Lesions and peripheral blood of cutaneous leishmaniasis patients have HLA-DR-expressing neutrophils with antigen presenting cell-like characteristics, which retain morphologic and functional characteristics of neutrophils.
Keywords: Leishmania, neutrophil, granulocyte
Abstract
The protozoan Leishmania braziliensis causes cutaneous leishmaniasis (CL) in endemic regions. In murine models, neutrophils (PMNs) are recruited to the site of infection soon after parasite inoculation. However, the roles of neutrophils during chronic infection and in human disease remain undefined. We hypothesized that neutrophils help maintain a systemic inflammatory state in subjects with CL. Lesion biopsies from all patients with CL tested contained neutrophils expressing HLA-DR, a molecule thought to be restricted to professional antigen-presenting cells. Although CL is a localized disease, a subset of patients with CL also had circulating neutrophils expressing HLA-DR and the costimulatory molecules CD80, CD86, and CD40. PMNs isolated from a low-density leukocyte blood fraction (LD-PMNs) contained a higher percentage of HLA-DR+ PMNs than did normal-density PMNs. In vitro coculture experiments suggested LD-PMNs do not suppress T cell responses, differentiating them from MDSCs. Flow-sorted HLA-DR+ PMNs morphologically resembled conventional PMNs, and they exhibited functional properties of PMNs. Compared with conventional PMNs, HLA-DR+ PMNs showed increased activation, degranulation, DHR123 oxidation, and phagocytic capacity. A few HLA-DR+ PMNs were observed in healthy subjects, and that proportion could be increased by incubation in either inflammatory cytokines or in plasma from a patient with CL. This was accompanied by an increase in PMN hladrb1 mRNA, suggesting a possible connection between neutrophil “priming” and up-regulation of HLA-DR. These data suggest that PMNs that are primed for activation and that also express surface markers of antigen-presenting cells emerge in the circulation and infected tissue lesions of patients with CL.
Introduction
The protozoan Leishmania Vianna braziliensis commonly causes tegumentary leishmaniasis in Latin America, leading to either CL with localized skin ulcers and/or disseminated cutaneous manifestations [1, 2]. Leishmania spp. promastigotes are introduced into mammalian skin by a sand fly vector, after which they are internalized by local or infiltrating phagocytes, including PMNs, monocytes, macrophages, or DCs [3–5]. Promastigotes convert to intracellular amastigote forms, which reside intracellularly long term in the host [3]. Adaptive immunity with a predominant Th1-type CD4 T cell response is required for cure or prevention of symptomatic disease. However, the type 1 CD4 T cell response is also responsible for the pathologic changes of CL and its disseminated, inflammatory complications [6, 7]. The optimal balance of curative vs. pathologic inflammation, both systemically and at the local site of the CL lesions, remains incompletely understood.
PMNs are the most abundant leukocytes in human blood. PMNs are the first infiltrating cells to arrive at the site of promastigote inoculation, and they are present in acute and chronic CL lesions [3, 5, 8, 9]. PMNs are able to phagocytose parasites in the dermis, but rather than eradicate the infection, they may promote infection by facilitating uptake by macrophages and DCs at the local infection site [10, 11]. It remains to be explored whether and how, local and/or systemic neutrophils might be altered during chronic leishmaniasis.
Studies of human neutrophil populations have identified diverse PMN subsets, including LD-PMNs [12] and PMNs with the capacity to suppress T cell activity [13]. Additional reports document cells that express markers of both PMNs and APCs in some inflammatory or infectious conditions [14–17]. These and analogous findings in mouse models have led investigators to question whether these unusual human neutrophils function more like APCs, neutrophils, or a hybrid cell type [18].
Neutrophils are prominent at early time points in murine leishmaniasis [4, 5]. Because we also observed neutrophils during chronic murine leishmaniasis (unpublished observations), we hypothesized that PMNs would exhibit unique properties in chronic human leishmaniasis and might contribute to the systemic inflammatory state of CL. We therefore investigated peripheral blood and lesion-infiltrating neutrophils in Brazilian patients with CL. Our studies revealed a fraction of PMNs which stained positive for HLA-DR+ and exhibited characteristics of both conventional PMNs and APCs. These data lead us to hypothesize a role for these unique PMNs in promoting or maintaining the chronic inflammatory state observed during active CL.
MATERIALS AND METHODS
Human subjects
Brazilian patients with CL seeking treatment at the leishmaniasis treatment center in Corte de Pedra, Bahia, in northeast Brazil, were recruited into the current study [19]. Diagnostic criteria for CL included a characteristic, ulcerated skin lesion and a positive, delayed-type hypersensitivity LST (≥5 mm induration at 48–72 h). These criteria are specific for a diagnosis of CL in this highly endemic region [1]. Thirty-two of the 54 subjects had additional, confirmatory parasitologic tests, including biopsy from the lesion’s edge, parasite culture, or quantitative PCR of DNA extracted from lesions (Table 1). Normal, healthy Brazilian endemic controls were recruited among individuals living in Corte de Pedra. Endemic control subjects had no physical signs characteristic of CL, and they lacked a history or scar suggestive of CL. Additional healthy blood donors were recruited from the Iowa population, for the purpose of testing properties of leukocytes.
TABLE 1.
Diagnosis and characteristics of 54 patients with CL recruited into the study after presumptive diagnosis of CL based on patient history and presence of characteristic skin lesion
| Parameters | Patients, No. (%) | Positive results, No. (%) |
|---|---|---|
| Patients with CL | 54 (100) | |
| Median/mean age (y) | 25/28.2 | – |
| Males, No. (%) | 28 (64) | – |
| Diagnostic test (CL patients only) | ||
| LST (all patients) | 54 | 54 (100) |
| Confirmatory test | ||
| Culture | 9 | 7 (77.8) |
| Histopathology | 17 | 14 (82.4) |
| PCR (kDNA) | 23 | 19 (82.6) |
| Confirmatory tests ≥1 | 31 | 30 (96.7) |
| Healthy controls (Brazil) | 23 (100) | |
| Age | – | – |
| Males, No. (%) | 9 (43) | – |
LST was administered and biopsy collected for confirmatory tests. kDNA, kinetoplast DNA.
Table 1 describes the number, gender, and diagnostic testing of study patients with CL and the gender of healthy controls. No subjects had other recognized underlying diseases.
All human studies were performed according to approved ethical standards. Procedures were approved by institutional review boards at the University of Iowa, the Universidade Federal da Bahia, the U.S. National Institutes of Health, and the Brazilian CONEP. The Brazilian institutional review board is registered with the U.S. National Institutes of Health.
Abs and flow cytometry
Abs against human CD66b (PE-Cy7 and APC, clone G10F5), MPO (FITC, MPO455-8E6), lactoferrin (unlabeled, B97), HLA-DR (PE-Cy5, L243), active CD11b (PE, CBRM1/5), CD62L (FITC, DREG54), CD63 (PE, H5C6), CD40 (FITC, 5C3), CD14 (PerCP Cy5.5, M5E2), and CD3 (PeCy5, SK7) were obtained from BioLegend (San Diego, CA, USA). Anti-CD80 (FITC, L307) or CD86 (PE, 2231 [FUN-1]) were from BD Biosciences (Franklin Lakes, NJ, USA). Polyclonal donkey anti-rabbit IgG (PE), anti-mouse IgG (FITC, poly 4060), and anti-mouse IgG (FITC, poly 4060) were purchased from eBioscience (San Diego, CA, USA).
Cells were first stained with Abs against surface proteins by incubation at 4°C. Unless stated otherwise, erythrocytes were then lysed, and leukocytes were fixed with Lyse/Fix solution (eBioscience). Cells stained for intracellular granules omitted the Lyse/Fix and were incubated in 2% paraformaldehyde followed by permeabilization with Perm Buffer (BD Bioscience). After staining and fixation, samples were analyzed using a FACSVerse flow cytometer (BD Bioscience) equipped with a single 488 wave-length laser (Brazil) or a 4-laser LSR II flow cytometer (BD Bioscience) (Iowa). Experiments directly comparing fixation methods (unfixed whole blood or cells fixed with 4% paraformaldehyde or 1× Lyse/Fix) did not result in any unexpected fluorescence that might confound signals from fluorescent Abs against surface markers (data not shown). Analyses were performed using FlowJo software (Tree Star, Ashland, OR, USA).
Blood counts
Peripheral blood differential cell counts were performed microscopically on Wright-Giemsa stained blood smears from patients with CL or control subjects.
Leukocyte preparations
Whole blood.
With the exception of the dextran separation method (below), whole blood for flow cytometry staining was drawn in heparinized tubes and stained directly.
Lesion-isolated PMNs.
To analyze neutrophils recruited to cutaneous lesions, punch biopsies were taken from the lesion border of patients with confirmed CL. Biopsies were treated with collagenase (Liberase; F. Hoffmann-La Roche, Basel, Switzerland) for 90 min at 37°C and 5% CO2, dissociated, passed through a 50-µm Medicon filter (BD Biosciences), and washed by centrifugation. Cell suspensions from human skin were stained with flow cytometry Abs directly ex vivo.
Cell sorting.
HLA-DR+ CD66b+ PMNs from control Iowa subjects were separated from HLA-DR− PMNs in whole blood using a FACS Aria II (BD Bioscience). Cells were applied to glass slides (Cytospin 4; Thermo Fisher Scientific, Waltham, MA, USA), fixed, and stained with Wright Giemsa (Hema 3; Thermo Fisher Scientific).
Low- and normal-density neutrophils.
Histopaque Ficoll 1077 (Sigma-Aldrich, St. Louis, MO, USA) was used to separate low-density leukocytes, including PBMCs from whole blood. The low-density fraction above the Ficoll also contained LD-PMNs as described [20]. Normal-density neutrophils were recovered from below the Ficoll layer after a brief hypotonic lysis in dH2O (30 s, room temperature).
Dextran separation of neutrophils.
For some experiments, neutrophils were isolated using a standard dextran sedimentation protocol for comparison with whole blood, low-density, and normal-density neutrophils. Briefly, whole blood was incubated in an equal volume of 3% dextran/PBS (18 min, room temperature), precipitating most erythrocytes [21]. After removing the pelleted erythrocyte layer, the remaining leukocyte-enriched supernatant was resuspended in buffer without cations, and separated into low-density (PBMCs) versus normal-density neutrophil fractions with Histopaque Ficoll 1077, as described above.
T cell proliferation
After Ficoll separation of leukocytes by density sedimentation, CD3 microbeads and MS columns (Miltenyi Biotech, San Diego, CA, USA) were used to isolate T cells from the low-density leukocyte fraction containing most of the mononuclear cells. CD15 microbeads were then used to separate the remaining low-density leukocytes into 2 populations, one enriched for LD-PMNs (>90% LD-PMNs), and the other depleted of LD-PMNs (<1% LD-PMNs). CD3+ T cells were stained with CFSE (Sigma-Aldrich) and suspended in RP10 (RPMI 1640 medium, 10% heat-inactivated FBS, 2 mM l-glutamine, 100 U penicillin/ml, 50 µg gentamicin/ml), supplemented with 100 units/ml of IL-2. Then, 5 × 105 T cells were incubated at 37°C and 5% CO2 for 72 h with CD3/CD28-expressing Dynabeads (Thermo Fisher Scientific) as a positive control or with 5 µg/ml of SLA plus a 1:1 ratio of autologous low-density leukocytes that were either enriched for LD-PMNs or were depleted of LD-PMNs. Bead-enriched T cells from healthy controls were also incubated with CD3/CD28 beads or LD-PMN–depleted PBMCs in the presence of SLA. SLA was prepared from cultured L. braziliensis as described [22]. After 72 h, cells were stained for CD3 and CD4 and analyzed by flow cytometry.
DHR123 fluorescence
To activate PMNs, 10 μg of 0.5 mM PMA (Sigma-Aldrich) was added to 200 µl of heparinized whole blood mixed 1:1 with RP10. After 5 min incubation at 37°C with 5% CO2, 10 µM DHR123 (Sigma-Aldrich) was added. After an additional 15 min at 37°C, at 5% CO2, cells were transferred to 4°C, stained, and fixed. DHR123 fluorescence in single cells was detected by flow cytometry.
Phagocytosis assay
Stationary phase Leishmania infantum promastigotes were labeled with 10 µM CFSE. Zymosan particles (Sigma-Aldrich) and L. infantum were opsonized in 5% fresh human serum (10 min, 37°C, 5% CO2); 100 μl whole blood, diluted 1:1 in PBS/1% FBS, was incubated with 5 × 105 opsonized L. infantum or zymosan for 30 min at 37°C and 5% CO2 and stained with CD66b and HLA-DR for flow cytometry. The rate of phagocytosis was calculated as the percentage of CD66b+ PMNs that were positive for CFSE or for opsonized zymosan autofluorescence.
Neutrophil priming and plasma replacement
To examine neutrophil priming in vitro, 100 µl of whole blood was incubated in RP10 supplemented with TNF (10 ng/ml), GM-CSF (20 ng/ml), IFN-γ (20 ng/ml) (PeproTech, Rocky Hill, NJ, USA), or LPS (10 ng/ml) (Sigma-Aldrich). Additional neutrophils were incubated with 5 × 105 serum-opsonized L. infantum or zymosan. After 2 h at 37°C and 5% CO2, cells were washed and stained for flow cytometry. To test replacement of healthy donor plasma with CL patient plasma, 100 µl of whole blood from healthy Brazilian endemic controls was centrifuged for 10 min at 1500 rpm, and the plasma was removed. Cells were suspended in 100 µl of plasma from patients with CL and was incubated for 2 h at 37°C with 5% CO2 and stained for flow cytometry.
Single-cell detection of hladrb1 mRNA
Intracellular transcripts in single cells were examined by flow cytometry using a PrimeFlow assay according to the manufacturer’s protocol (Affymetrix, Santa Clara, CA, USA). Briefly, healthy donor whole blood was primed by incubation in recombinant cytokine TNF (10 ng/ml) and GM-CSF (20 ng/ml) separately or in combination. After 2 h at 37°C and 5% CO2, cells were stained for surface CD66b, CD15, and HLA-DR; fixed; and permeabilized. Oligonucleotides complementary to 2 sites on the hladrb1 mRNA and to sequences designed to hybridize with subsequent “amplifiers” were added to the cells. This was followed by hybridization to “preamplifier” and fluorescence marker–labeled “amplifier” DNA probes. Cells were washed and analyzed by flow cytometry.
Statistics
Statistical comparison between patient and control groups was performed using an unpaired Welch’s t test to account for differences in variances and sample sizes. A paired Student’s t test was used to compare matched samples from the same patient. ANOVA and correlation coefficients were used for indicated tests. Statistical analyses were performed using GraphPad Software (La Jolla, CA, USA).
RESULTS
HLA-DR+ PMNs detected in patients with CL
Many studies of PMNs in leishmaniasis have focused on early local events at the site of Leishmania inoculation in murine models [11, 23]. We undertook a study of the phenotype of PMNs in Brazilian patients with active, untreated CL. Gating strategies for flow cytometry are shown in Supplemental Fig. 1.
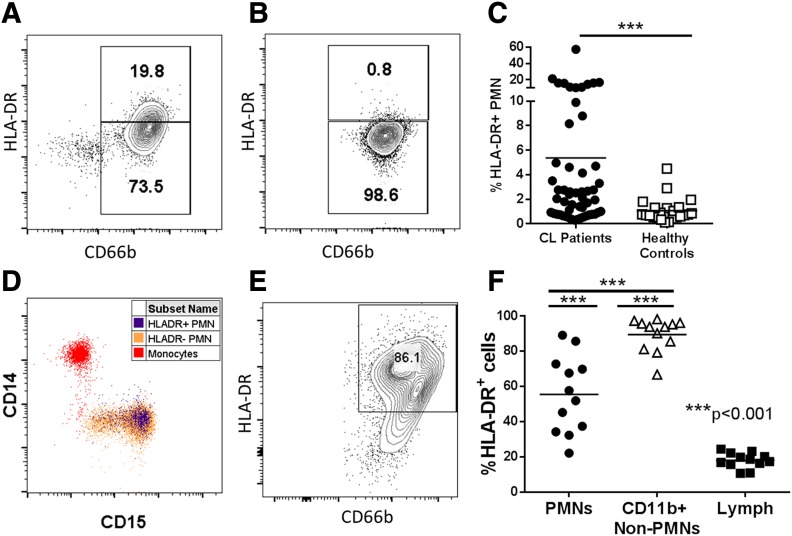
Surprisingly, there was a population of cells staining positive for both neutrophil markers and HLA-DR, a molecule believed to be present only on APCs in whole blood of many CL subjects (Fig. 1A and B). In contrast, <1% of PMNs from control subjects stained positive for HLA-DR (Fig. 1C). Costaining for monocyte-specific CD14 and for another neutrophil marker CD15 revealed no discernable contamination of CD66b+ PMNs with monocytes (Fig. 1D). PMNs isolated from 12 skin biopsies of patients with CL lesions also showed high amounts of surface HLA-DR (Fig. 1E and 1F). Although only a subset of patients had HLA-DR+ PMNs in peripheral blood, the CL lesions of all patients examined contained PMNs expressing HLA-DR (Fig. 1F).
Figure 1. A subset of Brazilian patients with CL has HLA-DR–expressing PMNs in the circulation and cutaneous lesions.
(A and B) Whole blood was isolated from Brazilian patients with CL or endemic healthy controls and stained directly ex vivo for HLA-DR and CD66b. Granulocytes were gated by forward (FSC) and side scatter (SSC) and on single cells resulting in representative flow plots showing HLA-DR expression on CD66b+ neutrophils from a patient with CL and a healthy endemic control, respectively. (C) Quantification of HLA-DR+ expression on total patients vs. endemic controls (unpaired Welch’s t test). (D) Expression of the monocyte marker CD14 and neutrophil marker CD15 is shown on monocytes (red), HLA-DR+ PMNs (blue), or HLA-DR− PMNs (orange). (E) Flow plot showing HLA-DR expression on CD66b+CD15+CD11b+ PMNs from lesions of patients with CL. (F) Quantification of surface HLA-DR on PMNs (CD15+CD66b+CD11b+), non-PMN myeloid cells (CD11b+CD66b−), and lymphocytes (CD11b− FSC, SSC) in lesions from patients with CL.
There were no significant correlations between sex, age, or size of CL lesion and the abundance of HLA-DR on PMNs in the peripheral blood, quantified as G-MFI (Supplemental Fig. 2). Thus, we did not measure a clinical characteristic that correlates with higher abundance of circulating HLA-DR PMNs.
Sorted HLA-DR+ PMNs have the appearance of neutrophils
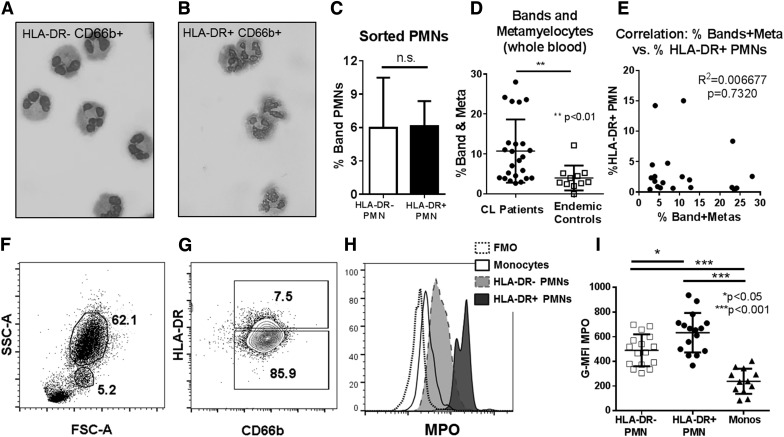
A small proportion (0.2–0.8%) of circulating neutrophils from healthy control donors stained positive for HLA-DR. To verify that HLA-DR+CD66b+ cells were truly neutrophils, we isolated these cells using FACS and examined them by light microscopy. These slides verified that both HLA-DR+ and HLA-DR− neutrophil populations had the multilobed nuclei typical of normal, mature PMNs (Figs. 2A and B).
Figure 2. Sorted HLA-DR+ PMN from healthy donors have morphologic and biochemical characteristics of neutrophils.
(A and B) Micrographs showing cytospun, FACS-isolated HLA-DR− or HLA-DR+ PMN (Diff Quik stain). (C) Quantification of band neutrophils in FACS-isolated HLA-DR+ vs. HLA-DR− fractions from healthy blood donors (n = 4; paired Student’s t test). (D) Quantification of the total percentage of band and metamyelocyte (Meta) neutrophil forms in blood smear differential counts from patients with CL compared with endemic healthy controls (unpaired students t test). (E) Correlation plot between the percentage of HLA-DR+ PMN vs. the percentage of bands plus metamyelocytes in peripheral blood smears, showing a lack of correlation between the abundance of immature neutrophil forms and the abundance of HLA-DR+ PMN in patients with CL (P = 0.732). (F and G) Representative flow cytometry plots showing gating on granulocytes (62.1%) and monocytes (5.2%) in the whole blood of a patient with CL (F), and gating on HLA-DR+ and HLA-DR− CD66b+ neutrophils in a patient with CL (G). (H) Histogram showing intracellular staining of HLA-DR+ PMNs, HLA-DR− PMNs, and monocytes for MPO. Plots are compared with the fluorescence minus one (FMO) staining control lacking MPO Abs. (I) Quantification of G-MFI of HLA-DR− PMNs, HLA-DR+ PMNs, and monocytes in patients with CL (statistical analysis, 1-way ANOVA with Tukey’s posttest analysis).
HLA-DR+ PMNs are not immature neutrophils
It was hypothesized that HLA-DR+ PMNs correspond to immature neutrophils released from bone marrow [24]. To examine that, we quantified immature (band, metamyelocyte) vs. mature neutrophil forms among sorted PMNs from healthy control donors. There was no difference in the percentage of immature neutrophils between sorted HLA-DR+ and HLA-DR− PMN populations (Fig. 2C). Study of peripheral blood differential leukocyte counts from patients with CL showed significantly increased percentages of total bands and metamyelocytes compared with healthy endemic controls, an expected result in this chronic, infectious disease (Fig. 2D). Nonetheless, there was no correlation between the percentage of immature neutrophil forms (bands and metamyelocytes) and the magnitude of HLA-DR expression on PMNs (Fig. 2E). These data suggest that high HLA-DR expression is not a marker of immature neutrophils.
Granulocytes other than neutrophils can express MHC class II and act as APCs [25]. However, there were no significant differences in circulating basophils, eosinophils, or mast cells between patients with higher (>5%) vs. lower (<5%) proportions of HLA-DR+ PMNs or between healthy controls and patients with CL (Supplemental Fig. 3). Taken together, the above findings suggest that HLA-DR+ PMNs were unlikely to be monocytes, basophils, eosinophils, or immature neutrophil forms.
HLA-DR+ PMN granule contents
As further verification that HLA-DR+ are neutrophils, we examined markers of intracellular granules. MPO is found in greatest abundance in neutrophil azurophilic granules and at low levels in monocyte granules. As expected, whole blood intracellular staining showed significantly more intracellular MPOs in all PMNs, including HLA-DR+ PMNs, than monocytes in neutrophil populations from CL subjects (Fig. 2F–I). Furthermore, there was significantly more intracellular MPO in HLA-DR+ than HLA-DR− PMNs in both CL subjects (Fig. 2H–I) and endemic healthy controls (Supplemental Fig. 5A). We were also able to verify that total neutrophil populations had intracellular neutrophil elastase and lactoferrin (Supplemental Fig. 4). Although technical limitations in flow cytometric staining at the Brazilian facility made it impossible to directly compare neutrophil elastase and lactoferrin in HLA-DR+ and HLA-DR− PMNs, patients with CL with a high proportion (>5%) of HLA-DR+ PMNs had equivalent amounts of these intracellular compounds as those with a low percentage (<5%) of HLA-DR+ PMNs (Supplemental Fig. 4).
Dextran sedimentation depletes HLA-DR+ PMNs
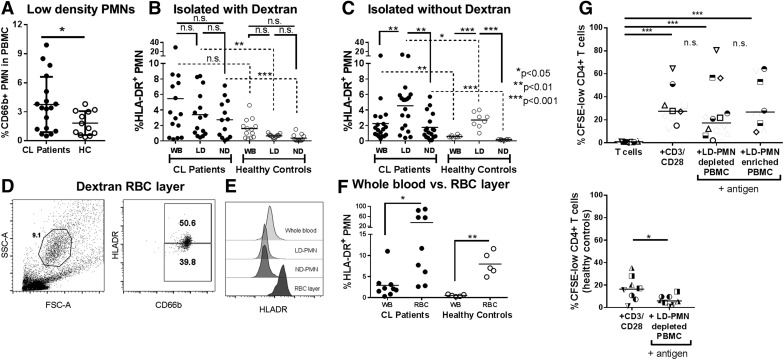
Separation of whole blood cells over a density gradient results in leukocyte fractions from which LD-PMNs or ND-PMNs can be isolated. When whole blood was centrifuged over a Ficoll gradient, patients with CL had significantly more CD66b+ LD-PMNs in the total low-density fraction than did healthy control Brazilian subjects (Fig. 3A) [12]. Typically, PMNs are purified from whole blood using a dextran solution to sediment erythrocytes before a Ficoll density gradient [21]. Surprisingly, when leukocytes from density fractions of either patients with CL or healthy controls were stained for HLA-DR, cells isolated after a dextran-sedimentation step were apparently depleted of HLA-DR+ PMNs in purified fractions of LD or ND (Fig. 3B). In contrast, leukocytes separated on a Ficoll density gradient from whole blood without the use of dextran showed significant enrichment of HLA-DR+ PMNs in the LD-PMN fractions of both patients with CL and healthy controls (Fig. 3C). Thus, the HLA-DR+ PMN phenotype seemed to be highly enriched in the LD-PMN fraction but preferentially lost during dextran sedimentation. To determine the fate of these cells during the dextran sedimentation procedure, we stained dextran-precipitated erythrocyte layers for leukocyte markers (Fig. 3D–F). The erythrocyte layer from CL subjects contained granulocytes that stained positive for CD66b and HLA-DR (Fig. 3D), whereas HLA-DR+ CD66b+ PMNs from the same samples were depleted from the LD- and ND-PMN fractions (see example in Fig. 3D and E). This observation was confirmed by the combined results of 9 CL subjects and 5 healthy controls (Fig. 3F). Consequently, dextran was omitted from all subsequent neutrophil isolation procedures in this study.
Figure 3. Dextran sedimentation of whole blood depletes HLA-DR+ PMN.
(A) Comparison of the percentage of LD-PMNs (staining positive for CD66b) among total low-density cell fractions after centrifugation over a Ficoll gradient of blood from patients with CL or endemic healthy controls (HCs; statistical analysis, unpaired Student’s t test). (B and C) Percentages of HLA-DR+ PMNs in peripheral blood of patients with CL (filled circles) vs. endemic HCs (open circles) was quantified in whole blood (WB, directly after blood draw) or in the low-density (LD) and normal-density (ND) layers after density separation over a Ficoll gradient. (B) The percentages of HLA-DR+ neutrophils in LD or ND fractions after sedimentation of erythrocytes in a 3% dextran solution. (C) The percentages of HLA-DR+ neutrophils in LD or ND fractions in blood preparations that were not exposed to dextran (statistical analysis between paired CL and HC samples used paired Student’s t test; comparisons between CL and HC samples used unpaired Welch’s t test to account for differences in variance and sample size). (D) Flow cytometric staining of the erythrocyte-enriched layer that was precipitated by incubation of whole blood in 3% dextran solution, a layer typically discarded during this PMN isolation procedure. Cells with typical granulocyte forward and side scatter were gated on and examined for both CD66b and HLA-DR staining. (E) Comparison of surface-stained neutrophils in blood preparations from a single patient with CL: Histograms show surface HLA-DR on PMNs from whole blood (WB), the dextran-exposed low-density fraction, the dextran-exposed ND PMN fraction, and the dextran-precipitated RBC layers after dextran sedimentation and Ficoll centrifugation procedures. (F) Comparison of the percentage of HLA-DR+ PMNs in whole blood compared with the RBC layer after dextran sedimentation of blood cells from patients with CL and endemic HCs (statistical analysis, paired Student’s t test). (G) T cell proliferation assay: purified, CFSE-labeled CD3+ T cells were incubated with control CD3/CD28 beads, LD leukocytes depleted of LD-PMNs (primarily mononuclear cells) or LD leukocytes enriched for LD-PMNs (mononuclear cells and PMNs) in the presence of SLA (right panel). HC T cells were incubated with either CD3/CD28 beads or SLA plus autologous PBMCs depleted of LD-PMNs (statistical analyses: 1-way ANOVA with Tukey’s posttest).
LD-PMNs do not suppress T cell proliferation in vitro
Low-density neutrophils have previously been described in several disease states, including systemic lupus erythematosus and cancer [12, 26]. We sought to determine whether LD-PMNs from patients with CL could influence T cell responses using an in vitro proliferation assay. CD3+ T cells were purified with magnetic beads from the low-density leukocyte fractions (after centrifugation over Ficoll) from patients with CL and controls. Subsequently, we used anti-CD15 magnetic beads to divide the remaining low-density fraction into 2 populations: one enriched (>90%) for LD-PMNs, and the other depleted (<1%) of LD-PMNs. Both fractions contained proportions of the other low-density leukocytes, including NK cells and known APC populations (monocytes, B cells, NK cells, and any DCs present). Total CD3+ T cells from patients with CL or from healthy subjects were CFSE-labeled and incubated with positive control stimulatory CD3/CD28-coated beads, with LD-PMN–depleted PBMCs or LD-PMN–enriched PBMCSs in the presence of SLA as a source of parasite antigen. After 72 h, cells were stained for surface CD3 and CD4 (Fig. 4G) and studied for evidence of proliferation (CFSE dilution).
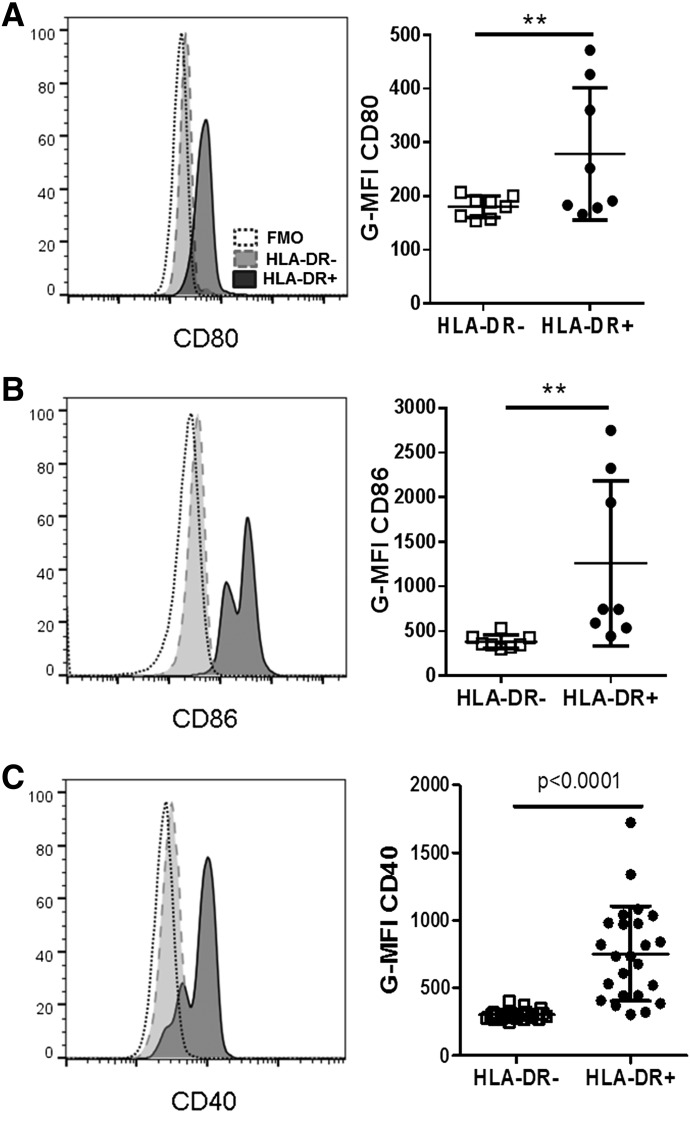
Figure 4. HLA-DR+ PMNs express costimulatory molecules.
(A, C, and E) Representative histograms of surface CD80 (A), CD86 (C), or CD40 (E) expression on CD66b+ HLA-DR+ (solid line, dark fill) vs. CD66b+ HLA-DR− (dashed line, gray fill) neutrophils and a fluorescence minus one (FMO) staining control (dotted line, no fill). Histograms show typical results in the whole blood of a patient with CL. (B, D, and G) Quantification of G-MFI for stains of CD66b+ HLA-DR− PMNs or CD66b+ HLA-DR+ PMNs compared with FMO controls in a series of patients with CL (statistical analyses: paired Student’s t test). **P < 0.001 in panels A and B.
The data showed there was no proliferation of isolated T cells without added APCs. T cells did proliferate when incubated with positive-control anti-CD3/anti-CD28 beads, as expected. Furthermore, T cell proliferation was observed in T cells that were incubated with parasite antigen (SLA) plus either of the low-density leukocyte populations. Importantly, there were no significant differences between proliferation of T cells incubated in fractions that were enriched for LD-PMNs or depleted of LD-PMNs. To show that proliferation indicated an antigen-specific response of SLA-infected subjects, negative control T cells from healthy endemic controls did not proliferate when autologous mononuclear cells were incubated with SLA, whereas they did proliferate with CD3/CD28 beads (Fig. 4G).
Because column-“purified” HLA-DR+ PMNs from LD fractions contained other APCs, no conclusions can be drawn about these cells’ antigen-presenting capacity. Nevertheless, the contrast between LD-PMN–enriched vs. LD-PMN–depleted fractions supports the conclusion that LD-PMNs from patients with CL do not suppress T cell proliferation in vitro.
Costimulatory molecule expression
The data above presented evidence that HLA-DR+ PMNs in whole blood exhibited the morphologic and at least some biochemical characteristics of neutrophils. Because they display surface HLA-DR, we examined whether they also displayed additional markers of APCs, such as costimulatory molecules. Indeed, the data showed that HLA-DR+ PMNs expressed significantly more surface CD80, CD86, and CD40 than HLA-DR− PMNs did from the same subjects (Fig. 4A–F). Similarly, the few HLA-DR+ PMNs in healthy control subjects expressed greater amounts of CD80 and CD86 than did the HLA-DR− PMNs from the same subjects (Supplemental Fig. 5B and C).
Neutrophil activation and function
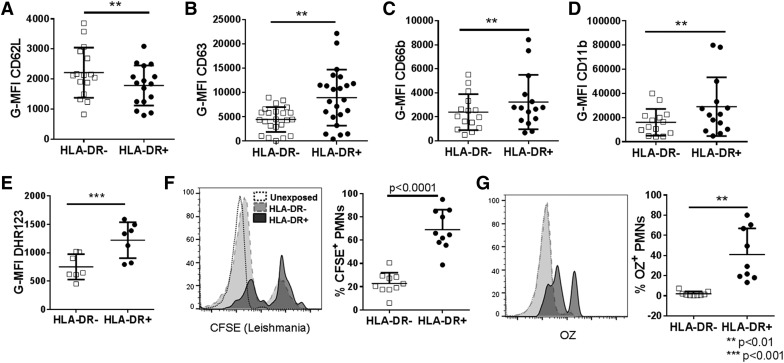
In addition to HLA-DR, whole blood neutrophils of patients with CL were stained for 4 markers of activation: 1) CD62L (l-selectin), which is shed from the membrane upon activation (Fig. 5A); 2) CD63 (protein in azurophilic granules); 3) CD66b (specific granules); and 4) active CD11b (specific and gelatinase granules) (Figs. 5B–D). The latter 3 are neutrophil granule membrane proteins that migrate to the surface membrane upon granule exocytosis [20]. By all measures, the activation state of HLA-DR+ PMNs was greater than that of HLA-DR− PMNs. We observed this same trend in HLA-DR+ PMNs from healthy controls (Supplementary Fig. 5D–G).
Figure 5. Activation, degranulation, and phagocytosis by HLA-DR+ vs. HLA-DR− PMNs.
Graphs show mean fluorescence intensity quantification of PMN activation markers HLA-DR+ and HLA-DR− PMNs from patients with CL. (A) CD62L is decreased on activated PMNs. CD63 (found on primary granules) (B), CD66b (found on secondary granules) (C), and CD11b (found on secondary and tertiary granules) (D) are increased after PMN degranulation. (E) Increased DHR123 fluorescence results from increased MPO and H2O2 in PMNs. (F and G) Phagocytosis assays: representative histograms show increased phagocytosis of CFSE-labeled Leishmania promastigotes (F) or opsonized zymosan (G) by HLA-DR+ PMNs compared with HLA-DR− PMNs isolated from healthy control patients. OZ, optimized zymosan. **P < 0.001, ***P < 0.0001.
We questioned whether HLA-DR+ PMNs exhibited neutrophil-specific functions. DHR123 freely diffuses into leukocytes where it can be oxidized to a form that emits fluorescence. DHR123 oxidation occurs in the presence of H2O2 and a peroxidase such as MPO [27]. When there is constant MPO concentration, superoxide can dismute to H2O2 and react with MPO and DHR123, resulting in a fluorescent DHR signal that is proportional to the amount of NADPH activity. We stimulated CL patient whole blood briefly with PMA in the presence of DHR123 [28, 29]. HLA-DR+ PMNs oxidized significantly more DHR123 than did HLA-DR− PMNs from the same subjects when stimulated with PMA (Fig. 5E). A similar increase in DHR123 fluorescence was also seen in HLA-DR+ PMN from healthy controls (Supplementary Fig. 5H). Because DHR123 fluorescence depends on MPO and because HLA-DR+ PMNs have significantly more intracellular MPO than HLA-DR− PMNs do (Fig. 2H and I), we cannot be certain whether the increased DHR123 oxidation reflects different MPO concentrations, increased NADPH oxidase activity, or both.
The phagocytic capacity of different neutrophil types was compared by incubating healthy donor whole blood with serum-opsonized L. infantum promastigotes or zymosan particles at 37°C for 30 min. Phagocytosis was detected as CD66b+ neutrophils costaining with CFSE-labeled parasites or autofluorescent zymosan according to flow cytometry. The data revealed that HLA-DR+ PMNs took up more L. infantum and more opsonized zymosan than did HLA-DR− PMNs (Fig. 5F and G).
Data in Fig. 3 and Supplemental Fig. 5 illustrate clearly that the major difference between HLA-DR+ PMNs in patients with CL, compared with healthy controls, is the significantly greater proportion and number of HLA-DR+ PMNs in subjects with CL. The density or other characteristics of HLA-DR+ neutrophils from different donors seems to be similar, whether derived from subjects with high (CL) or low (control) concentrations of these cells.
Neutrophil priming promotes PMN surface expression of HLA-DR
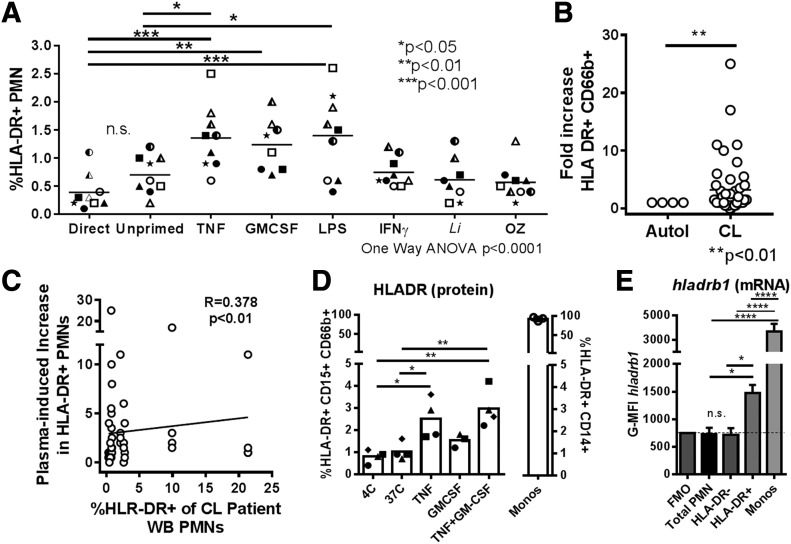
The enhanced activation and functional capacity of HLA-DR+ PMNs suggested a primed phenotype. Therefore, we investigated whether alternate priming signals [30–33] would augment neutrophil HLA-DR expression. We incubated whole blood from healthy donors for 2 h in conditions known to induce priming in vitro (TNF, LPS) [33], with cytokines implicated in PMN MHC class II expression (GM-CSF, IFN-γ) [24] or with opsonized L. infantum or zymosan under conditions allowing phagocytosis. Only TNF and LPS incubation caused a significant increase in PMN surface HLA-DR compared with cells incubated for the same length of time without exogenous stimuli. Furthermore, GM-CSF caused a significant increase in HLA-DR compared with cells stained directly ex vivo (Fig. 6A). There was no effect of IFN-γ, parasites, or zymosan on neutrophil HLA-DR expression. Thus, there seemed to be some degree of specificity to the signals inducing PMNs to increase surface HLA-DR expression in vitro.
Figure 6. Priming whole blood results in increased surface HLA-DR and hladrb1 mRNA in healthy blood donor PMNs.
(A) Healthy donor whole blood was incubated with priming, inflammatory cytokines, or other stimuli at 37°C. After 2 h, blood was stained for CD66b and HLA-DR. The graph shows the percentage of HLA-DR+ PMNs in plots. Cells from each donor are indicated by unique symbols (statistical analysis: 1ne-way ANOVA with Dunnett’s posttest comparing all conditions to control). (B) Plasma from healthy donor whole blood was removed and replaced with CL patient plasma or autologous plasma. Cells were incubated for 2 h at 37°C, with 5% CO2, followed by staining for surface with HLA-DR on PMNs. (C) Plot showing significant correlation between the expression of HLA-DR on PMNs of patients with CL (x axis) and the increase in HLA-DR seen in healthy donor cells when incubated in that patient’s plasma (y axis). (D and E) After 2 h priming in cytokines, similar to that in panel A, cells were stained for surface HLA-DR (D). The same cells were incubated with a probe hybridizing to intracellular hladrb1 mRNA, which was detected by flow cytometry using PrimeFlow assay (E). The graph shows a plot of the means ± sd MFI corresponding to the hladrb1 mRNA probe in neutrophils staining as HLA-DR− vs. neutrophils staining as HLA-DR+ for the conditions shown in panel D.
Plasma from patients with CL contains higher concentrations of inflammatory cytokines, including TNF and IFN-γ, than found in plasma from uninfected control subjects [6]. Based on the hypothesis that circulating cytokines might induce neutrophil HLA-DR expression, we incubated healthy donor whole blood cells with plasma from patients with CL for 2 h at 37°C. Compared with cells incubated in autologous plasma, plasma from patients with CL induced a significant increase in surface HLA-DR on PMNs (Fig. 6B). Furthermore, there was a significant correlation between the ability of a patient’s plasma to increase HLA-DR on healthy donor PMNs and the proportion of that same patient’s PMNs that expressed HLA-DR (P < 0.01; Fig. 6C).
We approached the question of whether the observed, increased neutrophil HLA-DR was derived from endogenous or newly synthesized neutrophil stores or was acquired from other cells in an experiment designed to detect steady-state abundance of hladrb1 mRNA. Confounding studies of neutrophil gene expression, even a small amount of contamination with more transcriptionally active hematopoietic cells such as monocytes could greatly skew results. We therefore used PrimeFlow analysis to detect hladrb1 mRNA in individual cells by flow cytometry. Whole blood from healthy donors was primed for 2 h with TNF or GM-CSF, as described for the experiments shown in Fig. 6D. Incubation in TNF significantly increased the expression of HLA-DR on neutrophils. Permeabilization and intracellular hybridization enabled us to discern that hladrb1 mRNA was significantly increased in HLA-DR+ PMNs and monocytes compared with total whole blood PMNs or HLA-DR− PMNs (Fig. 6E). These findings are consistent with the intracellular synthesis of hladrb1 mRNA resulting in HLA-DR surface expression, even after a short incubation period.
DISCUSSION
Studies of leishmaniasis in mouse models have revealed that neutrophils have a role in shuttling the Leishmania spp. parasite through host cells during the first hours of infection [4, 5, 34]. Relatively less is known about human infection or about the potential role for neutrophils throughout the chronic phases of leishmaniasis. The current study was based on the hypothesis that neutrophils in the circulation contribute to maintenance of a systemic inflammatory state in subjects with CL. Study of individuals in an endemic region of northeast Brazil revealed increased expression of HLA-DR on neutrophils in lesions and in the circulating blood of patients with CL caused by L. braziliensis. Whereas HLA-DR+ PMNs were observed in lesions of all subjects examined, HLA-DR+ PMNs were observed in the circulation of only some. These unusual cells phenotypically resembled neutrophils according to both flow cytometry for surface markers (CD66b+ CD15+ CD14−) and characteristic morphologic features under light microscopy. Further examination showed some neutrophil characteristics were enhanced in HLA-DR+ PMNs, including increased MPO content and a greater capacity for phagocytosis, compared with HLA-DR− PMNs from the same individual. HLA-DR+ PMNs also displayed DC-like properties, expressing costimulatory molecules CD80, CD86, and CD40.
A few reports showed increased circulating HLA-DR+ PMNs in patients with autoimmune disorders (Wegener’s granulomatosis, rheumatoid arthritis) [15, 35, 36], after experimental IFN-γ treatment [37] or in the wounds but not the blood of patients with persistent Staphylococcus aureus infection [17, 38]. It has also been reported that MHC class II expression on neutrophils is enhanced during prolonged in vitro culture with IFN-γ, GM-CSF, IL-4, or acidification [24, 39, 40]. Importantly, our study of patients with CL showed a high proportion of HLA-DR+ PMNs corresponded to the LD-PMNs. LD-PMNs have recently been described in subjects with visceral leishmaniasis and other conditions [12]. LD-PMNs from patients with systemic lupus erythematosus are known to produce type I interferons, which may contribute to inflammation and disease progression [41]. In contrast, LD-PMNs from patients with cancer have a suppressive phenotype that can impair T cell proliferation in vitro [26, 42]. Data presented in the current study show that LD-PMNs from patients with CL contain a substantial proportion of the HLA-DR+ PMNs. LD-PMNs did not suppress T cell proliferation in vitro.
It has not, to our knowledge, previously been demonstrated that cells staining as HLA-DR+ PMNs exhibit morphologic and functional characteristics of neutrophils. Herein, we showed that PMNs expressing MHC Class II are indeed neutrophils according to their morphology, granule contents, and function. A very small population of HLA-DR+ PMNs from healthy hosts had the same characteristics, suggesting these cells may be expanded, rather than emerge de novo, during chronic leishmaniasis.
The localized pathologic inflammatory tissue damage observed during tegumentary CL results primarily from vigorous CD4 and CD8 T cell responses [7, 43]. Neutrophils migrate in large numbers into the skin in the first hours of Leishmania spp. infection and are found in both early and late CL lesions in both mice and human hosts [43]. The presence of HLA-DR+ PMNs in all CL lesion biopsies examined and a similar phenotype seen in the systemic compartment, adds a novel potential role for these cells in the generalized inflammatory state accompanying the localized disease.
Rather unexpectedly, HLA-DR+ PMNs displayed a greater degree of activation (CD62L cleavage), degranulation (granule surface markers), MPO, and phagocytosis than did the HLA-DR− PMNs from the same subject. Because increased activation is characteristic of primed neutrophils, this raised a possible connection between neutrophil priming and HLA-DR expression. Consistently, exposure to known neutrophil priming conditions [33, 44] revealed that incubation in TNF or LPS induced HLA-DR expression on PMNs in vitro. Study of the subgroup of patients with CL having HLA-DR+ PMNs in their circulation revealed their plasma samples were able to induce HLA-DR expression on PMNs from healthy subjects. It is logical to hypothesize that exposure to inducing agents, such as TNF, in the tissue induces HLA-DR expression locally on all neutrophils migrating to the infection site, and that neutrophil HLA-DR expression on circulating neutrophils is induced only in subjects with elevated serum levels of priming cytokines. Several published studies document cytokine/chemokine levels in the serum of patients with CL. Patients with L. braziliensis infections have higher levels of CXCL10, CCL4, and soluble TNF receptor II compared with controls [6, 45]. Elevated serum levels of TNF, IL-2, IL-4, IL-6, and IL-17 are reported in subjects with New World CL from a variety of species [46]. Serum levels of TNF, IL-6, and IL-1β were also elevated in subjects with CL from Leishmania major [47]. Thus, there are ample cytokines that could be responsible for increased neutrophil HLA-DR, although TNF is primarily suspected.
The increase in HLA-DR–expressing PMNs in CL could reflect the emergence or prolonged survival of a distinct HLA-DR–expressing neutrophil subset or increased expression of HLA-DR on the surface of many neutrophils already in the circulation. Shift of the entire PMN population toward increased HLA-DR expression, observed in flow cytometry histograms, argues against a separate PMN subset and in favor of some mechanism leading to up-regulation of HLA-DR on the neutrophil surface. Mechanisms accounting for the latter could include de novo synthesis, movement of HLA-DR from a preexisting intracellular pool to the neutrophil surface, passive acquisition from nearby cells (documented for bovine neutrophils [48]), or trogocytic acquisition of membrane molecules after phagocytosis of other APCs, as documented in T cells [49]. Study of gene expression in HLA-DR+ neutrophils is a challenge because even a few contaminating monocytes would falsely indicate HLA-DR gene expression. Nonetheless, we were able to detect mRNA in single cells by flow cytometry and found a significant increase in hladrb1 mRNA in HLA-DR+ PMNs. This suggests neutrophils are capable of de novo HLA-DR synthesis, although this does not preclude the simultaneous contribution of several mechanisms to increase HLA-DR abundance.
It is becoming increasingly clear that neutrophils exhibit phenotypic and functional heterogeneity, both in the circulation and the tissues into which they migrate [13, 26, 50]. Because HLA-DR+ PMNs are depleted during isolation procedures that include a dextran sedimentation step provides at least one possible explanation for the lack of studies showing APC-like neutrophils, first described almost 30 y ago [24]. Our data have illuminated PMNs with a primed phenotype and APC-like surface markers. There are reports of PMN subtypes that suppress T cell responses, including G-MDSCs and PMNs arising during LPS-induced endotoxemia [13]. The fact that LD-PMNs from patients with CL did not suppress T cell proliferation in vitro argues that they are not the same as G-MDSCs. HLA-DR+ PMNs are increased among low-density leukocytes, but their properties differed from LD-PMNs that are expanded in other conditions. For instance, LD-PMNs from patients with systemic lupus erythematosus patients produce type I IFNs and contribute to inflammation and disease progression [41], and LD-PMNs from patients with cancer suppress T cell responses [26]. Thus, neutrophils of low density are induced by a variety of conditions and should not be considered as a single neutrophil phenotype. Clearly, there is much to be discovered about the longevity and plasticity of these various neutrophil phenotypes. In patients with CL, the ultimate effect of expanded numbers of circulating, partially activated neutrophils that express molecules characteristic of APCs remains to be shown.
AUTHORSHIP
R.E.D. designed and performed experiments and wrote the manuscript. S.Sharma, J.C., P.C., and F.N. performed experiments. P.S., S.Sundar, O.B., and E.M.C. designed experiments. M.E.W. designed experiments and wrote the manuscript.
Supplementary Material
ACKNOWLEDGMENTS
These studies were funded in part by a Tropical Medicine Research Center from the U.S. National Institutes of Health (Grant P50 AI-30639 to O.B., E.M.C., and M.E.W. and Grants R01 AI076233 and R01 AI045540), a Merit Review grant from the Department of Veterans’ Affairs (Grant 5I01BX001983), and a Major Project Award and Travel grant from the Stanley Foundation/University of Iowa. The authors are grateful to Dr. Luiz Henrique, Ednaldo Lago, Neuza Lago, and the staff at the Corte de Pedra Health Post for their help with recruitment and diagnosis of subjects. We are grateful to Drs. Lucas Carvalho and Sara Passos of the Federal University of Bahia for help planning and processing flow cytometry studies performed in Salvador, Brazil. We also thank the Flow Cytometry Core Facility at the University of Iowa for cell sorting. Drs. William Nauseef and Lee-Ann Allen provided helpful feedback regarding neutrophil biology.
Glossary
- CL
cutaneous leishmaniasis
- DC
dendritic cell
- DHR123
dihydrorhodamine 123
- G-MDSC
granulocytic myeloid-derived suppressor cell
- G-MFI
geometric mean fluorescence index
- HLA-DR
human leukocyte antigen D-related allele
- LD-PMN
low-density polymorphonuclear leukocyte
- LST
Leishmania skin test
- MHC class II
major histocompatibility class II antigen
- MPO
myeloperoxidase
- ND
normal density
- PMN
polymorphonuclear leukocyte
- SLA
soluble Leishmania antigen
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Martins A. L., Barreto J. A., Lauris J. R., Martins A. C. (2014) American tegumentary leishmaniasis: correlations among immunological, histopathological and clinical parameters. An. Bras. Dermatol. 89, 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schriefer A., Wilson M. E., Carvalho E. M. (2008) Recent developments leading toward a paradigm switch in the diagnostic and therapeutic approach to human leishmaniasis. Curr. Opin. Infect. Dis. 21, 483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaye P., Scott P. (2011) Leishmaniasis: complexity at the host-pathogen interface. Nat. Rev. Microbiol. 9, 604–615. [DOI] [PubMed] [Google Scholar]
- 4.Thalhofer C. J., Chen Y., Sudan B., Love-Homan L., Wilson M. E. (2011) Leukocytes infiltrate the skin and draining lymph nodes in response to the protozoan Leishmania infantum Chagasi. Infect. Immun. 79, 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters N. C., Egen J. G., Secundino N., Debrabant A., Kimblin N., Kamhawi S., Lawyer P., Fay M. P., Germain R. N., Sacks D. (2008) In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 321, 970–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Oliveira C. I., Brodskyn C. I. (2012) The immunobiology of Leishmania braziliensis infection. Front. Immunol. 3, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novais F. O., Carvalho L. P., Graff J. W., Beiting D. P., Ruthel G., Roos D. S., Betts M. R., Goldschmidt M. H., Wilson M. E., de Oliveira C. I., Scott P. (2013) Cytotoxic T cells mediate pathology and metastasis in cutaneous leishmaniasis. PLoS Pathog. 9, e1003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribeiro-Gomes F. L., Sacks D. (2012) The influence of early neutrophil–Leishmania interactions on the host immune response to infection. Front. Cell. Infect. Microbiol. 2, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearson R. D., Steigbigel R. T. (1981) Phagocytosis and killing of the protozoan Leishmania donovani by human polymorphonuclear leukocytes. J. Immunol. 127, 1438–1443. [PubMed] [Google Scholar]
- 10.Van Zandbergen G., Klinger M., Mueller A., Dannenberg S., Gebert A., Solbach W., Laskay T. (2004) Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J. Immunol. 173, 6521–6525. [DOI] [PubMed] [Google Scholar]
- 11.Ribeiro-Gomes F. L., Peters N. C., Debrabant A., Sacks D. L. (2012) Efficient capture of infected neutrophils by dendritic cells in the skin inhibits the early anti-leishmania response. PLoS Pathog. 8, e1002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carmona-Rivera C., Kaplan M. J. (2013) Low-density granulocytes: a distinct class of neutrophils in systemic autoimmunity. Semin. Immunopathol. 35, 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pillay J., Kamp V. M., van Hoffen E., Visser T., Tak T., Lammers J. W., Ulfman L. H., Leenen L. P., Pickkers P., Koenderman L. (2012) A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J. Clin. Invest. 122, 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng S., Matsushima H., Okamoto T., Yao Y., Lu R., Takashima A. (2013) Reciprocal regulation of development of neutrophil-dendritic cell hybrids in mice by IL-4 and interferon-γ. PLoS One 8, e82929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hänsch G. M., Radsak M., Wagner C., Reis B., Koch A., Breitbart A., Andrassy K. (1999) Expression of major histocompatibility class II antigens on polymorphonuclear neutrophils in patients with Wegener’s granulomatosis. Kidney Int. 55, 1811–1818. [DOI] [PubMed] [Google Scholar]
- 16.Iking-Konert C., Ostendorf B., Sander O., Jost M., Wagner C., Joosten L., Schneider M., Hänsch G. M. (2005) Transdifferentiation of polymorphonuclear neutrophils to dendritic-like cells at the site of inflammation in rheumatoid arthritis: evidence for activation by T cells. Ann. Rheum. Dis. 64, 1436–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner C., Iking-Konert C., Hug F., Stegmaier S., Heppert V., Wentzensen A., Hänsch G. M. (2006) Cellular inflammatory response to persistent localized Staphylococcus aureus infection: phenotypical and functional characterization of polymorphonuclear neutrophils (PMN). Clin. Exp. Immunol. 143, 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsushima H., Geng S., Lu R., Okamoto T., Yao Y., Mayuzumi N., Kotol P. F., Chojnacki B. J., Miyazaki T., Gallo R. L., Takashima A. (2013) Neutrophil differentiation into a unique hybrid population exhibiting dual phenotype and functionality of neutrophils and dendritic cells. Blood 121, 1677–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jirmanus L., Glesby M. J., Guimarães L. H., Lago E., Rosa M. E., Machado P. R., Carvalho E. M. (2012) Epidemiological and clinical changes in American tegumentary leishmaniasis in an area of Leishmania (Viannia) braziliensis transmission over a 20-year period. Am. J. Trop. Med. Hyg. 86, 426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacy P. (2006) Mechanisms of degranulation in neutrophils. Allergy Asthma Clin. Immunol. 2, 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nauseef W. M. (2014) Isolation of human neutrophils from venous blood. Methods Mol. Biol. 1124, 13–18. [DOI] [PubMed] [Google Scholar]
- 22.Carvalho A. M., Magalhães A., Carvalho L. P., Bacellar O., Scott P., Carvalho E. M. (2013) Immunologic response and memory T cells in subjects cured of tegumentary leishmaniasis. BMC Infect. Dis. 13, 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aga E., Katschinski D. M., van Zandbergen G., Laufs H., Hansen B., Müller K., Solbach W., Laskay T. (2002) Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J. Immunol. 169, 898–905. [DOI] [PubMed] [Google Scholar]
- 24.Gosselin E. J., Wardwell K., Rigby W. F., Guyre P. M. (1993) Induction of MHC class II on human polymorphonuclear neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-γ, and IL-3. J. Immunol. 151, 1482–1490. [PubMed] [Google Scholar]
- 25.Kambayashi T., Laufer T. M. (2014) Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat. Rev. Immunol. 14, 719–730. [DOI] [PubMed] [Google Scholar]
- 26.Sagiv J. Y., Michaeli J., Assi S., Mishalian I., Kisos H., Levy L., Damti P., Lumbroso D., Polyansky L., Sionov R. V., Ariel A., Hovav A. H., Henke E., Fridlender Z. G., Granot Z. (2015) Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Reports 10, 562–573. [DOI] [PubMed] [Google Scholar]
- 27.Nauseef W. M. (2014) Detection of superoxide anion and hydrogen peroxide production by cellular NADPH oxidases. Biochim. Biophys. Acta 1840, 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mócsai A. (2013) Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J. Exp. Med. 210, 1283–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolaczkowska E., Kubes P. (2013) Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175. [DOI] [PubMed] [Google Scholar]
- 30.Friedrichs B., Neumann U., Schuller J., Peck M. J. (2014) Cigarette-smoke-induced priming of neutrophils from smokers and non-smokers for increased oxidative burst response is mediated by TNF-α. Toxicol. In Vitro 28, 1249–1258. [DOI] [PubMed] [Google Scholar]
- 31.Holle J. U., Windmöller M., Lange C., Gross W. L., Herlyn K., Csernok E. (2013) Toll-like receptor TLR2 and TLR9 ligation triggers neutrophil activation in granulomatosis with polyangiitis. Rheumatology (Oxford) 52, 1183–1189. [DOI] [PubMed] [Google Scholar]
- 32.Naegele M., Tillack K., Reinhardt S., Schippling S., Martin R., Sospedra M. (2012) Neutrophils in multiple sclerosis are characterized by a primed phenotype. J. Neuroimmunol. 242, 60–71. [DOI] [PubMed] [Google Scholar]
- 33.Hallett M. B., Lloyds D. (1995) Neutrophil priming: the cellular signals that say ‘amber’ but not ‘green’. Immunol. Today 16, 264–268. [DOI] [PubMed] [Google Scholar]
- 34.McFarlane E., Perez C., Charmoy M., Allenbach C., Carter K. C., Alexander J., Tacchini-Cottier F. (2008) Neutrophils contribute to development of a protective immune response during onset of infection with Leishmania donovani. Infect. Immun. 76, 532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iking-Konert C., Vogt S., Radsak M., Wagner C., Hänsch G. M., Andrassy K. (2001) Polymorphonuclear neutrophils in Wegener’s granulomatosis acquire characteristics of antigen presenting cells. Kidney Int. 60, 2247–2262. [DOI] [PubMed] [Google Scholar]
- 36.Cross A., Bucknall R. C., Cassatella M. A., Edwards S. W., Moots R. J. (2003) Synovial fluid neutrophils transcribe and express class II major histocompatibility complex molecules in rheumatoid arthritis. Arthritis Rheum. 48, 2796–2806. [DOI] [PubMed] [Google Scholar]
- 37.Reinisch W., Tillinger W., Lichtenberger C., Gangl A., Willheim M., Scheiner O., Steger G. (1996) In vivo induction of HLA-DR on human neutrophils in patients treated with interferon-γ. Blood 87, 3068. [PubMed] [Google Scholar]
- 38.Takashima A., Yao Y. (2015) Neutrophil plasticity: acquisition of phenotype and functionality of antigen-presenting cell. J. Leukoc. Biol. 98, 489–496. [DOI] [PubMed] [Google Scholar]
- 39.Oehler L., Majdic O., Pickl W. F., Stöckl J., Riedl E., Drach J., Rappersberger K., Geissler K., Knapp W. (1998) Neutrophil granulocyte-committed cells can be driven to acquire dendritic cell characteristics. J. Exp. Med. 187, 1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pliyev B. K., Sumarokov A. B., Buriachkovskaia L. I., Menshikov M. (2011) Extracellular acidosis promotes neutrophil transdifferentiation to MHC class II-expressing cells. Cell. Immunol. 271, 214–218. [DOI] [PubMed] [Google Scholar]
- 41.Denny M. F., Yalavarthi S., Zhao W., Thacker S. G., Anderson M., Sandy A. R., McCune W. J., Kaplan M. J. (2010) A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J. Immunol. 184, 3284–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He G. X., Zhang H. H., Zhou J. X., Wang B. B., Chen Y. H., Kong Y. X., Xie X. W., Wang X. Y., Fei R., Wei L., Chen H. S., Zeng H. (2015) Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 34, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dantas M. L., Oliveira J. M., Carvalho L., Passos S. T., Queiroz A., Guimarães L. H., Machado P., Carvalho E., Arruda S. (2014) Comparative analysis of the tissue inflammatory response in human cutaneous and disseminated leishmaniasis. Mem. Inst. Oswaldo Cruz 109, 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brandt E., Petersen F., Flad H. D. (1992) Recombinant tumor necrosis factor-α potentiates neutrophil degranulation in response to host defense cytokines neutrophil-activating peptide 2 and IL-8 by modulating intracellular cyclic AMP levels. J. Immunol. 149, 1356–1364. [PubMed] [Google Scholar]
- 45.Vargas-Inchaustegui D. A., Hogg A. E., Tulliano G., Llanos-Cuentas A., Arevalo J., Endsley J. J., Soong L. (2010) CXCL10 production by human monocytes in response to Leishmania braziliensis infection. Infect. Immun. 78, 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Espir T. T., Figueira Lde. P., Naiff Mde. F., da Costa A. G., Ramalho-Ortigão M., Malheiro A., Franco A. M. (2014) The role of inflammatory, anti-inflammatory, and regulatory cytokines in patients infected with cutaneous leishmaniasis in Amazonas State, Brazil. J. Immunol. Res. 2014, 481750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Latifynia A., Khamesipour A., Bokaie S., Khansari N. (2012) Antioxidants and proinflamatory cytokines in the sera of patients with cutaneous leishmaniasis. Iran. J. Immunol. 9, 208–214. [PubMed] [Google Scholar]
- 48.Whale T. A., Beskorwayne T. K., Babiuk L. A., Griebel P. J. (2006) Bovine polymorphonuclear cells passively acquire membrane lipids and integral membrane proteins from apoptotic and necrotic cells. J. Leukoc. Biol. 79, 1226–1233. [DOI] [PubMed] [Google Scholar]
- 49.Zhou G., Ding Z. C., Fu J., Levitsky H. I. (2011) Presentation of acquired peptide-MHC class II ligands by CD4+ regulatory T cells or helper cells differentially regulates antigen-specific CD4+ T cell response. J. Immunol. 186, 2148–2155. [DOI] [PubMed] [Google Scholar]
- 50.Scapini P., Cassatella M. A. (2014) Social networking of human neutrophils within the immune system. Blood 124, 710–719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.