4, 269–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stockwell R. A. (1967) The cell density of human articular and costal cartilage. J. Anat. 101, 753–763 [PMC free article] [PubMed] [Google Scholar]
- 7.Dowthwaite G. P., Bishop J. C., Redman S. N., Khan I. M., Rooney P., Evans D. J. R., Haughton L., Bayram Z., Boyer S., Thomson B., Wolfe M. S., Archer C. W. (2004) The surface of articular cartilage contains a progenitor cell population. J. Cell Sci. 117, 889–897 [DOI] [PubMed] [Google Scholar]
- 8.Jiang Y., Tuan R. S. (2015) Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat. Rev. Rheumatol. 11, 206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozhemyakina E., Zhang M., Ionescu A., Ayturk U. M., Ono N., Kobayashi A., Kronenberg H., Warman M. L., Lassar A. B. (2015) Identification of a Prg4-expressing articular cartilage progenitor cell population in mice. Arthritis Rheumatol. 67, 1261–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura E., Nguyen M. T., Mackem S. (2006) Kinetics of tamoxifen-regulated Cre activity in mice using a cartilage-specific CreER(T) to assay temporal activity windows along the proximodistal limb skeleton. Dev. Dyn. 235, 2603–2612 [DOI] [PubMed] [Google Scholar]
- 11.Livet J., Weissman T. A., Kang H., Draft R. W., Lu J., Bennis R. A., Sanes J. R., Lichtman J. W. (2007) Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450, 56–62 [DOI] [PubMed] [Google Scholar]
- 12.Snippert H. J., van der Flier L. G., Sato T., van Es J. H., van den Born M., Kroon-Veenboer C., Barker N., Klein A. M., van Rheenen J., Simons B. D., Clevers H. (2010) Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144 [DOI] [PubMed] [Google Scholar]
- 13.Kaucka M., Ivashkin E., Gyllborg D., Zikmund T., Tesarova M., Kaiser J., Xie M., Petersen J., Pachnis V., Nicolis S. K., Yu T., Sharpe P., Arenas E., Brismar H., Blom H., Clevers H., Suter U., Chagin A. S., Fried K., Hellander A., Adameyko I. (2016) Analysis of neural crest-derived clones reveals novel aspects of facial development. Sci. Adv. 2, e1600060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tesařová M., Zikmund T., Kaucká M., Adameyko I., Jaroš J., Paloušek D., Škaroupka D., Kaiser J. (2016) Use of micro computed-tomography and 3D printing for reverse engineering of mouse embryo nasal capsule. J. Instrum. 11, C03006 [Google Scholar]
- 15.Simons B. D., Clevers H. (2011) Strategies for homeostatic stem cell self-renewal in adult tissues. Cell 145, 851–862 [DOI] [PubMed] [Google Scholar]
- 16.Yang L., Tsang K. Y., Tang H. C., Chan D., Cheah K. S. (2014) Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc. Natl. Acad. Sci. USA 111, 12097–12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Archer C. W. (1994) Skeletal development and osteoarthritis. Ann. Rheum. Dis. 53, 624–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pribylová E., Hert J. (1971) Proliferation zones in articular cartilage of young rabbits. Folia Morphol. (Praha) 19, 233–241 [PubMed] [Google Scholar]
- 19.Mankin H. J. (1962) Localization of tritiated thymidine in articular cartilage of rabbits. J. Bone Joint Surg. Am. 44, 682–688 [Google Scholar]
- 20.Hunziker E. B., Kapfinger E., Geiss J. (2007) The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthritis Cartilage 15, 403–413 [DOI] [PubMed] [Google Scholar]
- 21.Yasuhara R., Ohta Y., Yuasa T., Kondo N., Hoang T., Addya S., Fortina P., Pacifici M., Iwamoto M., Enomoto-Iwamoto M. (2011) Roles of β-catenin signaling in phenotypic expression and proliferation of articular cartilage superficial zone cells. Lab. Invest. 91, 1739–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duque A., Rakic P. (2011) Different effects of bromodeoxyuridine and [3H]thymidine incorporation into DNA on cell proliferation, position, and fate. J. Neurosci. 31, 15205–15217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollard D. R., Baran M. M., Bachvarova R. (1976) The effect of 5-bromodeoxyuridine on cell division and differentiation of preimplantation mouse embryos. J. Embryol. Exp. Morphol. 35, 169–178 [PubMed] [Google Scholar]
- 24.Kolb B., Pedersen B., Ballermann M., Gibb R., Whishaw I. Q. (1999) Embryonic and postnatal injections of bromodeoxyuridine produce age-dependent morphological and behavioral abnormalities. J. Neurosci. 19, 2337–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollibaugh J. T. (1988) Limitations of the [3H]thymidine method for estimating bacterial productivity due to thymidine metabolism. Mar. Ecol. (Berl.) 43, 19–30 [Google Scholar]
- 26.Korr H., Kurz C., Seidler T. O., Sommer D., Schmitz C. (1998) Mitochondrial DNA synthesis studied autoradiographically in various cell types in vivo. Braz. J. Med. Biol. Res. 31, 289–298 [DOI] [PubMed] [Google Scholar]
- 27.Breunig J. J., Arellano J. I., Macklis J. D., Rakic P. (2007) Everything that glitters isn’t gold: a critical review of postnatal neural precursor analyses. Cell Stem Cell 1, 612–627 [DOI] [PubMed] [Google Scholar]
- 28.Klein A. M., Nakagawa T., Ichikawa R., Yoshida S., Simons B. D. (2010) Mouse germ line stem cells undergo rapid and stochastic turnover. Cell Stem Cell 7, 214–224 [DOI] [PubMed] [Google Scholar]
- 29.Clayton E., Doupé D. P., Klein A. M., Winton D. J., Simons B. D., Jones P. H. (2007) A single type of progenitor cell maintains normal epidermis. Nature 446, 185–189 [DOI] [PubMed] [Google Scholar]
- 30.Doupé D. P., Klein A. M., Simons B. D., Jones P. H. (2010) The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Dev. Cell 18, 317–323 [DOI] [PubMed] [Google Scholar]
- 31.Wang B., Zhao L., Fish M., Logan C. Y., Nusse R. (2015) Self-renewing diploid Axin2+ cells fuel homeostatic renewal of the liver. Nature 524, 180–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang M., Mani S. B., He Y., Hall A. M., Xu L., Li Y., Zurakowski D., Jay G. D., Warman M. L. (2016) Induced superficial chondrocyte death reduces catabolic cartilage damage in murine posttraumatic osteoarthritis. J. Clin. Invest. 126, 2893–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lepper C., Partridge T. A., Fan C.-M. (2011) An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138, 3639–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoedel K. B., Morcos M. N., Zerjatke T., Roeder I., Grinenko T., Voehringer D., Göthert J. R., Waskow C., Roers A., Gerbaulet A. (2016) The bulk of the hematopoietic stem cell population is dispensable for murine steady-state and stress hematopoiesis. Blood 128, 2285–2296 [DOI] [PubMed] [Google Scholar]
- 35.Tian H., Biehs B., Warming S., Leong K. G., Rangell L., Klein O. D., de Sauvage F. J. (2011) A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478, 255–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rompolas P., Mesa K. R., Greco V. (2013) Spatial organization within a niche as a determinant of stem-cell fate. Nature 502, 513–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Driskell I., Oeztuerk-Winder F., Humphreys P., Frye M. (2015) Genetically induced cell death in bulge stem cells reveals their redundancy for hair and epidermal regeneration. Stem Cells 33, 988–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van der Flier L. G., van Gijn M. E., Hatzis P., Kujala P., Haegebarth A., Stange D. E., Begthel H., van den Born M., Guryev V., Oving I., van Es J. H., Barker N., Peters P. J., van de Wetering M., Clevers H. (2009) Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell 136, 903–912 [DOI] [PubMed] [Google Scholar]
- 39.Tata P. R., Mou H., Pardo-Saganta A., Zhao R., Prabhu M., Law B. M., Vinarsky V., Cho J. L., Breton S., Sahay A., Medoff B. D., Rajagopal J. (2013) Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature 503, 218–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pardo-Saganta A., Tata P. R., Law B. M., Saez B., Chow R. Dz., Prabhu M., Gridley T., Rajagopal J. (2015) Parent stem cells can serve as niches for their daughter cells. Nature 523, 597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurth T. B., Dell’accio F., Crouch V., Augello A., Sharpe P. T., De Bari C. (2011) Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis Rheum. 63, 1289–1300 [DOI] [PubMed] [Google Scholar]
- 42.Rountree R. B., Schoor M., Chen H., Marks M. E., Harley V., Mishina Y., Kingsley D. M. (2004) BMP receptor signaling is required for postnatal maintenance of articular cartilage. PLoS Biol. 2, e355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koyama E., Shibukawa Y., Nagayama M., Sugito H., Young B., Yuasa T., Okabe T., Ochiai T., Kamiya N., Rountree R. B., Kingsley D. M., Iwamoto M., Enomoto-Iwamoto M., Pacifici M. (2008) A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev. Biol. 316, 62–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shwartz Y., Viukov S., Krief S., Zelzer E. (2016) Joint development involves a continuous influx of Gdf5-positive cells. Cell Reports 15, 2577–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rhee D. K., Marcelino J., Baker M., Gong Y., Smits P., Lefebvre V., Jay G. D., Stewart M., Wang H., Warman M. L., Carpten J. D. (2005) The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J. Clin. Invest. 115, 622–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogawa H., Kozhemyakina E., Hung H. H., Grodzinsky A. J., Lassar A. B. (2014) Mechanical motion promotes expression of Prg4 in articular cartilage via multiple CREB-dependent, fluid flow shear stress-induced signaling pathways. Genes Dev. 28, 127–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kahn J., Shwartz Y., Blitz E., Krief S., Sharir A., Breitel D. A., Rattenbach R., Relaix F., Maire P., Rountree R. B., Kingsley D. M., Zelzer E. (2009) Muscle contraction is necessary to maintain joint progenitor cell fate. Dev. Cell 16, 734–743 [DOI] [PubMed] [Google Scholar]
- 48.Ray A., Singh P. N., Sohaskey M. L., Harland R. M., Bandyopadhyay A. (2015) Precise spatial restriction of BMP signaling is essential for articular cartilage differentiation. Development 142, 1169–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Bari C., Dell’Accio F., Tylzanowski P., Luyten F. P. (2001) Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 44, 1928–1942 [DOI] [PubMed] [Google Scholar]