Microfluidic techniques employed to measure neutrophil motility after interactions with platelets identify patterns of spontaneous and oscillatory neutrophil migration, mediated by CD62P-CD162 interactions.
Keywords: chemotaxis, platelet-leukocyte aggregate, innate immune system
Abstract
Neutrophils are traditionally regarded as the "first responders" of the immune system. However, recent observations revealed that platelets often respond earlier to recruit and activate neutrophils within sites of injury and inflammation. Currently, platelet–neutrophil interactions are studied by intravital microscopy. Although such studies provide exceptional, physiologic in vivo data, they are also laborious and have low throughput. To accelerate platelet–neutrophil interaction studies, we have developed and optimized an ex vivo microfluidic platform with which the interactions between platelets and moving neutrophils are measured at single-cell level in precise conditions and with high throughput. With the use of this new assay, we have evaluated changes in neutrophil motility upon direct contact with platelets. Motility changes include longer distances traveled, frequent changes in direction, and faster neutrophil velocities compared with a standard motility response to chemoattractant fMLP. We also found that the neutrophil–platelet direct interactions are transient and mediated by CD62P–CD162 interactions, localized predominantly at the uropod of moving neutrophils. This "crawling," oscillatory neutrophil behavior upon platelet contact is consistent with previous in vivo studies and validates the use of this new test for the exploration of this interactive relationship.
Introduction
Activation of the acute innate immune response is complex, consisting of both cellular and soluble components. In tissue injury and infection, neutrophils were long thought to be the first and most robust responders, being recruited by both the pathogenic stimulus and the tissue resident cells [1, 2]. Platelets are increasingly recognized as key players in the innate immune responses, being activated by tissue injury and several infections before neutrophils and subsequently, recruiting and activating them [3–5]. Platelets can be activated in hundreds of milliseconds, faster than neutrophils activate [6]. Platelet adhesion to pathogens or injured vessel walls results in platelet activation and has been described to facilitate binding to neutrophils, the release of neutrophil chemotactic substances, and the formation of neutrophil extracellular traps [3, 7–9].
In this study, we optimized and validated a microfluidic platform that enabled us to examine platelet–neutrophil interactions ex vivo with single-cell resolution. We found that upon contact with PRPt, neutrophils migrate spontaneously in the absence of chemoattractant gradients. Neutrophils appear to associate and dissociate frequently from platelets during migration, and the interactions are mediated by CD62P. In straight channels, neutrophils move in an oscillatory pattern between platelets, similar to the crawling behavior described in vivo [5].
MATERIALS AND METHODS
Platelet preparation
Blood was collected from healthy volunteers in accordance with Institutional Review Board protocols (2009-P-000295). Platelets were prepared from blood collected into 2.9 ml trisodium citrate Vacutainer tubes (Sarstedt, Nümbrecht, Germany). PRPt was prepared by centrifugation of the whole blood at 210 g, 22°C, for 20 min. The PRPt supernatant was gently pipetted into 2 tubes. In 1 tube, 20% vol ACD solution (Boston Bioproducts, Ashland, MA, USA) was added, and the PRPt was incubated at 37°C until the start of the experiment. The second tube was centrifuged at 1900 g, 22°C, for 10 min. The PPP was removed, leaving a platelet pellet. IMDM buffer with 20% FBS was used to resuspend the platelet pellet, and ACD was added to the PPP and the platelet suspension. Platelet cell membrane was stained with 1:1000 calcein green (Thermo Fisher Scientific, Waltham, MA, USA). Total platelet count was performed on all samples, with acceptable purity defined as <2% WBC or RBC contaminant. PPP acellularity (<2%) was also verified using a hemocytometer. The PRP platelet count ranged from 25 to 45 × 109 platelets/ml, whereas the WP count ranged from 15 to 25 × 109 platelets/ml; decreased platelet count in WP was most likely a result of platelet loss during the washing step. All plasma preparations were kept at 37°C until the start of the experiment.
Neutrophil isolation
Blood was collected in ACD BD Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ, USA). Neutrophils were isolated from whole blood using a negative-selection protocol, as previously described [10]. In brief, neutrophils were isolated using a density gradient with HetaSep (Stemcell Technologies, Vancouver, BC, Canada) and then purified with EasySep Human Neutrophil Kit (Stemcell Technologies), following the manufacturer's protocol. Neutrophil purity was assessed to be >98%, and cell count was performed using a hemocytometer. Neutrophils were subsequently resuspended in IMDM with 20% FBS (Thermo Fisher Scientific) for a total neutrophil concentration of 5–6 × 106 cells/ml. The cellular nucleic acid was stained with 1:2000 Hoechst Dye 33342 (Thermo Fisher Scientific) for 20 min, followed by 1 wash step. For experiments that evaluated the effect of blocking CD162, neutrophils were also stained with 1:200 mouse anti-human CD162 (clone KPL1; BioLegend, San Diego, CA, USA) for 20 min, followed by 1 wash step. Samples were processed within 1 h of blood draw and were maintained at 37°C.
Design and fabrication of the microfluidic devices
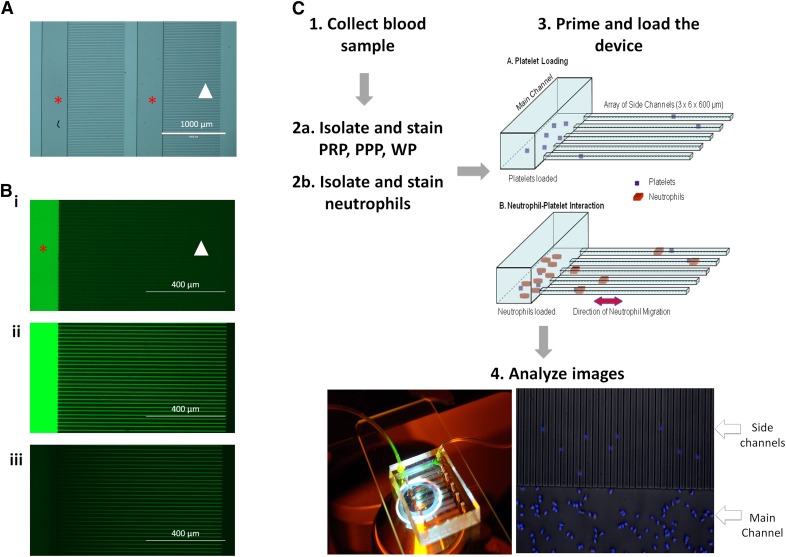
The microfluidic devices were manufactured using standard microfabrication techniques. In brief, a 2-layer photoresist design (SU-8; MicroChem, Newton, MA, USA), with a first and second layer (3 and 50 μm thick, respectively), was patterned on 1 silicon wafer via sequentially aligned photolithography masks and processing cycles, according to the manufacturer's protocols. The resulting patterned wafer was then used as a mold to produce PDMS (Thermo Fisher Scientific) devices, which were subsequently, irreversibly bonded to glass slides (1 × 3 in.; Thermo Fisher Scientific). Gradients were generated between an array of orthogonal side channels (6 μm width, 800 μm height), primed with the chemoattractant and serving as the source (of chemoattractant), and a central channel (500 μm width, 50 μm height), serving as the sink (Fig. 1A).
Figure 1. Microfluidic device work flow.
(A) Photomicrograph of the microfluidic device before priming and loading. Each main channel (red star) is connected to a parallel array of side channels (white arrowhead). (B) The main channels of the device is loaded with fluorescein (i). One end of the main channel is clamped off, and fluorescein is pushed into the side channels (white arrowhead), displacing the air and filling them with dye (ii). The main channel is opened and is flushed with buffer, creating a fluorescein concentration gradient within the device (iii). (C) Schematic of neutrophil migration protocol. Blood is collected (1) and aliquoted into 2 samples: 1 for neutrophil isolation and 1 for platelet isolation. Experimental conditions, PRP, PPP, and WP, are prepared via differential centrifugation and stained with calcein (2a), and neutrophils are collected via negative isolation and stained with Hoechst dye (2b). The microfluidic device is primed using the plasma preparation (3A), and then the neutrophils are loaded into the main channel (3B). Image analysis is then performed on time-lapse fluorescence microscopy of the neutrophils in the different experimental conditions (4). fMLP is used as a positive control that triggers persistent neutrophil migration.
Priming and loading the microfluidic devices
The microfluidic devices were loaded with various solutions uniformly or used to generate chemical gradients, as previously described [10, 11]. To generate gradients, a 2-step process was involved. The microfluidic devices were primed with various solutions: media (negative control), fMLP (100 nM, positive control), PRPt, PPP, or WP. Because the side channels are closed at the distal end, when the main channel is washed with media, a linear gradient is formed along the length of the side channel (Fig. 1B). To load a device, a 1 ml syringe filled with solution was connected to 1 port of the device and the solution pushed through the main channel, and then the outlet port was clamped off. With the application of steady pressure to the syringe, the solution was infused into the device, displacing the air into the PDMS. Once the solution was infused, the neutrophils were loaded into the main channel of the device and allowed to settle by clamping off both ports of the device.
Neutrophil motility analysis
Neutrophil migration was recorded, starting within 15 min of neutrophil loading, using time-lapse imaging on a fully automated Nikon Ti-E microscope with the biochamber at 37°C and 80% humidity and in the presence of 5% carbon dioxide gas. Images of the neutrophils were acquired automatically, every 2.5–3.5 min for up to 2 h, from at least 5 distinct locations on each of 4 microfluidic devices run in parallel in the same experiment. ImageJ manual tracking software (U.S. National Institutes of Health, Bethesda, MD, USA) was used for the analysis of neutrophil migration and behavior. Previous reports showed that when neutrophils are mechanically confined to the smaller side channels during migration, the cell trajectories and velocities can be measured more easily and with higher precision than any of the current assays [10].
Platelet–neutrophil interactions
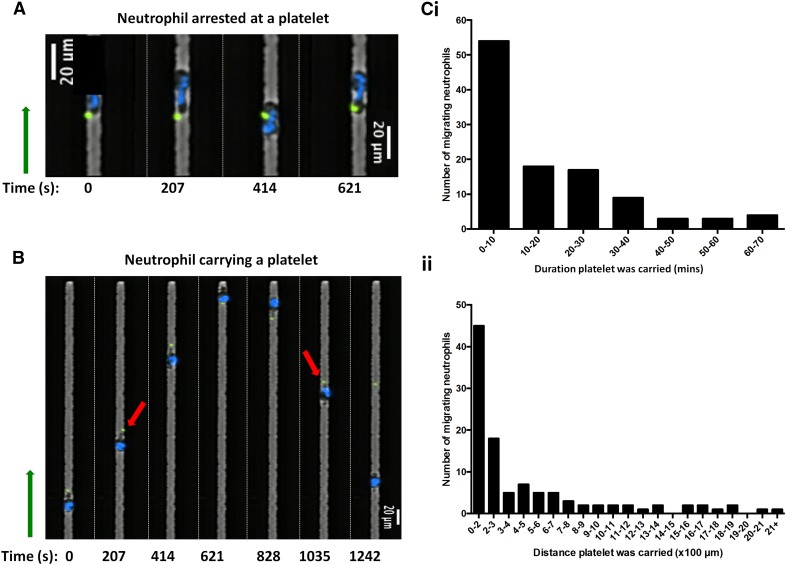
Platelet–neutrophil interactions were analyzed using multiple parameters. For each side channel in which there was neutrophil migration, the total number of platelets within the channel was counted; this included platelets that were attached to neutrophils as they entered from the main channel. Neutrophils traveling in channels containing no platelets were not included in statistical analyses for platelet interactions. Neutrophil–platelet interactions were categorized as “arrested” or “carrying.” Arrested contact involved a migrating neutrophil stopping at the position of the platelet, in contact with the platelet, for >1 time frame (i.e., at least 2.5 min; Fig. 2A). Platelet carrying occurred when neutrophils and platelets moved together through the channel for at least 1 time frame (Fig. 2B). The platelet–neutrophil interaction was further categorized by the number of times that a neutrophil carried a platelet, the total distance it carried the platelet, and the duration of time a platelet was carried. The mean and sd of each of these conditions were calculated for all groups that had contact with platelets, and Student's 2-tailed t tests were carried out to compare the behavior of the PRPc with the PRPn.
Figure 2. Definition of platelet–neutrophil interactions.
(A) Montage of time-lapse fluorescent imaging of the platelet–neutrophil interaction. A neutrophil (Hoechst, blue) in PRP is shown to stop in place and interact with a platelet (calcein, green) for 10.35 min. (B) A neutrophil is shown migrating down a side channel toward a platelet and then attaching and carrying the platelet on its uropod (red arrows) as it changes directions. Direction toward the blind end of the side channel is indicated by the green arrow. (C) Graphs of the total amount of neutrophils migrating as a function of duration of time that a platelet was carried by a neutrophil (i) and the total distance that a platelet was carried by a neutrophil (ii).
Flow cytometry
Phlebotomy was performed with a 24-gauge butterfly needle, and blood was collected into an 8.5 ml ACD BD Vacutainer tube (Becton Dickinson). Isolated neutrophils were stained with 1:200 mouse anti-human CD162 antibodies (clone KPL1; BioLegend) and incubated with PRP. For the negative control, neutrophils were neither stained with anti-CD162 nor incubated with PRP. For the positive control, neutrophils were not stained with anti-CD162 and were incubated with PRP. All samples were stained with 1:200 mouse anti-human CD66b (clone G10F5; BD Biosciences, San Jose, CA, USA) and 1:200 mouse anti-human CD41 (clone HIP8; BioLegend) markers. Data were obtained through the Amnis ImageStreamX Mark II imaging flow cytometer and INSPIRE software (EMD Millipore, Billerica, MA, USA). The accompanying IDEAS software (EMD Millipore) was used to perform data analysis.
Statistical analysis
For determination of the neutrophil phenotype upon interaction with platelets, all cells in each condition (fMLP, PRPt, PPP, and WP) were manually tracked, and the total distance traveled, time spent in a side channel, and average velocity were calculated for each individual neutrophil. Histograms were prepared for all conditions and the skewness, intermediate quartile range, and 95% CI were reported. The number of directional changes a neutrophil made within a channel described neutrophil oscillatory behavior. A change in directionality was defined as when a neutrophil traveled for a minimum of 50 µm in one direction after a turn. Changes in direction were calculated for each neutrophil in all conditions. Arrested contact with a platelet was defined as a neutrophil stopping and interacting with a platelet for >1 time frame (2–3.5 min) and traveling a distance of <50 µm in any direction. The mean and sd for each condition was calculated and compared across conditions (fMLP, PRPt, PRPc, PRPn, PPP, and WP) using Student's 2-tailed t tests, with P < 0.05 considered significant. The experiment was performed for a total of 5 biologic replicates for interdonor comparison. Four intradonor samples were compared and were not found to be significantly different. The same analysis was performed on the samples that were blocked with anti-CD162 antibody, and this was performed on 3 biologic replicates. Statistical analysis was performed using Excel (Microsoft, Redmond, WA, USA).
The flow cytometry experiments to determine the effectiveness of the anti-CD162 antibody were conducted on 3 biologic replicates. Each neutrophil that had a platelet attached was defined as a PLA. The percentage of PLAs in each was calculated, and the number of neutrophils that were determined to be PLAs was compared between experimental conditions. The percent of "PLA inhibition" was determined by calculating the difference in the percent of PLAs formed with and without anti-CD162 antibody. Statistical analysis was performed using Excel (Microsoft).
RESULTS AND DISCUSSION
Neutrophil–platelet interactions and their role in the initiation of innate immune responses are under intense scrutiny [5]. However, previous studies were limited by the techniques available. Whereas intravital microscopy has contributed a great deal of knowledge regarding this relationship, it has its limitations as well, including the use of live animals, highly technical equipment, and microsurgical training [12]. Additionally, although the use of in vivo experiments has an essential role in exploring physiology, complex mechanistic studies can sometimes be better teased apart under highly controlled conditions, restricting the number of variables. The ex vivo assay we have developed for the study of the platelet–neutrophil interaction using microfluidic devices fills this void, enabling precise control of soluble and cellular inputs and analysis at the single-cell level. With the use of this assay, we studied the effect of various platelet-containing solutions on neutrophil motility and the changes of neutrophil motility after direct, physical interactions with platelets (Fig. 1C).
Spontaneous migration of neutrophils in the absence of chemoattractant
Spontaneous neutrophil migration into the side channels occurred in the absence of a chemoattractant. Surprisingly, neutrophils migrated into the side channels for both the PRPt and PPP (231 and 182 total neutrophils, respectively), equivalent to the migration of neutrophils when exposed to fMLP (201 total neutrophils). Neutrophil migration in the WP was rare (18 total neutrophils). Interestingly, the average velocity of the migrating neutrophils in the PRPt and PPP was 28.4 ± 5.8 μm/min and 29.5 ± 5.8 μm/min, respectively, compared with the fMLP [19.5 ± 9.1 μm/min (Table 1)]. With the evaluation of the representative histogram curves of average velocity for all conditions, the neutrophils in fMLP were clustered on the left, whereas the neutrophils in PRPt and PPP were clustered toward the right (Supplemental Fig. 1 and Supplemental Table 1). This implies that PRPt and PPP stimulation of neutrophils results in neutrophils moving at higher velocities than those stimulated by fMLP. This phenomenon is not seen with platelets without the plasma component (WP). This is notable, as fMLP is one of the most potent neutrophil chemoattractants currently known [13].
TABLE 1.
Comparison of neutrophil migration and interaction with platelets
| Condition | No. of neutrophils migrating | Number of directional changes,a % | Total distance traveled (μm)b | Velocity μm/min | Time spent in channel (min) | Neutrophil arrest near plateletc | Neutrophil carrying plateletd | Total distance platelet carried (μm)b | Time platelet carried (min)b | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3+ | |||||||||
| fMLP | 201 | 24.9 | 68.7 | 4.5 | 2.0 | 1047.7 ± 460.3 | 19.5 ± 9.1 | 64.0 ± 36.2 | NA | NA | NA | NA |
| PRPt | 231 | 14.7 | 48.5 | 2.2 | 34.6 | 1695.7 ± 811.0 | 28.4 ± 5.8 | 61.4 ± 29.8 | 20.1% | 20.1%, 39.1% | 468.3 ± 507.1 | 16.1 ± 16.4 |
| PRPc | 83 | 7.2 | 37.4 | 4.8 | 50.6 | 2029.7 ± 812.9 | 29.1 ± 5.7 | 71.5 ± 28.0 | 20.1% | 38.6%, 74.7% | 468.3 ± 507.1 | 16.1 ± 16.4 |
| PRPn | 148 | 18.9 | 54.7 | 0.7 | 25.7 | 1508.4 ± 749.8 | 28.0 ± 5.9 | 55.7 ± 29.4 | NA | NA | NA | NA |
| PPP | 182 | 20.9 | 51.7 | 2.2 | 25.3 | 1470.3 ± 736.8 | 29.5 ± 5.8 | 53.0 ± 30.4 | 0.0% | 1.1% | 475.3 ± 517.3 | 17.4 ± 19.7 |
| WP | 18 | 23.5 | 76.5 | 0.0 | 5.9 | 1155.5 ± 510.1 | 27.0 ± 7.6 | 44.6 ± 22.9 | 0.0% | 0.0% | NA | NA |
NA, Not applicable.
Presented as percent of all neutrophils moving within 2 h observation.
Means (±sd).
Percent of neutrophils in channels that contained at least 1 platelet, which arrested at the position of a platelet for >1 time frame.
Percent of neutrophils in channels with platelets, which carried a platelet once or more for at least 1 time frame.
These data suggest that there are factors within the plasma that result in spontaneous neutrophil migration. These soluble factors may include the following: exosomes, microparticles, members of the coagulation and fibrinolysis cascade, complement, or the leukotriene family [13–17]. The discovery and analysis of plasma factors that may stimulate neutrophils are beyond the scope of this paper but are important and should be pursued in future studies. Additionally, although there are minimal platelets in PPP, and spontaneous chemotaxis is seen in the PRPt without direct contact with platelets, there may be platelet activation during both processing of the plasma, as well as the plasma loading into the microfluidic device. This may result in a number of factors being released by the platelets, including microparticles and exosomes, many of which have been shown to result in neutrophil activation [18]. The lack of spontaneous migration observed in the WP group provides a useful negative control for the presence of ACD and suggests that if platelet activation is responsible for neutrophil migration, then additional plasma-derived components must also be required to stimulate this activity.
Frequent directional changes by neutrophils after interactions with platelets
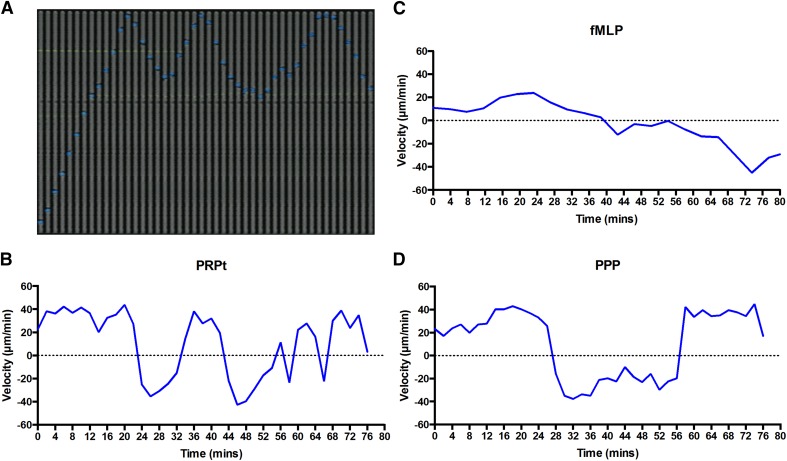
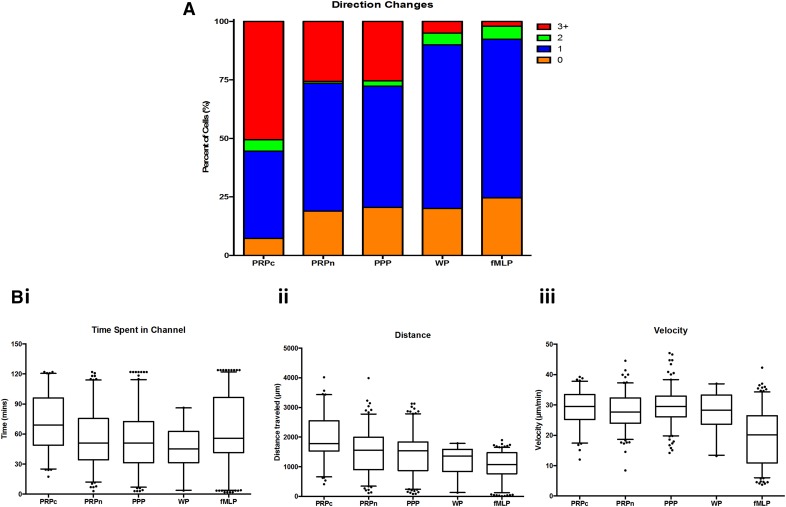
Neutrophils in PRPt changed directions in the side channels more frequently than those in PPP or in fMLP (Fig. 3). The plotting of representative neutrophil velocities versus time showed a clear phenotypic difference among PRPt, PPP, and fMLP (Fig. 3B–D). Additionally, neutrophils that came into contact with a platelet often displayed an oscillatory behavior, switching directions within the side channel at a higher rate than those without platelet contact (Fig. 4A and Table 1). A large proportion of neutrophils in PRPt and PPP changed in direction 3 or more times (34.6% and 25.3%, respectively; Fig. 4A, Table 1, and Supplemental Video 1). By comparison, the majority neutrophils in fMLP and WP changed directions only once (68.7% and 76.5%, respectively) and usually at the end of the channels, as reported previously [19] (Fig. 4A, Table 1, and Supplemental Video 2). The difference in proportions of neutrophils changing directions 3 or more times was statistically significant between fMLP and PRPt (P < 0.01; Supplemental Table 2). These data show that there is a unique oscillatory phenotype present in neutrophils when exposed to PRP and PPP, as opposed to WP and fMLP. The PRPc neutrophils made significantly more directional changes in the side channel than the PRPn neutrophils (50.6% and 25.7%, respectively; P < 0.01), and this was in the absence of any exogenous chemoattractant (Table 1 and Supplemental Table 1). This oscillatory behavior seen in neutrophils exposed to plasma and exacerbated by direct contact with platelets is similar to the crawling behavior observed in vivo [5].
Figure 3. Neutrophil directionality.
Neutrophil behavior was evaluated in microfluidic devices, with individual velocities recorded and analyzed. (A) A montage of time-lapse (207 s) fluorescent imaging shows a representative neutrophil (Hoechst, blue) in PRPt (calcein, green), migrating and interacting in a side channel. (B) The velocity of this individual neutrophil toward the end of the channel was tracked and displayed on a graph over time. The migratory behavior of a representative neutrophil is also displayed for fMLP (C) and PPP (D).
Figure 4. Neutrophil phenotype in various conditions.
Neutrophil phenotype was evaluated in all 4 conditions (PRPc, PRPn, PPP, WP, and fMLP). (A) Bar graph represents the percent of neutrophils that displayed a certain number of directional changes (orange, 0; blue, 1; green, 2; red, 3+). (B) Box scatter plots showing the 95% CIs are shown for time spent in the side channel (i), the distance traveled within the side channel (ii), and the velocity (iii).
Time and distance traveled by neutrophils after interactions with platelets
The time spent in the channel by neutrophils also revealed differences between neutrophil migration patterns in each condition. Neutrophils exposed to PRPt, PPP, and fMLP spent an average of 61.4 ± 29.8, 53.0 + 30.4, and 64.0 + 36.2 min in the side channels, respectively (Fig. 4Bi and Table 1). The distributions for fMLP and WPs demonstrated bimodality, indicating that the behavior of these neutrophils is bimodal (Supplemental Fig. 1 and Supplemental Table 1). Conversely, the distributions for PRPt and PPP followed a normal distribution curve. The distance traveled in the side channels is also influenced by both PRP and the interaction with platelets. The average total distance traveled in the PRPt and PPP was 1695.7 ± 811.0 μm and 1470.3 ± 736.8 μm, respectively, compared with the fMLP, 1047.7 ± 460.3 μm (Fig. 4Bii and Table 1). Statistical comparisons among all conditions are summarized in Supplemental Table 2. Overall, the neutrophil phenotype of fMLP was similar to that of WP, whereas the phenotype of PRPn was similar to that of PPP, with the exception of the velocity of the neutrophil. When comparing PRPc with PRPn, it is apparent that with the exception of the velocity of the neutrophil, contact with platelets does significantly change the migration behavior of the neutrophil.
Neutrophils migrate faster after direct, physical interaction with platelets
Direct platelet–neutrophil interactions resulted in either the arrest of neutrophil motility after contact with a platelet (20.1% of interactions) or in the neutrophil carrying the platelet (39.1% of interactions; Table 1). The duration of time that a neutrophil carried a platelet along the side channel was indicative of the transient nature of the platelet–neutrophil interactions, with most neutrophils carrying platelets for <4 min over a distance of ∼120 µm. Surprisingly, some neutrophils carried platelets for up to 1 h and for >2100 µm (Fig. 2C). Occasionally, neutrophils were noted to carry a platelet from the main channel into a side channel; these neutrophils exhibited a similar phenotype to the neutrophils that came into contact with a platelet within the side channel. Initial contact with the platelet mostly involved the leading edge of the neutrophil as a result of the spatial constraints within the side channels of the device. Interestingly, however, the neutrophils that did attach to and carry platelets along the channels appeared to bind at the uropod (the trailing edge). This is consistent with previously published literature establishing that 2 of the main receptors involved in the platelet–neutrophil interaction are CD62P, on the surface of the activated platelets and, CD162, on the uropod of the activated neutrophil [5, 20, 21]. Further experiments to validate the binding of the platelet to the neutrophil uropod are described later, in the section concerning neutrophil phenotypes following blockage of CD162.
Direct contact with platelets had an effect on the time spent in the side channel, the total distance traveled, and the velocity, with neutrophils in the PRPc group having faster velocities and spending longer time in the channels than the PRPn group, 71.5 ± 28.0 and 55.7 ± 29.4 min, 2029.7 ± 812.9 and 1508.4 ± 749.8 μm, and 29.1 ± 5.7 and 28.0 ± 5.9 μm/min, respectively (Fig. 4Bi–iii and Table 1). Histograms of these behaviors were also plotted (Supplemental Fig. 1 and Supplemental Table 1). The difference between PRPc and PRPn was statistically significant for both the total time spent in the channel (P < 0.01) and the total distance traveled (P < 0.01; Supplemental Table 2). These observations indicate that interactions between neutrophils and platelets result in an altered neutrophil phenotype, making them significantly more mobile than neutrophils that simply migrate into the channels.
The platelet–neutrophil interaction is mediated by CD62P–CD162 interactions
To verify further the use of this assay and validate the importance of the CD62P–CD162 interaction in the platelet–neutrophil interaction, CD162 was blocked. The blocking of the CD162 on neutrophils resulted in a decrease in the amount of PLA formation ex vivo (Supplemental Fig. 2A). Flow cytometry experiments showed that coincubation of isolated neutrophils with PRP resulted in an increase in PLA formation (1.3 ± 0.9% compared with 39.4 ± 17.4%); however, when neutrophils were treated with the anti-CD162 antibody before coincubation with PRP, formation of PLAs decreased (39.4 ± 17.4% compared with 14.1 ± 1.8%; Supplemental Fig. 2B and C). We also compared the amount of PLA formation before and after neutrophil isolation (4.0 ± 0.8% compared with 1.3 ± 0.9%) to show that there was minimal PLA formation with the blood collection technique and that neutrophils resulting from the negative-isolation technique used were primarily not bound to platelets (Supplemental Fig. 2B and C).
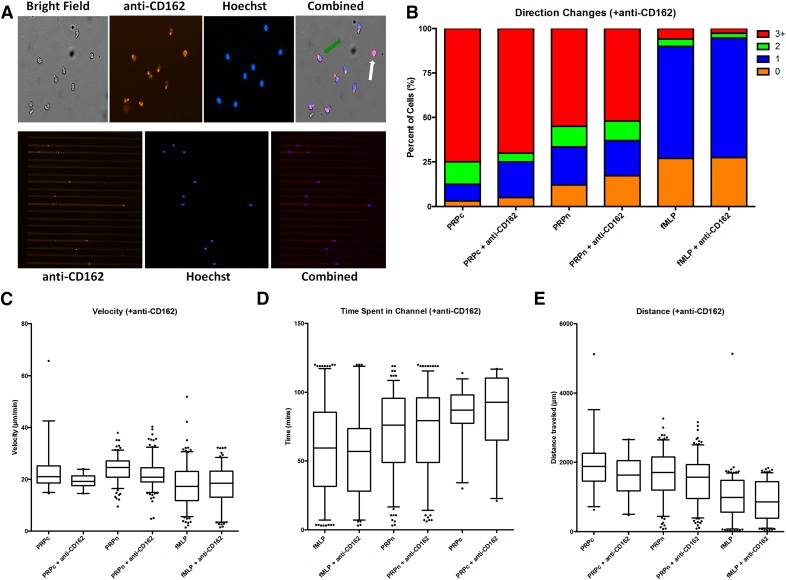
The microfluidic assay was then run using CD162-blocked neutrophils to determine whether a change in platelet–neutrophil interaction would be observed. Activation and polarization of CD162 at the uropod of the neutrophil were clearly visualized in both the main channel and the side channel of the microfluidic device (Fig. 5A). There was no significant difference in migration behaviors for the neutrophils with and without CD162 blocked upon exposure to fMLP (Fig. 5 and Supplemental Tables 3 and 4). There was a significant difference in migration behavior between neutrophils in PRP with and without blocking of CD162 for both the total distance traveled (P < 0.01) and the average velocity (P < 0.01; Supplemental Table 4). As expected, there was also a significant decrease in the amount of neutrophils that bound to and carried platelets when CD162 was blocked in PRP (Table 2). There was no significant different in neutrophil migration behavior for neutrophils that did not come into contact with a platelet (PRPn and PRPn + anti-CD162), although there were significant differences in behavior with the neutrophils that came into contact with platelets (PRPc and PRPc + anti-CD162; Fig. 5B–E and Supplemental Tables 3 and 4). These findings demonstrate that blocking CD162 on neutrophils does significantly inhibit initial platelet–neutrophil binding, although if a platelet were bound, then neutrophils displayed a phenotype consistent with platelet interaction.
Figure 5. Neutrophil phenotype after treatment with anti-CD162 antibody.
(A) Neutrophils in the microfluidic device, stained with both Hoechst (blue) and anti-CD162 antibody (red). The upper 4 photos show the neutrophil within the main channel. Activation of the neutrophil is characterized by the polarization of the uropod and the localization of the anti-CD162 staining (green arrow). Neutrophils that are not activated are not polarized and display CD162 staining diffusely throughout their cell membrane (white arrow). (B) Bar graph represents the percent of neutrophils that displayed a certain number of directional changes (orange, 0; blue, 1; green, 2; red, 3+). Neutrophil phenotype was evaluated in all 4 conditions (fMLP, fMLP + anti-CD162, PRP, PRPc + anti-CD162). Box scatter plots showing the 95% CI are shown for velocity (C), time spent in the side channel (D), and the distance traveled within the side channel (E).
TABLE 2.
Comparison of neutrophil migration and platelet interaction in the presence of anti-CD162 antibodies
| PRPca | PRPc + anti-CD162a | Pb | |
|---|---|---|---|
| Distance platelet was carried (µm) | 449.1 ± 113.2 | 214.0 ± 64.2 | 0.19 |
| Time duration platelet was carried (min) | 16.0 ± 3.5 | 10.4 ± 2.9 | 0.32 |
| % Neutrophils carrying platelets | 17.4 | 7.6 | 0.01 |
| % Neutrophils in arrested contact with platelets | 8.3 | 6.3 | 0.47 |
| Time duration of arrested platelet contact (min) | 4.9 ± 2.3 | 5 ± 2.8 | 0.94 |
Means (±sd).
Two-tailed Student's t test between the PRPc and PRPc + anti-CD162 groups.
Relevance of platelet–neutrophil interaction to the pathology of disease
With the use of this new microfluidic technique, we observed spontaneous migration of neutrophils to both PRPt and PPP, although the average velocity of neutrophils in PRPt was almost double of that in fMLP. This suggests that either soluble components or microparticles within the plasma result in spontaneous neutrophil migration. Additionally, there were significant differences in the number of neutrophil directional changes, velocity, and total time spent in the side channels, dependent on whether the neutrophils made direct contact with platelets. This spontaneous migration and oscillatory phenotype has been reported before in the context of sepsis after burn injuries [10]. This is the first time that this behavior has been recapitulated in an ex vivo system. Platelets can play both pro- and anti-inflammatory roles. For instance, CD62P–CD162 interactions can instigate the activation and crawling of neutrophils, and neutrophils can use platelet arachidonic acid for the biosynthesis of leukotrienes; these events can take place in both the circulatory system as well as in peripheral organs [5, 22]. A better understanding of neutrophil–platelet interactions could help advance our understanding of neutrophil dysfunction and mechanisms leading to sepsis [23–25].
Our observations of the interactions between platelets and moving neutrophils, in conditions of mechanical confinement, are suggestive of a mechanism by which neutrophils may transport platelets inside tissues. This biologic property is likely relevant to certain disease states, when platelets do not always remain within the circulatory system. On many occasions, platelets have been noted to be present in extravascular spaces, suggesting a mechanism for platelet diapedesis. Platelets and neutrophils have been observed in the skin of rats after intradermal injection of the platelet-activating factor and in the sinusoidal and perisinusoidal Disse spaces in the liver of mice and rats after injection of LPS [26–30]. The recent study by Zuchtriegel et al. [31], investigating the binding of CD162 by CD62P, showed that this interaction leads to ERK1/2 MAPK-dependent conformational changes of leukocyte integrins, promoting the successive extravasation of neutrophils and monocytes. Importantly, this study also revealed that platelet-directed, spatiotemporally organized crosstalk between platelets and leukocytes is essential in the trafficking of neutrophils toward sites of inflammation. One hypothesis emerging from this literature suggests that neutrophils mediate transport of platelets and that the role of this behavior in disease should be studied in more detail. These interactions may also serve a protective function during inflammation and injury, as suggested by the biosynthesis of inflammation resolution mediators, such as Maresin 1 [32]. In our study, some neutrophils in the PRPt were noted to migrate from the main channel into the side channel with a platelet already adhered, demonstrating that this ex vivo technique could potentially provide a platform for the study of platelet extravasation secondary to neutrophil diapedesis.
Conclusions
The development of microfluidic techniques for the study of platelet–neutrophil interactions at a single-cell level can provide new insights into innate immune cell behavior. In this study, phenotypic changes of the neutrophil upon platelet binding are identified and described in detail. Future applications of the assay presented here can be broadened to explore the effect of drugs, molecules, and various disease states on the neutrophil–platelet interaction.
AUTHORSHIP
G.H.F. and D.I. developed the hypothesis and designed the experiments. G.H.F., A.L., F.E., and J.J. performed the experiments and analyzed the results. G.H.F., J.G.F., R.G.T, and D.I. reviewed the results and prepared the manuscript.
Supplementary Material
ACKNOWLEDGMENTS
Financial support was provided by The U.S. National Institutes of Health through grants T32-0D019078-27 and P30-ES002109 (to J.G.F.) and GM092804 and AI113937 (to D.I.). All microfabrication procedures were performed at the BioMEMS Resource Center (EB002503). The authors acknowledge Alyssa Terestre for the final review and editing of this manuscript.
Glossary
- ACD
acid citrate dextrose
- CD62P
P-selectin
- CD162
P-selectin glycoprotein ligand-1/selectin P ligand
- CI
confidence interval
- PDMS
polymidemethylsiloxane
- PLA
platelet-leukocyte aggregate
- PPP
platelet-poor plasma
- PRP
platelet-rich plasma
- PRPc
neutrophils that made direct contact with platelets
- PRPn
neutrophils that did not make direct contact with platelets
- PRPt
platelet-rich plasma
- WP
washed platelet
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Kim N. D., Luster A. D. (2015) The role of tissue resident cells in neutrophil recruitment. Trends Immunol. 36, 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar V., Sharma A. (2010) Neutrophils: Cinderella of innate immune system. Int. Immunopharmacol. 10, 1325–1334. [DOI] [PubMed] [Google Scholar]
- 3.Engelmann B., Massberg S. (2013) Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 13, 34–45. [DOI] [PubMed] [Google Scholar]
- 4.Semple J. W., Freedman J. (2010) Platelets and innate immunity. Cell. Mol. Life Sci. 67, 499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sreeramkumar V., Adrover J. M., Ballesteros I., Cuartero M. I., Rossaint J., Bilbao I., Nácher M., Pitaval C., Radovanovic I., Fukui Y., McEver R. P., Filippi M. D., Lizasoain I., Ruiz-Cabello J., Zarbock A., Moro M. A., Hidalgo A. (2014) Neutrophils scan for activated platelets to initiate inflammation. Science 346, 1234–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frojmovic M. M. (1999) Platelet biorheology: adhesive interactions in flow. In Handbook of Platelet Physiology and Pharmacology (Rao G. H., ed.), Springer Science and Business Media, New York, 315-341. [Google Scholar]
- 7.Ghasemzadeh M., Hosseini E. (2013) Platelet-leukocyte crosstalk: linking proinflammatory responses to procoagulant state. Thromb. Res. 131, 191–197. [DOI] [PubMed] [Google Scholar]
- 8.Hurley S. M., Kahn F., Nordenfelt P., Mörgelin M., Sørensen O. E., Shannon O. (2015) Platelet-dependent neutrophil function is dysregulated by M protein from Streptococcus pyogenes. Infect. Immun. 83, 3515–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim K. H., Barazia A., Cho J. (2013) Real-time imaging of heterotypic platelet-neutrophil interactions on the activated endothelium during vascular inflammation and thrombus formation in live mice. J. Vis. Exp., e50329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones C. N., Moore M., Dimisko L., Alexander A., Ibrahim A., Hassell B. A., Warren H. S., Tompkins R. G., Fagan S. P., Irimia D. (2014) Spontaneous neutrophil migration patterns during sepsis after major burns. PLoS One 9, e114509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler K. L., Ambravaneswaran V., Agrawal N., Bilodeau M., Toner M., Tompkins R. G., Fagan S., Irimia D. (2010) Burn injury reduces neutrophil directional migration speed in microfluidic devices. PLoS One 5, e11921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu N., Lei X., Liu L. (2011) Tracking neutrophil intraluminal crawling, transendothelial migration and chemotaxis in tissue by intravital video microscopy. J. Vis. Exp., e3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boneschansker L., Yan J., Wong E., Briscoe D. M., Irimia D. (2014) Microfluidic platform for the quantitative analysis of leukocyte migration signatures. Nat. Commun. 5, 4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakagawa H., Komorita N. (1993) Complement component C3-derived neutrophil chemotactic factors purified from exudate of rat carrageenin-induced inflammation. Biochem. Biophys. Res. Commun. 194, 1181–1187. [DOI] [PubMed] [Google Scholar]
- 15.Patrick R. A., Hollers J. C., Liu D. Y., Giese B. H., Smith C. W. (1980) Effects of human complement component 1 inactivator on neutrophil chemotaxis and chemotactic deactivation. Infect. Immun. 28, 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paegelow I., Trzeczak S., Böckmann S., Vietinghoff G. (2002) Migratory responses of polymorphonuclear leukocytes to kinin peptides. Pharmacology 66, 153–161. [DOI] [PubMed] [Google Scholar]
- 17.Schuliga M. (2015) The inflammatory actions of coagulant and fibrinolytic proteases in disease. Mediators Inflamm. 2015, 437695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yáñez-Mó M., Siljander P. R., Andreu Z., Zavec A. B., Borràs F. E., Buzas E. I., Buzas K., Casal E., Cappello F., Carvalho J., Colás E., Cordeiro-da Silva A., Fais S., Falcon-Perez J. M., Ghobrial I. M., Giebel B., Gimona M., Graner M., Gursel I., Gursel M., Heegaard N. H., Hendrix A., Kierulf P., Kokubun K., Kosanovic M., Kralj-Iglic V., Krämer-Albers E. M., Laitinen S., Lässer C., Lener T., Ligeti E., Linē A., Lipps G., Llorente A., Lötvall J., Manček-Keber M., Marcilla A., Mittelbrunn M., Nazarenko I., Nolte-’t Hoen E. N., Nyman T. A., O’Driscoll L., Olivan M., Oliveira C., Pállinger É., Del Portillo H. A., Reventós J., Rigau M., Rohde E., Sammar M., Sánchez-Madrid F., Santarém N., Schallmoser K., Ostenfeld M. S., Stoorvogel W., Stukelj R., Van der Grein S. G., Vasconcelos M. H., Wauben M. H., De Wever O. (2015) Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 4, 27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamza B., Wong E., Patel S., Cho H., Martel J., Irimia D. (2014) Retrotaxis of human neutrophils during mechanical confinement inside microfluidic channels. Integr. Biol. (Camb.) 6, 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu T., Zhang L., Geng Z. H., Wang H. B., Wang J. T., Chen M., Geng J. G. (2007) P-Selectin cross-links PSGL-1 and enhances neutrophil adhesion to fibrinogen and ICAM-1 in a Src kinase-dependent, but GPCR-independent mechanism. Cell Adhes. Migr. 1, 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore K. L., Patel K. D., Bruehl R. E., Li F., Johnson D. A., Lichenstein H. S., Cummings R. D., Bainton D. F., McEver R. P. (1995) P-Selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J. Cell Biol. 128, 661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antoine C., Murphy R. C., Henson P. M., Maclouf J. (1992) Time-dependent utilization of platelet arachidonic acid by the neutrophil in formation of 5-lipoxygenase products in platelet-neutrophil co-incubations. Biochim. Biophys. Acta 1128, 139–146. [DOI] [PubMed] [Google Scholar]
- 23.Gawaz M., Dickfeld T., Bogner C., Fateh-Moghadam S., Neumann F. J. (1997) Platelet function in septic multiple organ dysfunction syndrome. Intensive Care Med. 23, 379–385. [DOI] [PubMed] [Google Scholar]
- 24.Opal S. M., Esmon C. T. (2003) Bench-to-bedside review: functional relationships between coagulation and the innate immune response and their respective roles in the pathogenesis of sepsis. Crit. Care 7, 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russwurm S., Vickers J., Meier-Hellmann A., Spangenberg P., Bredle D., Reinhart K., Lösche W. (2002) Platelet and leukocyte activation correlate with the severity of septic organ dysfunction. Shock 17, 263–268. [DOI] [PubMed] [Google Scholar]
- 26.Endo Y., Nakamura M. (1993) Active translocation of platelets into sinusoidal and Disse spaces in the liver in response to lipopolysaccharides, interleukin-1 and tumor necrosis factor. Gen. Pharmacol. 24, 1039–1053. [DOI] [PubMed] [Google Scholar]
- 27.Endo Y., Nakamura M. (1992) The effect of lipopolysaccharide, interleukin-1 and tumour necrosis factor on the hepatic accumulation of 5-hydroxytryptamine and platelets in the mouse. Br. J. Pharmacol. 105, 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura M., Shibazaki M., Nitta Y., Endo Y. (1998) Translocation of platelets into Disse spaces and their entry into hepatocytes in response to lipopolysaccharides, interleukin-1 and tumour necrosis factor: the role of Kupffer cells. J. Hepatol. 28, 991–999. [DOI] [PubMed] [Google Scholar]
- 29.Pons F., Rossi A. G., Norman K. E., Williams T. J., Nourshargh S. (1993) Role of platelet-activating factor (PAF) in platelet accumulation in rabbit skin: effect of the novel long-acting PAF antagonist, UK-74,505. Br. J. Pharmacol. 109, 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pirotzky E., Page C. P., Roubin R., Pfister A., Paul W., Bonnet J., Benveniste J. (1984) PAF-acether-induced plasma exudation in rat skin is independent of platelets and neutrophils. Microcirc. Endothelium Lymphatics 1, 107–122. [PubMed] [Google Scholar]
- 31.Zuchtriegel G., Uhl B., Puhr-Westerheide D., Pörnbacher M., Lauber K., Krombach F., Reichel C. A. (2016) Platelets guide leukocytes to their sites of extravasation. PLoS Biol. 14, e1002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdulnour R. E., Dalli J., Colby J. K., Krishnamoorthy N., Timmons J. Y., Tan S. H., Colas R. A., Petasis N. A., Serhan C. N., Levy B. D. (2014) Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc. Natl. Acad. Sci. USA 111, 16526–16531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.