Abstract
Background
Non-Hispanic blacks and Hispanics with end-stage renal disease have a lower risk of death than non-Hispanic whites, but data on racial/ethnic variation in cardiovascular outcomes for non-dialysis-dependnet chronic kidney disease (CKD) are limited.
Study Design
Prospective cohort
Setting & Participants
3785 adults with entry estimated glomerular filtration rate 20-70 ml/min/1.73 m2 enrolled in the CRIC (Chronic Renal Insufficiency Cohort) Study.
Predictors
Race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic).
Outcomes
Cardiovascular outcomes (atherosclerotic events [myocardial infarction, stroke, or peripheral arterial disease] and heart failure), and a composite of each cardiovascular outcome or all-cause death.
Measurements
Multivariable Cox proportional hazards.
Results
During median follow-up of 6.6 years, we observed 506 atherosclerotic events, 551 heart failure events, and 692 deaths. In regression analyses, there were no significant differences in atherosclerotic events between the three racial/ethnic groups. In analyses stratified by clinical site, non-Hispanic blacks had higher risk for heart failure events (HR, 1.59; 95% CI, 1.29-1.95) which became non-significant after adjustment for demographic factors and baseline kidney function. In contrast, Hispanics had a similar risk for heart failure events as non-Hispanic whites. In analyses stratified by clinical site, compared to non-Hispanic whites, non-Hispanic blacks were at similar risk for atherosclerotic event or death. However, after further adjustment for cardiovascular risk factors, medications, and mineral metabolism markers, non-Hispanic blacks had a 17% lower risk of the outcome (HR, 0.83; 95% CI, 0.69-0.99) than non-Hispanic whites while there was no significant association with Hispanic ethnicity.
Limitations
Hispanics were largely recruited from a single center, and the study was underpowered to evaluate the association between Hispanic ethnicity and mortality.
Conclusions
There were no significant racial/ethnic differences in the adjusted risk of atherosclerotic or heart failure outcomes. Future research is needed to better explain the reduced risk for atherosclerotic event or death in non-Hispanic blacks compared to non-Hispanic whites.
Keywords: race, ethnicity, survival paradox, racial disparities, racial/ethnic variation, dialysis, atherosclerotic cardiovascular events, heart failure, end-stage renal disease (ESRD), Hispanic, CRIC (Chronic Renal Insufficiency Cohort), Hispanic CRIC
Chronic kidney disease (CKD) increases the risks for cardiovascular disease1 and of cardiovascular and all-cause mortality.2-4 The etiology of the high burden of cardiovascular disease in patients with CKD is not completely understood. Traditional and non-traditional cardiovascular risk factors are highly prevalent in patients with CKD and are believed to play an important role in the pathogenesis of cardiovascular disease in patients with CKD.5, 6 Among individuals receiving long-term dialysis therapy, the risk of cardiovascular events and death appears to vary systematically across racial/ethnic groups. It is well established that non-Hispanic blacks and Hispanics with end-stage renal disease (ESRD) receiving dialysis have a lower risk for cardiovascular events and death than non-Hispanic whites.7-10 This phenomenon is often referred to as the black or Hispanic “survival paradox” stemming from opposite observations in the US general population.10, 11 Although these racial disparities are poorly understood, it is likely that they result from a complex interaction between socio-cultural, genetic and environmental factors.12
Existing data about racial/ethnic disparities in non-dialysis-dependent CKD patients in regard to cardiovascular events and mortality are inconclusive. Analyses of data from NHANES (National Health and Nutrition Examination Survey) have shown that blacks/African Americans with CKD younger than 65 years are more likely to die than white individuals.13 In contrast, a recently published study using data from the Kidney Early Evaluation Program (KEEP) found no difference in mortality between African Americans and whites.14 Data from Kaiser Permanente Northern California suggest that Hispanics with CKD have reduced all-cause mortality and cardiovascular events compared with non-Hispanic whites.15 The importance of exploring racial/ethnic differences in cardiovascular risk in CKD is further reinforced by a study by Arce et al which found that patterns of association between eGFR and cardiovascular risk differed in non-Hispanic black and white postmenopausal women enrolled in the Women’s Health Initiative Study.16
The comprehensive longitudinal clinical and demographic data collected by the prospective CRIC (Chronic Renal Insufficiency Cohort) Study provide an opportunity to extend and deepen our knowledge about potential racial/ethnic variation in cardiovascular outcomes in individuals with reduced kidney function. Our goal was to compare the risk of atherosclerotic cardiovascular events and heart failure between three major racial/ethnic groups and understand potentially contributing factors.
Methods
Study Population
The CRIC and Hispanic CRIC are ongoing prospective, observational studies of risk factors for progression of CKD and cardiovascular disease. The design, methods and baseline characteristics of study participants have been previously published.17, 18 Major eligibility criteria included adults aged 21-74 years with mild-to-moderate CKD (estimated glomerular filtration rate [eGFR] range, 20-70 ml/min/1.73m2). As previously reported, CRIC included 170 Hispanics and 3,289 non-Hispanics recruited at seven clinical centers across the United States from May 2003 through March 2007, while Hispanic CRIC included 327 Hispanics recruited at the University of Illinois at Chicago and Chicago metropolitan area from October 2005 through June 2008 (Table S1, available as online supplementary material).19 Exclusion criteria included inability to consent, institutionalization, pregnancy, severe heart failure (New York Heart Association Class III or IV), and certain other severe chronic conditions as noted previously.17, 18, 20 The current analyses include 3785 participants whose self-reported race/ethnicity was non-Hispanic white, non-Hispanic black, or Hispanic. The study protocol was approved by the Institutional Review Board of participating centers (University of Illinois at Chicago approval number 2003-0149) and is in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all participants.
Predictor
Race/ethnicity was self-reported and recorded at the baseline visit. For the purpose of these analyses, it was categorized as follows: non-Hispanic white, non-Hispanic black, and Hispanic.
Covariates
Detailed information on sociodemographics, medical history, and medications were obtained at baseline by self-reported questionnaires.18 Diabetes mellitus was defined by a fasting glucose level ≥126 mg/dL or use of insulin or oral hypoglycemic medications; hypertension, by a systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mmHg, or use of antihypertensive medications. Prior cardiovascular disease was assessed by self-reported history of heart failure, myocardial infarction, coronary revascularization, cerebrovascular accident, peripheral artery revascularization or amputation. The GFR was estimated annually using a CRIC-specific equation which includes serum creatinine, cystatin C, age, gender, and race.21 A 24-hour urine sample collected at study entry was used to measure protein and excretion.
Outcomes
Our main outcomes included atherosclerotic cardiovascular events (myocardial infarction [MI], stroke, or peripheral artery disease); heart failure and two composite outcomes: atherosclerotic event or death from any cause, and heart failure or death from any cause. Ascertainment of ESRD was confirmed by cross-linkage of participants with the US Renal Data System.17 Cardiovascular events were adjudicated by two independent physician reviewers using hospital records. Hospitalizations were self-reported by participants every 6 months by telephone or at in-person follow-up visits. Study personnel identified possible cardiovascular events by reviewing hospital billing codes. Two independent reviewers adjudicated cardiovascular events using hospital records and standardized criteria. Criteria for heart failure events, which were adapted from the Framingham Heart Study criteria,22 included a combination of symptoms (dyspnea on exertion, paroxysmal nocturnal dyspnea, and orthopnea) accompanied by ≥1 of the following findings: 1) radiographic evidence of pulmonary edema or pulmonary congestion; 2) physical examination with two or more findings (rales, S3 gallop, jugular venous distention or peripheral edema); 3) invasive hemodynamic or echocardiographic evidence of heart failure (pulmonary capillary wedge pressure >18 mm Hg, cardiac index <2.0 L/min per square meter of body surface area or left ventricular ejection fraction ≤35%). Criteria for MI included a combination of chest pain, electrocardiography abnormalities, and elevated cardiac biomarkers. Peripheral vascular disease procedures were ascertained using International Classification of Diseases, Ninth Revision, codes. Two neurologists adjudicated cerebrovascular accidents.17 Deaths were ascertained from reports by next of kin, death certificates, hospital records, and linkage with the Social Security Death Master File. Participants were followed up until the occurrence of death, voluntary withdrawal from the study, or May 2012 when the database was locked for analysis. Median follow-up duration was 6.6 (interquartile range [IQR], 5.4-7.6) years. Follow-up for the main analyses was censored at the onset of ESRD because of likely differences in the pathogenesis of cardiovascular events or death in patients after the initiation of long-term dialysis therapy or receipt of a kidney transplant. In addition, we conducted the following sensitivity analyses: 1) follow-up time not censored at the onset of ESRD; and 2) death as a competing risk for atherosclerotic events.23
Statistical Analysis
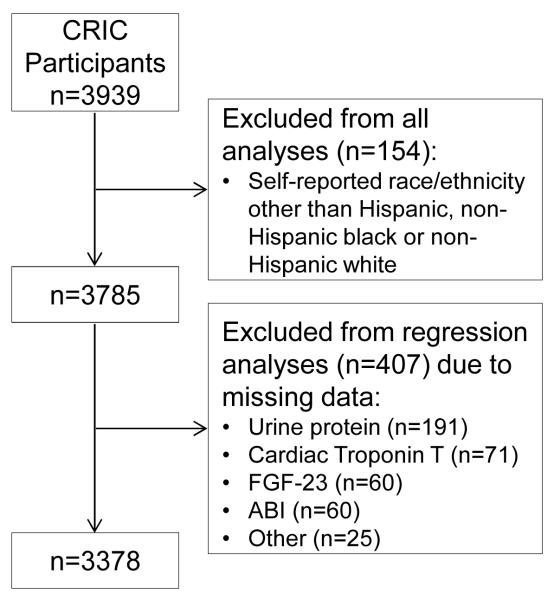
Descriptive statistics were summarized as mean ± standard deviation for continuous variables and frequency (proportion) for categorical variables. If the data distributions were skewed, natural logarithmic transformations were conducted, and/or data were presented as median (IQRs). Chi-squared and analysis of variance tests were used to compare categorical and continuous variables, respectively. Event rates (per 100 person-years) for the time-to-event outcomes were calculated as the ratio of the number of participants reaching the event divided by the total person-years of follow-up before an event or until censoring, and controlling for age, gender, baseline eGFR, diabetes status, and clinical site to account for study design. Cox proportional hazards models were used to examine the association between race/ethnicity and each outcome. For each outcome of interest, we fitted a series of nested regression models that adjusted for the following factors at baseline which were chosen a priori: Model 1 (clinical site); Model 2 includes Model 1 plus sociodemographic characteristics (age, gender, annual income, educational attainment) and nephrology care; Model 3 includes all the variables in Model 2 plus kidney function (eGFR and proteinuria); Model 4 is equal to Model 3 plus traditional cardiovascular risk factors (prior cardiovascular disease, diabetes mellitus, systolic blood pressure, LDL cholesterol, HDL cholesterol, body mass index, ankle-brachial index, tobacco use) and cardiovascular-related medication use (aspirin or antiplatelet agent, angiotensin-converting enzyme [ACE]-inhibitor or angiotensin receptor blocker [ARB], β-blocker, statin); Model 5 is equal to model 4 plus measures of bone and mineral metabolism (serum calcium, serum phosphorus, calcium phosphorus product, fibroblast growth factor 23 [FGF-23], parathyroid hormone [PTH]); Model 6 is equal to model 4 plus measures of anemia and inflammation (hemoglobin level and high-sensitivity C-reactive protein [CRP]); and Model 7 is equal to model 4 plus markers of subclinical ischemia (cardiac troponin I and T levels). We evaluated for effect modification of age, gender, and eGFR on the association between race/ethnicity and each of the outcomes. Absence of violation of the proportional hazards assumption was confirmed in all final models using Schoenfeld residuals and log-log plots. Eleven percent of individuals were excluded from regression models due to missing covariate values. The primary variables that had missing data were urine protein (n=191), troponin T (n=71), FGF-23 (n=60), lowest ankle brachial index (n=60), and other (n=25), leaving 3378 individuals with complete data on all variables (Figure 1). In order to include all of the subjects in the analysis, we used multiple imputation with a total of 10 imputed data sets. Chained equations (full conditional models) were specified, with logistic regression for categorical variables and linear regression for continuous variables. Multiple imputation was implemented in SAS PROC MI and the results were combined using PROC MIANALYZE. All analyses were performed using SAS 9.3 (SAS Institute Inc, Cary, NC).
Figure 1.
Analytic Cohort Flowchart
Results
Baseline Characteristics by Race/Ethnicity
For the 3785 participants included in these analyses, mean age was 58 ± 11 (standard deviation) years, 45% were women; 1638 (43%), non-Hispanic white; 1650 (44%), non-Hispanic black; and 497 (13%), Hispanic. In the Hispanic category, 9 individuals identified themselves as black. In addition, 48% had diabetes, mean eGFR was 45 ±17 ml/min/1.73 m2, and median proteinuria level was 0.18 g/24 hr.
As compared with non-Hispanic whites, both non-Hispanic blacks and Hispanics had lower socioeconomic status, were less likely to be insured, had a higher prevalence of diabetes, higher blood pressure, lower eGFR, more proteinuria, and were more likely have abnormal markers of bone and mineral metabolism (Table 1). In addition, compared with non-Hispanic whites, non-Hispanic blacks were more likely to have a history of heart failure, stroke and be current smokers. As compared with non-Hispanic whites, Hispanics were younger, and were less likely to have a history of MI/revascularization, be current smokers, and receive nephrology care. In terms of cardiovascular disease-related medication use, Hispanics were less likely to be on aspirin or antiplatelet therapy than non-Hispanic whites and blacks. In comparison with non-Hispanic whites, non-Hispanic blacks were more likely to be receiving an ACE-inhibitor or ARB but were less likely to be receiving β-blockers and statins.
Table 1.
Demographic and Clinical Characteristics by Race/Ethnicity
| Variable | 1: Non-Hispanic White |
2: Non-Hispanic Black |
3: Hispanic | P value 1 vs. 2 |
P value 1 vs. 3 |
|---|---|---|---|---|---|
| Number | 1638 | 1650 | 497 | ||
| Age (y) | 58.9 ±11.0 | 58.1 ±10.6 | 56.3 ±11.7 | 0.03 | <0.001 |
| Female sex | 656 (40.0%) | 844 (51.2%) | 209 (42.1%) | <0.001 | 0.4 |
| Annual Income | <0.001 | <0.001 | |||
| ≤$20,000 | 254 (15.5%) | 646 (39.2%) | 313 (63.0%) | ||
| $20,001 - $50,000 | 416 (25.4%) | 417 (25.3%) | 92 (18.5%) | ||
| $50,001 - $100,000 | 455 (27.8%) | 215 (13.0%) | 24 (4.8%) | ||
| >$100,000 | 295 (18.0%) | 62 (3.8%) | 12 (2.4%) | ||
| Missing | 218 (13.3%) | 310 (18.8%) | 56 (11.3%) | ||
| Education | <0.001 | <0.001 | |||
| < High school | 90 (5.5%) | 437 (26.5%) | 293 (59.0%) | ||
| High school graduate | 291 (17.8%) | 366 (22.2%) | 71 (14.3%) | ||
| Some college | 467 (28.5%) | 567 (34.4%) | 78 (15.7%) | ||
| ≥ College graduate | 790 (48.2%) | 280 (17.0%) | 55 (11.1%) | ||
| Insurance Status | <0.001 | <0.001 | |||
| Yes | 1446 (88.3%) | 1340 (81.2%) | 328 (66.0%) | ||
| No | 48 (2.9%) | 96 (5.8%) | 113 (22.7%) | ||
| Missing | 144 (8.8%) | 214 (13.0%) | 56 (11.3%) | ||
| Nephrology care at baseline | 0.2 | <0.001 | |||
| Yes | 1131 (69.0%) | 1107 (67.1%) | 265 (53.3%) | ||
| No | 507 (31.0%) | 543 (32.9%) | 232 (46.7%) | ||
| Medical History | |||||
| Diabetes | 649 (39.6%) | 848 (51.4%) | 335 (67.4%) | <0.001 | <0.001 |
| Current Smoker | 155 (9.5%) | 320 (19.4%) | 29 (5.8%) | <0.001 | 0.01 |
| MI/Prior revascularization | 376 (23.0%) | 361 (21.9%) | 90 (18.1%) | 0.5 | 0.02 |
| Congestive Heart Failure | 117 (7.1%) | 217 (13.2%) | 37 (7.4%) | <0.001 | 0.8 |
| Stroke | 118 (7.2%) | 227 (13.8%) | 37 (7.4%) | <0.001 | 0.9 |
| Peripheral Artery Disease |
105 (6.4%) | 117 (7.1%) | 35 (7.0%) | 0.4 | 0.6 |
| Baseline visit measure | |||||
| Systolic BP (mmHg) | 121.8 ±18.6 | 132.9 ±23.1 | 136.1 ±23.7 | <0.001 | <0.001 |
| Diastolic BP (mmHg) | 69.0 ±11.4 | 73.7 ±13.8 | 72.6 ±12.8 | <0.001 | <0.001 |
| Body Mass Index (kg/m2) | 31.2 ±7.6 | 33.4 ±8.3 | 31.6 ±6.6 | <0.001 | 0.2 |
| Serum Creatinine (mg/dL) |
1.7 ±0.5 | 2.0 ±0.7 | 2.0 ±0.7 | <0.001 | <0.001 |
| eGFR (mL/min/1.73 m2) | 47.7 ±17.1 | 43.5 ±16.3 | 39.0 ±15.2 | <0.001 | <0.001 |
| Urine Protein (g/24 hr) | 0.1 [0.1 - 0.5] | 0.2 [0.1-1.1] | 0.7 [0.1 -3.3] | <0.001 | <0.001 |
| Cholesterol | |||||
| Total (mg/dL) | 180.1 ±41.9 | 185.6 ±45.7 | 189.5 ±53.7 | <0.001 | <0.001 |
| LDL (mg/dL) | 99.4 ±32.1 | 106.1 ±37.2 | 103.7 ±40.0 | <0.001 | 0.01 |
| HDL (gm/dL) | 47.1 ±15.2 | 49.3 ±16.1 | 43.1 ±12.9 | <0.001 | <0.001 |
| Calcium (mg/dl) | 9.2 ±0.5 | 9.2 ±0.5 | 9.0 ±0.5 | 0.4 | <0.001 |
| Phosphate (mg/dL) | 3.6 ±0.6 | 3.8 ±0.7 | 4.0 ±0.7 | <0.001 | <0.001 |
| FGF-23 (RU/ml) | 136.4 [91.5- 217.7] |
150.4 [95.6 - 258.7] |
163.2 [113.8 - 265.8] |
0.005 | <0.001 |
| Total PTH (pg/ml) | 43.0 [30.4 - 68.6] | 67.2 [41.1.-114.9] | 62.0 [41.0-102.0] | <0.001 | <0.001 |
| C-reactive protein (mg/L) | 2.2 [0.9-5.2] | 3.3 [1.3-8.2] | 2.5 [1.0-5.7] | <0.001 | 0.1 |
| Hemoglobin (g/dL) | 13.2 ±1.7 | 12.2 ±1.7 | 12.1 ±1.9 | <0.001 | <0.001 |
| Troponin T (pg/mL) | 15.3 ±19.6 | 23.2 ±34.4 | 28.0 ±35.9 | <0.001 | <0.001 |
| Troponin I (pg/mL) | 8.0±18.4 | 17.9±98.9 | 12.6±44.0 | <0.001 | <0.001 |
| Aspirin or antiplatelet agent use |
748 (46.0%) | 710 (43.3%) | 155 (31.4%) | 0.1 | <0.001 |
| ACE inhibitor or ARB use | 1089 (66.9%) | 1164 (71.1%) | 332 (67.3%) | 0.01 | 0.9 |
| β-Blocker use | 726 (44.6%) | 899 (54.9%) | 236 (47.9%) | <0.001 | 0.2 |
| Statin use | 936 (57.5%) | 852 (52.0%) | 274 (55.6%) | 0.002 | 0.4 |
Note: Values for categorical variables are given as number (percentage); values for continuous variables, as mean ± standard deviation or median [interquartile range]. Conversion factors for units: calcium in mg/dL to mmol/L, ×0.2495; cholesterol in mg/dL to mmol/L, ×0.02586; creatinine in mg/dL to μmol/L, ×88.4. Abbreviations: BP, blood pressure; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; FGF-23, fibroblast growth factor 23; PTH, parathyroid hormone; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; HDL, high-density lipoprotein; LDL, low-density lipoprotein
Outcomes
During a median follow-up of 6.6 (IQR, 5.4-7.6) years, participants had 506 atherosclerotic events, 551 heart failure events, 692 deaths, 999 atherosclerotic event or death, and 1010 heart failure or death. The aggregate retention by racial/ethnic groups for this analytic cohort was 89.1% non-Hispanic white, 87.2% non-Hispanic black, and 89.2% Hispanic.
Atherosclerotic Events
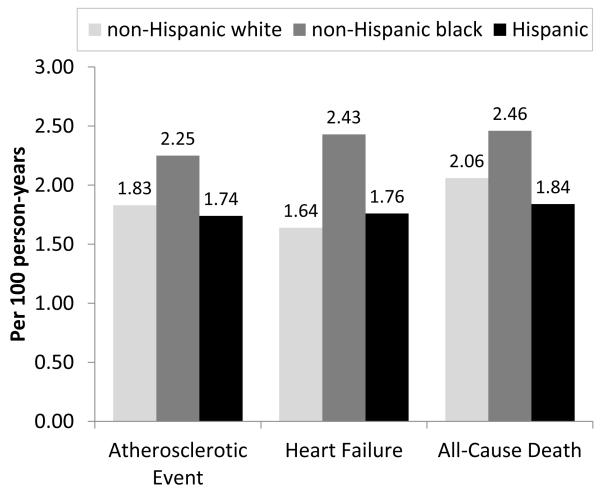
Adjusted atherosclerotic event rates were similar in non-Hispanics whites, non-Hispanic-blacks, and Hispanics (1.83, 2.25, and 1.74 per 100 person-years, respectively; Figure 2). In Cox regression models censored for ESRD and adjusted for clinical center, compared to non-Hispanic whites, there was no significant difference in atherosclerotic event rates (expressed per person-years) for non-Hispanic blacks (HR, 1.08; 95% CI, 0.87-1.34; Table 2) and this did not change after accounting for various potential confounders. Similarly, in Cox models adjusted for clinical center, compared with non-Hispanic whites, there was no significant difference in atherosclerotic event rates for Hispanics (HR, 1.07; 95% CI, 0.63-1.82) and this association remained non-significant with adjustment for potential confounders.
Figure 2.
Event rates by race/ethnicity adjusted for age, gender, baseline eGFR, diabetes, and clinical site.
Table 2.
Association Between Race/Ethnicity and Atherosclerotic and Heart Failure Events
| Atherosclerotic Event | Heart Failure | |||
|---|---|---|---|---|
| Model | Non-Hispanic Black vs. Non-Hispanic White |
Hispanic vs. Non- Hispanic White |
Non-Hispanic Black vs. Non-Hispanic White |
Hispanic vs. Non- Hispanic White |
| 1 | 1.08 (0.87-1.34) | 1.07 (0.63-1.82) | 1.59 (1.29-1.95) | 1.23 (0.77-1.96) |
| 2 | 1.03 (0.82-1.29) | 1.11 (0.64-1.91) | 1.36 (1.09-1.70) | 1.08 (0.67-1.74) |
| 3 | 0.92 (0.73 -1.16) | 0.92 (0.53-1.59) | 1.20 (0.96-1.50) | 0.81 (0.49-1.32) |
| 4 | 0.83 (0.65-1.05) | 0.96 (0.55-1.69) | 1.13 (0.90-1.42) | 0.81 (0.49-1.34) |
| 5 | 0.81 (0.63-1.04) | 0.94 (0.53-1.65) | 1.13 (0.90-1.44) | 0.79 (0.48-1.30) |
| 6 | 0.79 (0.61-1.01) | 0.94 (0.54-1.65) | 1.05 (0.83-1.32) | 0.78 (0.47-1.28) |
| 7 | 0.81 (0.63-1.03) | 0.98 (0.56-1.72) | 1.08 (0.86-1.36) | 0.84 (0.51-1.38) |
Note: Values are given as adjusted hazard ratio (95% confidence interval). Model 1: Clinical site; Model 2: Model 1 + sociodemographic characteristics, nephrology care; Model 3: Model 2+ baseline estimated glomerular filtration rate, proteinuria; Model 4: Model 3 + baseline cardiovascular risk factors, cardiovascular medication; Model 5: Model 4 + baseline markers mineral metabolism; Model 6: Model 4 + measures of anemia and inflammation (hemoglobin and C-reactive protein); Model 7: Model 4 + measures of subclinical ischemia (cardiac troponin I and T)
Heart Failure Events
Heart failure event rates were higher in non-Hispanic blacks than in non-Hispanic whites and Hispanics (2.43 vs. 1.64 and 1.76 per 100 person-years, respectively; Figure 2). In Cox models adjusted for clinical center, compared with non-Hispanic whites, non-Hispanic blacks were at higher risk for heart failure events (HR, 1.59; 95% CI, 1.29-1.95; Table 2). This association remained significant after adjustment for sociodemographic factors and nephrology care (HR, 1.36; 95% CI, 1.09-1.70) but was no longer significant after adjustment for baseline eGFR and proteinuria. In Cox models adjusted for clinical center, compared with non-Hispanic whites, there was no significant difference in heart failure event rates for Hispanics (HR, 1.23; 95% CI, 0.77-1.96) and this association remained non-significant with adjustment for potential confounders.
Cardiovascular Event or All-Cause Death Composite Outcomes
Rates of all-cause death were similar in non-Hispanics whites, non-Hispanic-blacks, and Hispanics (2.06, 2.46, and 1.84 per 100 person-years, respectively; Figure 1). The corresponding rates of the composite atherosclerotic or death outcome were 3.45, 4.33, and 3.22 per 100 person-years, respectively. The corresponding rates of the composite heart failure or death outcome were 3.24, 4.42, and 3.14 per 100 person-years, respectively. In multivariable analyses adjusted for clinic center, we found no significant difference in the risk for the composite of atherosclerotic event or death between non-Hispanic blacks and whites (Table 3). However, after additional adjustment for cardiovascular risk factors, medications, and markers of mineral metabolism, non-Hispanics blacks had a 17% lower adjusted rate of the composite outcome (HR, 0.83; 95% CI, 0.69-0.99). In contrast, the risk for this composite outcome in Hispanics was similar to non-Hispanic whites in both unadjusted and fully adjusted analyses. In multivariable analyses adjusted for clinic center, we found that non-Hispanic blacks were at higher risk or the composite of heart failure or death compared with non-Hispanic whites (HR, 1.37; 95% CI, 1.17-1.60). This association was attenuated and no longer significant after further adjustment. We found no significant difference in the risk for this outcome between Hispanics and non-Hispanic whites.
Table 3.
Association Between Race/Ethnicity and Cardiovascular Events or All-Cause Death
| Atherosclerotic Event or All-Cause Death | Heart Failure or All-Cause Death | |||
|---|---|---|---|---|
| Model | Non-Hispanic Black vs. Non-Hispanic White |
Hispanic vs. Non-Hispanic White |
Non-Hispanic Black vs. Non-Hispanic White |
Hispanic vs. Non-Hispanic White |
| 1 | 1.15 (0.98-1.35) | 1.07 (0.74-1.55) | 1.37 (1.17-1.60) | 1.19 (0.84-1.68) |
| 2 | 1.06 (0.89-1.25) | 1.06 (0.72-1.56) | 1.18 (1.00-1.39) | 1.04 (0.73-1.49) |
| 3 | 0.97 (0.82-1.15) | 0.90 (0.61-1.34) | 1.09 (0.92-1.28) | 0.84 (0.59-1.22) |
| 4 | 0.87 (0.73-1.04) | 0.93 (0.62-1.38) | 1.01 (0.85-1.20) | 0.84 (0.58-1.22) |
| 5 | 0.83 (0.69-0.99) | 0.89 (0.60-1.33) | 0.99 (0.83-1.18) | 0.81 (0.56-1.18) |
| 6 | 0.84 (0.70-1.00) | 0.93 (0.62-1.38) | 0.95 (0.80-1.14) | 0.82 (0.56-1.19) |
| 7 | 0.83 (0.69-1.00) | 0.96 (0.65-1.43) | 0.96 (0.80-1.14) | 0.86 (0.59-1.25) |
Note: Values are given as adjusted hazard ratio (95% confidence interval). Model 1: Clinical site; Model 2: Model 1 + sociodemographic characteristics, nephrology care; Model 3: Model 2+ baseline estimated glomerular filtration rate, proteinuria; Model 4: Model 3 + baseline cardiovascular risk factors, cardiovascular medication; Model 5: Model 4 + baseline markers mineral metabolism; Model 6: Model 4 + measures of anemia and inflammation (hemoglobin and C-reactive protein); Model 7: Model 4 + measures of subclinical ischemia (cardiac troponin I and T)
Sensitivity Analyses
In sensitivity analyses where the follow-up time was not censored at the onset of ESRD, the findings were similar to those in the main analysis. For the outcome of atherosclerotic events, including death as a competing risk did not alter the results. We found no evidence of effect modification by age, gender, and eGFR.
Discussion
In this large diverse prospective cohort of adults with mild-to-moderate CKD, we found no significant racial/ethnic differences in the risk for atherosclerotic events. Non-Hispanic blacks had higher rates of heart failure events. However, after adjustment for sociodemographic factors and baseline kidney function, there was no significant difference in risk for incident heart failure. In addition, we found that compared with non-Hispanic whites, non-Hispanic blacks had a reduced risk for the composite of atherosclerotic event or all-cause death compared with non-Hispanic whites. The risk for the composite of cardiovascular outcomes or death was similar between Hispanics and non-Hispanic whites.
A striking finding of our study was that non-Hispanic blacks with CKD had lower risk for atherosclerotic event or death prior to ESRD than non-Hispanic whites, after accounting for various cardiovascular risk factors and markers of mineral metabolism. Several other studies have found a lower risk of death in non-Hispanic blacks with CKD as compared to non-Hispanic whites.24-26 In a sample of over 500,000 veterans with CKD, blacks had an 18% lower risk of all-cause death compared to whites.26 Similar findings were reported in an analysis of a large Medicare sample population examining long-term survival of individuals after incident MI.24 However, other studies did not observe a survival advantage for blacks.13, 27, 28 In a pooled analysis of the Atherosclerosis Risk in Communities Study, Cardiovascular Health Study, Framingham Heart Study, and the Framingham Offspring Study, Weiner et al found that blacks with CKD were at higher risk for death than whites with CKD.4 However, CRIC participants had more advanced CKD than individuals in these cohorts and this difference may, in part, explain the discordant findings. Future work is needed to better elucidate reasons for a potential survival advantage in blacks in the setting of CKD. For example, one potential explanation for these observations is that only the healthiest black patients survive to more advanced CKD stages and this may not be fully captured through measures of traditional and selected non-traditional risk factors. Nevertheless, our findings that the risk for death was lower after adjustment for traditional cardiovascular risk factors and medications also suggest that strategies aimed at improving access to care and reducing cardiovascular risk could potentially diminish racial disparities in outcomes.
We found that non-Hispanic blacks and non-Hispanic whites were at similar risk for heart failure events contrasts with observations from some general population studies that suggest non-Hispanic blacks are at higher risk for heart failure.29, 30 However, similar to our findings, in the Multi-Ethnic Study of Atherosclerosis, a higher incidence of heart failure among blacks was primarily related to differences in the prevalence of hypertension, diabetes, and socioeconomic status compared to whites.31
At baseline, we found a greater burden of risk factors among Hispanics compared with non-Hispanic whites (i.e., lower eGFR, higher proteinuria, greater abnormality in lipids and markers of bone mineral metabolism). After adjustment for these factors, we found no significant differences in the risk for atherosclerotic cardiovascular events or death between Hispanics and non-Hispanic whites. This contrasts with multiple general population studies reporting that Hispanic ethnicity is associated with a survival advantage despite a higher burden of cardiovascular risk factors among Hispanics. This phenomenon has been referred to as the “Hispanic paradox.”32 Our results also differ from a previous study by Peralta et al of 39,550 patients with CKD stages 3-4 from Kaiser Permanente Northern California, a large integrated health care delivery system, in which Hispanic ethnicity was associated with lower adjusted rates of cardiovascular events and death compared to non-Hispanic whites.33 One potential explanation could be that the Hispanics in CRIC had lower socioeconomic status, less access to care, or lower quality of care as well as more advanced CKD at study entry. Supporting this, Hall et al found that in a low-income community health network, Hispanics had a similar risk of death as non-Hispanics whites.34
Our study has several strengths including the prospective design, long-term follow-up, detailed characterization of a wide range of patient features, and systematic ascertainment and adjudication of cardiovascular events. However, our study also has several limitations. The findings from this cohort of volunteer participants may not be fully generalizable to all US non-Hispanic blacks or Hispanics with CKD. Since the majority of Hispanic participants were recruited from a single center, we were not able to comprehensively evaluate differences between Hispanic background groups. Nonetheless, the composition of Hispanic participants is reflective of the heterogeneity of the US Hispanic population in terms of country of origin, education, income, and primary language.19, 35 In the future, the large ongoing population-based Hispanic Community Health Study/Study of Latinos is likely to provide substantial additional insights into the risk of cardiovascular events in adults with CKD across the major US Hispanic background groups.36 Additional limitations of the study included inclusion of a volunteer cohort rather than a representative population-based sample, which may affect generalizability; lack of data about specific cause of kidney disease; and missing data for income and health insurance in greater than 5% of participants, which precluded their inclusion in multivariable models.
In conclusion, we found that there were no significant racial/ethnic differences in the risk of atherosclerotic or heart failure outcomes in this cohort of individuals with CKD. In addition, we found that in adjusted models, non-Hispanic blacks had a lower risk for the composite of atherosclerotic event or death compared with non-Hispanic whites. Future work is needed to better understand reasons underlying racial/ethnic variation in death in CKD.
Supplementary Material
Acknowledgements
Support: Funding for the CRIC Study was obtained under a cooperative agreement from the US National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award National Institutes of Health (NIH)/National Center for Advancing Translational Sciences UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland General Clinic Research Center M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the NIH and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago Clinical and Translational Science Award UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, Kaiser Permanente Northern California NIH/National Center for Research Resources University of California San Francisco–Clinical and Translational Science Institute UL1 RR-024131. Drs Lash, Ricardo, and Lora are funded by the NIDDK (grants K24DK092290, K23DK094829, and K23DK091313, respectively). Dr Wolf is supported by grants R01DK076116, R01DK081374, R01DK094796, K24DK093723, R21DK100754, and U01DK099930 from the NIH, and a Strategically Focused Research Network Center Grant from the American Heart Association.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: JPL, ACR, ASG; data acquisition: ACR, JPL, RD, MF, JF, JH, MK, CL, AO, MR, SS, MW, JTW, ASG; data analysis/interpretation: ACR, JPL, RD, MF, JF, JH, MK, CL, AO, MR, SS, MW, JTW, ASG; statistical analysis: JPL, ACR, ASG, JR, KT; supervision or mentorship: JPL, ASG. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. JPL and ASG take responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Peer Review: Evaluated by 3 external reviewers, a Statistical Editor, a Co-Editor, and the Editor-in-Chief.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42(5):1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Muntner P, He J, Hamm L, Loria C, Whelton PK. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol. 2002;13(3):745–753. doi: 10.1681/ASN.V133745. [DOI] [PubMed] [Google Scholar]
- 4.Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15(5):1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 5.Collins AJ, Li S, Gilbertson DT, Liu J, Chen SC, Herzog CA. Chronic kidney disease and cardiovascular disease in the Medicare population. Kidney Int Suppl. 2003;(87):S24–S31. doi: 10.1046/j.1523-1755.64.s87.5.x. [DOI] [PubMed] [Google Scholar]
- 6.Sarnak MJ, Coronado BE, Greene T, et al. Cardiovascular disease risk factors in chronic renal insufficiency. Clin Nephrol. 2002;57(5):327–335. doi: 10.5414/cnp57327. [DOI] [PubMed] [Google Scholar]
- 7.Yan G, Norris KC, Yu AJ, et al. The relationship of age, race, and ethnicity with survival in dialysis patients. Clin J Am Soc Nephrol. 2013;8(6):953–961. doi: 10.2215/CJN.09180912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young BA, Rudser K, Kestenbaum B, Seliger SL, Andress D, Boyko EJ. Racial and ethnic differences in incident myocardial infarction in end-stage renal disease patients: The USRDS. Kidney Int. 2006;69(9):1691–1698. doi: 10.1038/sj.ki.5000346. [DOI] [PubMed] [Google Scholar]
- 9.Held PJ, Pauly MV, Diamond L. Survival analysis of patients undergoing dialysis. JAMA. 1987;257(5):645–650. [PubMed] [Google Scholar]
- 10.Arce CM, Goldstein BA, Mitani AA, Winkelmayer WC. Trends in relative mortality between Hispanic and non-Hispanic whites initiating dialysis: a retrospective study of the US Renal Data System. Am J Kidney Dis. 2013;62(2):312–321. doi: 10.1053/j.ajkd.2013.02.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Renal Data System, USRDS . 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2013. USRDS 2013. [Google Scholar]
- 12.Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney Int. 2005;68(3):914–924. doi: 10.1111/j.1523-1755.2005.00485.x. [DOI] [PubMed] [Google Scholar]
- 13.Mehrotra R, Kermah D, Fried L, Adler S, Norris K. Racial differences in mortality among those with CKD. J Am Soc Nephrol. 2008;19(7):1403–1410. doi: 10.1681/ASN.2007070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jolly SE, Burrows NR, Chen SC, et al. Racial and ethnic differences in mortality among individuals with chronic kidney disease: results from the Kidney Early Evaluation Program (KEEP) Clin J Am Soc Nephrol. 2011;6(8):1858–1865. doi: 10.2215/CJN.00500111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peralta CA, Katz R, DeBoer I, et al. Racial and Ethnic Differences in Kidney Function Decline among Persons without Chronic Kidney Disease. Journal of the American Society of Nephrology. 2011;22(7):1327–1334. doi: 10.1681/ASN.2010090960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arce CM, Rhee JJ, Cheung KL, et al. Kidney Function and Cardiovascular Events in Postmenopausal Women: The Impact of Race and Ethnicity in the Women's Health Initiative. Am J Kidney Dis. 2016;67(2):198–208. doi: 10.1053/j.ajkd.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14(7 Suppl 2):S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 18.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer MJ, Go AS, Lora CM, et al. CKD in Hispanics: Baseline Characteristics From the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC Studies. American Journal of Kidney Diseases. 2011;58(2):214–227. doi: 10.1053/j.ajkd.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer MJ, Go AS, Lora CM, et al. CKD in Hispanics: Baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC Studies. Am J Kidney Dis. 2011;58(2):214–227. doi: 10.1053/j.ajkd.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson AH, Yang W, Hsu CY, et al. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012;60(2):250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88(1):107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 23.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 24.Newsome BB, McClellan WM, Coffey CS, Allison JJ, Kiefe CI, Warnock DG. Survival advantage of black patients with kidney disease after acute myocardial infarction. Clin J Am Soc Nephrol. 2006;1(5):993–999. doi: 10.2215/CJN.01251005. [DOI] [PubMed] [Google Scholar]
- 25.Kovesdy CP, Anderson JE, Derose SF, Kalantar-Zadeh K. Outcomes associated with race in males with nondialysis-dependent chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(5):973–978. doi: 10.2215/CJN.06031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovesdy CP, Quarles LD, Lott EH, et al. Survival advantage in black versus white men with CKD: effect of estimated GFR and case mix. Am J Kidney Dis. 2013;62(2):228–235. doi: 10.1053/j.ajkd.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Hernandez GT, O'Hare AM. White/black racial differences in risk of end-stage renal disease and death. Am J Med. 2009;122(7):672–678. doi: 10.1016/j.amjmed.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derose SF, Rutkowski MP, Levin NW, et al. Incidence of end-stage renal disease and death among insured African Americans with chronic kidney disease. Kidney Int. 2009;76(6):629–637. doi: 10.1038/ki.2009.209. [DOI] [PubMed] [Google Scholar]
- 29.Gurwitz JH, Magid DJ, Smith DH, et al. The complex relationship of race to outcomes in heart failure with preserved ejection fraction. Am J Med. 2015;128(6):591–600. doi: 10.1016/j.amjmed.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franciosa JA, Ferdinand KC, Yancy CW. Treatment of heart failure in African Americans: a consensus statement. Congest Heart Fail. 2010;16(1):27–38. doi: 10.1111/j.1751-7133.2009.00118.x. [DOI] [PubMed] [Google Scholar]
- 31.Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168(19):2138–2145. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franzini L, Ribble JC, Keddie AM. Understanding the Hispanic paradox. Ethn Dis. 2001;11(3):496–518. [PubMed] [Google Scholar]
- 33.Peralta CA, Shlipak MG, Fan D, et al. Risks for end-stage renal disease, cardiovascular events, and death in Hispanic versus non-Hispanic white adults with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2892–2899. doi: 10.1681/ASN.2005101122. [DOI] [PubMed] [Google Scholar]
- 34.Hall YN, Choi AI, Chertow GM, Bindman AB. Chronic kidney disease in the urban poor. Clin J Am Soc Nephrol. 2010;5(5):828–835. doi: 10.2215/CJN.09011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.US Census Bureau [Accessed February 9, 2016];Hispanic Heritage Month 2010: Sept 15-Oct 15. 2011 http://www_census_gov/newsroom/releases/archives/facts_for_features_special_editions/cb10-ff17_html.
- 36.Ricardo AC, Flessner MF, Eckfeldt JH, et al. Prevalence and Correlates of CKD in Hispanics/Latinos in the United States. Clin J Am Soc Nephrol. 2015;10(10):1757–1766. doi: 10.2215/CJN.02020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.