Abstract
Purpose
To screen for and characterize compounds that protect corneal endothelial cells against unfolded protein response (UPR) and oxidative stress.
Methods
Bovine corneal endothelial cells (BCECs) were treated for 48 hours with 640 compounds from a Food and Drug Administration (FDA)-approved drug library and then challenged with thapsigargin or H2O2 to induce UPR or oxidative stress, respectively. Cell viability was measured using the CellTiter-Glo survival assay. Selected “hits” were subjected to further dose-response testing, and their ability to modulate expression of UPR and oxidative stress markers was assessed by RT-PCR, Western blot, and measurement of protein carbonyl and 8-hydroxydeoxyguanosine (8-OHdG) adducts in immortalized human corneal endothelial cells (iHCECs).
Results
Forty-one drugs at 20 μM and 55 drugs at 100 μM increased survival of H2O2-challenged cells, and 8 drugs at 20 μM and 2 drugs at 100 μM increased survival of thapsigargin-challenged cells, compared with untreated control cells. Nicergoline, ergothioneine, nimesulide, oxotremorine, and mefenamic acid increased survival of both H2O2- and thapsigargin-challenged cells. Oxotremorine altered DNA damage inducible 3 (CHOP) gene expression, glucose-regulated protein 78 kDa (GRP78) and activating transcription factor 4 (ATF4) protein expression, and protein carbonyl and 8-OHdG levels. Mefenamic acid altered GRP78 protein expression and protein carbonyl and 8-OHdG levels.
Conclusions
Oxotremorine and mefenamic acid are potential survival factors for corneal endothelial cells under UPR and oxidative stress. The described assay can be further expanded to screen additional drugs for potential therapeutic effect in corneal endothelial diseases such as Fuchs' endothelial corneal dystrophy.
Keywords: drug screening, corneal endothelial cells, unfolded protein response, oxidative stress, Fuchs' endothelial corneal dystrophy
Fuchs' endothelial corneal dystrophy (FECD) is a common cause of corneal vision loss characterized by corneal endothelial (CE) cell loss and abnormalities in Descemet membrane.1–3 Early disease stages are asymptomatic, but in the disease course corneal edema and loss of vision occur approximately 20 years after onset.4 Fuchs' endothelial corneal dystrophy is among the leading causes of primary corneal transplant surgery in Europe and the United States, and so far there is no definitive conservative (nonsurgical) treatment.5 Evidence of oxidative stress in the pathogenesis of FECD has been described.6,7 Unfolded protein response (UPR) activation leads to endothelial cell apoptosis in Fuchs' dystrophy and may play a central pathogenic role in this disease.8,9
Koh and Waschek10 have described the survival effects of vasoactive intestinal peptide for cultured corneal endothelial cells undergoing oxidative stress with H2O2. High-throughput screening (HTS) is being used more commonly to identify novel therapeutic compounds.11 Given the previous evidence for endoplasmic reticulum (ER) and oxidative stress in the pathogenesis of FECD, we sought to develop an efficient system to screen a commercially available drug library of Food and Drug Administration (FDA)-approved drugs for improved survival of cultured corneal endothelial cells undergoing ER and oxidative stress. Positive drugs were further tested for dose-response effects and alterations in ER and oxidative stress specific markers.
The approach described in the present work identified mefenamic acid as a drug with positive and specific survival effects against ER and oxidative stress in cultured corneal endothelial cells. This method could be extended to identify drugs with similar effects on known or yet to be elucidated pathophysiologic mechanisms in corneal endothelial diseases. The overall purpose of this study was to apply a rational approach to drug discovery/screening, which could yield additional agents with the potential to alter the disease course and form the basis of nonsurgical treatments for these disorders.
Methods
Cell Culture
Primary cultures of bovine corneal endothelial cells (BCECs) from freshly enucleated globes were established by scraping Descemet membrane of excised corneas with a surgical blade and resuspending cells in Dulbecco's modification of Eagle's minimum essential medium (DMEM; Cellgro, Manassas, VA, USA) supplemented with 10% fetal calf serum, antibiotic/antimycotic solution (10,000 units of penicillin [base], 10,000 μg of streptomycin [base], and 25 μg of amphotericin B/mL; Invitrogen, Grand Island, NY, USA) and 50 μg/mL of gentamicin (Invitrogen). Cells were maintained at 37°C with 5% CO2 in air on a 6-well plate (Cytoone, Orlando, FL, USA). Once confluence was reached (usually 7–10 days), the primary cultures were subcultured to 96-well plates (Cytoone) in the same medium.
Immortalized human corneal endothelial cell line (iHCECs)12 was kindly provided by Ula Jurkunas, MD (Schepens Eye Research Institute, Massachusetts Eye and Ear Infirmary, Harvard Medical School, Boston, MA, USA). Cells were grown in OptiMEM-1 (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 8% fetal calf serum, antibiotic/antimycotic solution (10,000 units of penicillin [base], 10,000 μg of streptomycin [base], and 25 μg of amphotericin B/mL; Invitrogen), 50 μg/mL of gentamicin (Invitrogen), 5 ng/mL of epidermal growth factor (EGF; Sigma-Aldrich Corp., St. Louis, MO, USA), 20 ng/mL of nerve growth factor (NGF; Alfa Aesar, Haverhill, MA, USA), 100 μg of pituitary extract (Alfa Aesar), 20 μg/mL of ascorbic acid (Sigma-Aldrich Corp.), 200 mg/L of calcium chloride (Sigma-Aldrich Corp.), and 0.08% of chondroitin sulfate (Sigma-Aldrich Corp.). Cells were maintained at 37°C with 5% CO2 in air on a 6-well plate and subcultured every 2 days. Once confluence was reached, iHCECs were subcultured to 96-well plates (Cytoone) in the same medium.
Cytotoxic Assay With H2O2 and Thapsigargin
At 48-hours post-seeding BCECs and iHCECs in triplicate 96-well plates, cells were treated with 100-μL medium containing thapsigargin (0, 6, 10, 20, 30, 50, and 100 μM for BCECs; 0, 2.5, 5.0, 7.5, 10.0, 12.5, 15.0, 17.5, 20.0, 25.0, and 30.0 μM for iHCECs; Sigma-Aldrich Corp.) for 24 hours or H2O2 (0, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, and 0.9 mM for BCECs; 0, 0.1, 0.125, 0.15, 0.175, 0.2, 0.225, 0.25, 0.30, and 0.35 mM for iHCECs; Thermo Fisher Scientific) for 4 hours at 37°C in a humidified 5% CO2 atmosphere. Thapsigargin was initially resuspended in dimethyl sulfoxide (DMSO; Sigma-Aldrich Corp.) at a stock concentration of 10 mM. Lethal dose (LD50) values of both thapsigargin and H2O2 were determined by calculating mean cell viability (see below) of triplicate wells relative to untreated cells.
Compound Library
Bovine corneal endothelial cells were used in these experiments. A commercially available FDA-approved drug library was obtained (Enzo Life Science, Inc., Farmingdale, NY, USA) consisting of 640 drugs and bioactive compounds. All drugs were dissolved in 100% DMSO at 10-mM concentration and all have known biological activity. Each drug was tested in triplicate at a 1:100 dilution (100 μM with 1% DMSO) or a 1:500 dilution (20 μM with 0.2% DMSO) in cell culture medium. DMSO-treated control cells were tested in triplicate at 1% or 0.2% DMSO in cell culture medium. After pretreatment with drug for 48 hours, medium in all wells was replaced with thapsigargin (25 μM) in fresh medium for 24 hours or H2O2 (0.6 mM) in fresh medium for 4 hours at 37°C in a humidified 5% CO2 atmosphere. For the compound library screening and all subsequent experiments, drugs tested were dissolved in DMSO. Thus, no drug-treated control cells were incubated in equivalent concentrations of DMSO as experimental cells to avoid artifactitious results from effect of the drug vehicle.
Luminescence Assay
Bovine corneal endothelial cells were used in these experiments. At the end of incubation with drug and toxin (thapsigargin or H2O2), cell viability was determined by adding to each well 100 μL of CellTiter-Glo luminescent reagent (Promega, Madison, WI, USA), which produces light in direct proportion to the amount of ATP and viable cells present. Luminescence was measured in relative light units using a FLUO star OPTIMA luminometer (Optima, Tokyo, Japan). Drugs associated with increased luminescence (cell survival) compared with no drug-treated controls exposed to H2O2 and thapsigargin were selected for further study. Results were calculated as mean luminescence of treated replicates/mean luminescence of control replicates ×100. Control replicates were cultured in cell culture medium without H2O2, thapsigargin, or other additional agents. Level of cell protection for each agent (proportion of increased cell survival) against oxidative stress or UPR was assessed as grade 0 (no protection), less than 5%; grade 1 (low protection), 5% to 24%; grade 2 (medium protection), 25% to 44%; grade 3 (high protection), 45% to 69%; grade 4 (very high protection), 70% or more compared with cells with H2O2 or thapsigargin and no additional agent.11
Dose Response Assays
After initial selection of drugs with medium to high levels of protection (grade 2–3), additional dose response assays were performed with BCECs to determine the effective dose range for each protective drug and to determine the efficacy of an optimal dose of drug against oxidative stress and UPR. The following drugs were tested (all from Sigma-Aldrich Corp.): oxotremorine, mefenamic acid, nimesulide, and diclofenac. Each drug was resuspended in DMSO at a stock concentration of 10 mM and diluted in cell culture medium. Oxotremorine, mefenamic acid, and nimesulide were retested in quadruplicate at 0.0-, 0.3-, 1.0-, 3.0-, 10.0-, 30.0-, 60.0- (oxotremorine and mefenamic acid only), and 100-μM (nimesulide only) concentrations for both H2O2 and thapsigargin. Diclofenac was tested in quadruplicate at 0.0-, 0.05-, 0.1-, 0.2-, 0.3-, and 1.0-μM concentrations for H2O2 and 0.0-, 0.3-, 1.0-, 3.0-, 10.0-, 30.0-, 100.0-, and 300.0-μM concentrations for thapsigargin.
Dose response assays were performed under the same H2O2 or thapsigargin conditions as used above in the compound library screening. Negative control cells with no drug treatment and no H2O2 or thapsigargin conditioning were also included to determine baseline cell viability (luminescence). Relative cell viability (%) for each drug and concentration was calculated as mean luminescence in H2O2 or thapsigargin conditioned cells/mean luminescence in negative controls × 100.
Additionally, iHCECs were used to confirm the effectiveness of oxotremorine and mefenamic acid. Oxotremorine was tested in quadruplicate at 0.0- and 60.0-μM concentrations for H2O2 (0.18 mM) and 0.0- and 200.0-μM concentrations for thapsigargin (27.5 μM). Mefenamic acid was tested in quadruplicate at 0.0- and 30.0-μM concentrations for H2O2 (0.18 mM) and 0.0- and 20.0-μM concentrations for thapsigargin (27.5 μM).
Real-Time PCR
Immortalized HCECs cultured in triplicate 6-well plates were pretreated with 50.0-μM oxotremorine, 20.0-μM mefenamic acid, or no drug (only 0.5% and 0.2% DMSO, respectively) for 48 hours followed by treatment with thapsigargin (2.5 μM) for 24 hours at 37°C in a humidified 5% CO2 atmosphere. RNA samples were extracted from iHCECs with TRIzol (Ambion, Austin, TX, USA) using the RNAeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. A 100-ng quantity of RNA was reverse transcribed using a High Capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). Real time-PCR was performed using the TaqMan Gene Expression Master Mix (2×; Applied Biosystems), preamplified cDNA products (diluted 1:20; 5 uL), nuclease free water, and human-specific primers (Supplementary Table S1; Applied Biosystems) in a 20-μL reaction volume. A no-template control was included in each experiment to confirm the absence of DNA contamination. All assays used similar amplification efficiency, and a ΔCT experimental design was used for relative quantification. Data analysis was performed using StepOne software (Version 2.2; Applied Biosystems). Data were normalized to GAPDH and are expressed as relative expression compared with cells untreated with thapsigargin, oxotremorine, or mefenamic acid.
Western Blot
Immortalized HCECs cultured in triplicate 6-well plates were pretreated with 50.0-μM oxotremorine, 20.0-μM mefenamic acid, or no drug for 48 hours followed by treatment with thapsigargin (2.5 μM) for 24 hours at 37°C in a humidified 5% CO2 atmosphere. Cells were lysed with ice-cold Tissue Protein Extraction Reagent (Thermo Fisher Scientific) containing protease inhibitor (1%) and EDTA (1%). Total protein concentration was measured using a protein assay kit (Thermo Fisher Scientific) and each sample was adjusted to 20 μg/mL. Proteins were subjected to SDS-PAGE (Mini-PROTEAN TGX Gels; Bio-Rad, Hercules, CA, USA) and transferred to polyvinvlidene fluoride membranes (The PerfectMembrane; GenHunter Corporation, Nashville, TN, USA) that had been soaked in methanol for 1 minute. After blocking with 5% milk for 1 hour, the membranes were then incubated overnight with rabbit anti-ATF4 antibody (1:1000 dilution; Cell Signaling Technology, Danvers, MA, USA), or rabbit anti-GRP78 antibody (1:1000; Cell Signaling Technology). The membranes were then incubated with horseradish peroxidase-conjugated donkey anti-rabbit IgG antibodies (1:10,000 dilution; Cell Signaling Technology) for 30 minutes. The membrane was washed with tris buffered saline with Tween 20 (TBST; Cell Signaling Technology) and antigen was detected using ECL solution (Pierce Biotechnology, Inc., Rockford, IL, USA). Membranes were stripped with Restore Western blotting stripping buffer (Thermo Fisher Scientific) using manufacturer's instructions, reblocked with 5% milk for 1 hour, and restained for GAPDH using rabbit anti-GAPDH antibody (HRP conjugate, 1:10000; Cell Signaling Technology) with 1-hour incubation at room temperature.
ELISA of Oxidative Stress Markers
Immortalized HCECs cultured in triplicate 6-well plates were pretreated with 50.0-μM oxotremorine, 20.0-μM mefenamic acid, or no drug for 48 hours followed by treatment with H2O2 (0.8 mM) for 4 hours at 37°C in a humidified 5% CO2 atmosphere. Protein carbonyls and 8-hydroxydeoxyguanosine (8-OHdG) were evaluated as oxidative stress markers. Protein carbonyls in iHCECs were measured using ELISA (OxiSelect Protein Carbonyl ELISA Kit; Cell Biolabs, San Diego, CA, USA) according to the manufacturer's protocols. Treated cells were lysed with ice-cold Tissue Protein Extraction Reagent (Thermo Fisher Scientific) containing protease inhibitor (1%) and EDTA (1%). Total protein concentration was measured using a protein assay kit (Thermo Fisher Scientific) and each sample was diluted to 10 μg/mL. 8-OHdG in iHCECs was measured by DNA extraction (QIAamp DNA Mini Kit; Qiagen) followed by ELISA (OxiSelect Oxidative DNA Damage ELISA Kit; Cell Biolabs).
Statistical Analysis
Statistical analysis was performed with two-tailed Student's t-test using SPSS (version 16.0; IBM, Armonk, NY, USA) or JMP (version 10.0.2; SAS, Cary, NC, USA) and P less than 0.05 was considered statistically significant.
Results
Preliminary Cytotoxic Assay
Cytotoxic effects of BCECs exposed to H2O2 for 4 hours and thapsigargin for 24 hours showed a dose-dependent response. The LD50 values for H2O2 and thapsigargin against BCECs were 0.6 mM and 25.6 μM, respectively (Supplementary Figs. S1A, S1B). In a similar way, the LD50s for H2O2 and thapsigargin against iHCECs were 0.18 mM and 16.5 μM (data not shown).
Initial Screen
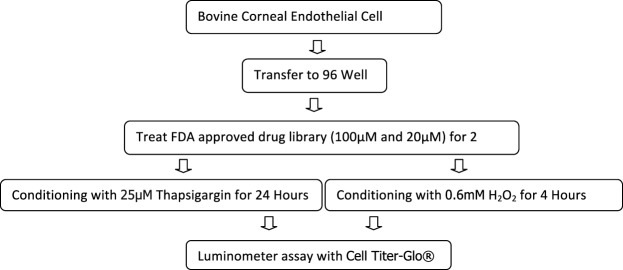
Bovine corneal endothelial cells were used in this screening test. For a flow chart of the initial screening process (Fig. 1). Initial screening of the 640 drug library using conditions established by the preliminary cytotoxic assay demonstrated 55 drugs at 100 μM and 41 at 20 μM with moderate to high levels of increased cell survival (grade 2–3) against H2O2 conditioning. Against thapsigargin conditioning, two drugs at 100 μM and eight at 20 μM demonstrated moderate to high levels of increased cell survival. Overall, 14 drugs demonstrated increased cell survival with both concentrations of drug tested showing a grade 2 to 3 effect against either oxidative stress or UPR (Supplementary Table S2). Five drugs (ergothioneine, nicergoline, nimesulide, mefenamic acid, and oxotremorine) were found to demonstrate a grade 2 to 3 survival effect at one concentration against both oxidative stress and UPR (Supplementary Table S2).
Figure 1.
Protocol for screening drugs with corneal endothelial cell survival effect against unfolded protein response and oxidative stress.
Dose Response Assays
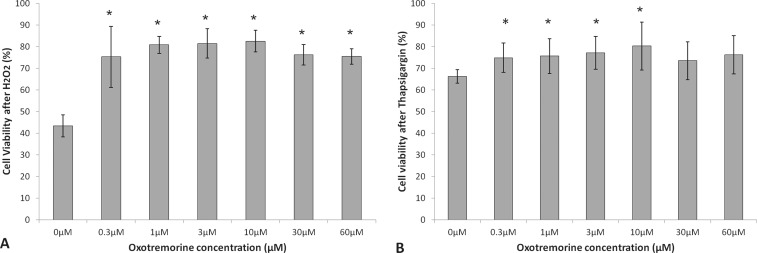
After initial screening of the FDA drug library, dose-response assays with BCECs were performed for the five compounds with survival effects against both of oxidative stress and UPR. Nicergoline and ergothioneine did not exhibit a clear response (data not shown), but oxotremorine, mefenamic acid, and nimesulide exhibited a significant dose response. Cell viability of 0.3- (75.2 ± 14.1%, P = 0.002), 1.0- (80.8 ± 4.0%, P = 0.00001), 3.0- (81.4 ± 6.7%, P = 0.00005), 10.0- (82.5 ± 5.0%, P = 0.00003), 30.0- (76.2 ± 4.7%, P = 0.00004), and 60.0-μM (75.4 ± 3.6%, P = 0.00002) oxotremorine-treated cells against oxidative stress was significantly increased compared with 0.0-μM control (43.3 ± 5.1%; Fig. 2A). Cell viability of 0.3- (74.8 ± 6.7%, P = 0.01), 1.0- (75.6 ± 8.0%, P = 0.02), 3.0- (77.1 ± 7.6%, P = 0.02), and 10.0-μM (80.2 ± 11.0%, P = 0.01) oxotremorine-treated cells against UPR was significantly increased compared with 0.0-μM control (66.3 ± 3.1%; Fig. 2B). Overall oxotremorine demonstrated a maximal effect at 10.0 μM of 90.5% increased cell viability against H2O2 and a maximal effect at 10.0 μM of 21.0% increased cell viability against thapsigargin.
Figure 2.
Cell viability of oxotremorine-treated BCECs after H2O2 (A) or thapsigargin- (B) induced stress. Results are expressed as mean ± SD. *P < 0.05.
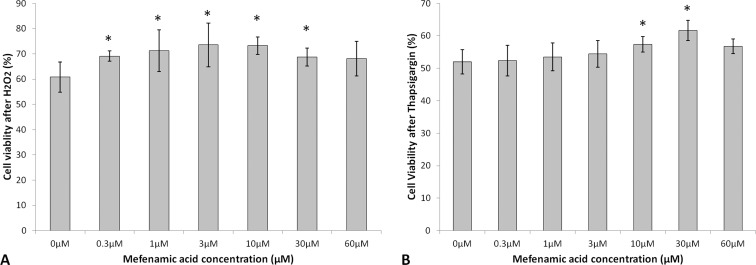
Cell viability of 0.3- (69.1 ± 2.0%, P = 0.02), 1.0- (71.2 ± 8.2%, P = 0.01), 3.0- (73.6 ± 8.7%, P = 0.01), 10.0- (73.2 ± 3.5%, P = 0.005), and 30.0-μM (68.7 ± 3.4%, P = 0.03) mefenamic acid–treated cells against oxidative stress was significantly increased compared with 0.0-μM control (60.8 ± 6.0%; Fig. 3A). Cell viability of 10.0- (57.4 ± 2.4%, P = 0.04) and 30.0-μM (61.7 ± 3.1%, P = 0.04) mefenamic acid–treated cells against UPR was significantly increased compared with 0.0-μM control (51.9 ± 3.7%; Fig. 3B). Overall, mefenamic acid demonstrated a maximal effect at 3.0 μM of 21.1% increased cell viability against H2O2 and a maximal effect at 30.0 μM of 18.9% increased cell viability against thapsigargin.
Figure 3.
Cell viability of mefenamic acid–treated BCECs after H2O2 (A) or thapsigargin- (B) induced stress. Results are expressed as mean ± SD. *P < 0.05.
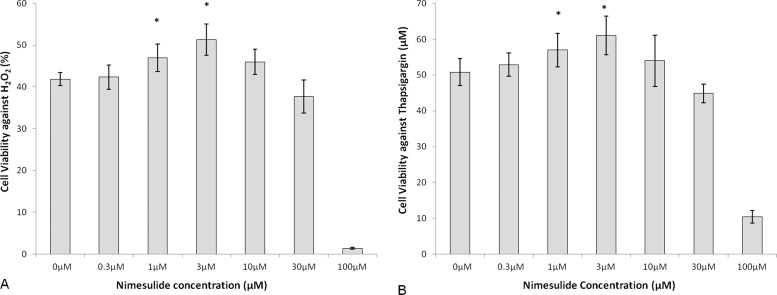
Cell viability of 1.0- (47.0 ± 3.3%, P = 0.01) and 3.0-μM (51.3 ± 3.8%, P = 0.001) nimesulide-treated cells against oxidative stress was significantly increased compared with 0.0-μM control (41.9 ± 1.5%; Fig. 4A). Cell viability of 1.0- (57.0 ± 4.6%, P = 0.04) and 3.0-μM (61.0 ± 5.4%, P = 0.01) nimesulide-treated cells against UPR was significantly increased compared with 0.0-μM control (50.8 ± 3.8%; Fig. 4B). Overall, nimesulide showed a maximal effect at 3.0 μM of 22.4% increased cell viability against H2O2 and 20.1% increased cell viability against thapsigargin.
Figure 4.
Cell viability of nimesulide-treated bovine corneal endothelial cells after H2O2 (A) or thapsigargin- (B) induced stress. Results are expressed as mean ± SD. *P < 0.05.
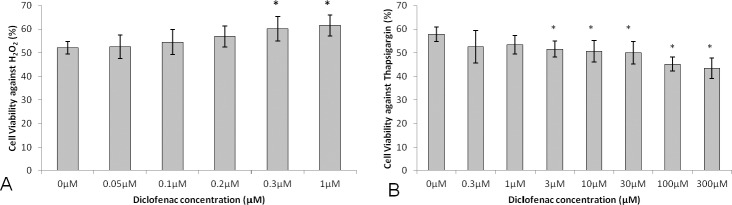
Based on the above results indicating a protective effect of the nonsteroidal anti-inflammatory drugs (NSAIDs) mefenamic acid and nimesulide on BCECs exposed to oxidative stress and UPR, we selected diclofenac, a highly potent NSAID13 for additional dose-response testing. Cell viability of 0.3- (60.1 ± 5.2%, P = 0.01) and 1.0-μM (61.6 ± 4.4%, P = 0.005) diclofenac-treated cells against oxidative stress was significantly increased compared with 0.0-μM control (52.1 ± 2.7%; Fig. 5A). However, cell viability of 3.0- (51.5 ± 3.4%, P = 0.01), 10.0- (50.6 ± 4.6%, P = 0.02), 30.0- (50.0 ± 4.7%, P = 0.01), 100.0- (45.2 ± 2.9%, P = 0.0005), and 300.0-μM (43.4 ± 4.2%, P = 0.0007) diclofenac-treated cells against UPR was significantly decreased compared with 0.0-μM control (57.8 ± 3.1%; Fig. 5B). Overall diclofenac demonstrated a maximal effect at 1.0 μM of 18.2% increased cell viability against H2O2 and a maximal effect at 300 μM of 24.9% decreased cell viability against thapsigargin.
Figure 5.
Cell viability of diclofenac-treated bovine corneal endothelial cells after H2O2 (A) or thapsigargin- (B) induced stress. Results are expressed as mean ± SD. *P < 0.05.
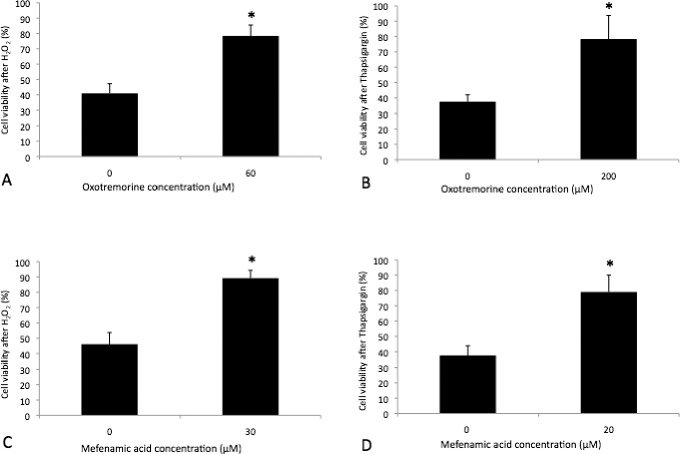
In addition, we sought to characterize potential cell protective effects of oxotremorine and mefenamic acid on HCECs using iHCECs. Cell viability of 60.0-μM (78.4 ± 7.3%, P = 0.0002) oxotremorine-treated cells against oxidative stress was significantly increased compared with 0.0-μM control (40.9 ± 6.3%; Fig. 6A). Cell viability of 200.0-μM (78.4 ± 15.4%, P = 0.03) oxotremorine-treated cells against UPR was significantly increased compared with 0.0-μM control (37.5 ± 4.5%; Fig. 6B). Cell viability of 30.0-μM (89.2 ± 5.1%, P = 0.004) mefenamic acid–treated cells against oxidative stress was significantly increased compared with 0.0-μM control (46.1 ± 7.1%; Fig. 6C). Cell viability of 20.0-μM (79.0 ± 11.0%, P = 0.009) mefenamic acid–treated cells against UPR was significantly increased compared to 0.0-μM control (37.6 ± 6.3%; Fig. 6D).
Figure 6.
Cell viability of oxotremorine-treated iHCECs after H2O2 (A) or thapsigargin- (B) and mefenamic acid–treated iHCECs after H2O2 (C) or thapsigargin- (D) induced stress. Results are expressed as mean ± SD. *P < 0.05.
Effects of Oxotremorine and Mefenamic Acid on UPR Markers
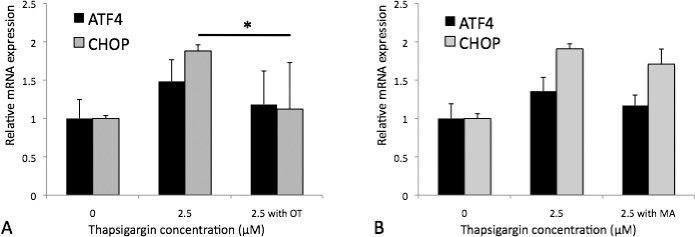
To assess mRNA level effects of oxotremorine and mefenamic acid on UPR markers in HCECs, iHCECs were pretreated with 50.0-μM oxotremorine or 20.0-μM mefenamic acid, exposed to thapsigargin, and real-time PCR was performed for ATF4 and CHOP (Supplementary Table S1), two commonly used markers of UPR.14,15 For thapsigargin-exposed iHCECs, oxotremorine treatment showed a trend for reduced ATF4 mRNA expression to 118.2 ± 43.7% (P = 0.23) compared with untreated controls (148.3 ± 28.3%) and reduced CHOP mRNA expression to 112.3 ± 60.6% (P = 0.024) compared with untreated controls (188.1 ± 8.0%; Fig. 7A). For thapsigargin-exposed iHCECs, mefenamic acid treatment showed trends for reduced ATF4 mRNA expression to 117.0 ± 13.6% (P = 0.10) compared with untreated controls (135.6 ± 18.1%) and reduced CHOP mRNA expression to 170.9 ± 19.7% (P = 0.06) compared with untreated controls (190.9 ± 6.5%; Fig. 7B).
Figure 7.
Reverse transcription–PCR results of 50-μM oxotremorine (OT) (A) and 20-μM mefenamic acid (MA)- (B) treated iHCECs after thapsigargin-induced stress. Results are expressed as mean ± SD. *P < 0.05.
To assess protein level effects of oxotremorine and mefenamic acid on UPR markers in HCECs, iHCECs were pretreated with 50.0-μM oxotremorine or 20.0-μM mefenamic acid, exposed to thapsigargin, and Western blot was performed for ATF4 and GRP78, which are also commonly used markers of UPR.14,15 For thapsigargin-exposed iHCECs, oxotremorine treatment increased GRP78 protein expression to 894.6 ± 123.7% (P = 0.013) compared with untreated controls (462.6 ± 121.9%) and decreased ATF4 protein expression to 3943.6 ± 307.7% (P = 0.039) compared with untreated controls 6465.3 ± 1413.3% (Fig. 8A). For thapsigargin-exposed iHCECs, mefenamic acid treatment increased GRP78 protein expression to 455.0 ± 154.9% (P = 0.038) compared with untreated controls (168.0 ± 52.7%) and showed a trend for decreased ATF4 protein expression to 19354.7 ± 10697.4% (P = 0.44) compared with untreated controls (27061.5 ± 11133.2%; Fig. 8B).
Figure 8.
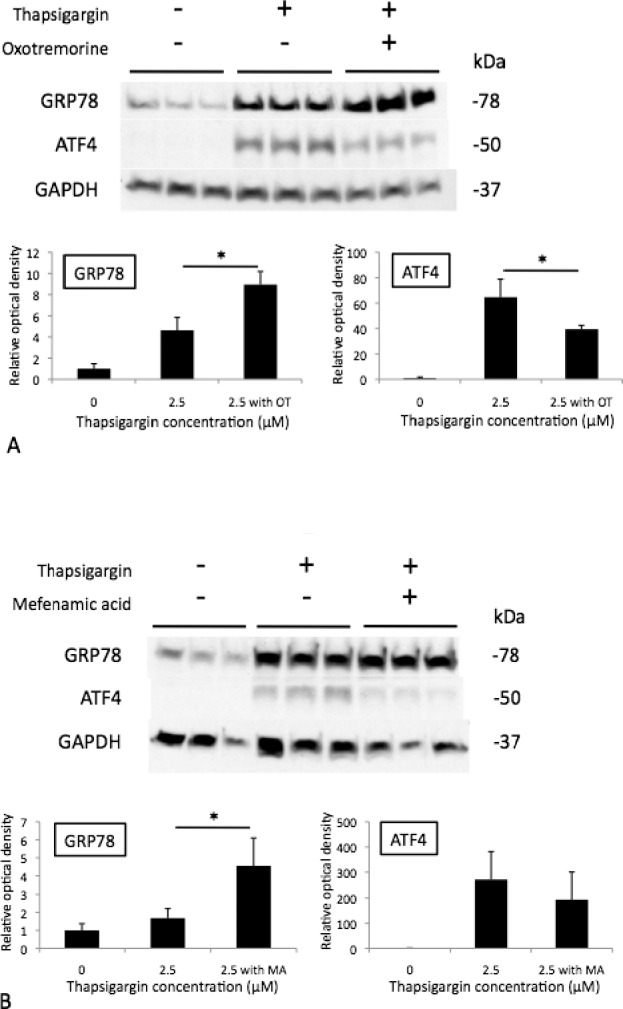
Western blot of 50-μM oxotremorine (OT) (A) and 20-μM mefenamic acid (MA)- (B) treated iHCECs after thapsigargin-induced stress. Results are expressed as mean ± SD. *P < 0.05.
Effects of Oxotremorine and Mefenamic Acid on Oxidative Stress Markers
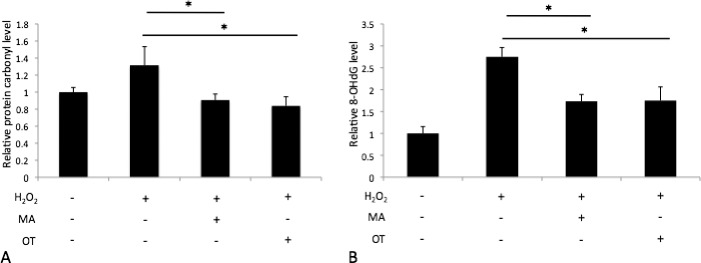
To assess effects of oxotremorine and mefenamic acid on oxidative stress in HCECs, iHCECs were pretreated with 50.0-μM oxotremorine or 20.0-μM mefenamic acid, exposed to H2O2, and protein carbonyl and 8-OHdG levels were measured by ELISA. For H2O2-exposed iHCECs, mefenamic acid treatment reduced protein carbonyl levels to 90.7 ± 7.1% (P = 0.037), and oxotremorine treatment reduced protein carbonyl levels to 83.8 ± 10.7% (P = 0.028) compared with untreated controls (131.5 ± 21.9%; Fig. 9A). For H2O2-exposed iHCECs, mefenamic acid treatment reduced 8-OHdG levels to 173.3 ± 15.7% (P = 0.0008), and oxotremorine treatment reduced 8-OHdG levels to 175.0 ± 31.2% (P = 0.0051) compared with untreated controls (275.0 ± 21.2%; Fig. 9B).
Figure 9.
Protein carbonyl (A) and 8-OHdG (B) semiquantification of 50-μM OT and 20-μM MA-treated iHCECs after H2O2-induced stress. Results are expressed as mean ± SD. *P < 0.05.
Discussion
Fuchs' endothelial corneal dystrophy results from primary loss of corneal endothelial cells and is a common cause of corneal vision loss. Current definitive therapy includes endothelial keratoplasty, and there is no nonsurgical treatment to delay or prevent disease progression. Multiple studies using keratoplasty tissue have shown evidence of oxidative stress in FECD.6,7 We have shown evidence of unfolded protein response activation in FECD patient corneas and in a mouse model of FECD.8,9 Thus, we sought to develop a system to screen for drugs with corneal endothelial cell survival effects against oxidative stress and UPR. The link between oxidative stress and UPR has been established in multiple reports. Holtz et al.16 described oxidative stress-triggered UPR in cell death evoked by Parkinson mimetics. Tumor necrosis factor-alpha has been shown to induce UPR in a reactive oxygen species (ROS) dependent fashion.17 Tagawa et al.18 demonstrated endoplasmic reticulum (ER) stress–mediated apoptosis is induced by cigarette smoke via an ROS-dependent mechanism.19
Our results demonstrate a dose response effect of the muscarinic receptor agonist, oxotremorine, and the NSAIDs, mefenamic acid and nimesulide, against oxidative stress and UPR in a BCEC culture model. Although bovine cells are commonly used in studies of the corneal endothelium,20–22 their use is a potential shortcoming of our system. However, our previous experience with primary cultured human corneal endothelium23–25 indicated potential difficulties obtaining adequate numbers of phenotypically consistent cells for the high throughput scale of these experiments. Thus, we used immortalized corneal endothelial cells12 to confirm dose-responsive survival and further characterize the effects of oxotremorine and mefenamic acid against ER and oxidative stress in human cells. Another potential shortcoming of our system is the use of pretreatment with pharmacologic agents prior to the application of cellular stress. We chose this design expecting it to maximize effects on cell survival. However, clinical situations would likely include the possibility of initiating drug therapy in patients with moderate to advanced disease (i.e., after intermediate to severe levels of cell stress were already established). Thus, follow-up studies in cultured HCECs with pre- and post-stress application of candidate drugs are warranted prior to drawing further implications in diseases such as FECD. A further shortcoming of our work involves short-term responses to relatively high doses of cytotoxic agents. Such a model, though convenient for high throughput analysis, may have limited relevance to FECD, which more likely involves lower levels of chronic cellular stress.
Stimulation of muscarinic receptors provides substantial protection from DNA damage, oxidative stress, and mitochondrial impairment.26 Oxotremorine prevents camptothecin-induced mitochondrial cytochrome c release, Bcl-2 depletion, and Bax accumulation.26 In addition, activation of muscarinic receptors blocks caspase 3 activation.27 The mechanism by which oxotremorine increases survival of cultured corneal endothelial cells treated with the UPR inducer, thapsigargin, is not well understood. Thapsigargin blocks the ability of the cell to pump calcium into the ER, which results in ER damage.28 Oxotremorine has also been shown to cause an increase in intracellular free Ca2+.29 Thus, one could speculate that oxotremorine would synergize with thapsigargin to cause increased cytotoxicity mediated by intracellular calcium levels. This result was not observed in our cell survival data for reasons yet to be elucidated.
Mefenamic acid decreases production of the free radical nitric oxide and reduces cytochrome c release from mitochondria induced by Aβ1-42, Swe-APP, or APP-CTs in neuronal cells.30 Similarly, our results show significantly decreased oxidative stress markers in mefenamic acid–treated iHCECs exposed to H2O2. Thus, our results indicate, for the first time to our knowledge, the ability of mefenamic acid to upregulate the UPR marker GRP78 and to alleviate cytotoxicity caused by the UPR inducer, thapsigargin.
COX-2 inhibitors such as nimesulide act as antioxidants to correct lipid peroxidation products such as malondialdehyde (MDA).31 Unfolded protein response due either to expression of viral surface proteins or drugs can stimulate the expression of COX-2 through the nuclear factor kappa-B and pp38 kinase pathways.32 Thus, NSAIDs may represent an attractive agent to reduce both oxidative stress and UPR in corneal cells in vivo. Additional NSAIDs in our drug library showed a partial protective effect against either H2O2 (celecoxib, diclofenac, fenoprofen, ketoprofen, niflumic acid, piroxicam, and tolfenamic acid) or thapsigargin (aceclofenac). Diclofenac was selected as a potent NSAID for additional follow-up studies in our ER and oxidative stress model system.13 Our results indicate positive cell survival effects against oxidative stress, but decreased survival against UPR (Fig. 5). Diclofenac previously has been shown to reduce superoxide and peroxide production as well as to reduce glutathione depletion in human lens epithelial cells exposed to oxidative stress.13 However, diclofenac also activates the PERK pathway of the UPR, which induces the expression of the proapoptotic GADD153/CHOP.33 Thus, our results indicate differential effects of individual NSAIDs on the responses to ER versus oxidative stress in corneal endothelial cells.
Chiu et al.34 recently used a high throughput assay system and elegant mechanistic studies to identify the NSAID, glafenine, as being able to reduce retention of SLC4A11 mutant protein in the ER and increase post-ER trafficking to the cell membrane. Properly trafficked mutant SLC4A11 was further shown to retain partial function.34 These authors suggested that prostaglandin synthesis may contribute to ER retention of mutant proteins, and a further inference of this work would be that NSAIDs could reduce UPR levels for certain misfolded mutant proteins. Our work using a high throughput drug library screen to identify the positive survival effects of multiple NSAIDS (Supplementary Table S2) against the UPR inducer thapsigargin supports these earlier findings by Chiu et al.34
To our knowledge, this is the first system for screening drugs with corneal endothelial cell survival effect against ER and oxidative stress. Based on our results, it is tempting to speculate that NSAIDs such as mefenamic acid, nimesulide, or others may have potential efficacy as a survival factor for corneal endothelial cells under these stressors in vivo. Our screening system also could be used as the basis of an expanded approach to identify additional therapeutic targets for corneal endothelial diseases.
Supplementary Material
Acknowledgments
Supported by grants from the J. Willard and Alice S. Marriott Foundation (Bethesda, MD, USA), Margaret Andrews, Edward Colburn, Lorraine Collins, Richard Dianich, Mary Finegan, Barbara and Peter Freeman, Stanley Friedler, MD, Ida Jeffries, Herbert Kasoff, Diane Kemker, Jean Mattison, Florenz Ourisman, Lee Silverman, Norman Tunkel, PhD, the National Eye Institute EY 019874 (all to ASJ; Bethesda, MD, USA), Japan Eye Bank Association (Tokyo, Japan) and EBAA Richard Lindstrom Research Grant (TT; Washington, DC, USA), and generous support from the Guerrieri Family Foundation (Salisbury, MD, USA).
Disclosure: E.C. Kim, None; T. Toyono, None; C.A. Berlinicke, None; D.J. Zack, None; U. Jurkunas, None; T. Usui, None; A.S. Jun, None
References
- 1. Borderie VM,, Baudrimont M,, Vallée A,, Ereau TL,, Gray F,, Laroche L. Corneal endothelial cell apoptosis in patients with Fuchs' dystrophy. Invest Ophthalmol Vis Sci. 2000; 41: 2501–2505. [PubMed] [Google Scholar]
- 2. Jun AS. One hundred years of Fuchs' dystrophy. Ophthalmology. 2010; 117: 859–860. [DOI] [PubMed] [Google Scholar]
- 3. Vedana G,, Villarreal G,, Jun AS. Fuchs endothelial corneal dystrophy: current perspectives. Clin Ophthalmol. 2016; 10: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Elhalis H,, Azizi B,, Jurkunas UV. Fuchs endothelial corneal dystrophy. Ocul Surf. 2010; 8: 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Afshari NA,, Pittard AB,, Siddiqui A,, Klintworth GK. Clinical study of Fuchs corneal endothelial dystrophy leading to penetrating keratoplasty: a 30-year experience. Arch Ophthalmol. 2006; 124: 777–780. [DOI] [PubMed] [Google Scholar]
- 6. Jurkunas UV,, Bitar MS,, Funaki T,, Azizi B. Evidence of oxidative stress in the pathogenesis of Fuchs endothelial corneal dystrophy. Am J Pathol. 2010; 177: 2278–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Azizi B,, Ziaei A,, Fuchsluger T,, Schmedt T,, Chen Y,, Jurkunas UV. p53-regulated increase in oxidative-stress–induced apoptosis in Fuchs endothelial corneal dystrophy: a native tissue model. Invest Ophthalmol Vis Sci. 2011; 52: 9291–9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Engler C,, Kelliher C,, Spitze AR,, Speck CL,, Eberhart CG,, Jun AS. Unfolded protein response in Fuchs endothelial corneal dystrophy: a unifying pathogenic pathway? Am J Ophthalmol. 2010; 149: 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jun AS,, Meng H,, Ramanan N,, et al. An alpha 2 collagen VIII transgenic knock-in mouse model of Fuchs endothelial corneal dystrophy shows early endothelial cell unfolded protein response and apoptosis. Hum Mol Genet. 2011; 21: 384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koh SW,, Waschek JA. Corneal endothelial cell survival in organ cultures under acute oxidative stress: effect of VIP. Invest Ophthalmol Vis Sci. 2000; 41: 4085–4092. [PubMed] [Google Scholar]
- 11. Zhang J,, Chung T,, Oldenburg K. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999; 4: 67–73. [DOI] [PubMed] [Google Scholar]
- 12. Schmedt T,, Chen Y,, Nguyen TT,, Li S,, Bonanno JA,, Jurkunas UV. Telomerase immortalization of human corneal endothelial cells yields functional hexagonal monolayers. PLoS One. 2012; 7: e51427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petersen A,, Carlsson T,, Karlsson JO,, Zetterberg M. Intracellular effects of NSAIDs/ASA in oxidatively stressed human lens epithelial cells in culture. Ophthalmic Res. 2008; 40: 77–85. [DOI] [PubMed] [Google Scholar]
- 14. Malhi H,, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011; 54: 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang C,, Jiang K,, Gao D,, et al. Clusterin protects hepatocellular carcinoma cells from endoplasmic reticulum stress induced apoptosis through GRP78. PLoS One. 2013; 8: e55981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holtz WA,, Turetzky JM,, Jong Y-JI,, O'Malley KL. Oxidative stress-triggered unfolded protein response is upstream of intrinsic cell death evoked by parkinsonian mimetics. J Neurochem. 2006; 99: 54–69. [DOI] [PubMed] [Google Scholar]
- 17. Xue X,, Piao J-H,, Nakajima A,, et al. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J Biol Chem. 2005; 280: 33917–33925. [DOI] [PubMed] [Google Scholar]
- 18. Tagawa Y,, Hiramatsu N,, Kasai A,, et al. Induction of apoptosis by cigarette smoke via ROS-dependent endoplasmic reticulum stress and CCAAT/enhancer-binding protein-homologous protein (CHOP). Free Radic Biol Medicine. 2008; 45: 50–59. [DOI] [PubMed] [Google Scholar]
- 19. Huang S-S,, Cheng H,, Tang C-M,, et al. Anti-oxidative, anti-apoptotic, and pro-angiogenic effects mediate functional improvement by sonic hedgehog against focal cerebral ischemia in rats. Exp Neurol. 2013; 247: 680–688. [DOI] [PubMed] [Google Scholar]
- 20. Teo BKK,, Goh KJ,, Ng ZJ,, Koo S,, Yim EKF. Functional reconstruction of corneal endothelium using nanotopography for tissue-engineering applications. Acta Biomater. 2012; 8: 2941–2952. [DOI] [PubMed] [Google Scholar]
- 21. Wang T-J,, Wang I-J,, Lu J-N,, Young T-H. Novel chitosan-polycaprolactone blends as potential scaffold and carrier for corneal endothelial transplantation. Mol Vis. 2012; 18: 255–264. [PMC free article] [PubMed] [Google Scholar]
- 22. Nguyen TT,, Bonanno JA. Bicarbonate, NBCe1, NHE, and carbonic anhydrase activity enhance lactate-H+ transport in bovine corneal endothelium. Invest Ophthalmol Vis Sci. 2011; 52: 8086–8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Engler C,, Kelliher C,, Speck CL,, Jun AS. Assessment of attachment factors for primary cultured human corneal endothelial cells. Cornea. 2009; 28: 1050–1054. [DOI] [PubMed] [Google Scholar]
- 24. Engler C,, Kelliher C,, Wahlin KJ,, Speck CL,, Jun AS. Comparison of non-viral methods to genetically modify and enrich populations of primary human corneal endothelial cells. Mol Vis. 2009; 15: 629–637. [PMC free article] [PubMed] [Google Scholar]
- 25. Suh LH,, Zhang C,, Chuck RS,, et al. Cryopreservation and lentiviral-mediated genetic modification of human primary cultured corneal endothelial cells. Invest Ophthalmol Vis Sci. 2007; 48: 3056–3061. [DOI] [PubMed] [Google Scholar]
- 26. De Sarno P,, Shestopal SA,, King TD,, Zmijewska A,, Song L,, Jope RS. Muscarinic receptor activation protects cells from apoptotic effects of dna damage, oxidative stress, and mitochondrial inhibition. J Biol Chem. 2003; 278: 11086–11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leloup C,, Michaelson DM,, Fisher A,, Hartmann T,, Beyreuther K,, Stein R. M1 muscarinic receptors block caspase activation by phosphoinositide 3-kinase- and MAPK/ERK-independent pathways. Cell Death Differ. 2000; 7: 825–833. [DOI] [PubMed] [Google Scholar]
- 28. Rogers TB,, Inesi G,, Wade R,, Lederer WJ. Use of thapsigargin to study Ca2+ homeostasis in cardiac cells. Biosci Rep. 1995; 15: 341–349. [DOI] [PubMed] [Google Scholar]
- 29. Grudt TJ,, Usowicz MM,, Henderson G. Ca2+ entry following store depletion in SH-SY5Y neuroblastoma cells. Mol Brain Res. 1996; 36: 93–100. [DOI] [PubMed] [Google Scholar]
- 30. Joo Y,, Kim H-S,, Woo R-S,, et al. Mefenamic acid shows neuroprotective effects and improves cognitive impairment in in vitro and in vivo Alzheimer's disease models. Mol Pharmacol. 2006; 69: 76–84. [DOI] [PubMed] [Google Scholar]
- 31. Yesilot S,, Ozer MK,, Bayram D,, Oncu M,, Karabacak HI,, Cicek E. Effects of aspirin and nimesulide on tissue damage in diabetic rats. Cytokine. 2010; 52: 163–167. [DOI] [PubMed] [Google Scholar]
- 32. Hung J-H,, Su I-J,, Lei H-Y,, et al. Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of NF-kappaB and pp38 mitogen-activated protein kinase. J Biol Chem. 2004; 279: 46384–46392. [DOI] [PubMed] [Google Scholar]
- 33. Franceschelli S,, Moltedo O,, Amodio G,, Tajana G,, Remondelli P. In the Huh7 hepatoma cells diclofenac and indomethacin activate differently the unfolded protein response and induce er stress apoptosis. Open Biochem J. 2011; 5: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chiu AM,, Mandziuk JJ,, Loganathan SK,, Alka K,, Casey JR. High throughput assay identifies glafenine as a corrector for the folding defect in corneal dystrophy–causing mutants of SLC4A11 assay for SLC4A11 folding correctors. Invest Ophthalmol Vis Sci. 2015; 56: 7739–7753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.