Abstract
Tumor exosomes are emerging as antitumor immunity regulators; however, their effects on secondary exosome secretion by distal organs have not been explored. We have previously demonstrated that suppression of exosomes at the distal tumor site of pancreatic ductal adenocarcinoma (PDAC) ablated the development of salivary biomarker profile. Here, we explore the function of salivary exosomes from tumor-bearing mice in immune surveillance. We provide evidence that salivary exosomes from mice with PDAC exhibit a suppressive effect that results in reduced tumor-killing capacity by NK cells. Salivary exosomes from mice with PDAC where pancreatic tumors were engineered to suppress exosome biogenesis failed to suppress NK cell cytotoxic potential against tumor cells, as opposed to salivary exosomes from mice with PDAC with normal tumor exosome biogenesis. These results reveal an important and previously unknown mechanism of antitumor immune regulation and provide new insights into our understanding of the alterations of this biofluid during tumor development.—Katsiougiannis, S., Chia, D., Kim, Y., Singh, R. P., Wong, D. T. W. Saliva exosomes from pancreatic tumor–bearing mice modulate NK cell phenotype and antitumor cytotoxicity.
Keywords: extracellular vesicles, immune surveillance, cancer
Exosomes are small, nanometer-sized vesicles (30–150 nm) of endocytic origin that are released in the extracellular milieu by several cell types (1) under physiological and pathological conditions, including tumors and inflammation (2–4). These vesicles are capable of mediating local and systemic cell communication via horizontal transfer of information to recipient cells (5). Their role in tumor development is a result of the ability of tumor cell–derived exosomes to modulate and mold the host microenvironment as well as distal cell targets, which results in tumor progression and metastasis (2, 6, 7). Exosomes are present in all body fluids (8), including saliva, and have recently received attention because of their potential role as a new type of tumor biomarker (9, 10). Whereas several groups have studied the potential of exosome biomarkers in body fluids, such as saliva, urine, and CSF, we have only limited knowledge about the downstream function of body fluid exosomes in the context of disease.
The ability of tumors to escape from the host immune system has long been considered an obstacle to cancer immunotherapy (11). Human and animal studies support the existence of profound immune suppression in pancreatic ductal adenocarcinoma (PDAC) driven by defective or absent inflammatory cells, tumor-promoting immune cells, and immunosuppressive cell types (12). NK cells (13) represent a distinct lymphocyte subset with a natural ability to kill tumor cells (14–16). In patients and animal models, impaired NK cells or NK cell deficiency have been associated with an increased incidence of various types of cancer (17, 18). NK cells use activating receptors, such as NK group 2D (NKG2D), to recognize neoplastically transformed cells and eliminate them in the process of immune surveillance (11). Mice that are deficient for NKG2D are more susceptible to primary tumorigenesis, which confirms the crucial role of NKG2D in tumor immune surveillance (17).
We have previously demonstrated that suppression of exosome secretion by pancreatic cancer cells altered the transcriptomic profile of mouse saliva (19), which suggests that tumor exosomes are responsible for shuttling tumor biomarkers into saliva. We therefore asked whether salivary exosomes transfer information to the host immune system via the gastrointestinal tract to modulate immune surveillance. To investigate this, we chose PDAC as a model to examine the possible effects of salivary exosomes on immune surveillance during the development of pancreatic tumors.
In this article, we show that the effect of salivary exosomes on NK cells represents an important mechanism of escape from NK cell–mediated immunity and is at play in PDAC. We demonstrate that saliva from patients with PDAC decreases activation levels of peripheral NK cells, which renders them less cytotoxic against pancreatic tumor cells. We further show that salivary exosomes are the mediators of this mechanism. These findings describe an important immunomodulatory function of salivary exosomes and provide new insights into our understanding of the alterations of saliva during tumor development in favor of NK cell antitumor immunity escape.
MATERIALS AND METHODS
Animals
Six- to 8-wk-old C57BL/6 female mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Mice were housed at the Division of Laboratory Animal Medicine (University of California, Los Angeles).
Tumor cell lines and in vivo tumor model
Panc02, a C57BL/6 murine PDAC cell line induced by methylcholanthrene, was a generous gift from Dr. Guido Eibl (David Geffen School of Medicine, University of California, Los Angeles, USA). Cells were cultured in McCoy’s 5A medium with 10% fetal calf serum (USA Scientific, Ocala, FL, USA) and penicillin/streptomycin (Thermo Fisher Scientific, Waltham, MA, USA). All cells were maintained in an atmosphere of 5% CO2 at 37°C.
Plasmids pEGFP-C1 [green fluorescent protein (GFP)] and pEGFP-C1-Rab11-DN (dominant-negative; Addgene plasmid 12678; 4 μg; DN-Rab11-GFP) were transfected into Panc02 that was cultured in 6-well plates at ∼85% confluency using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s protocol, as previously described (19). Before injection, GFP or DN-Rab11-GFP Panc02 cells were resuspended in sterile cold PBS. Mice were anesthetized and pancreatic tumor was induced via orthotopic injection into the head of the pancreas with 0.5 × 106 cells in PBS in a total volume of 50 μl. Animals were sutured and monitored postoperatively. Mice were salivated and euthanized after primary tumors were confirmed at 14 d.
Reagents
The following reagents were used: McCoy’s 5A medium, RPMI 1640 medium (American Type Culture Collection; Manassas, VA, USA), fetal bovine serum (USA Scientific), penicillin/streptomycin, HyClone Dulbecco’s PBS (Thermo Fisher Scientific), SuperSignal West Dura Chemiluminescent substrate (Thermo Fisher Scientific), ECL anti-mouse and anti-rabbit IgG horseradish peroxidase–coupled secondary Abs (GE Healthcare, Marlborough, MA, USA), ketamine, xylazine, pilocarpine, and Lipofectamine XLT (Thermo Fisher Scientific).
Isolation of mouse saliva
Mice were administered an intramuscular injection of 1 μl/kg body weight of ketamine/xylazine solution (60 and 8 mg/ml ketamine and xylazine, respectively) for anesthesia. Pilocarpine subcutaneously (0.05 mg pilocarpine/100 g body weight) was administered between the ears to induce salivation. Mice were positioned above the 1.5 ml collection tubes, and a glass micropipette was inserted in the cheek, where saliva was allowed to pool for 15–20 min. Samples were stored in −80°C until analysis.
Salivary exosome purification and fractionation
Exosomes were purified from mouse saliva by differential centrifugation and ultracentrifugation: 1500 g for 10 min, 17,000 g for 15 min, and 120,000 g overnight. Ultracentrifugation was performed at 4°C by using a Beckman XE-90 Optima with SW-55-Ti rotor (Beckman Coulter, Brea, CA, USA). Finally, exosomes were resuspended in the same volume of PBS as the starting volume of mouse saliva. For the exosome-depleted fraction, after the final step of ultracentrifugation, we pooled supernatants from all previous centrifugation steps to include all nonexosome constituents in the exosome-depleted saliva samples. Exosomes were further analyzed by electron microscopy, NanoSight (NanoSight, Salisbury, United Kingdom), and Western blot to determine purity and size distribution.
Electron microscopy
Exosomes were adsorbed for 1 min to a carbon-coated grid that was rendered hydrophilic by 30-s exposure to glow discharge. Excess liquid from the sample was removed by using a filter paper, and samples were stained with 0.75% uranyl acetate for 30 s. After excess uranyl formate was removed with filter paper, grids were examined in a TF20 electron microscope (FEI, Hillsboro, OR, USA) that was running EM-Menu4 software, and images were recorded with a Tietz F415MP CCD camera (Tietz Video and Image Processing Systems, Gauting, Germany). All experiments were performed at the Electron Imaging Center for Nanomachines in the California NanoSystems Institute.
Nanotracking analysis
Approximately 100 µl mouse salivary exosome suspension that was diluted in a final volume of 1 ml was loaded into the sample chamber of a NanoSight NS300 (NanoSight) using a sterile syringe. The system calculated size by tracking the Brownian motion of individual nanoparticles detected by scattered laser light as a function of dispersing medium viscosity and temperature. Samples were measured 5 times and analyzed by using Nanoparticle Tracking Analysis Software (NanoSight).
SDS-PAGE and Western blot
Purified exosome lysates were prepared by resuspension of the ultracentrifugation pellets to PBS that contained PMSF, aprotinin, and leupeptin (Sigma-Aldrich, St. Louis, MO, USA). Exosome lysates were resuspended in Laemmli sample buffer (62.5 mM Tris-HCl, pH 6.8, 2% w/v SDS, 50 mM dithiothreitol, 0.01% w/v bromphenol blue) and analyzed by SDS-PAGE using 4–20% Mini-Protean TGX precast gels. Blocking of PVDF membrane was performed in 1% nonfat dry milk in PBS 0.1%–Tween-20 overnight at 4°C. Anti-CD9 Ab (Bio-Rad, Hercules, CA, USA) was used at 1:1000 as well as anti-Calnexin Ab from Abcam (Cambridge, United Kingdom) at 1:2000, diluted in 1% nonfat dry milk in PBS 0.1%–Tween-20. Primary Abs were incubated at room temperature for 2 h with gentle agitation. PBS 0.1%–Tween-20 was used to wash PVDF membrane 3 times at 5 min. Appropriate horseradish peroxidase–coupled secondary Abs (GE Healthcare) were incubated with membranes for 1 h at room temperature, followed by 3 washes. Membranes were then incubated with SuperSignal West Dura chemiluminescence substrate (Thermo Fisher Scientific) for 5 min and visualized on the Bio-Rad Chemidoc MP system.
Ex vivo whole-blood culture
Naive mice were euthanized in a CO2 chamber, followed by cervical dislocation, and whole blood was immediately collected using cardiac puncture technique and diluted at 1:10 in RPMI 1640 that was supplemented with 1% penicillin/streptomycin and 50 IU/ml heparin (APP Pharmaceuticals, Lake Zurich, IL, USA). Of diluted whole blood, 150 μl was added to each well of a 96-well culture plate. Then, 10 μl of whole mouse saliva from each animal group (PBS, GFP, DN-Rab11-GFP) was added to wells, and the plate was placed in an atmosphere of 5% CO2 at 37°C. After 48 h of culture, whole-blood samples were collected, and red blood cells were lysed with 1× red blood cell lysis buffer (eBioscience, San Diego, CA, USA). Samples were stained with appropriate Abs and analyzed by using flow cytometry within 2 h of processing.
PKH67 labeling of exosomes
Salivary exosomes were labeled with PKH67 green fluorescent membrane-like dye (Sigma-Aldrich) according to the manufacturer’s protocol. Labeled exosomes were washed in medium that was supplemented with exosome-free FBS (System Biosciences, Palo Alto, CA, USA) and collected by ultracentrifugation, as previously described. PKH67-labeled exosomes were added to purified mouse NK cells in 96-well plates in the presence of RPMI 1640 and 10% exosome-free FBS, complemented with suboptimal concentration of IL-2 (100 U/ml; Thermo Fisher Scientific), and incubated for 4 h at 5% CO2 and 37°C. NK cells were stained with anti-CD49b BV421, and internalization of exosomes was assessed by using Amnis ImageStreamx MarkII (Amnis, Seattle, WA, USA), which combined the sensitivity of flow cytometry with the detailed imagery and structural information of microscopy.
In vivo NK cytotoxicity
Healthy mice were primed via oral gavage with saliva from the 3 groups (PBS, GFP, DN-Rab11-GFP) and were left undisturbed for 48 h. After priming, all animals were intravenously injected with 1 × 106 Panc02 cells in 100 μl cold PBS through the tail vein. Four hours later, mice were euthanized in CO2 chamber, followed by cervical dislocation. For single-cell suspensions, spleens were harvested, mechanically dissociated and filtered through 70-μm cell strainers (BD Biosciences, San Jose, CA, USA), and washed with staining buffer (PBS that contained 1% bovine serum albumin, 2 mmol EDTA). Red blood cells were lysed with 1× red blood cell lysis buffer. All samples were analyzed by using flow cytometry within 2 h of processing.
NK cell isolation from splenocytes
NK cells were negatively isolated from healthy mouse splenocytes by using the EasySep NK cell isolation kit (StemCell Technologies, Vancouver, BC, Canada) according to the manufacturer’s instructions. Purity and viability of isolated cells were established and were always >90%.
In vitro NK cytotoxicity
NK cells were incubated in RPMI 1640 and 10% exosome-free FBS and complemented with suboptimal concentrations of IL-2 (100 U/ml; Thermo Fisher Scientific) overnight before cytotoxicity assay. NK cells were then tested for cytotoxic activity against the parental Panc02 cell line (direct cytotoxicity) after having been cultured for 48 h in the presence of 10 μl whole or fractionated saliva from the 3 animal groups (PBS, GFP, DN-Rab11-GFP) in 100 μl of total volume per well in a round-bottom 96-well plate. For the exosomal fraction of saliva, we used exosomes that were reconstituted in PBS and the volume used corresponded to the same amount of saliva. These cytotoxic tests were all performed in 4-h assays. Measured parameters were degranulation (CD107a) by CD49+ cells and the percentage of dead target cells vs. live cells in CD49− population stained with 7-aminoactinomycin (7AAD; Cayman Chemicals, Ann Arbor, MI, USA). The respective effector/target ratios are indicated in the figure legends.
Flow cytometry and fluorochrome-conjugated Abs
All experiments were performed using the LSRFortessa X-20 SORP cytometer running FACSDiva Software (BD Biosciences). Anti-CD49b clone DX5 conjugated with BV421 was purchased from BD Biosciences. Anti-CD69 conjugated with FITC, anti-CD314 (NKG2D) conjugated with PE, and anti-CD107a (LAMP-1) conjugated with allophycocyanin were from BioLegend (San Diego, CA, USA). Anti-granzyme B conjugated with PE/Cy7 and anti-perforin conjugated with FITC were from eBioscience. Isotype-matched negative-control Abs labeled with BV421, FITC, PE, allophycocyanin, or PE/Cy7 were all from BioLegend. For adjustment of the multicolor flow cytometry settings and to eliminate false-positive signal, we used positive and negative control compensation beads (CompBeads; BD Biosciences). For the study of intracellular proteins, cells were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) for 15 min at room temperature, followed by permeabilization with 0.1% Saponin/PBS/1% bovine serum albumin for 20 min on ice. For all samples, a minimum prespecified total cell number of 200,000 cells/event (splenocytes) were acquired. Data were compensated and analyzed with FlowJo software (v 10.0.8; Tree Star, Ashland, OR, USA).
Study approval
The Office of Animal Research Committee (known locally as the Chancellor’s Animal Research Committee), in conjunction with the Institutional Animal Care and Use Committee, University of California, Los Angeles, approved all experimental protocols used in this study. All mice were treated, monitored, and euthanized in accordance with the approved animal protocol (2013-004-03I).
Statistical analysis
P values presented were based on 2-tailed Student’s t tests (α = 0.05). Values of P < 0.05 were considered significant. All quantitative data are represented as means ± sem. All graphs were made, and statistical analyses were performed, by R-2.7.0 Prism (GraphPad Software, La Jolla, CA, USA).
RESULTS
Saliva from tumor-bearing mice decreases NK cell activation level and triggers down-regulation of surface NKG2D in vivo
We established a mouse PDAC model to study the effect of saliva on immune surveillance. To test whether saliva from tumor-bearing mice has immunomodulatory effects, Panc02 mouse model was used for hypothesis testing by implanting the pancreatic cancer cell line Panc02 into the head of the pancreas of the syngeneic host C57BL/6 mice and allowing tumor to develop over 14 d. This time point resulted in a well-developed primary tumor without virtually metastasized organs. We have described that orthotopic injections of Panc02 cells that stably expressed GFP or DN-Rab11-GFP into mouse pancreases successfully induced pancreatic cancer (19). DN-Rab11-GFP, which suppresses exosome biogenesis, is an engineered Panc02 cell line that is stably transfected with the DN exosome membrane protein Rab11, which allowed us to monitor the effects of exosomes. As a control, mice received PBS (vehicle control group) into the pancreas in lieu of cancer cells. After 14 d of tumor development, saliva from these animals was collected and stored for oral gavaging experiments.
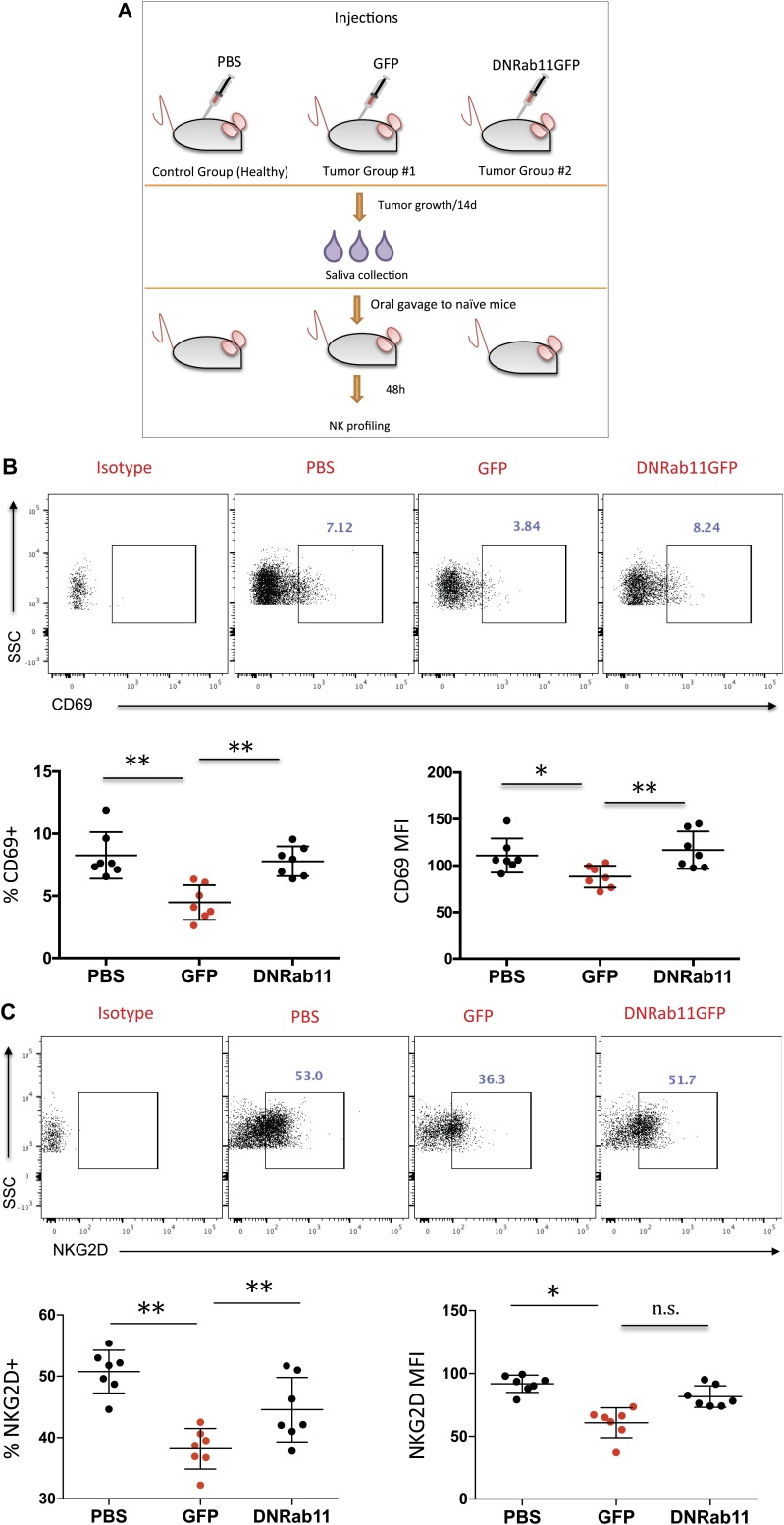
To investigate a potential role of saliva from tumor-bearing animals in NK cell biology, we introduced saliva from PBS, GFP, and DN-Rab11-GFP groups into naïve syngeneic mice via oral gavage (Fig. 1A). After 48 h, we collected splenocytes and examined the expression of CD69 and NKG2D on NK cells. We found that GFP (tumor) saliva resulted in a 45.7% reduction (P = 0.0011) of CD69 and 24.8% reduction (P = 0.0001) of NKG2D surface expression on NK cells compared with PBS saliva (Fig. 1B, C). When saliva from animals with exosome suppression at the distal tumor (DN-Rab11-GFP) was introduced, expression levels of CD69 and NKG2D did not differ significantly from PBS saliva group (Fig. 1B, C). These data demonstrated that saliva from tumor-bearing mice decreased the activation levels of peripheral NK cells, thereby inhibiting immune surveillance against cancer cells. Furthermore, given the lack of a similar effect when we introduced DN-Rab11-GFP saliva, our data suggest that there is direct crosstalk between tumor cell exosomes and the downstream effect of saliva.
Figure 1.
Tumor saliva decreases NK cell activation level and down-regulates surface NKG2D in vivo. A) Schematic of the experiment performed to analyze the effect of tumor saliva on mouse NK cells in vivo. Mouse saliva (100 μl) from the 3 groups was orally gavaged into healthy mice, and 48 h later, spleens were collected and analyzed for CD69+ and NKG2D+ expression by NK cells. B, C) Flow cytometry for CD69+ (B) and NKG2D+ (C) gated in the CD49b+ population (S1) after exposure to PBS saliva, cancer saliva (GFP), or reduced-exosome saliva (DN-Rab-11GFP). Data show the average relative frequency of all CD69+ and NKG2D+ cells in the lymphocyte population (left) and the absolute median fluorescence intensity (MFI) of the same cells (right). Each group represents n = 7 mice and each dot represents 1 individual mouse. n.s., not significant; SSC, side scatter. Data are means ± sem. *P < 0.05, **P < 0.01, 2-tailed Student’s t test.
Saliva from tumor-bearing mice decreases NK cell activation level and triggers down-regulation of surface NKG2D ex vivo
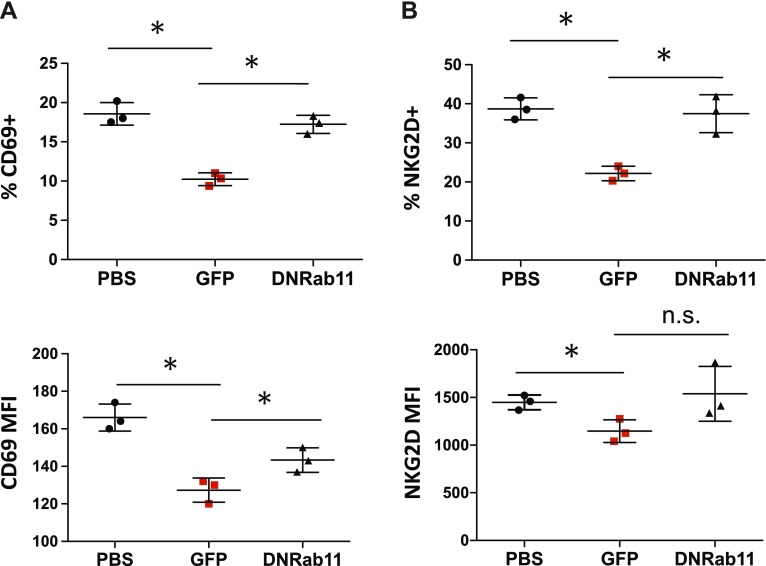
To substantiate our in vivo results and as additional proof that NK cell alterations are saliva-induced, we studied the effect of tumor saliva on NK cell phenotype ex vivo by using peripheral blood from naïve animals. Whole-blood cultures were treated with saliva from the 3 different groups in the presence of IL-2 and were analyzed for expression of CD69 and NKG2D. GFP (tumor), but not DN-Rab11-GFP, saliva resulted in a 43.8% reduction (P = 0.0009) of cell surface CD69 expression in the CD49b population (Fig. 2A, B) compared with PBS group by 48 h. Moreover, and in concordance with our in vivo results, NK cells that interacted with GFP saliva down-regulated their surface NKG2D by 42.7% (P = 0.001) after 48 h in coculture, whereas NK cells that were cultured with DN-Rab11-GFP saliva in whole-blood culture did not differ significantly from the PBS saliva-treated group (Fig. 2A–C). Taken together, these data strongly support that saliva from tumor-bearing mice exerts immunosuppressive effects on NK cells as indicated by the significant down-regulation of the surface proteins CD69 and NKG2D. In addition, and given the effects of DN-Rab11-GFP saliva, this phenotype is mainly controlled by tumor cell exosome biogenesis.
Figure 2.
Tumor saliva reduces surface NKG2D and CD69 expression of NK cells in vitro. CD69 (A) and NKG2D (B) expression in CD49b+ cells after 48-h exposure to control saliva (PBS), cancer saliva (GFP) and reduced-exosome cancer saliva (DN-Rab-11GFP). Upper graphs indicate the average relative frequency of all CD69+ and NKG2D+ cells in the lymphocyte population and lower graphs show the mean fluorescentce intensity (MFI) of the same cells. Representative flow cytometry plots of CD69+ and NKG2D+ cells are shown in Supplemental Fig. S2. Data are from 3 independent experiments (means ± sem). *P < 0.05, 2-tailed Student’s t test.
Saliva from tumor-bearing mice decreases the cytotoxic potential of NK cells in vivo
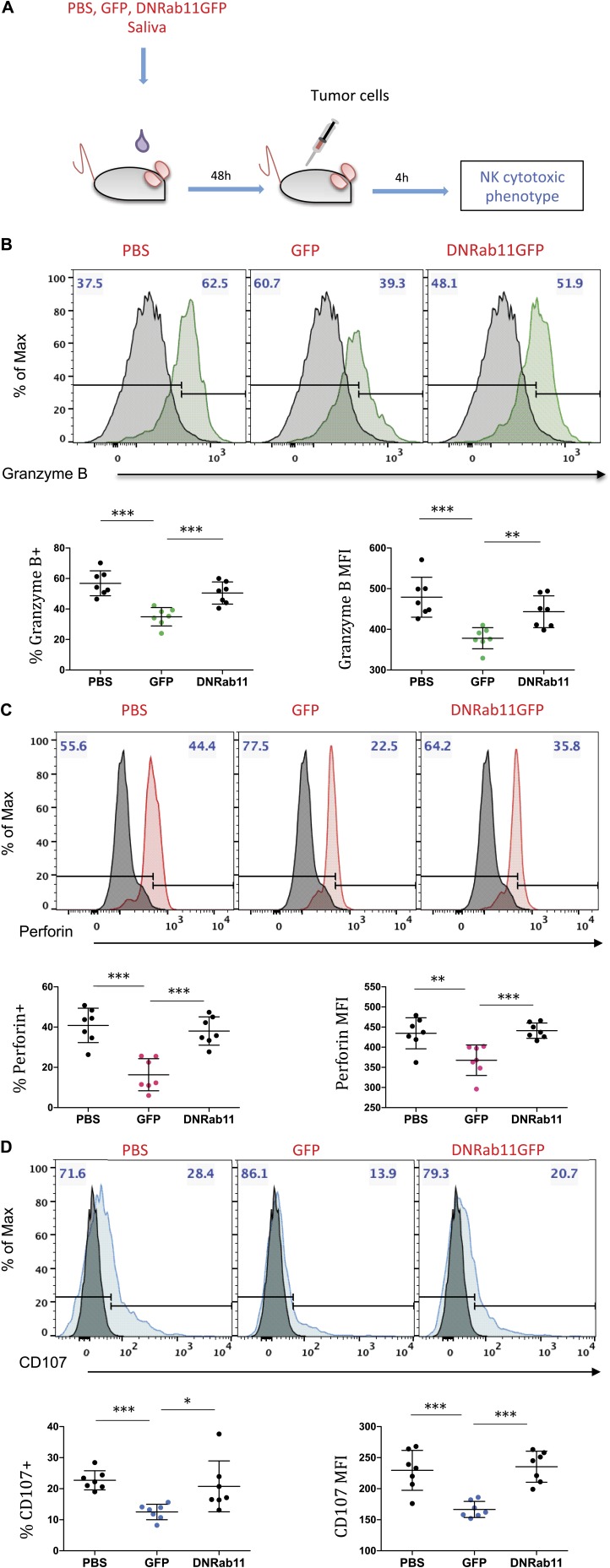
We next determined whether these surface expression modifications were associated with altered NK cell functions. CD69 and NKG2D are functional triggering molecules on NK cells and are capable of mediating cytotoxicity (20, 21). We asked the following important translational question: do changes in expression levels of CD69 and NKG2D in NK cells—the prime effectors of the innate immune system—have a functional effect on their tumor cell killing activity? We hypothesized that saliva from tumor-bearing animals might decrease NK cytotoxic activity. To test this, 48 h after saliva oral gavage, we intravenously injected parental tumor cells (Panc02) to trigger immune surveillance and induce NK-mediated cell killing. Four hours later, we collected splenocytes and analyzed them by using flow cytometry (Fig. 3A) to assay for expression of CD107 (degranulation index), granzyme B, and perforin, which correlates with NK killing activity. We observed that NK cells from animals that were orally gavaged with GFP saliva had decreased expression of CD107 (44.9% P = 0.0001), granzyme B (38.5%, P = 0.0001), and perforin (59.9% P = 0.0001) when compared with PBS saliva group 4 h after intravenous injection of tumor cells (Fig. 3B–D). In line with our findings for CD69 and NKG2D, saliva from the DN-Rab11-GFP animals had effects similar to the PBS group, which suggested that suppression of the exosome biogenesis at the distal tumor alters the immunomodulatory effects of tumor saliva on peripheral NK cells. Taken together, these results support that saliva from tumor-bearing mice decreases the cytotoxic potential of NK cells against the target pancreatic tumor cells, and this effect is ablated when exosome production at the tumor site is suppressed.
Figure 3.
Saliva from tumor-bearing mice decreases the cytotoxic potential of NK cells in vivo. A) Schematic of the experimental design to evaluate NK cell cytotoxicity against Panc02 tumor cells. B–D) Flow cytometry for granzyme B (B), perforin (C), and CD107 (D) in CD49b+ population (S1) of mouse splenocytes 4 h after tumor cell injection. Mice were orally gavaged with the indicated saliva group 48 h before injections. Gray histograms represent isotype-matched negative controls. Data show the proportion of positive cells and median fluorescence intensity (MFI) as indicated. Histograms are representative of 7 animals. Each group represents n = 7 mice and each dot represents 1 individual animal. Data are means ± sem. *P < 0.05; **P < 0.01; ***P < 0.001, 2-tailed Student’s t test.
Exosomes are abundantly present in mouse saliva
We next sought to investigate the salivary component that mediates these alterations of NK phenotype, which results in a compromised tumor killing activity. We have previously demonstrated that suppression of exosome secretion by pancreatic cancer cells altered the transcriptomic cargo of saliva (19). Because the majority of RNA in saliva is concentrated in exosomes (22, 23), we hypothesized that salivary exosomes might be mediators of the described NK alterations. Salivary exosomes represent a mechanism of tumor escape only if their effect on peripheral NK cells modulates NK killing capacity.
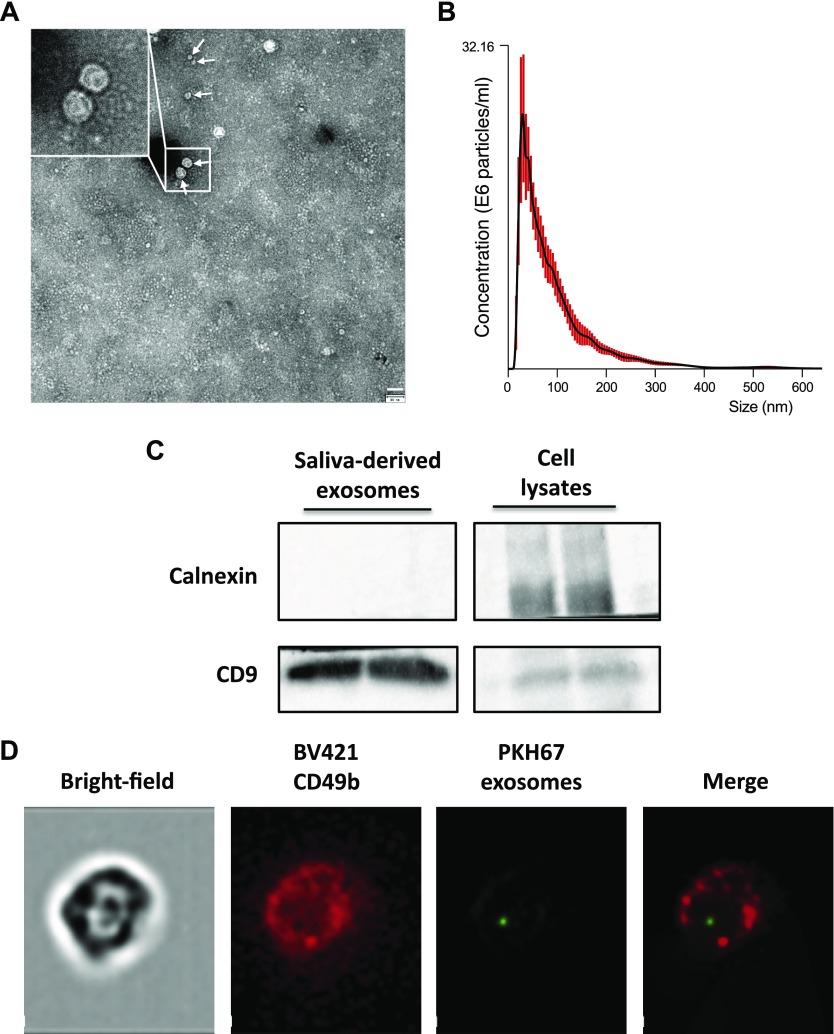
To explore this mechanism, we first performed a characterization of mouse salivary microvesicles content. Microvesicles were isolated from mouse saliva by using ultracentrifugation as described in Materials and Methods and were studied by transmission electron microscopy, which demonstrated rounded particles of 30–150 nm (Fig. 4A). Exosomal expression of the tetraspanin CD9 surface antigen, a well-described exosomal marker (24), was subsequently evaluated and confirmed by using Western blot (Fig. 4C). In addition, and as a control, we tested the expression of calnexin, an endoplasmic reticulum membrane protein, to exclude any cellular membrane contamination of our samples. Size distribution of the salivary microvesicles was further analyzed by nanoparticle tracking analysis. Microvesicle distribution was physically homogeneous, ranging from 30 to 150 nm with a peak at 50 nm (Fig. 4B). This size range corresponded to the exosome subclass of microvesicles, and, therefore, our data demonstrate that exosomes are abundantly present in mouse saliva.
Figure 4.
Characterization of saliva that contained exosomes and the ability to be transferred to NK cells. A) Mouse saliva contains exosomes. Exosomes were isolated by ultracentrifugation and analyzed with transmission electron microscopy. Arrows indicate the typical exosomes. Scale bar, 80 nm. B) NanoSight particle analysis displaying the size distribution of mouse saliva-derived exosomes. C) Western blot of salivary exosome proteins using anti-CD9 (positive marker) and anti-calnexin (negative marker) Abs. Panco2 cell lysates served as positive controls. D) PKH67-prelabeled salivary exosomes were coincubated for 3 h with purified mouse NK cells stained with BV421-CD49b Ab. Magnification: ×60.
Salivary exosomes are transferred to NK cells
To investigate the potential role of salivary exosomes, we next examined whether they could be transferred to NK cells. To gain insight into exosome uptake, PKH67 fluorescently labeled exosomes that were isolated from mouse saliva were cocultured with primary mouse NK cells. We detected salivary PKH67-labeled exosomes in the cytoplasm and on the cell membrane (Fig. 4D and Supplemental Fig. S3) of mouse CD49b cells. These results indicate that salivary exosomes are transferred into mouse primary NK cells, which is suggestive of a potential role in regulating NK biology.
Salivary exosomes from tumor-bearing mice decrease NK cell cytotoxicity against tumor cells
Our findings on exosome incorporation by NK cells support the hypothesis that salivary exosomes from tumor-bearing animals may actively mediate NK cell deactivation; therefore, we next examined the ability of healthy and tumor salivary exosomes to modulate NK cell biology.
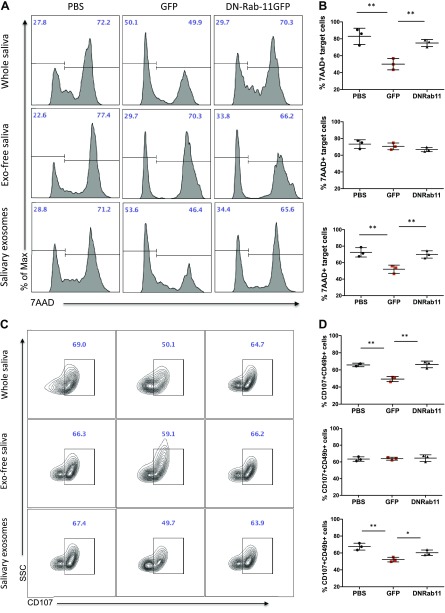
First, to determine whether tumor saliva induction of decreased NK cytotoxic potential was restricted to the above in vivo experimental setup, we used whole, unfractionated mouse saliva to establish an ex vivo cytotoxicity system. We tested the effect of tumor saliva on the antitumor activity of purified mouse primary NK cells. To investigate this effect, we treated NK cells with saliva from the 3 groups for 48 h, which corresponded to the time in which CD69 and NKG2D were significantly down-regulated. After this treatment, primed NK cells were cocultured with target cells at an effector:target cell ratio of 25:1 for 4 h in a round-bottom 96-well plate. Similar to our in vivo system, coculture of NK cells with GFP saliva resulted in a 39.6% reduction (P = 0.0082) of killed Panc02 target cells compared with PBS and DN-Rab11-GFP saliva (Fig. 5A, B). As an additional marker of specific NK killing, we assessed the degranulation levels of NK cells by measuring the expression of CD107a on their surface. Treatment of NK cells with GFP saliva resulted in a 25.1% decrease of cells that were positive for degranulation marker CD107a, which indicated less NK killing activity (P = 0.0064; Fig. 5C, D).
Figure 5.
Salivary exosomes from tumor-bearing mice decrease NK cell cytotoxicity. Purified mouse NK cells were treated with saliva fractions from the 3 groups as indicated for 48 h and were subsequently cocultured with Panc02 tumor cells in a 25:1 effector:target ratio. A) Flow cytometry for 7AAD+ target cells (killed Panc02 cells, gated in CD49b− population). B) Data show the average relative frequency of 7AAD+CD49b− cells in the indicated groups/treatments. C) Flow cytometry for CD107+ effector cells (degranulation status of NK cells, gated in CD49b+ population). D) Data show the average relative frequency of CD107+CD49b+ cells in the indicated groups/treatments. SSC, side scatter. Data are from 3 independent experiments (means ± sem). *P < 0.05, **P < 0.01, 2-tailed Student’s t test.
Then, to test involvement of salivary exosomes in this phenotype, we performed exosome depletion of mouse saliva by using ultracentrifugation. By using the different saliva fractions, we treated primary mouse NK cells for 48 h and we subsequently evaluated their cytotoxic potential. As shown in Fig. 5, exosome-depleted saliva fractions from the 3 animal groups had similar effects on NK antitumor killing, as indicated by the number of dead Panc02 cells and the degranulation marker CD107. Of note, the percentages of target tumor cells that were killed by NK cells treated with exosome-depleted saliva did not differ from those of PBS and DN-Rab11-GFP whole saliva–treated NK cells. These data suggested that exosomes are critical to the suppression of cytotoxic activity of NK cells by GFP saliva.
To further confirm this finding, we exposed normal NK cells to purified salivary exosomes from the 3 animal groups. According to our characterization studies, the ultracentrifugation pellet corresponds to the exosomal fraction of mouse saliva. Exosomes from GFP animals resulted in a significantly decreased antitumor activity of NK cells, which was indicated by 28.2% fewer 7AAD+ target cells (P = 0.0092) and 22.7% fewer CD107+ effector cells (P = 0.0056) compared with PBS salivary exosomes (Fig. 5). When we exposed normal NK cells to exosomes from DN-Rab11-GFP saliva, the killing activity did not differ from that of the PBS group. This observation indicates that, first, salivary exosomes from tumor-bearing animals down-regulate the cytotoxic potential of normal NK cells against tumor cells and this effect was confirmed by evaluating the number of dead cells (7AAD+ cells in the CD49b− population) as well as the level of NK degranulation (CD49+CD107+). Second, suppression of exosome biogenesis in the distal tumor (DN-Rab11-GFP) is reflected in the salivary exosomes and results in an increased saliva-induced antitumor activity of NK cells compared with the GFP tumor group.
In summary, our data suggest that saliva from tumor-bearing mice has immunomodulatory properties, and exosome suppression at the distal pancreatic tumor is reflected by reversal of immune suppression of NK cells.
DISCUSSION
Nonserum bodily fluids, such as saliva, urine, and CSF, have long been known to contain exosomes, and their cargo can be used to develop disease biomarkers (10, 23). Although many investigations have explored the differential expression of several biomolecules in these exosomes, to date nothing is known about their downstream role in the biology of the body. This work contributes to a growing understanding of the role and importance of salivary exosomes, not only as biomarker containers, but also as key players in the suppression of NK cell–mediated immune surveillance. Here, we describe a previously unidentified set of interactions between tumor cell–derived exosomes, salivary exosomes, and NK cells during pancreatic cancer development, a component of the body’s immune surveillance. By using in vivo and ex vivo techniques, we show that salivary exosomes from pancreatic tumor–bearing mice interact with peripheral NK cells to reduce their activation levels and eventually to suppress their antitumor cytotoxic capacity. This immune compromise is ablated when tumor exosome biogenesis is suppressed at the distal site of the primary tumor, which is suggestive of a systemic connection with salivary glands and, therefore, with salivary exosomes.
Our lab has previously defined a mechanism for saliva biomarker appearance during systemic tumorigenesis (19). Lau et al. demonstrated that suppression of exosomes at the distal tumor site from pancreatic cancer cells ablated the development of the respective salivary biomarker profile, which suggested that exosomes are responsible for shuttling tumor biomarkers to saliva. Moreover, we and others have previously demonstrated that systemic diseases, including tumors, can be detected by discriminatory salivary biomarkers (13, 23, 25–28). Despite successfully discovering, verifying, and validating discriminatory salivary biomarkers for cancers, including pancreatic, breast, and lung, why a tumor that develops distally from the oral cavity could alter the salivary exosomes is not yet known. This study demonstrates that salivary exosomes have a definitive effect on immune surveillance during tumor development via direct interaction with NK cells.
To investigate the effects of pancreatic tumor–derived exosomes on salivary gland–derived exosomes, we used a previously established mouse model that allows us to suppress exosome biogenesis at the primary tumor. We have demonstrated that orthotopic injections of Panc02 cells that stably expressed GFP or DN-Rab11-GFP into mouse pancreases successfully induced pancreatic cancer (19). Stable overexpression of the DN form of Rab11 significantly inhibits the secretion of exosomes by Panc02 cells. For control, we injected sterile PBS into mouse pancreases to ensure that the differences in salivary exosome biology described are the result of the development of pancreatic cancer, rather than the inflammatory response from the surgery procedure during orthotopic injections. By using the DN-Rab11-GFP Panc02 cell line, which is defective in its ability to fully secrete exosomes, we were able to induce mouse pancreatic tumors with hindered exosome secretion.
By oral gavage of saliva from tumor-bearing mice to naïve mice, we, for the first time to our knowledge, demonstrated that tumor saliva has a definitive immunoregulatory effect on immune surveillance. We found that after GFP tumor saliva administration, peripheral NK cells decreased their activation levels as indicated by down-regulation of their surface receptors NKG2D and CD69. Furthermore, we demonstrated that animals that were exposed to tumor saliva had reduced NK cytotoxic activity after intravenous challenge with tumor cells. Of interest, suppression of exosome biogenesis from the primary tumor ablated the low activation phenotype, as indicated by lower CD69 and NKG2D expression, and reversed the cytotoxic potential of peripheral NK cells. Our present work suggests that tumor-derived exosomes are shuttles that travel through the vasculature of the body and trigger salivary gland cells to modulate their exosome release. This secondary exosome biogenesis seems to be of significance for the suppression of immune surveillance by saliva.
In an attempt to confirm that salivary exosomes are mediators of the described effects, we isolated and characterized the microvesicle content of mouse saliva. By using electron microscopy, nanoparticle tracking analysis, and Western blot, we showed that the vast majority of mouse salivary particles are exosomes. Moreover, we examined whether salivary exosomes can be taken up by NK cells. By using fluorescence labeling, we demonstrated that salivary exosomes are transferred to purified mouse NK cells. Finally, by using different fractions of mouse saliva from the 3 groups, we confirmed that salivary exosomes are mediators of NK phenotype alterations. Hence, through these findings we provide a mechanism to support our working model as to why tumor-derived exosomes relay information to the salivary exosomes, altering their downstream effects on peripheral NK cells.
In the present study, we used an animal model, which is unique in its ability to test the connection between exosome biogenesis at the distal tumor and salivary exosome biology. However, one of the limitations of this mouse model is that during the early phase of pancreatic tumor development in the absence of metastasis, we did not observe any significant differences in tumor weight or volume between GFP and DN-Rab11-GFP groups. We reason that this observation is likely a result of the noncomplete knockdown of tumor exosome biogenesis, which, in our model, is approximately 40% (19). Furthermore, the direct effect of tumor exosomes on the immune system via the vasculature should not be neglected. Our data clearly suggest that even a partial exosome suppression at the distal tumor results in a differential effect of salivary exosomes on NK cytotoxic activity.
Although the membrane vesicles of tumor cells have been suggested to contribute to horizontal propagation of several signals that are involved in immune suppression and metastasis (6, 7, 29), our study is the first, to our knowledge, to show that tumor exosomes alter the biology of salivary exosomes, which, in turn, suppress immune surveillance and renders NK cells less cytotoxic against cancer cells. Of note, we show that suppression of exosome biogenesis at the pancreatic tumor ablates the effect of tumor salivary exosomes on NK cells.
Here, we propose a new mechanism that enhances the ability of tumors to escape immune surveillance via crosstalk between tumor exosomes and salivary exosomes. Collectively, our data identify salivary exosomes as functional components during tumor development, not only carrying tumor biomarkers, but also having a definitive downstream effect on the cytotoxic potential of NK cells.
ACKNOWLEDGMENTS
The authors thank Dr. David Elashoff (University of California, Los Angeles) for advice on the animal numbers and statistical analysis during the study design. Flow cytometry experiments were performed on equipment in the Jonsson Comprehensive Cancer Center Flow Cytometry Core. Panc02, the mouse pancreatic cancer cell line, was a generous gift from Dr. Guido Eibl (David Geffen School of Medicine, University of California, Los Angeles). Nanoparticle tracking experiments were performed by using the NanoSight equipment in the Eduardo Marbán lab (Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA). This work was funded, in part, by the Hirschberg Foundation for Pancreatic Cancer Research, and by U.S. National Institutes of Health, Exploratory/Developmental Cooperative Agreement Phase I Grant UH2-TR000923. D.T.W.W. is cofounder of RNAmeTRIX, a molecular diagnostic company. He holds equity in RNAmeTRIX and serves as a company Director and Scientific Advisor. The University of California also holds equity in RNAmeTRIX. Intellectual property invented by D.T.W.W. and which was patented by the University of California has been licensed to RNAmeTRIX.
Glossary
- 7AAD
7-amino-actinomycin D
- DN
dominant-negative
- GFP
green fluorescent protein
- NKG2D
NK group 2D
- PDAC
pancreatic ductal adenocarcinoma
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. Katsiougiannis designed and performed the experiments, wrote the manuscript with contributions from all authors, and was responsible for statistical analysis after consulting with Dr. David Elashoff; D. Chia and Y. Kim provided scientific insights in exosome immunobiology and played a major role in the interpretation of the results and the editing of the article; R. P. Singh provided scientific insight and technical assistance in analyzing the flow cytometry data; and D. T. W. Wong directed all the work and edited the manuscript; all authors reviewed the manuscript and provided scientific input.
REFERENCES
- 1.Kowal J., Arras G., Colombo M., Jouve M., Morath J. P., Primdal-Bengtson B., Dingli F., Loew D., Tkach M., Théry C. (2016) Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 113, E968–E977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whiteside T. L. (2016) Exosomes and tumor-mediated immune suppression. J. Clin. Invest. 126, 1216–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buzas E. I., György B., Nagy G., Falus A., Gay S. (2014) Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 10, 356–364 [DOI] [PubMed] [Google Scholar]
- 4.György B., Szabó T. G., Pásztói M., Pál Z., Misják P., Aradi B., László V., Pállinger E., Pap E., Kittel A., Nagy G., Falus A., Buzás E. I. (2011) Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell. Mol. Life Sci. 68, 2667–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanada M., Bachmann M. H., Hardy J. W., Frimannson D. O., Bronsart L., Wang A., Sylvester M. D., Schmidt T. L., Kaspar R. L., Butte M. J., Matin A. C., Contag C. H. (2015) Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc. Natl. Acad. Sci. USA 112, E1433–E1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoshino A., Costa-Silva B., Shen T. L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., Di Giannatale A., Ceder S., Singh S., Williams C., Soplop N., Uryu K., Pharmer L., King T., Bojmar L., Davies A. E., Ararso Y., Zhang T., Zhang H., Hernandez J., Weiss J. M., Dumont-Cole V. D., Kramer K., Wexler L. H., Narendran A., Schwartz G. K., Healey J. H., Sandstrom P., Labori K. J., Kure E. H., Grandgenett P. M., Hollingsworth M. A., de Sousa M., Kaur S., Jain M., Mallya K., Batra S. K., Jarnagin W. R., Brady M. S., Fodstad O., Muller V., Pantel K., Minn A. J., Bissell M. J., Garcia B. A., Kang Y., Rajasekhar V. K., Ghajar C. M., Matei I., Peinado H., Bromberg J., Lyden D. (2015) Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peinado H., Alečković M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G., Hergueta-Redondo M., Williams C., García-Santos G., Ghajar C., Nitadori-Hoshino A., Hoffman C., Badal K., Garcia B. A., Callahan M. K., Yuan J., Martins V. R., Skog J., Kaplan R. N., Brady M. S., Wolchok J. D., Chapman P. B., Kang Y., Bromberg J., Lyden D. (2012) Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18, 883–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raposo G., Stoorvogel W. (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melo S. A., Luecke L. B., Kahlert C., Fernandez A. F., Gammon S. T., Kaye J., LeBleu V. S., Mittendorf E. A., Weitz J., Rahbari N., Reissfelder C., Pilarsky C., Fraga M. F., Piwnica-Worms D., Kalluri R. (2015) Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523, 177–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An T., Qin S., Xu Y., Tang Y., Huang Y., Situ B., Inal J. M., Zheng L. (2015) Exosomes serve as tumour markers for personalized diagnostics owing to their important role in cancer metastasis. J. Extracell. Vesicles 4, 27522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morvan M. G., Lanier L. L. (2016) NK cells and cancer: you can teach innate cells new tricks. Nat. Rev. Cancer 16, 7–19 [DOI] [PubMed] [Google Scholar]
- 12.Clark C. E., Hingorani S. R., Mick R., Combs C., Tuveson D. A., Vonderheide R. H. (2007) Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 67, 9518–9527 [DOI] [PubMed] [Google Scholar]
- 13.Zhang L., Xiao H., Zhou H., Santiago S., Lee J. M., Garon E. B., Yang J., Brinkmann O., Yan X., Akin D., Chia D., Elashoff D., Park N. H., Wong D. T. (2012) Development of transcriptomic biomarker signature in human saliva to detect lung cancer. Cell. Mol. Life Sci. 69, 3341–3350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Artis D., Spits H. (2015) The biology of innate lymphoid cells. Nature 517, 293–301 [DOI] [PubMed] [Google Scholar]
- 15.Pross H. F., Lotzová E. (1993) Role of natural killer cells in cancer. Nat. Immun. 12, 279–292 [PubMed] [Google Scholar]
- 16.Vivier E., Raulet D. H., Moretta A., Caligiuri M. A., Zitvogel L., Lanier L. L., Yokoyama W. M., Ugolini S. (2011) Innate or adaptive immunity? The example of natural killer cells. Science 331, 44–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerra N., Tan Y. X., Joncker N. T., Choy A., Gallardo F., Xiong N., Knoblaugh S., Cado D., Greenberg N. M., Raulet D. H. (2008) NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 28, 571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orange J. S. (2013) Natural killer cell deficiency. J. Allergy Clin. Immunol. 132, 515–525, quiz 526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau C., Kim Y., Chia D., Spielmann N., Eibl G., Elashoff D., Wei F., Lin Y. L., Moro A., Grogan T., Chiang S., Feinstein E., Schafer C., Farrell J., Wong D. T. (2013) Role of pancreatic cancer-derived exosomes in salivary biomarker development. J. Biol. Chem. 288, 26888–26897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gras Navarro A., Björklund A. T., Chekenya M. (2015) Therapeutic potential and challenges of natural killer cells in treatment of solid tumors. Front. Immunol. 6, 202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borrego F., Robertson M. J., Ritz J., Peña J., Solana R. (1999) CD69 is a stimulatory receptor for natural killer cell and its cytotoxic effect is blocked by CD94 inhibitory receptor. Immunology 97, 159–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallo A., Tandon M., Alevizos I., Illei G. G. (2012) The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One 7, e30679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J., Wei F., Schafer C., Wong D. T. (2014) Detection of tumor cell-specific mRNA and protein in exosome-like microvesicles from blood and saliva. PLoS One 9, e110641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jakobsen K. R., Paulsen B. S., Bæk R., Varming K., Sorensen B. S., Jørgensen M. M. (2015) Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma. J. Extracell. Vesicles 4, 26659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong D. T. (2015) Salivary extracellular noncoding RNA: emerging biomarkers for molecular diagnostics. Clin. Ther. 37, 540–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elashoff D., Zhou H., Reiss J., Wang J., Xiao H., Henson B., Hu S., Arellano M., Sinha U., Le A., Messadi D., Wang M., Nabili V., Lingen M., Morris D., Randolph T., Feng Z., Akin D., Kastratovic D. A., Chia D., Abemayor E., Wong D. T. (2012) Prevalidation of salivary biomarkers for oral cancer detection. Cancer Epidemiol. Biomarkers Prev. 21, 664–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humeau M., Vignolle-Vidoni A., Sicard F., Martins F., Bournet B., Buscail L., Torrisani J., Cordelier P. (2015) Salivary microRNA in pancreatic cancer patients. PLoS One 10, e0130996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L., Xiao H., Karlan S., Zhou H., Gross J., Elashoff D., Akin D., Yan X., Chia D., Karlan B., Wong D. T. (2010) Discovery and preclinical validation of salivary transcriptomic and proteomic biomarkers for the non-invasive detection of breast cancer. PLoS One 5, e15573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa-Silva B., Aiello N. M., Ocean A. J., Singh S., Zhang H., Thakur B. K., Becker A., Hoshino A., Mark M. T., Molina H., Xiang J., Zhang T., Theilen T. M., García-Santos G., Williams C., Ararso Y., Huang Y., Rodrigues G., Shen T. L., Labori K. J., Lothe I. M., Kure E. H., Hernandez J., Doussot A., Ebbesen S. H., Grandgenett P. M., Hollingsworth M. A., Jain M., Mallya K., Batra S. K., Jarnagin W. R., Schwartz R. E., Matei I., Peinado H., Stanger B. Z., Bromberg J., Lyden D. (2015) Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826 [DOI] [PMC free article] [PubMed] [Google Scholar]