Abstract
Purpose
Epithelial-mesenchymal transition (EMT) of lens epithelial cells (LECs) is a key pathologic mechanism underlying cataract. Two members of the transforming growth factor-β (TGFβ) superfamily, TGFβ and bone morphogenetic protein-7 (BMP-7) have functionally distinct roles in EMT. While TGFβ is a potent inducer of EMT, BMP-7 counteracts the fibrogenic activity of TGFβ. We examine the modulating effect of BMP-7 on TGFβ-induced EMT in LECs.
Methods
Rat lens epithelial explants were treated exogenously with TGFβ2 alone or in combination with BMP-7 for up to 5 days. Expression levels of E-cadherin, β-catenin, α-smooth muscle actin (α-SMA), and phosphorylated downstream Smads were determined using immunofluorescence and Western blotting. Reverse transcriptase quantitative PCR (RT-qPCR) was used to study gene expression levels of EMT markers and downstream BMP target genes, including the Inhibitors of differentiation (Id).
Results
Transforming growth factor-β2 induced LECs to transdifferentiate into myofibroblastic cells. Addition of BMP-7 suppressed TGFβ2-induced α-SMA protein levels and mesenchymal gene expression, with retention of E-cadherin and β-catenin expression to the cell membrane. Addition of BMP-7 prevented lens capsular wrinkling and cellular loss associated with TGFβ2-induced EMT over the 5-day treatment period. The inhibitory effect of BMP-7 was accompanied by an early induction of pSmad1/5 and suppression of TGFβ2-induced pSmad2/3. Treatment with TGFβ2 alone suppressed gene expression of Id2/3 and addition of BMP-7 restored Id2/3 expression.
Conclusions
Exogenous administration of BMP-7 abrogated TGFβ2-induced EMT in rat lens epithelial explants. Understanding the complex interplay between the TGFβ- and BMP-7–associated Smad signaling pathways and their downstream target genes holds therapeutic promise in cataract prevention.
Keywords: bone morphogenetic protein (BMP), transforming growth factor-beta (TGFβ), epithelial-mesenchymal transition (EMT), cataract
Cataract, an opacification of the ocular lens, is the leading cause of blindness worldwide.1 Although cataract surgery restores vision initially, a common postoperative complication is posterior capsular opacification (PCO). Advances in surgical techniques and intraocular lens design have reduced the prevalence of PCO, yet it remains a significant and uneradicated problem.2 Currently, cataract surgery and Nd:YAG laser capsulotomy are the only effective treatments for cataract and PCO, respectively; however, they carry vision-related surgical complications and place major personal and financial burden on the aging population.2 Hence, there is interest in the development of noninvasive pharmacologic alternatives to maintain lens transparency.
There is compelling evidence implicating epithelial-mesenchymal transition (EMT) in the pathogenesis of anterior subcapsular cataract (ASC) and PCO. During EMT, polarized lens epithelial cells (LECs) relinquish their cobblestone morphology and transdifferentiate into spindle-shaped myofibroblastic cells that elongate and migrate across the lens capsule.3 Transforming growth factor-β (TGFβ) has been studied extensively as a key inducer of this lens EMT in vitro3,4 and in vivo.5–7 While TGFβ induces aberrant lens cell behavior, other growth factors found in the ocular media, such as bone morphogenetic protein-7 (BMP-7) regulate normal cellular processes in the lens. Specifically, BMP-7 and its receptors have been identified as key regulators of lens induction,8–10 gap-junction mediated communication,11 and lens fiber differentiation12–14—all critical elements for proper lens development, maintenance, and function. Moreover, human genetic mutations in BMP-7 have resulted in severe eye defects, such as anophthalmia, in addition to kidney and skeletal defects.15
There is a growing body of literature supporting the role of BMP-7 in antagonizing TGFβ-driven EMT.16 Several studies unequivocally demonstrate that exogenous administration of BMP-7 enhanced the recovery rate of TGFβ-induced renal fibrosis.17–19 Adenoviral gene transfer of BMP-7 suppressed TGFβ-driven corneal scarring in a mouse alkali burn-induced injury model.20 In a human model of primary open angle glaucoma, BMP-7 antagonized TGFβ-induced expression of extracellular matrix proteins in trabecular meshwork cells.21 In the lens, however, there is a paucity of literature investigating the potential inhibitory role of BMP-7. Adenoviral-mediated overexpression of BMP target genes, Inhibitors of differentiation (Id)-2 and Id3, suppressed TGFβ-induced EMT in a murine lens epithelial cell line.22 However, adenoviral-mediated expression of BMP-7 in situ delayed but did not completely inhibit EMT in a mouse lens injury-induced model.23
We describe experiments in vitro investigating the role of BMP-7 in modulating TGFβ-induced EMT in the lens. This is the first time exogenous administration of BMP-7 has been used to counteract TGFβ-induced EMT in the lens epithelial explant system. To establish further evidence of the opposing effects of BMP-7 and TGFβ, we examined differences in activation of their respective downstream Smad signaling proteins. We found that inhibition of EMT by BMP-7 resulted in an increased phosphorylation of BMP-7–responsive Smads (Smad1/5) and a concurrent reduction in phosphorylation of TGFβ-responsive Smads (Smad2/3). Moreover, we showed that TGFβ suppresses expression of BMP-7 target genes, Id2/3, and the addition of BMP-7 restores these Id2/3 levels. Taken together, we highlighted the contrasting roles of different TGFβ superfamily members in the lens, in which tight regulation of BMP-7–responsive Smad-signaling and downstream target genes maintain the normal lens epithelial phenotype, and aberrant TGFβ-responsive Smad-signaling leads to the EMT underlying fibrotic cataract formation.
Methods
Animals
Ocular tissues were collected from postnatal-day-21 albino Wistar rats (Rattus norvegicus) that were euthanized by carbon dioxide asphyxiation and cervical dislocation. All animal procedures conformed to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research (United States) and the National Health and Medical Research Council (NHMRC) Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (Australia). All studies were approved by the Animal Ethics Committee of the University of Sydney (Australia).
Preparation of Lens Epithelial Explants
Lens epithelial explants were prepared as described previously.24 All tissue culture was performed in Medium 199 with Earle's salts (Life Technologies, Waltham, MA, USA), supplemented with 0.1% BSA (Sigma-Aldrich Corp., St. Louis, MO, USA), 0.1 μg/ml L-glutamine (Life Technologies), 100 IU/ml penicillin/100 μg/ml streptomycin (Life Technologies), and 2.5 μg/ml Amphostat B (Thermo Fisher Scientific, Waltham, MA, USA) at 37°C in a humidified 5% CO2 incubator.
The effects of BMP-7 on TGFβ-induced EMT were initially investigated by adding increasing doses (1, 5, 10, or 50 ng/ml) of recombinant human BMP-7 (R&D Systems, Minneapolis, MN, USA) to explants concurrently in the presence or absence of 200 pg/ml of recombinant human TGFβ2 (R&D Systems). Subsequent experiments were performed using 5 ng/ml of BMP-7 as this was the lowest inhibitory dosage of BMP-7. Lens epithelial cells were cultured for up to 5 days. For short-term exposure studies, TGFβ2 was added alone or in combination with BMP-7 for 2 hours after which the culture media was removed and replaced with fresh unsupplemented media. Cells in explants were viewed with phase-contrast microscopy (CK2; Olympus, Tokyo Japan) and photographed (Leica DFC-280; Leica Camera, Wetzlar, Germany) before being harvested for immunofluorescence, Western blot or RT-qPCR analysis.
Assessment of Capsular Wrinkling
Following a 5-day culture period, explants were rinsed in water for 1 hour to remove the overlying cells, exposing the underlying lens capsule. The bare lens capsule was viewed using phase-contrast microscopy and photographed as described above.
Immunofluorescence
Explants were fixed in 100% methanol for 45 seconds and rinsed four times in phosphate-buffered saline (PBS). This was followed by rinsing (3 × 5 minutes) in PBS supplemented with 0.1% (wt/vol) BSA (PBS/BSA). Explants then were incubated in 10% normal goat serum (NGS) diluted in PBS/BSA for 1 hour. Primary antibodies were diluted in PBS/BSA with 1.5% NGS and incubated overnight at 4°C in a humidified chamber. Anti-mouse antibodies specific for α-smooth muscle actin (α-SMA; A2547; monoclonal; Sigma-Aldrich Corp.), E-cadherin (4A2; monoclonal; Cell Signaling Technology, Danvers, MA, USA) and anti-rabbit antibodies specific for β-catenin (H-102, polyclonal; Santa Cruz Biotechnology, Dallas, TX, USA), and total-Smad2/3 (8685; monoclonal; Cell Signaling Technology) were all diluted at 1:100.
Following application of the primary antibody, explants were equilibrated to room temperature and any unbound primary antibody was removed by rinsing in PBS/BSA (3 × 5 minutes). The appropriate secondary antibody was diluted 1:1000 in PBS/BSA and applied to the explants for 2 hours at room temperature in a dark humidified chamber. E-cadherin and α-SMA were detected using goat anti-mouse Alexa-Fluor 488 (Cell Signaling Technology). Beta-catenin was detected using goat anti-rabbit Alexa-Fluor 594 (Abcam, Cambridge, MA, USA). Explants were rinsed (3 × 5 minutes) in PBS/BSA before counterstaining with 3 μg/ml bisbenzimide (Hoechst 33342; Sigma-Aldrich Corp.) for 2 minutes. Explants then were rinsed in PBS/BSA (3 × 5 minutes). Explants were mounted with 10% PBS (vol/vol) in glycerol and viewed with a Zeiss LSM-5Pa confocal microscope (Carl Zeiss AG, Jena, Germany).
SDS-Page and Western Blotting
Explants were rinsed in cold PBS and cell proteins were extracted in cold lysis buffer containing 2.5 mM EDTA, 25 mM Tris-HCl (pH 7.5), 0.375 NaCl, 250 mM sodium orthovandate, 10 mM sodium deoxycholate, a protease inhibitor cocktail tablet (Complete; Roche Applied Science, Penzburg, Germany) and a phosphatase inhibitor tablet (PhosStop; Roche Applied Science). Homogenized samples were rotated for 2 hours at 4°C to facilitate lysis. Lysates were precleared by centrifugation at 15,500g for 15 minutes at 4°C. Protein content of the supernatant was quantified using the Micro BCA protein assay reagent kit (Thermo Fisher Scientific).
Protein lysates were mixed in a 1:1 ratio with Laemmli sample buffer (BioRad Laboratories, Hercules, CA, USA). Up to 10 μg of lens explant protein extract was loaded onto 10% SDS-PAGE gels for electrophoresis for 1.5 hours at 200 V before being transferred onto an Immobilon polyvinylidene fluoride membrane (Merck Millipore, Billerica, MA, USA) for 2 hours at 100 V. The membrane then was incubated for 1 hour with a blocking solution of 5% (wt/vol) nonfat skim milk powder in 0.1% Tween-20 in Tris-buffered saline (TBST) for nonphosphorylated proteins and 2.5% BSA in TBST for phosphorylated proteins. Membranes were incubated overnight at 4°C with the primary antibody. Anti-mouse antibodies specific against α-SMA (A2547; monoclonal; Sigma-Aldrich Corp.), E-cadherin (4A2; monoclonal; Cell Signaling Technology), GAPDH (G8795; Sigma-Aldrich Corp.), and anti-rabbit antibodies specific against β-catenin (H-102; Santa Cruz Biotechnology), phospho-Smad2/3 (D27F4; monoclonal; Cell Signaling Technology), total-Smad2/3 (8685; monoclonal; Cell Signaling Technology), and phospho-Smad1/5 (41D10; monoclonal; Cell Signaling Technology) were all diluted at 1:1000 with the exception of α-SMA and β-catenin, which were diluted at 1:2000.
Membranes were rinsed with TBST (3 × 5 minutes) and incubated for 2 hours with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies, either goat anti-mouse HRP-conjugated IgG or goat anti-rabbit HRP-conjugated IgG (both diluted 1:5000 in TBST; Cell Signaling Technology). Membranes then were rinsed (3 × 10 minutes) in TBST and incubated for 2 minutes in Immobilon Western Chemiluminescent HRP Substrate (Merck Millipore). Chemiluminescence signals were captured using the ChemiDoc MP imaging system (BioRad Laboratories) and densitometric analysis was performed using ImageLab software (BioRad Laboratories).
Total RNA Extraction and cDNA Synthesis
Following a 24-hour treatment period, explants were rinsed in cold PBS and total RNA was extracted using the Isolate II RNA Micro Kit (Bioline, Alexandria, NSW, Australia) according to the manufacturer's instructions. Concentration and purity of RNA was measured using the Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific). Integrity of RNA was assessed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Only samples with 260/280 ratios greater than 2 and RNA integrity numbers greater than 8 were used for reverse transcription quantitative PCR (RT-qPCR) analysis.
Reverse Transcriptase-Quantitative PCR (RT-qPCR)
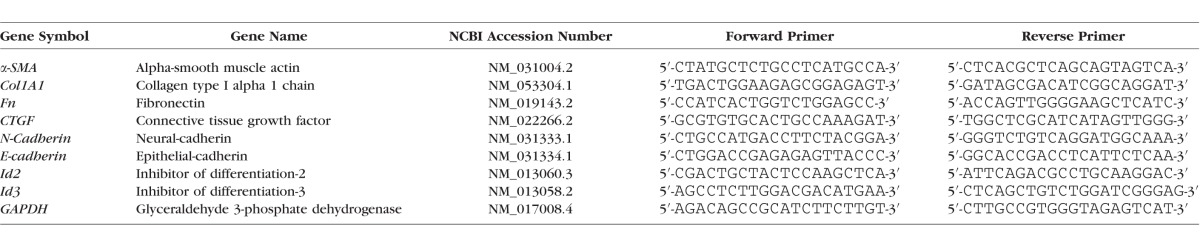
Total RNA (200 ng) was reverse-transcribed using the SensiFAST cDNA synthesis kit (Bioline) as per the manufacturer's instructions. Complementary DNA (cDNA) samples then were diluted 1:12 with nuclease-free water. Oligonucleotide primers (Table) were designed using Primer-BLAST to span the exon-exon junction.
Table.
Oligonucleotide Primers
All RT-qPCR reactions were performed using the SensiFAST SYBR No-ROX kit (Bioline). Reactions (10 μl) were set up in a LightCycler 480, 384-well plate (Roche Diagnostics Ltd., Forrenstrasse, Switzerland) using a Freedom EV075 robotic station with Freedom EVOware Standard 3.2 software (Tecan, Port Melbourne, VIC, Australia) consisting of 4 μl cDNA, 5 μl SYBR, and 300 nM forward and reverse primers. Reverse transcriptase qPCR analysis was done using the Roche LightCycler 480 (Roche Diagnostics Ltd.) under the following thermal cycling conditions: 95°C for 2 minutes followed by 45 cycles consisting of denaturation (95°C, 5 seconds), annealing (60°C, 10 seconds), and extension (72°C, 15 seconds). At the end of each run, melting curve profiles (95°C for 5 minutes, 60°C for 1 minute, and then slowly heating at 0.11°C/s up to 98°C with continuous measurement of fluorescence per 5°C) to confirm amplification of specific transcripts. Standard curves were generated by serially diluting (1:2), covering an appropriate concentration range. Relative gene expression was determined as a ratio of the gene of interest and GAPDH using the second derivative maximum method of the LightCycler software (Roche Diagnostics Ltd.). All reactions, including no-template controls and minus RT controls, were run in duplicate.
Statistical Analysis
Each experiment was performed at least three times and graphing was performed using GraphPad Prism version 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). Western blot data were analyzed using GraphPad Prism version 6.0 (GraphPad Software, Inc.) by 1- or 2-way ANOVA with post hoc Tukey's multiple comparisons test. Reverse transcriptase PCR data were analyzed using the Light Cycler software (Roche Diagnostics Ltd.) and GenEx version 6.0 (MultiD Analyses AB, Goteborg, Sweden) using an unpaired 2-tailed t-test. All data are presented as mean ± SEM and P < 0.05 was considered statistically significant.
Results
BMP-7 Blocks TGFβ2-Induced EMT of LECs in a Dose-Dependent Manner
Using phase-contrast microscopy, LECs in untreated explants remained as a cuboidal monolayer with a characteristic cobblestone-packed arrangement, indicative of a normal epithelial phenotype (Fig. 1A). At 48 hours, explants treated with TGFβ2 exhibited an elongate, spindle-shaped morphology, indicative of an EMT response (Fig. 1B). While the lowest dose of BMP-7 (1 ng/ml) in the presence of TGFβ2 resulted in similar elongation in many cells (Fig. 1C), higher doses of BMP-7 (from 5 ng/ml) displayed a retention of the normal epithelial phenotype, demonstrating suppression of TGFβ2-induced transdifferentiation (Figs. 1D–F).
Figure 1.
Bone morphogenetic protein-7 application inhibits TGFβ2-induced EMT in a dose-dependent manner. Following 48 hours of culture, untreated control explants (A) retained a normal cuboidal layer of epithelial cells, whereas cells in explants treated with TGFβ2 alone (B) displayed an elongate, spindle-shaped morphology (yellow arrows). Cotreatment with TGFβ2 and 1 ng/ml BMP-7 ([C], +TB1) also resulted in elongate cells (yellow arrows), whereas higher doses of BMP-7 (5, 10, and 50 ng/ml; +TB5, +TB10, +TB50) retained the normal lens epithelial phenotype (D–F). Images are representative of 3 independent experiments. Scale bar: 100 μm.
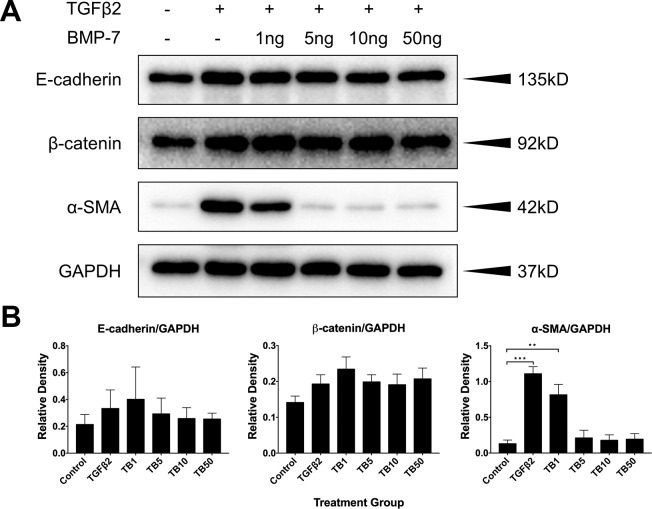
To further explore the suppressive effect of BMP-7 on TGFβ2-induced EMT, we examined the expression of epithelial and mesenchymal markers in these treatment groups at 48 hours using Western blotting (Fig. 2A). Addition of TGFβ2 to LECs significantly upregulated α-SMA expression compared to cells in control explants (P = 0.0001, Fig. 2B). Cotreatment of LECs with TGFβ2 and 1 ng/ml of BMP-7 significantly upregulated α-SMA levels compared to that seen in control explants (P = 0.0034, Fig. 2B), and exhibited a slight, but not significant reduction in α-SMA levels compared to TGFβ2 alone–treated LECs (P = 0.3314, Fig. 2B). Cotreatment of higher doses of BMP-7 with TGFβ2 resulted in no difference in α-SMA levels compared to cells in control explants (P = 0.9899 for 5 ng/ml, P = 0.9991 for 10 ng/ml, P = 0.9967 for 50 ng/ml; Fig. 2B). As a result of this, the lowest suppressive dose of BMP-7 (5 ng/ml) was chosen for all subsequent blocking experiments. There was no significant effect in the expression of epithelial markers, E-cadherin, and β-catenin between all treatment groups (P = 0.9293 for E-cadherin, P = 0.3341 for β-catenin; Fig. 2B).
Figure 2.
Bone morphogenetic protein-7 application inhibits TGFβ2-induced upregulation of α-SMA in a dose-dependent manner. Expression levels of epithelial and mesenchymal markers at 48 hours by Western blotting (A) and corresponding, relative densitometry analysis (B). In untreated control explants, lens epithelial cells showed minimal expression of the mesenchymal marker, α-SMA. Transforming growth factor-β2 alone and in combination with 1 ng/ml of BMP-7 significantly upregulated α-SMA expression compared to control explants. Complete inhibition of TGFβ2-induced α-SMA expression was evident with co-treatment of BMP-7 at higher doses (5 ng/ml and up), displaying no significant difference to control levels. No significant difference was observed in the levels of epithelial markers, E-cadherin, and β-catenin between all treatment groups. Representative Western blots from 3 independent experiments, with data presented relative to GAPDH, as the mean ± SEM. One-way ANOVA, Tukey's post hoc test **P < 0.01, ***P < 0.001.
BMP-7 Application Blocks TGFβ2-Mediated Incorporation of α-SMA Into Stress Fibers
We examined the localization of α-SMA in LECs using immunofluorescence confocal microscopy. Cells in control explants displayed low basal levels of α-SMA immunoreactivity (Fig. 3A), while cells in explants treated with TGFβ2 exhibited strong immunoreactivity for α-SMA-labeled stress fibers, characteristic of an EMT response (Fig. 3B). Cotreatment with BMP-7 (5 ng/ml) inhibited TGFβ2-mediated labeling of α-SMA to stress fibers and resembled cells in control explants (Fig. 3C). Similarly, treatment with BMP-7 (5 ng/ml) alone showed no difference in α-SMA-labeling compared to cells in control explants (Fig. 3D).
Figure 3.
Bone morphogenetic protein-7 application blocks TGFβ2-mediated α-SMA. Immunofluorescence confocal microscopy of control explants at 48 hours showed low levels of basal cytoplasmic α-SMA immunoreactivity (A), while explants treated with TGFβ2 exhibited strong immunoreactivity for α-SMA, colabeling to stress fibers (B). Cotreatment with BMP-7 (5 ng/ml) and TGFβ2 (C) and treatment with BMP-7 alone (D) showed no difference in α-SMA labeling compared to control explants. Images are representative of 3 independent experiments. Scale bar: 50 μm.
BMP-7 Application Facilitates Retention of Epithelial Membrane Markers
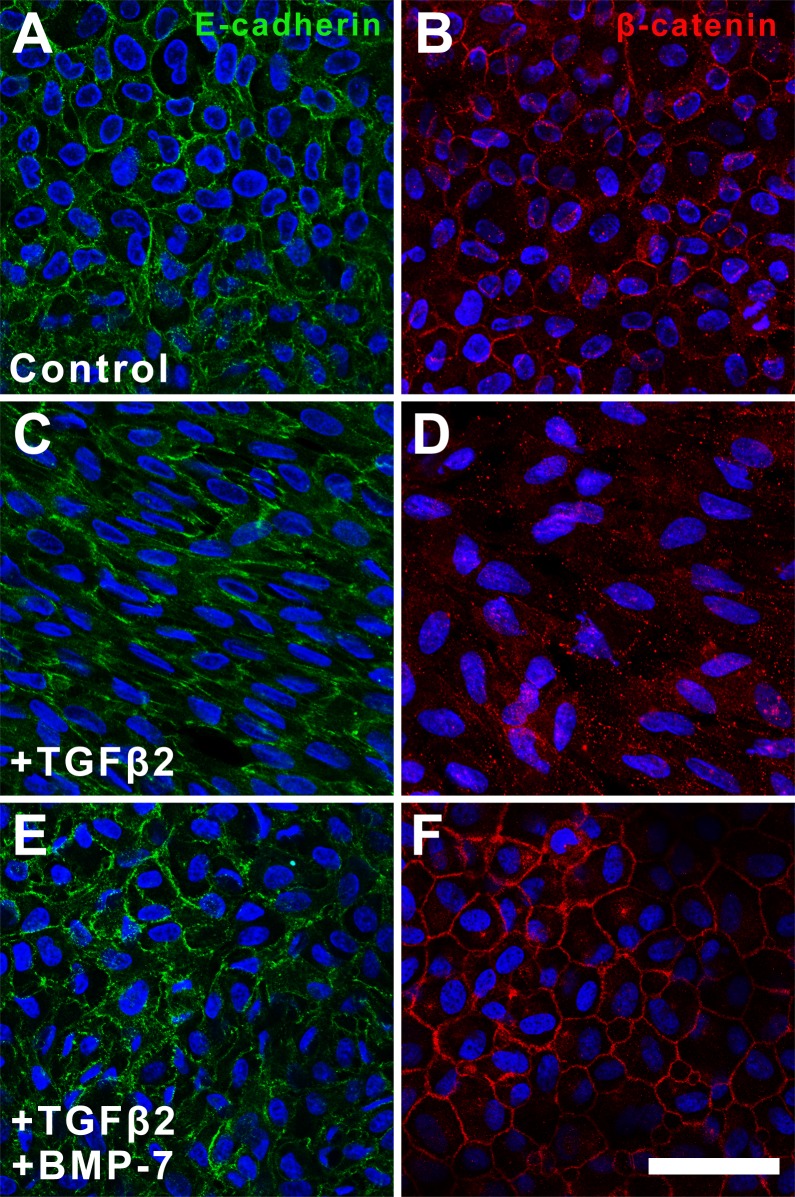
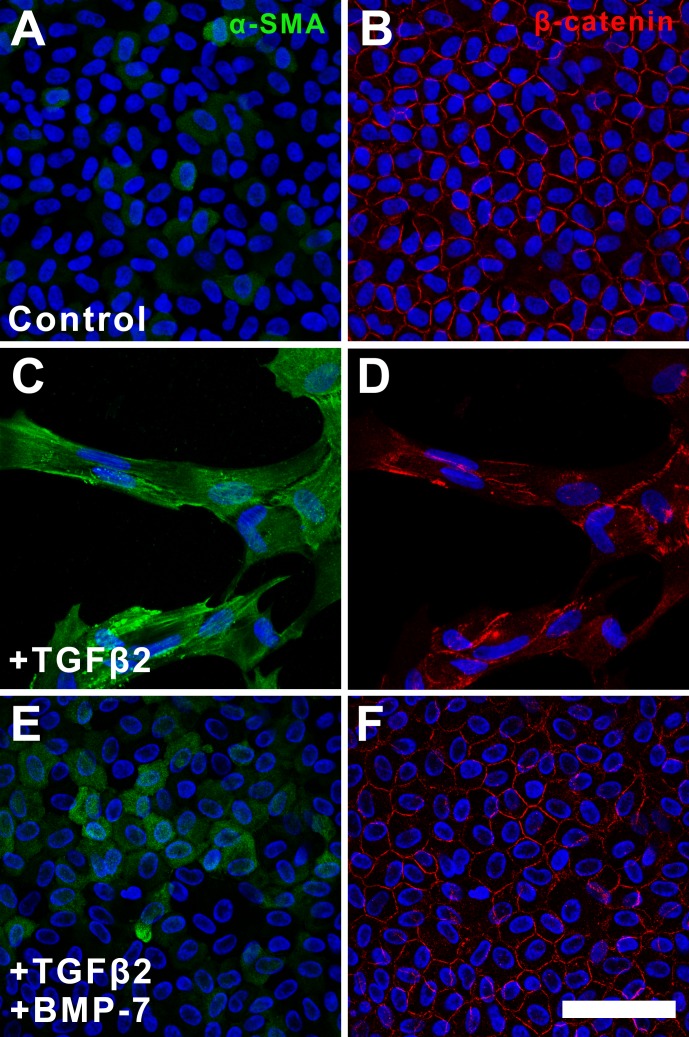
Since the protein levels of E-cadherin and β-catenin remained similar in all treatment groups, we next investigated for changes in their spatial localization. In untreated cells, E-cadherin (Fig. 4A) and β-catenin (Fig. 4B) were localized to the cell membranes highlighting the cobblestone-packed arrangement. Although E-cadherin remained localized to the cell membrane in TGFβ2-treated explants, the expression pattern lacked the normal cobblestone-packed arrangement, appearing unevenly distributed in an elongate pattern (Fig. 4C). This was in contrast to the lack of membranous localization of β-catenin in the same cells of these TGFβ2-treated explants (Fig. 4D). Specifically, β-catenin displayed a pronounced punctate labeling in the cell nuclei and cytoplasm following treatment with TGFβ2. Treatment with BMP-7 inhibited TGFβ2-induced dysregulation of these epithelial markers with retention of E-cadherin (Fig. 4E) and β-catenin (Fig. 4F) labeling to the membrane, again highlighting the cobblestone-packed arrangement of the LECs.
Figure 4.
Bone morphogenetic protein-7 application facilitates retention of epithelial membrane markers. Immunofluorescence confocal microscopy at 48 hours showed that E-cadherin (A) and β-catenin (B) were localized to the cell membrane in control explants. In TGFβ-treated explants, the expression pattern of E-cadherin (C) and β-catenin (D) appeared dysregulated and unevenly distributed. Treatment with BMP-7 inhibited TGFβ-induced dysregulation of epithelial markers and restored E-cadherin (E) and β-catenin (F) labeling to the membrane. Images are representative of 3 independent experiments. Scale bar: 50 μm.
BMP-7 Application Reduces TGFβ2-Induced Mesenchymal Gene Expression
Following treatment of LECs with TGFβ2 for 24 hours, RT-qPCR showed a significant upregulation of mesenchymal gene expression compared to control explants: α-SMA (P < 0.0001), Col1A1 (P < 0.0001), fibronectin (P < 0.0001), CTGF (P < 0.0001), and N-cadherin (P = 0.0003; Fig. 5). The addition of BMP-7 also significantly upregulated mesenchymal gene expression compared to control explants: α-SMA (P = 0.016), Col1A1 (P = 0.014), fibronectin (P = 0.0063), CTGF (P = 0.015), and N-cadherin (P = 0.0012); however, this was to a significantly lesser extent when compared to TGFβ2 alone: α-SMA (P = 0.0094), Col1A1 (P = 0.014), fibronectin (P = 0.0018), and CTGF (P = 0.0011, Fig. 5). Interestingly, the addition of BMP-7 did not significantly reduce N-cadherin expression (P = 0.86) compared to TGFβ2 alone. It should be noted that TGFβ2 only weakly upregulated CTGF and N-cadherin showing changes less than 2-fold. Gene expression of the epithelial marker, E-cadherin, did not change significantly among all treatment groups, corroborating the Western blot data.
Figure 5.
Bone morphogenetic protein-7 application blocks TGFβ-induced mesenchymal gene expression. Using RT-qPCR, treatment with TGFβ2 alone for 24 hours resulted in a significant upregulation of mesenchymal gene expression (α-SMA, Col1A1, fibronectin, CTGF, N-cadherin) compared to control. The addition of BMP-7 significantly reduced mesenchymal gene expression compared to TGFβ2 alone; however, a significant difference to control cells was still evident. Bone morphogenetic protein-7 did not significantly reduce N-cadherin expression compared to TGFβ2 alone. Gene expression of E-cadherin, also did not change significantly amongst all treatment groups. Data from 4 independent experiments are presented relative to GAPDH as fold change ± SEM. Unpaired t-test *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
BMP-7 Application Blocks TGFβ2-Induced EMT and Apoptosis
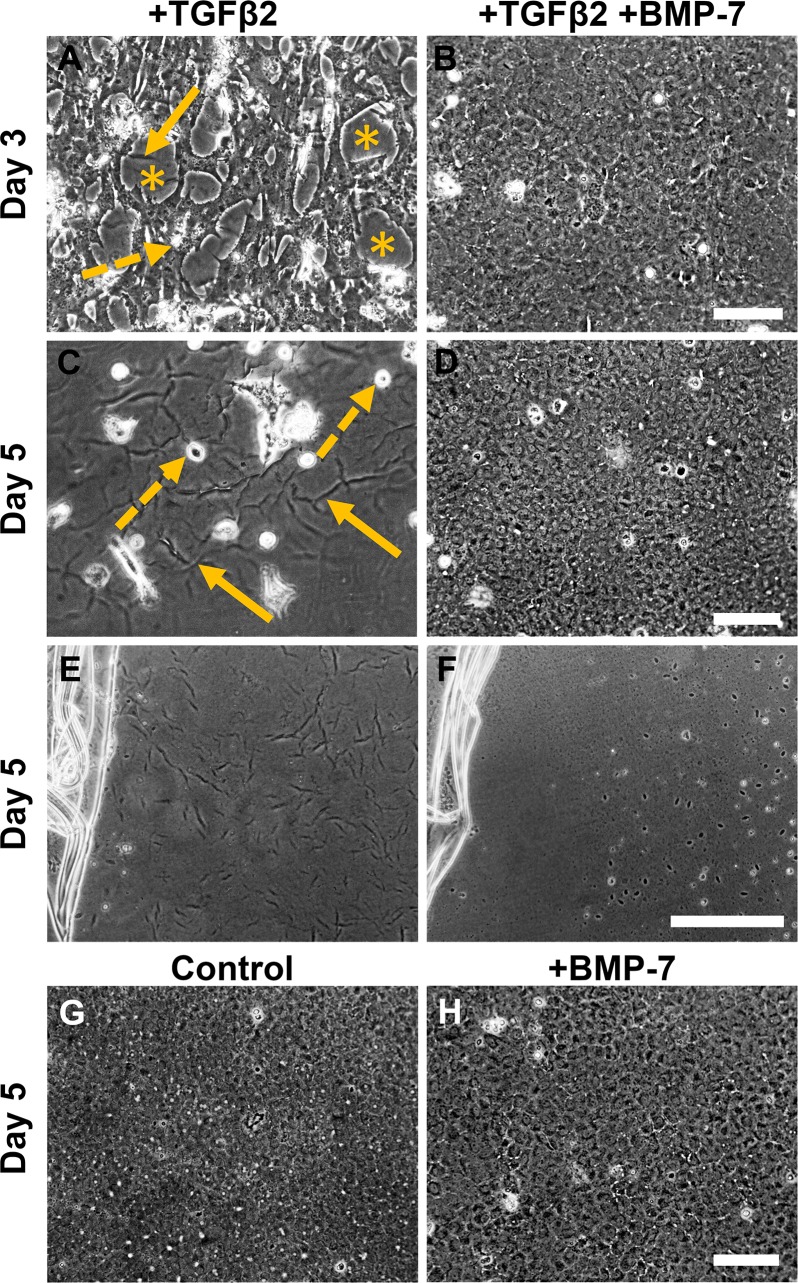
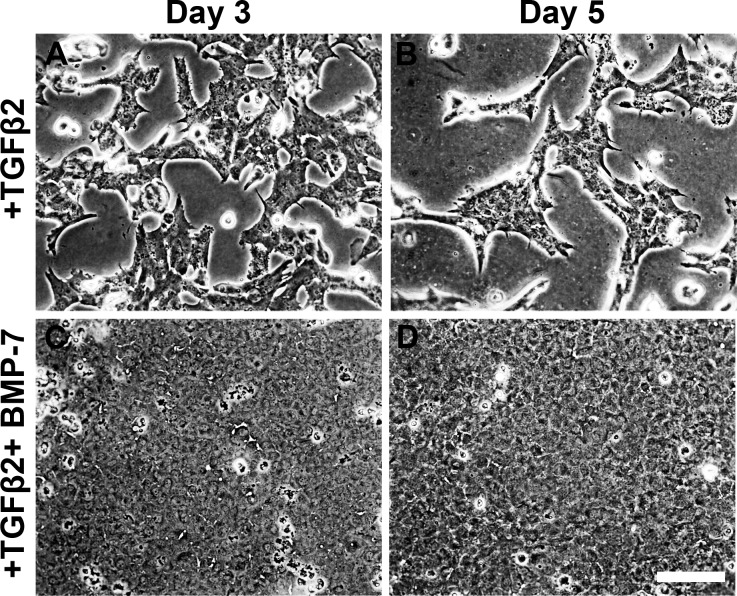
To investigate whether the inhibitory effect of BMP-7 extended beyond 2 days, we followed the progression of TGFβ2-induced EMT over a five-day culture period. Progressive cell loss was evident from day 3 of culture indicated by cellular blebbing (dashed-line arrows) and exposed areas of bare lens capsule (asterisks, Fig. 6A). The exposed lens capsule also accentuated the presence of lens capsular wrinkling (solid-line arrows, Fig. 6A). By day 5 of culture with TGFβ2, there was extensive cell loss and lens capsular wrinkling (Fig. 6B). Explants cotreated with BMP-7 (5 ng/ml) and TGFβ2 retained a confluent epithelial monolayer throughout the 5-day culture period (Figs. 6C, 6D), with no evidence of capsular wrinkling (Fig. 6F) when we removed the overlying cells, compared to the extensive lens capsular wrinkling in TGFβ2-treated alone explants (Fig. 6E). Untreated explants (Fig. 6G) and explants treated with BMP-7 alone (Fig. 6H) retained a monolayer of epithelial cells with no indication of cell loss (Figs. 6G, 6H).
Figure 6.
Bone morphogenetic protein-7 application blocks TGFβ2-induced EMT and apoptosis. Phase-contrast microscopy showed that treatment with TGFβ2 resulted in progressive cell loss from day 3 of culture (A), indicated by cellular blebbing (dashed-line arrows), exposed areas of bare lens capsule (asterisks), and resultant lens capsular wrinkling (solid-line arrows). By day 5 of culture (B), there is marked cell loss and more apparent lens capsular wrinkling (solid-line arrows). Explants cotreated with BMP-7 and TGFβ2 retained a confluent epithelial monolayer throughout the 5-day culture period (C–D), appearing similar to untreated control explants (E) as well as explants treated with BMP-7 alone (F). Images are representative of 3 independent experiments. Scale bar: 100 μm.
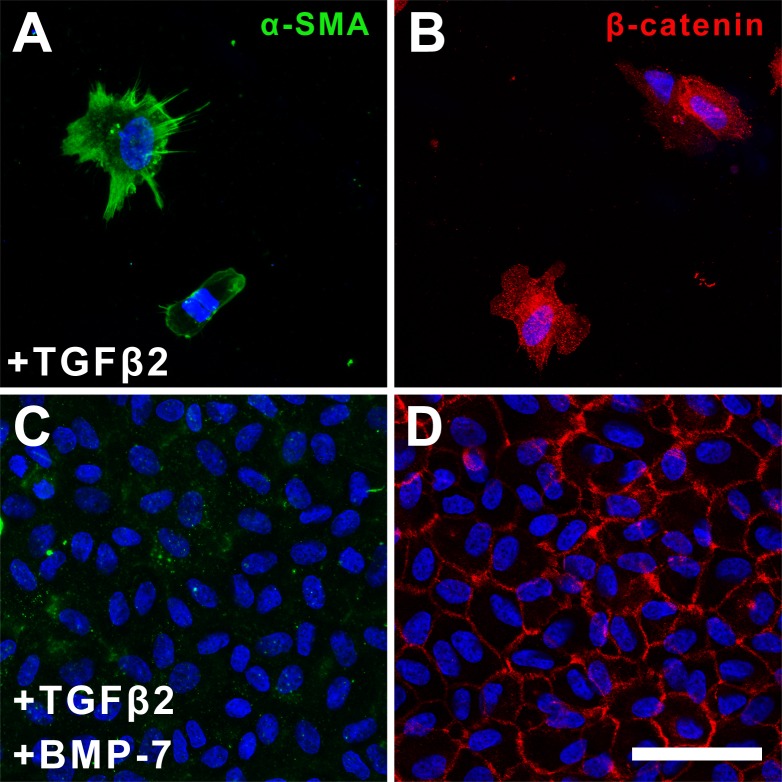
Immunofluorescence labeling of occasional cells remaining in explants treated with TGFβ2 at 5 days showed α-SMA–labeled stress-fibers (Fig. 7A) and punctate cytosolic β-catenin (Fig. 7B). In contrast, cells in explants cotreated with BMP-7 and TGFβ2 retained an epithelial phenotype, with little to no α-SMA label (Fig. 7C), and membranous β-catenin expression, highlighting their packed arrangement (Fig. 6D).
Figure 7.
Bone morphogenetic protein-7 application blocks TGFβ2-induced α-SMA expression at 5 days of culture. Immunofluorescence confocal microscopy of the occasional remaining cells in TGFβ2-treated explants at day 5 of culture showed stress-fibers labeled for α-SMA (A) and punctate cytosolic β-catenin labeling (B). Cells in explants cotreated with BMP-7 and TGFβ2 showed minimal α-SMA expression (C) and membranous β-catenin labeling (D). Images are representative of 3 independent experiments. Scale bar: 50 μm.
BMP-7 Application Modulates TGFβ2-Induced Smad Signaling
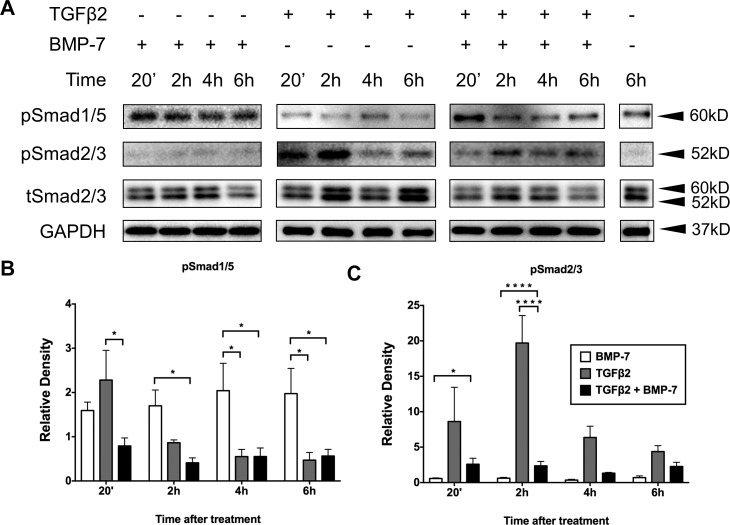
To examine the signaling pathways involved in mediating the inhibitory effect of BMP-7 on TGFβ2-induced EMT, we monitored for changes in the respective phosphorylated Smads during the first 6 hours following treatment (Fig. 8A). Treatment of LECs with BMP-7 alone induced a sustained upregulation of pSmad1/5 that was significantly higher than treatment with TGFβ2 alone at 2 (P = 0.0280), 4 (P = 0.0114), and 6 (P = 0.0161) hours (Fig. 8B). Cotreatment of cells with BMP-7 and TGFβ2 exhibited an early peak of pSmad1/5 signaling at 20 minutes, that was significantly higher than with TGFβ2 alone at this same time point (P = 0.0112; Fig. 8B). Lens epithelial cells treated with BMP-7 alone did not have altered basal pSmad2/3 levels. Treatment of LECs with TGFβ2 showed a marked increase of pSmad2/3, peaking at 2 hours compared to BMP-7 alone (P = 0.0313; Fig. 8C). Cotreatment with BMP-7 significantly reduced this 2-hour peak of TGFβ2-induced pSmad2/3 in LECs (P = 0.0048; Fig. 8C).
Figure 8.
Bone morphogenetic protein-7 application modulates TGFβ2-induced Smad signaling. Treatment with BMP-7 alone induced a sustained increase in pSmad1/5 that was significantly higher than treatment with TGFβ2 alone at 2, 4, and 6 hours (B). Cotreatment with BMP-7 and TGFβ2 exhibited an early peak of pSmad1/5 signaling at 20 minutes (B). Treatment with TGFβ2 showed a marked increase of pSmad2/3, peaking at 2 hours (C). Cotreatment with BMP-7 significantly reduced TGFβ2-driven activation of pSmad2/3 at 2 hours (C). Representative Western blots from 3 independent experiments (A) with data presented as mean ± SEM. Two-way ANOVA, Tukey's post hoc test *P < 0.05, **P < 0.01, and ***P < 0.001.
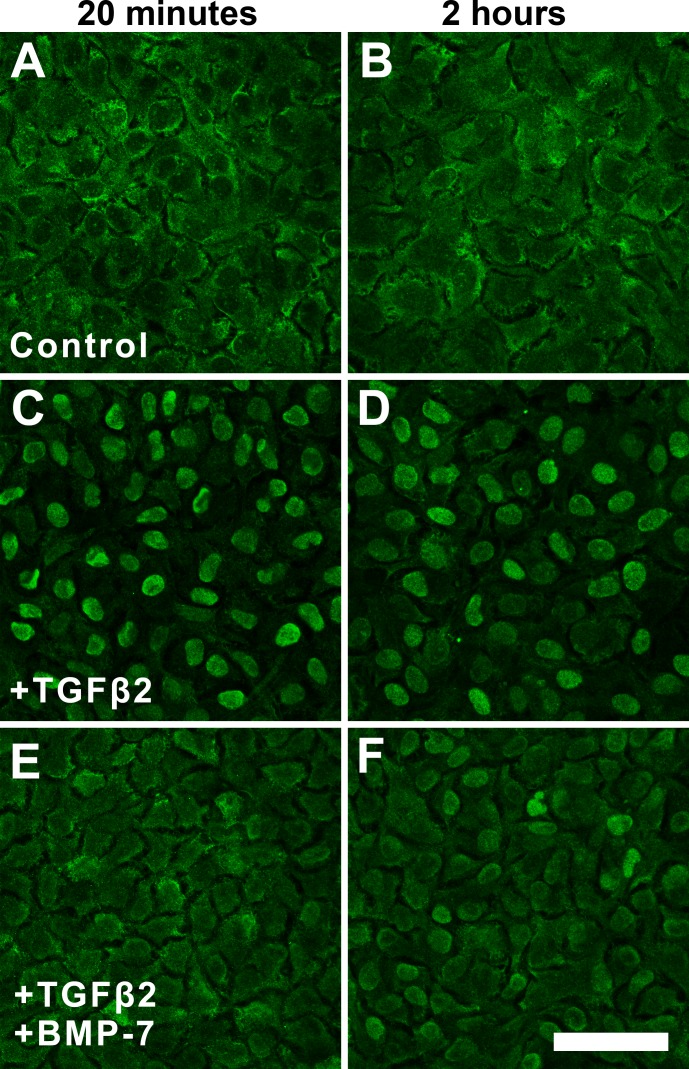
These findings were further corroborated with immunofluorescence analysis of total Smad2/3 (tSmad2/3) localization. Control explants displayed cytoplasmic labeling of tSmad2/3 at 20 minutes and 2 hours (Figs. 9A, 9B). Exposure of LECs to TGFβ2 for 20 minutes and 2 hours demonstrated localization of tSmad2/3 labeling to cell nuclei (Figs. 9C, 9D). The addition of BMP-7 at 20 minutes or 2 hours reduced TGFβ2-induced nuclear translocation of tSmad2/3, with retention of cytoplasmic tSmad2/3, similar to control explants (Fig. 9E); however, at 2 hours, some nuclear translocation of tSmad2/3 still was apparent (Fig. 9F).
Figure 9.
Bone morphogenetic protein-7 application modulates nuclear localization of tSmad2/3. Immunofluorescence confocal microscopy showed cytoplasmic labeling of tSmad2/3 at 20 minutes (A) and 2 hours (B) in control explants. Exposure of LECs to TGFβ2 for 20 minutes (C) and 2 hours (D) demonstrated nuclear localization of tSmad2/3. The addition of BMP-7 at 20 minutes retained cytoplasmic tSmad2/3 (E); however, some nuclear translocation of tSmad2/3 is evident at 2 hours (F). Images are representative of 3 independent experiments. Scale bar: 50 μm.
Short-Term Exposure to BMP-7 Blocks TGFβ2-Induced EMT and Apoptosis
Given that BMP-7 induced a peak of pSmad1/5 signaling at 20 minutes and suppression of TGFβ2-induced pSmad2/3 at 2 hours, we next asked whether the inhibitory effect of BMP-7 was dependent on this early time point. Lens epithelial cells exposed to TGFβ2 alone for 2 hours continued to undergo EMT over the 5-day culture period with characteristic cell elongation and cell loss, but interestingly, minimal lens capsular wrinkling (Figs. 10A, 10B). The addition of BMP-7 for 2 hours blocked TGFβ2-induced EMT with retention of a monolayer of epithelial cells over the entire culture period (Figs. 10C, 10D).
Figure 10.
Short-term exposure to BMP-7 blocks TGFβ2-induced EMT and apoptosis. Phase-contrast microscopy showed that LECs exposed to TGFβ2 alone for 2 hours underwent EMT over the 5-day culture period with characteristic cell elongation and cell loss (A, B). Cotreatment of TGFβ2 and BMP-7 for 2 hours retained a monolayer of epithelial cells over the 5-day culture period (C, D). Images are representative of 3 independent experiments. Scale bar: 100 μm.
Immunofluorescence labeling of control explants showed minimal α-SMA label (Fig. 11A) and membranous β-catenin expression (Fig. 11B). Explants treated with TGFβ2 at 5 days showed α-SMA–reactive stress-fibers (Fig. 11C) and punctate cytosolic β-catenin (Fig. 11D). Interestingly, LECs cotreated with BMP-7 and TGFβ2 for 2 hours displayed an upregulation in cytoplasmic α-SMA expression (Fig. 11E) despite retaining membranous β-catenin labeling highlighting the cobblestone cell arrangement (Fig. 11F).
Figure 11.
Short-term exposure to BMP-7 modulates TGFβ2-induced markers of EMT. Immunofluorescence confocal microscopy of control explants showed minimal α-SMA label (A) and membranous β-catenin expression (B). At day 5, explants exposed to TGFβ2 alone for 2 hours showed α-SMA–reactive stress-fibers (C) and punctate cytosolic β-catenin (D). Cotreatment of TGFβ2 and BMP-7 for 2 hours displayed an upregulation in cytoplasmic α-SMA expression (E) despite retaining membranous β-catenin expression (F). Images are representative of 3 independent experiments. Scale bar: 50 μm.
BMP-7 Inhibits TGFβ2-Induced Suppression of Id2 and Id3 Gene Expression
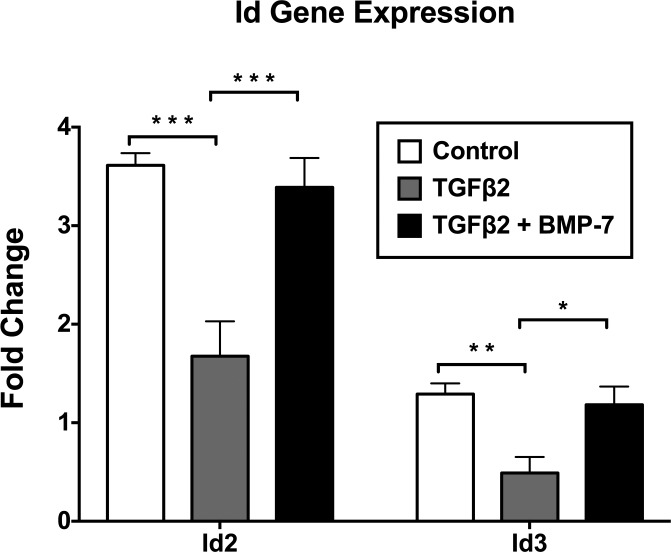
As the Id proteins are reported to be downstream effectors of BMP signaling, we investigated the gene expression of Id2 and Id3 using RT-qPCR. Treatment with TGFβ2 alone significantly downregulated the expression of Id2 (P = 0.00014) and Id3 (P = 0.0001) compared to levels in control explants. The addition of BMP-7 significantly inhibited TGFβ2-induced suppression of Id2 (P = 0.0023) and Id3 (P = 0.014), showing no significant difference from control cells for Id2 (P = 0.50) and Id3 (P = 0.62) (Fig. 12).
Figure 12.
Bone morphogenetic protein-7 inhibits TGFβ2-induced suppression of Id2 and Id3 gene expression. Using RT-qPCR, treatment with TGFβ2 alone significantly downregulated expression of Id2 and Id3 compared to control explants. The addition of BMP-7 significantly inhibited TGFβ2-induced suppression of Id2 and Id3 showing no significant difference to control explants for Id2 and Id3. Data from 4 independent experiments are presented relative to GAPDH as fold change ± SEM. Unpaired t-test *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
The present study showed, for the first time to our knowledge, the direct ability of BMP-7 in blocking TGFβ2-driven EMT in the rat lens explant system. We demonstrated that exogenous administration of BMP-7 inhibited TGFβ2-induced myofibroblastic transdifferentiation by blocking cell elongation and incorporation of α-SMA into stress fibers. Addition of BMP-7 suppressed TGFβ2-induced upregulation of mesenchymal gene expression of ECM markers including collagen 1A1, fibronectin, and connective tissue growth factor. Although N-cadherin was not suppressed by the addition of BMP-7, the fold change in expression of this gene was below 2 for all treatment groups. Furthermore, BMP-7 promoted membranous localization of epithelial markers, E-cadherin, and β-catenin, maintaining the cobblestone-packed arrangement of cells, compared to the dysregulated and punctate cytosolic expression of these markers in LECs treated with TGFβ2 alone. Hence, BMP-7 promotes the epithelial phenotype, emphasizing its role in maintaining lens cell integrity and polarity.
We showed that the inhibitory effect of BMP-7 extends over the entire culture period examined, with a monolayer of cuboidal LECs retained by day 5. The progressive cell elongation, cell blebbing, extensive cell loss, and capsular wrinkling observed in TGFβ2-induced EMT over 5 days has been well characterized in the rat lens epithelial explant system and mimics morphologic features of human ASC and PCO.3 The cell loss induced by TGFβ2 has been reported to occur through apoptosis, corroborating the apoptotic features typically observed in human ASC and PCO, such as chromatin margination and cytoplasmic condensation.25 In the present study, BMP-7 promoted cell survival by preventing TGFβ2-induced apoptosis. Moreover, BMP-7 prevented EMT-associated lens capsular winkling, a key feature of human PCO, highlighting its role in blocking myofibroblastic cell transdifferentiation whose contractility is believed to disrupt the surface integrity and optical properties of the lens capsule.26
To elucidate putative elements of the signaling cascade used by BMP-7 to antagonize TGFβ2-induced EMT, we investigated the expression of phosphorylated Smads. Both BMP-7 and TGFβ bind to distinct type II serine-threonine kinase receptors, which then form a complex with specific type I receptors.27 The activated receptor complex relays the signal to the cytoplasm by phosphorylating Smad2/3 in TGFβ signaling and Smad1/5/8 in BMP signaling.27 Phosphorylated Smads then hetero-oligomerize with Smad4, shared by TGFβ and BMP signaling, that translocates to the nucleus to regulate transcription of respective target genes.27
In this study, treatment with BMP-7 alone increased phosphorylated Smad1/5 with protein levels sustained over the 6-hour culture period, indicating that the Smad signaling pathway can be activated in rat LECs by exogenous BMP-7. Furthermore, in explants cotreated with BMP-7 and TGFβ2, we showed that BMP-7 induces a significant increase in pSmad1/5 signaling, and concurrently reduces TGFβ2-induced Smad2/3 phosphorylation. These findings were corroborated by immunofluorescence data demonstrating that the addition of BMP-7 suppressed TGFβ2-induced nuclear translocation of tSmad2/3 at least early in the culture period. This is consistent with previous studies demonstrating that the mechanism underlying the antagonistic effect of BMP-7 is through direct Smad-dependent inhibition of the TGFβ pathway, both in renal17 and pulmonary fibrosis.28 Interestingly, in the present study, activation of pSmad1/5 is evident only at 20 minutes and falls to basal levels thereafter, suggesting that TGFβ2 also may abrogate BMP-7 signaling to some extent, albeit at a later time-point.
In the present study, short-term treatment of LECs with TGFβ2 for 2 hours was sufficient to induce an EMT response over the 5-day treatment period. Our data supported human studies where LECs exposed to TGFβ2 for a brief 2-day period resulted in significant and persistent long-term fibrotic events over a long 28-day culture period.29 Cotreatment with TGFβ2 and BMP-7 for 2 hours blocked the EMT process with retention of an epithelial monolayer of LECs displaying membranous β-catenin localization. Interestingly, α-SMA expression still was upregulated despite the addition of BMP-7; however, it was not incorporated into stress fibers, hence, possibly unable to exert contractile activity. This suggests that the early surge in pSmad1/5 signaling may be sufficient to initiate downstream BMP-associated gene transcription, thus preventing subsequent activation of TGFβ2-mediated Smad signaling and resultant EMT. This finding has important ramifications in the prevention of PCO as it is the transient elevation of TGFβ during cataract surgery that gives rise to postoperative PCO many years later.30 It is promising to see that even a short exposure to BMP-7 is able to abrogate the potent effects of TGFβ2 over an extended culture period and may be a novel treatment for the prevention of PCO.
Communication between TGFβ and BMP-7 pathways also occurs further downstream of Smad proteins at the level of specific target genes. Using a renal epithelial cell line, Zeisberg et al.17 showed that TGFβ-induced phosphorylation of Smad2/3 and EMT led to reduced E-cadherin expression. Exogenous addition of BMP-7 increased pSmad1/5/8 and this directly blocked pSmad2/3, restoring E-cadherin promoter activity to control levels.17 Interestingly, in the present study, expression levels of epithelial markers, E-cadherin, and β-catenin did not reduce with TGFβ2 treatment. It has been reported that instead of being downregulated, E-cadherin and β-catenin can relocate from the membrane to the cytoplasm during EMT in human corneal epithelial cells.31 This is consistent with our study that shows a dysregulated cellular distribution of these epithelial markers during TGFβ2-mediated EMT. Since E-cadherin and β-catenin are not localized to the membrane in their normal cobblestone-packed arrangement, they can no longer fulfil their functional role as cell–cell adhesion molecules in maintaining epithelial cell integrity and intercellular junctions, hence promoting the EMT response.
In addition to the E-cadherin promoter gene, Id proteins also have been identified as downstream target genes mediating the antagonistic effect of BMP-7 on TGFβ-induced EMT.22,23,28 Id proteins have a crucial role in development and cancer biology, permitting proliferation and preventing premature terminal differentiation.32 Downregulation of Id proteins has been detected during TGFβ-induced EMT in a prostate cancer model.33 Bone morphogenetic protein-7 also has been shown to antagonize TGFβ-induced pulmonary fibrosis by inducing Id2 and Id3,28 while adenoviral gene transfer of Id2 and Id3 delayed injury-induced EMT of LECs in a mouse model.23 Mouse mutants deficient in Id2 and Id3 enabled BMP-7 to induce EMT, further highlighting the importance of BMP-induced Id proteins in antagonizing TGFβ-induced EMT.22 Interestingly, in our present study, BMP-7 does not upregulate Id2/3 gene expression but rather, is able to inhibit TGFβ2-induced Id2/3 gene suppression, maintaining Id2/3 gene expression at basal levels. Future research will focus on the importance of BMP-induced Id proteins in the lens, and examine the role of dysregulated Id expression in promoting EMT to potentially exacerbate cataract formation.
Using an in vivo capsular injury-induced mouse model, Saika et al.23 reported that BMP-7 upregulated pSmad1/5/8 signaling and inhibited pSmad2 signaling. Although adenoviral gene transfer of BMP-7 suppressed the expression of α-SMA, the EMT process was merely delayed and LECs eventually continued to undergo myofibroblastic transdifferentiation.23 In contrast, our study showed that the inhibitory effect of BMP-7 was maintained over the entire culture period. This may be attributed to differences in the model of EMT induction, as well as the administration of BMP-7. In our study, we induced EMT by exogenous addition of TGFβ2, whereas, the lens capsular injury-induced model of EMT relies on indirect activation of endogenous TGFβ in situ through physical damage to the lens that may activate more complex signaling pathways. It also is possible that the method of enhancing BMP-7 signaling may have a role; where exogenous administration of BMP-7 may be more effective at counteracting the effects of TGFβ compared to adenoviral-mediated overexpression of BMP-7.
The in vivo situation, no doubt, differs markedly from our lens explant system given LECs are in constant contact with a myriad of growth factors derived from the aqueous humor. These growth factors may interact with TGFβ and/or BMP-7 in an antagonistic or synergistic manner; thus, adding to the complexity of effectively inhibiting EMT. For example, fibroblast growth factor has been found to exacerbate the cataractogenic effect of TGFβ2 in cultured rat lenses.34 The major advantage of our rat lens epithelial explant system is that it enables the controlled application and study of growth factors in isolation and/or in combination, compared to the in vivo system. Our future research will involve characterizing the combined actions of other growth factors in the aqueous humor with TGFβ2 and/or BMP-7 in regulating the EMT process.35
Bone morphogenetic protein signaling is regulated by several secreted extracellular agonists and antagonists of BMPs.36 While molecules, such as chordin, noggin, gremlin, and DAN/Cerberus, inhibit the binding of BMP to its receptor, other molecules, such as Kielin/chordin-like protein, are enhancers of BMP signaling, increasing BMP binding to its receptor.36 Gremlin, an extracellular and intracellular BMP antagonist, has been shown to increase ECM synthesis in trabecular meshwork cells.37,38 In human lens epithelial cells, silencing gremlin effectively inhibited TGFβ2-induced EMT and ECM synthesis.39 It is possible that inhibition of gremlin may abolish the inhibitory effect of endogenous BMP on TGFβ2, thereby blocking EMT. By investigating the role of these extracellular regulators of BMP signaling in the lens, we can potentially augment the antagonistic effect of BMP-7 on TGFβ-induced EMT. Future studies should also aim to explore the role of other BMP isoforms found in the lens, including BMP-2 and BMP-4,14 in inhibiting EMT, to investigate whether the inhibitory capacity of BMP is unique to BMP-7. The expression levels of BMP agonists/antagonists, BMP receptors, and other BMP isoforms may influence the efficacy of BMP-7 in inhibiting TGFβ-induced EMT. Hence, further investigation is required to understand the tight regulation of BMP signaling before there is potential for clinical application.
Bone morphogenetic protein-7 is an endogenous growth factor that has a crucial role during lens embryogenesis and development. By exploiting this pre-existing BMP pathway in the lens through exogenous administration of recombinant human BMP-7 to lens epithelial explants, we have shown that TGFβ-driven EMT leading to cataract can be inhibited. Recombinant human BMP-7 already has been approved by the United States Food and Drug Administration for bone regeneration, and clinical trials of a BMP-7 analog have been launched to evaluate its efficacy on diabetic nephropathy.40 Bone morphogenetic protein-7, hence, shows promise as a novel pharmaceutical agent in the prevention of ASC and PCO. Future studies will focus on extending these findings to in vivo models, characterizing the complex regulation of BMP signaling and identifying specific BMP-7 target genes mediating its protective effects.
Acknowledgments
Supported by the National Institutes of Health (NIH; Bethesda, MD, USA; R01 EY0-3177; McAvoy and FJL) and the Rebecca L. Cooper Foundation, Australia (FJL), and by an Australian Postgraduate Award PhD scholarship and a Sydney Eye Hospital Foundation Postgraduate scholarship (DYS).
Disclosure: D.Y. Shu, None; M.C. Wojciechowski, None; F.J. Lovicu, None
References
- 1. Foster A,, Renikoff S. The impact of Vision 2020 on global blindness. Eye. 2005; 19: 1133–1135. [DOI] [PubMed] [Google Scholar]
- 2. Awasthi N,, Guo S,, Wagner BJ. Posterior capsular opacification: a problem reduced but not yet eradicated. Arch Ophthalmol. 2009; 127: 555–562. [DOI] [PubMed] [Google Scholar]
- 3. de Iongh RU,, Wederell E,, Lovicu FJ,, McAvoy JW. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs. 2005; 179: 43–55. [DOI] [PubMed] [Google Scholar]
- 4. Wormstone IM,, Tamiya S,, Anderson I,, Duncan G. TGF-beta2-induced matrix modification and cell transdifferentiation in the human lens capsular bag. Invest Ophthalmol Vis Sci. 2002; 43: 2301–2308. [PubMed] [Google Scholar]
- 5. Hales AM,, Chamberlain CG,, Dreher B,, McAvoy JW. Intravitreal injection of TGFβ induces cataract in rats. Invest Ophthalmol Vis Sci. 1999; 40: 3231–3236. [PubMed] [Google Scholar]
- 6. Lovicu FJ,, Schulz MW,, Hales AM,, et al. TGFbeta induces morphological and molecular changes similar to human anterior subcapsular cataract. Br J Ophthalmol. 2002; 86: 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robertson J,, Nathu Z,, Najjar A,, Dwivedi DJ,, Gauldie J,, West-Mays J. Adenoviral gene transfer of bioactive TGFbeta1 to the rodent eye as a novel model for anterior subcapsular cataract. Mol Vis. 2007; 27: 457–469. [PMC free article] [PubMed] [Google Scholar]
- 8. Luo G,, Hofmann C,, Bronckers AL,, Sohocki M,, Bradley A,, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995; 9: 2808–2820. [DOI] [PubMed] [Google Scholar]
- 9. Wawersik S,, Purcell P,, Rauchman M,, Dudley AT,, Robertson EJ,, Maas R. BMP7 acts in murine lens placode development. Dev Biol. 1999; 207: 176–188. [DOI] [PubMed] [Google Scholar]
- 10. Huang J,, Liu Y,, Filas B,, Gunhaga L,, Beebe DC. Negative and positive auto-regulation of BMP expression in early eye development. Dev Biol. 2015; 407: 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boswell PA,, Lein PJ,, Musil LS. Cross-talk between fibroblast growth factor and bone morphogenetic proteins regulates gap junction-mediated intercellular communication in lens cells. Mol Biol Cell. 2008; 19: 2631–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hung FC,, Zhao S,, Chen Q,, Overbeek PA. Retinal ablation and altered lens differentiation induced by ocular overexpression of BMP7. Vision Res. 2002; 42: 427–438. [DOI] [PubMed] [Google Scholar]
- 13. Belecky-Adams TL,, Adler R,, Beebe DC. Bone morphogenetic protein signalling and the initiation of lens fibre cell differentiation. Development. 2002; 129: 3795–3802. [DOI] [PubMed] [Google Scholar]
- 14. Boswell BA,, Overbeek PA,, Musil LS. Essential role of BMPs in FGF-induced secondary lens fibre differentiation. Dev Biol. 2008; 324: 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wyatt AW,, Osborne RJ,, Stewart H,, Ragge NK. Bone morphogenetic protein 7 (BMP7) mutations are associated with variable ocular, brain, ear, palate, and skeletal anomalies. Hum Mutat. 2010; 31: 781–787. [DOI] [PubMed] [Google Scholar]
- 16. Miyazono K,, Maeda S,, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005; 16: 251–263. [DOI] [PubMed] [Google Scholar]
- 17. Zeisberg M,, Hanai J,, Sugimoto H,, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003; 9: 964–968. [DOI] [PubMed] [Google Scholar]
- 18. Zeisberg M,, Bottiglio C,, Kumar N,, et al. Bone morphogenetic protein-7 inhibits progression of chronic renal fibrosis associated with two genetic mouse models. Am J Physiol Renal Physiol. 2003; 285: F1060–F1067. [DOI] [PubMed] [Google Scholar]
- 19. Morrissey J,, Hruska K,, Guo G,, Wang S,, Chen Q,, Klahr S. Bone morphogenetic protein-7 improves renal fibrosis and accelerates the return of renal function. J Am Soc Nephrol. 2002; 13: S14–S21. [PubMed] [Google Scholar]
- 20. Saika S,, Ikeda K,, Yamanaka O,, et al. Therapeutic effects of adenoviral gene transfer of bone morphogenetic protein-7 on a corneal alkali injury model in mice. Lab Investig. 2005; 85: 474–486. [DOI] [PubMed] [Google Scholar]
- 21. Fuchshofer R,, Yu AHL,, Welge-Lussen U,, Tam ER. Bone morphogenetic protein-7 is an antagonist of transforming growth factor-beta2 in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007; 48: 715–726. [DOI] [PubMed] [Google Scholar]
- 22. Kowanetz M,, Valcourt U,, Bergstrom R,, Heldin CH,, Moustakas A. Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor beta and bone morphogenetic protein. Mol Cell Biol. 2004; 24: 4241–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saika S,, Ikeda K,, Yamanaka O,, et al. Adenoviral gene transfer of BMP-7, Id2, or Id3 suppresses injury-induced epithelial-to-mesenchymal transition of lens epithelium in mice. Am J Physiol Cell Physiol. 2006; 290: C282–C289. [DOI] [PubMed] [Google Scholar]
- 24. West-Mays JA,, Pino G,, Lovicu FJ. Development and use of the lens epithelial explant system to study lens differentiation and cataractogenesis. Prog Retin Eye Res. 2010; 29: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maruno KA,, Lovicu FJ,, Chamberlain CG,, McAvoy J. Apoptosis is a feature of TGFβ-induced cataract. Clin Exp Optom. 2002; 85: 76–82. [DOI] [PubMed] [Google Scholar]
- 26. Marcantonio JM,, Rakic JM,, Vrensen GFJM,, Duncan G. Lens cell populations studied in human donor capsular bags with implanted intraocular lenses. Invest Ophthalmol Vis Sci. 2000; 41: 1130–1141. [PubMed] [Google Scholar]
- 27. Miyazono K,, Kamiya Y,, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010; 147: 35–51. [DOI] [PubMed] [Google Scholar]
- 28. Izumi N,, Mizuguchi S,, Inagaki Y,, et al. BMP-7 opposes TGFβ1-mediated collagen induction in mouse pulmonary myofibroblasts through Id2. Am J Physiol Lung Cell Mol Physiol. 2005; 290: L120–L126. [DOI] [PubMed] [Google Scholar]
- 29. Wormstone IM,, Anderson IK,, Eldred JA,, Dawes LJ,, Duncan G. Short-term exposure to transforming growth factor β induced long-term fibrotic responses. Exp Eye Res. 2006; 83: 1238–1245. [DOI] [PubMed] [Google Scholar]
- 30. Wallentin N,, Wickstrom K,, Lundberg C. Effect of cataract surgery on aqueous TGF-beta and lens epithelial cell proliferation. Invest Ophthalmol Vis Sci. 1998; 39: 1410–1418. [PubMed] [Google Scholar]
- 31. Kato N,, Shimmura S,, Kawakita T,, Miyashita H,, Ogawa Y,, Yoshida S,, et al. Beta-catenin activation and epithelial-mesenchymal transition in the pathogenesis of pterygium. Invest Ophthalmol Vis Sci. 2007; 48: 1411–1417. [DOI] [PubMed] [Google Scholar]
- 32. Yokota Y. Id and development. Oncogene. 2001; 20: 8290–8298. [DOI] [PubMed] [Google Scholar]
- 33. Ling MT,, Wang X,, Tsao SW,, Wong YC. Down-regulation of Id-1 expression is associated with TGFβ1-induced growth arrest in prostate epithelial cells. BBA-Gen Subjects. 2002; 1570: 145–152. [DOI] [PubMed] [Google Scholar]
- 34. Cerra A,, Mansfield KJ,, Chamberlain CG. Exacerbation of TGFβ-induced cataract by FGF-2 in cultured rat lenses. Mol Vis. 2003; 9: 689–700. [PubMed] [Google Scholar]
- 35. Iyengar L,, Patkunanathan B,, McAvoy JW,, Lovicu FJ. Growth factors involved in aqueous humour-induced lens cell proliferation. Growth Factors. 2009; 27: 50–62. [DOI] [PubMed] [Google Scholar]
- 36. Yanagita M. BMP antagonists: their roles in development and involvement in pathophysiology. Cytokine Growth Factor Rev. 2005; 16: 309–317. [DOI] [PubMed] [Google Scholar]
- 37. Wordinger RJ,, Fleenor DL,, Hellberg PE,, et al. Effects of TGF-β2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest Ophthalmol Vis Sci. 2007; 48 3: 1191–1200. [DOI] [PubMed] [Google Scholar]
- 38. Sethi A,, Jain A,, Zode GS,, Wordinger RJ,, Clark AF. Role of TGFbeta/Smad signaling in gremlin induction of human trabecular meshwork extracellular matrix proteins. Invest Ophthalmol Vis Sci. 2011; 52: 5251–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ma B,, Kang Q,, Qin L,, Cui L,, Pei C. TGF-β2 induces transdifferentiation and fibrosis in human lens epithelial cells via regulating gremlin and CTGF. Biochem Biophys Res Commun. 2014; 447: 689–695. [DOI] [PubMed] [Google Scholar]
- 40. Li RX,, Yiu WH,, Tang SCW. Role of bone morphogenetic protein-7 in renal fibrosis. Front Physiol. 2015; 6: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]