Abstract
Purpose
Reticular macular disease (RMD) is the highest-risk form of early age-related macular degeneration and also specifically confers decreased longevity. However, because RMD requires advanced retinal imaging for adequate detection of its characteristic subretinal drusenoid deposits (SDD), it has not yet been completely studied with respect to coronary artery disease (CAD), the leading cause of death in the developed world. Because CAD appears in middle age, our purpose was to screen patients aged 45–80 years, documented either with or without CAD, to determine if CAD is associated with RMD.
Design
A prospective cohort study of patients with documented CAD status and no known retinal disease in a clinical practice setting at one institution.
Subjects and Controls
76 eyes from 38 consecutive patients (23 with documented CAD, 15 controls documented without CAD; 47.4% female; mean age 66.7 years).
Methods
Patients were imaged with near infrared reflectance/spectral domain optical coherence tomography and assessed in masked fashion by two graders for the presence of SDD lesions of RMD and soft drusen.
Main Outcome Measures
Presence or absence of RMD/SDD and soft drusen.
Results
RMD was more frequent in patients with CAD vs. those without (Relative Risk (RR) =2.1, CI=1.08–3.95, P=0.03). There was no association of CAD with soft drusen.
Conclusions
A specific relationship between CAD and RMD suggests common systemic causes for both and warrants further study.
Keywords: Age-Related Macular Degeneration, Coronary Artery Disease, Reticular Macular Disease, Reticular Pseudo-Drusen
Age-related macular degeneration (AMD) is the most common cause of legal blindness in developed countries,[1] with severe visual loss due to the late phases of geographic atrophy (GA) of the retinal pigment epithelium (RPE) and choroidal neovascularization (CNV). Until recently, early AMD’s structural lesions were thought to consist entirely of drusen and pigment abnormalities, with large soft drusen under the RPE being the most significant predictor of progression.[2] However, rapid advances in clinical imaging over the past decade, including scanning laser ophthalmoscopy (SLO) for autofluorescence (AF) and near infrared reflectance (NIR-R) imaging, as well as high-resolution spectral domain optical coherence tomography (SD-OCT), have shown that reticular pseudodrusen, also known as reticular macular disease (RMD)[3] or subretinal drusenoid deposits (SDD),[4] are far more prevalent and confer a worse prognosis than previously realized.[3–6] The more general term, RMD, will be used here and includes SDD on SD-OCT[7] and the characteristic associated hypoautofluorescent and hyporeflectant patterns on en face AF and NIR-R imaging.[3] RMD has been shown prospectively to be associated with essentially double the rate of onset of both CNV[8, 9] and GA[9] compared to soft drusen and is highly prevalent in GA,[5] with rates reported between 65% for all GA[10] and 96% for the most common form, multilobular GA.[5]
The pathology of RMD is both subretinal and choroidal. SD-OCT and histopathology show that SDD consist of cholesterol-containing material between the RPE and the ellipsoid zone (EZ) of the photoreceptors.[11] RMD has also been shown to be associated with choroidal thinning[12] and has a spatially predictive value for the development of GA.[5, 13] Its marked association with the multilobular architecture of most GA is also indirect evidence for choroidal vascular bed involvement,[5] consistent with a disease continuum on which the lesions of RMD and the lobules of GA both lie. Indeed, the size correspondence of lobules of GA and choriocapillaris lobules, with clear pathophysiological inference, was noted by Maguire and Vine almost 30 years ago.[14] Our group first suggested, in 2009, based on hypoautofluorescent defects on indocyanine green angiography (ICGA), that RMD could be due to choroidal insufficiency.[3] Alternately, these lesions seen on ICGA could simply be blocking defects caused by SDD.[4] Additionally, RMD, but not soft drusen, has been shown to be associated with decreased longevity, with causality as yet undetermined[9] but suggesting systemic association. An article by Fleckenstein et al. recently pointed out an association of the diffuse trickling (DT) AF phenotype of GA with severe vascular disease, particularly coronary artery disease (CAD), as well as with choroidal thinning. The subjects with the DT phenotype all had extensive RMD, and it is known that this phenotype of GA is multilobular and rapidly progressive. The link with CAD suggests that all these processes are related.[15]
Against this background, several studies have linked AMD with cardiovascular disease. Some have noted patients with AMD to have a markedly increased risk of dying of complications of atherosclerotic disease, and others have noted a reciprocal relationship, with patients with atherosclerotic disease more likely to develop AMD. Thus, Tan et al. found that patients with early AMD <75 years of age at baseline had twice the risk of dying from complications of atherosclerotic disease than normal subjects.[16] For those with advanced AMD, the prognosis was much worse. Interestingly, these findings did not hold for subjects aged >=75 years. Duan et al.’s subsequent review of Medicare data involving 32,788 subjects found that subjects with AMD/CNV were 26% more likely to develop myocardial infarction (MI) events than subjects without AMD.[17] However, some inconsistencies remain in the literature. Another large study of Medicare beneficiaries with CNV found a rate of CAD/cerebrovascular accident (CVA) similar to that of matched controls,[18] and another study found that rates of MI or CVA were significantly lower in subjects with CNV than in controls.[19] Regarding lipids, one study suggested that CNV is associated with elevated high-density lipoprotein (HDL) cholesterol levels, a protective factor against MI and CVA.[20] Wong and colleagues published two studies looking at the risk of CVA and coronary heart disease in patients with AMD, and the 10-year risk for developing either outcome was higher in subjects with early AMD than in controls.[21, 22] Klein et al., in the Beaver Dam Eye Study, found increasing risk ratios for 10-year incident early AMD and CNV for each 10 mm Hg increase in both baseline systolic blood pressure and pulse pressure.[23]
The conflicting results of these studies suggest a possibility of unrecognized confounders. Because RMD requires advanced retinal imaging for optimal detection, and such imaging was not available in any of these studies, unrecognized RMD may partly explain the mixed associations between CAD and AMD found in these numerous investigations. This suggests that associations of AMD with cardiovascular disease should be tested again with the high-risk phenotype RMD in mind. Furthermore, if there is a common underlying process, perhaps inflammatory, responsible for both RMD and CAD, which can manifest as lethal CAD in middle age, then patients with CAD but without diagnosed macular disease might show signs of RMD as it is beginning. Thus, instead of an AMD population, we chose to probe a younger cardiac population. We undertook a prospective study of patients with CAD and controls aged 45–80 years, younger than typical AMD cohorts, to determine whether documented CAD was correlated with signs of previously undocumented RMD.
METHODS
This prospective cohort study was approved by the Institutional Review Board of New York University School of Medicine. The research was carried out in accordance with the Health Insurance Portability and Accountability Act of 1996 and adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all study participants, allowing the review of relevant medical records. Between April 2013 and December 2013, study participants were recruited consecutively from one cardiology practice at New York University School of Medicine.
Serial patients from the cardiology practice, whose CAD status was documented (CAD+ or CAD−), were considered for enrollment. In particular, patients classified as CAD− were required to be free of symptoms for their lifetime and designated as such by one of two cardiologists in the practice. The symptoms for which patients were excluded were specifically only those commonly associated with CAD, such as dyspnea or chest pain. However, the patients without CAD in this practice had a variety of vascular risk factors, including hypertension and hyperlipidemia, but specifically did not have valvular or other heart disease. Potential subjects who were then ascertained by interview to have no previously identified retinal disease underwent slit lamp exam and NIR-R/SD-OCT imaging obtained with the Spectralis HRA2 (Heidelberg Engineering, Inc., Carlsbad, California, USA). For each patient, 19 6-mm B-scans, each composed of 9 averaged OCT B-scans at 240 μm intervals, were obtained from within the macula. Patients who were found on imaging, despite negative interviews, to have other significant retinal or macular disorders not related to AMD, such as high myopia and diabetic retinopathy, were excluded. Patients with mild epiretinal membrane (ERM) or with mild cataracts not interfering with imaging were not excluded. All subjects were documented as to hypertension (Y/N) and smoking status (ever/never).
SD-OCT terminology and grading
We use the terminology of 4 reflectivity bands posterior to the outer nuclear layer (ONL) based on Spaide and Curcio,[24, 25] which has since been accepted and expanded by an international consortium[26] (Fig 1). For purposes of this study, RMD was defined by and was equivalent to the presence of SDD on SD-OCT. No angiography was performed. NIR-R SLO en face imaging was used for confirmation. Soft drusen were defined by their characteristic SD-OCT appearance.
Figure 1. Normal retina on spectral domain optical coherence tomography.
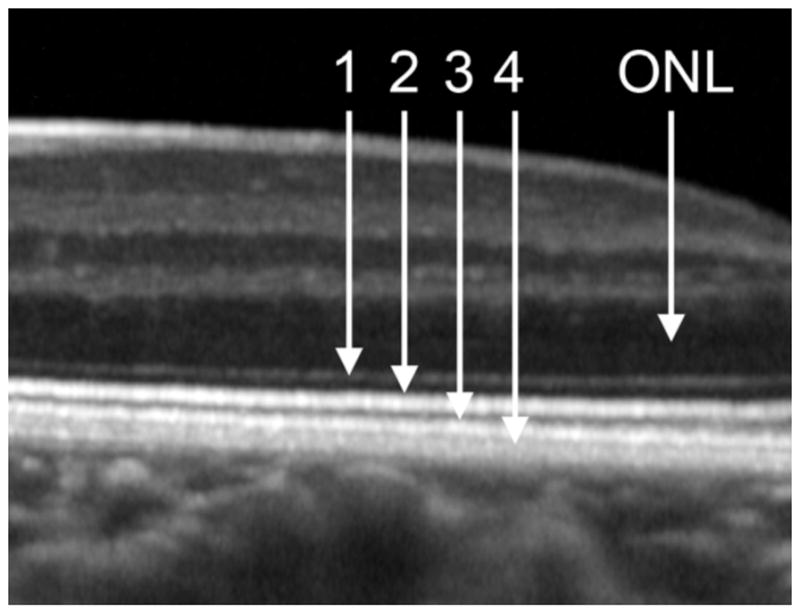
The 4 reflectivity bands beneath the dark outer nuclear layer (ONL) are, respectively, (1) the external limiting membrane (ELM); (2) the inner segment ellipsoid zone (EZ); (3) the interdigitated outer segments zone (IZ); and (4) the RPE/Bruch’s membrane (RPE/BrM).
To be graded positive for RMD, eyes had to have SD-OCT evidence of definite SDD lesions in more than 1 B-scan, with a total of at least 5 lesions between them, detected by 2 graders. In the cases where smaller numbers of lesions were found (<10), each was marked on a separate SD-OCT image for possible resolution or adjudication. Differences were resolved as necessary by a senior grader (RTS). NIR-R images were examined for corresponding lesions in registration with the SDD. To be graded positive for soft drusen, characteristic sub-RPE lesions and/or the similar pathology of RPE detachment were confirmed by SD-OCT. Hard drusen were not included. Contrasted examples of a normal SD-OCT and one with marked RMD/SDD are shown in Fig 2.
Figure 2. Spectral domain optical coherence tomography (SD-OCT) scans of a normal subject and a subject with advanced reticular macular disease (RMD) and coronary artery disease (CAD).
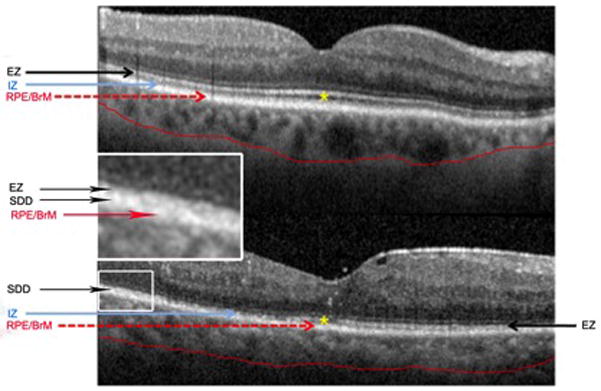
The inner segment ellipsoid zone (EZ) is indicated by black arrows; the interdigitated outer segments zone (IZ) is indicated by blue arrows. The subretinal space between the retinal pigment epithelium (RPE) and EZ is marked with yellow asterisks. Top: Normal SD-OCT image from a 75-year-old female with no known vascular or macular disease. The EZ is uniformly visualized, and the IZ appears just above the RPE. The subretinal space between the IZ and EZ is essentially clear, with a few tiny opacities less than 50 μm. Bottom: SD-OCT image from a 59-year-old male with known CAD and no previously identified eye disease. Subretinal drusenoid deposit (SDD) lesions of RMD are present. The subretinal space is partly obliterated by Stage 1 and 2 SDD. The nominal position of the IZ is marked, but the IZ itself is undetectable, whereas the EZ itself is largely disrupted temporally to the fovea. There are no soft drusen in either image. The central choroid is thinner in the lower image.
SDD staging was adapted from the system proposed by Zweifel et al.[4] The only modification was a more precise definition of Stage 1, which was warranted by those cases in which only Stage 1 lesions were found. Stage 1 SDD was defined as loss of interdigitated outer segments zone (IZ) and diffuse deposition of granular hyperreflective lesions at least 50 μm in diameter between the RPE and the EZ, or a single such lesion at least 100 μm in basal diameter, not altering the contour of the EZ. Granular lesions and a single larger 100 μm lesion together were counted as a single Stage 1 SDD. Stage 2 was defined as a mound of accumulated material sufficient to alter the contour of the EZ. Stage 3 was defined by thicker material that adopted a conical appearance and broke through the EZ. (For examples of a normal SD-OCT image and Stages 1, 2, and 3 SDD, see Fig 3; for an example of NIR-R lesions corresponding to Stage 2 SDD, see Fig 4.)
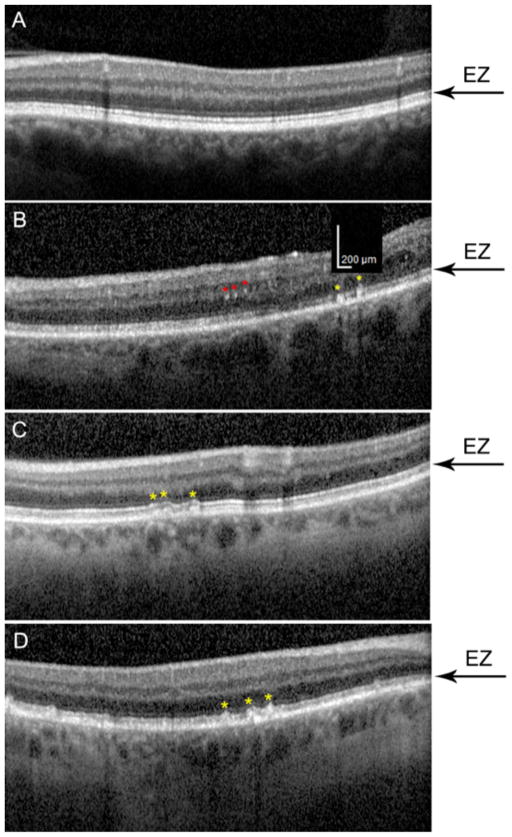
Figure 3. Stages of subretinal drusenoid deposits (SDD) as graded on spectral domain optical coherence tomography (SD-OCT) in study subjects.
SDD are indicated by yellow asterisks; the inner segment ellipsoid zone (EZ) is indicated by black arrows. The scale bar in B applies to all panels. A. Normal SD-OCT scan from a 69-year-old female with hypertension and documented without coronary artery disease (CAD). B. Stage 1 SDD in an SD-OCT scan from a 70-year-old female with documented CAD. The lesions are about 100 μm in diameter (see scale bar). The EZ contour is still flat. C. Stage 2 SDD in an SD-OCT scan from a 62-year-old female with hypertension and CAD. The EZ contour is elevated over each of three lesions but is unbroken. D. Stage 3 SDD in an SD-OCT scan from a 63-year-old male with CAD. The EZ is penetrated by the three SDD marked centrally.
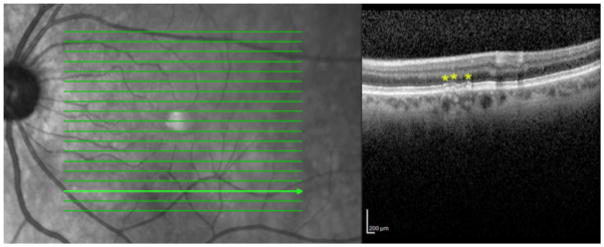
Figure 4. Reticular macular disease lesion correspondence between imaging modalities.
Left: Near infrared reflectance (NIR-R) image with scattered low-contrast hyporeflectant lesions from a 62-year-old female with coronary artery disease. Right: Spectral domain optical coherence tomography B scan through the retinal cross-section marked by the bright green line on the NIR-R scan, with Stage 2 subretinal drusenoid deposits (yellow asterisks). Hyporeflectant lesions are seen in the corresponding area of the NIR-R scan.
Statistical analysis
Relative risks for the incidence of RMD and soft drusen in the CAD+ and CAD− groups were calculated with MedCalc for Windows, version 12.5 (MedCalc Software, Ostend, Belgium).
RESULTS
Forty consecutive patients were screened. Two were excluded due to poor image quality. No subjects were excluded due to undiagnosed retinal disease. One subject with mild ERM was included. The study group consisted of 76 eyes from 38 consecutive patients (23 with documented CAD, 15 controls documented without CAD; 47.4% female; aged 45–80 years; mean age 66.7 years). There were more females in the CAD− group than in the CAD+ group (60.0% vs. 39.1%, respectively), which would be expected in the study age range. The subjects in the CAD+ group were slightly younger than those in the CAD− group (mean ages 66.5 and 67.1 years, respectively, not statistically significant). All subjects were Caucasian with the exception of 2 Asian and 2 Hispanic subjects in the CAD+ group and 1 Hispanic subject in the CAD− group. Approximately equal percentages of smokers were present among the subjects with CAD (12/23, or 52.2%) and those without CAD (7/15, or 47.8%). Slightly more subjects had hypertension in the CAD− group (9/15, or 60%) than in the CAD+ group (12/23, or 52.2%).
RMD was significantly more frequent in patients with CAD than in those without (19/23 vs. 6/15, RR=2.1, CI=1.08–3.95, P=0.03). There was no significant association between soft drusen and CAD. We found incidentally that 3/46 eyes of the 23 patients in the CAD+ group had adult-onset foveomacular vitelliform dystrophy (AOFVD), defined by the presence of pseudovitelliform subretinal material beneath the fovea. No control eyes had AOFVD.
The SDD in these cases were less often multitudinous and obvious Stage 2 and 3 lesions, as typically reported in older subjects with AMD, and were more often smaller numbers of Stage 1 and 2 lesions. Of the 25 subjects graded positive for SDD, only 5 had Stage 3 lesions. Of the 20 subjects that had only Stage 1 and 2 lesions, 7 had only 5–10 lesions total, and the remainder had >10 lesions.
DISCUSSION
In this pilot study, we have shown that the prevalence of RMD, as defined by SDD on SD-OCT, was significantly higher in a population that included middle-aged subjects with CAD than in a similar population without CAD. This association with CAD appears to be stronger than for AMD in general in the older populations studied in the literature. The prevalence of soft drusen, the standard hallmark of early AMD, was no different between these groups. In particular, we often observed characteristic SDD and NIR-R lesions of RMD in younger patients with CAD with no soft drusen. Notably, the SDD in these cases less commonly presented as clear-cut AMD, with multitudinous and obvious lesions such as are typically reported in older subjects with AMD, but required careful scrutiny of all B-scans for identification of smaller Stage 1 and 2 lesions.
These data, if confirmed in larger studies, suggest a stronger correlation between the RMD/SDD form of AMD specifically and CAD than has been demonstrated between AMD generally (early or advanced) and CAD. Furthermore, because RMD requires advanced retinal imaging for optimal detection, unrecognized RMD may partly explain the mixed associations between CAD and AMD found by numerous studies that did not use these methods. Likewise, while decreased choroidal blood flow has been measured in AMD, it is only the RMD/SDD form that shows structural vascular abnormality (choroidal thinning) on SD-OCT. The cause of this thinning is unknown, but defects in the posterior ciliary circulation that feeds the choroid are suspect. Thus, RMD, with lipid-laden SDD in the subretinal space and choroidal vascular pathology, shares common features with CAD, likewise a vascular disease with abnormal lipid deposits. These common pathogenic substrates suggest that further parallel investigation of both diseases is warranted.
An interesting and important separate issue is whether RMD itself is more than a subphenotype of AMD. Hyperreflective deposits in the subretinal space have been identified on SD-OCT in retinal degenerations distinct from AMD, including pseudoxanthoma elasticum with angioid streaks,[27] fundus albipunctatus,[28] and vitamin A deficiency retinopathy.[29] Whether these deposits are the SDD specifically characterized histologically and biochemically in AMD eyes by Curcio et al.[11] remains to be determined. Nonetheless, it is quite possible that SDD are a more general feature of some retinal degenerations, particularly those with abnormalities in the visual cycle.[28, 29] This question bears on the present study because we considered subjects who were younger than required for the usual definition of AMD (usually age >60 years) and who also had lesions of RMD in the absence of the classic AMD finding of soft drusen. What has been documented so far (in the Beaver Dam Eye Study) is that subjects with photographic evidence of RMD are at significant risk for AMD progression and hence are logically placed in the AMD spectrum.[9] However, we do not yet know whether our subjects with RMD, many of whom had only a handful of SDD lesions, are at risk for classic AMD, or in particular for late AMD. Therefore, although we suspect that indeed this is the case, it must be stated clearly that what we have shown is an association of CAD with SDD on SD-OCT in younger patients, but we have not proven that all these patients are on the AMD spectrum. As an alternate, extreme example of a non-AMD phenotype, photographic lesions typical of RMD have been seen in young females with pre-eclampsia,[30] but it is not known if SDD accompany them. These are all points that require clarification by future study.
There are several important differences between SDD and soft drusen, and this study identifies another. Visual function can be more impaired over SDD than over soft drusen.[31, 32] Of pathogenic import, SDD are abundant in the rod-rich perifovea, in contrast to soft drusen and their precursors, which are thickest under the fovea.[11] SDD contain unesterified cholesterol, with little of the esterified cholesterol that dominates conventional drusen.[33, 34] Thus SDD are not simply drusen in the wrong place but distinctive lesions. Why SDD appear to be more tied to CAD than soft drusen should be an illuminating subject in lipid metabolism.
As noted earlier, the RMD lesions in these younger patients are mostly early-stage lesions. This suggests that, while CAD and RMD may be linked by some common pathophysiology, the disease process in CAD can be mature, and lethal, by middle age, whereas in RMD it is mostly nascent. Indeed, among patients with AMD, those with RMD not only are older by several years in most studies, but also have a significantly later age of AMD onset than patients with soft drusen (83 vs. 70 years, respectively),[35] suggesting a long disease maturation process.
The study subjects with CAD and RMD were predominantly male (68.4%). This is in stark contrast to the female preponderance among subjects with AMD and RMD, which is about 85% in several studies that included, respectively, subjects with all forms of AMD,[3, 9] those with GA only,[13] those with unilateral CNV,[8] and those with early AMD only.[35] These data make sense, however, if we consider the significantly earlier mortality among men with CAD than among women with CAD, generally estimated to be about 10 years. If RMD is associated with lethal CAD in younger male patients, with women surviving to the age of AMD onset, then if our younger CAD population were reexamined in 15 years, one would expect the proportion of men to fall dramatically. Furthermore, the natural history of SDD lesion stage progression[36] suggests that many of these remaining subjects, who have survived middle age and had initial SD-OCT evidence of RMD, might progress to more clinically evident AMD and thus fall mainly into the female category of subjects with AMD and RMD, consistent with clinical observations. Indeed, Querques et al. found that, of SDD that progressed, all lesions graded as Stage 1 at baseline examination progressed to Stage 2 over 2 years, and most graded as Stage 2 at baseline progressed to Stage 3.[36] In advanced cases, of course, RMD lesions can also regress, leaving outer retinal atrophy. Querques et al. also found that 5/48 eyes of 33 patients with SDD showed coincident pseudovitelliform material (a sign of AOFVD) within the macular area. We incidentally also found that 3/46 eyes of the 23 patients in the CAD+ group, but none in the CAD− group, had AOFVD. One of these eyes had SDD elsewhere. The connection between the hyperautofluorescent subretinal material of AOFVD and hypoautofluorescent SDD is unclear. However, tracking of non-central pathologic material to the central macula is a well-known phenomenon, e.g., in peripheral exudative lesions. Perhaps hyperautofluorescent fluorophores from damaged outer segments track centrally, leaving hypoautofluorescent fluorophore-deficient RPE behind.
A very important question about RMD in AMD that remains unanswered is the cause of significantly earlier mortality in these subjects than in those with AMD generally. The very carefully done Beaver Dam Eye Study could find neither a CAD nor CVA history in these subjects to explain it, nor was an attributable cause of death determined. However, the vast majority of subjects with AMD/RMD are women, and the “atypical” (meaning different from men), or silent, presentation of CAD in women has been brought to the fore as a major public health concern in recent years. As women are much more likely than men to experience subclinical MI at all ages,[37] is it possible that many elderly women with RMD are dying of unrecognized CAD? CAD is still the leading cause of death in the elderly, and therefore, even without any evidence to connect it to RMD, this would be the best guess. More careful cardiac evaluation of older subjects with RMD in a controlled study is also warranted and could have specific implications for women’s health.
This study has several limitations, and the modest sample size is a major one. Indeed, this pilot study was underpowered to detect a significant difference between the two groups at an alpha = 0.05 level of significance, thereby increasing the possibility of a type I error in the results. A further consideration is that no correction, such as Bonferroni, was made for multiple measures (SDD and soft drusen) on the same subjects. However, this correction also comes with the risk of a type II error (false negative). In the case at hand, because the incidence of SDD was the important variable, we chose not to correct further for the non-significant ascertainment of soft drusen. Another limitation is that the groups were not matched for other independent RMD risk factors found by the Beaver Dam Eye Study, such as hypertension and smoking history,[9] which could have confounded associations with SDD. However, in patients with CAD and those without, smoking history was comparable at 52% vs. 47%, respectively. Hypertension was actually more common in the CAD− group than in the CAD+ group; thus, if this factor had any effect on our results, it would have been to weaken the association of CAD with RMD. Because the controls were symptom free, they had not usually undergone recent definitive stress testing to rule out occult CAD, another potential source of error. Future studies with larger numbers should include hypertension and other factors in multivariate analysis.
A major strength of the study, however, is the ascertainment of CAD status, positive or negative, by a cardiologist, a more reliable classification than simple patient self-report. Thus, all patients in the CAD group were symptomatic, with CAD confirmed by a cardiologist. All of our patients without CAD were lifetime asymptomatic and medically well-characterized. Other strengths include the prospective design, where unselected patients with no eye history were recruited from a cardiology practice, as well as the careful definition and ascertainment of RMD/SDD status by experienced graders, who used a previously established protocol based on SD-OCT. Finally, despite the relatively small numbers, the measured association of RMD and CAD was significant and, at the very least, suggests that cardiologists should consider retinal screening for patients with CAD in their practices.
Given that RMD/SDD is possibly a major hidden link between AMD and CAD, the leading causes of blindness and death, respectively, in the developed world, larger-scale studies are surely warranted to test this hypothesis and also to search for common pathophysiology. Because identifying patients at increased risk for both late AMD and CAD has great prognostic value, the association of RMD with CAD warrants further investigation from the standpoint of early detection, primary and secondary prevention, and public health initiatives.
Acknowledgments
Financial Support: This work was supported by an individual investigator research award from the Foundation Fighting Blindness, Columbia, MD (RTS); National Institutes of Health/National Eye Institute, Bethesda, MD, grants R01 EY015520 (RTS) and R01 EY06109 (CC); unrestricted funds from Research to Prevent Blindness, New York, NY (RTS, CC); and EyeSight Foundation of Alabama (CC). The funding organizations had no role in the design or conduct of this research.
ACRONYMS USED
- AF
autofluorescence
- AMD
age-related macular degeneration
- AOFVD
adult-onset foveomacular vitelliform dystrophy
- BrM
Bruch’s membrane
- CAD
coronary artery disease
- CNV
choroidal neovascularization
- CVA
cerebrovascular accident
- DT
diffuse trickling
- ELM
external limiting membrane
- ERM
epiretinal membrane
- EZ
ellipsoid zone
- GA
geographic atrophy
- HDL
high-density lipoprotein
- ICGA
indocyanine green angiography
- IZ
interdigitated outer segments zone
- MI
myocardial infarction
- NIR-R
near infrared reflectance
- ONL
outer nuclear layer
- RMD
reticular macular disease
- RPE
retinal pigment epithelium
- SDD
subretinal drusenoid deposits
- SD-OCT
spectral domain optical coherence tomography
- SLO
scanning laser ophthalmoscopy
Footnotes
Meeting Presentation: Poster presented at the American Academy of Ophthalmology Annual Meeting, October 2014.
Conflict of Interest: No conflicting relationship exists for any author. RTS is consultant to Ocata Therapeutics.
REFERENCES CITED
- 1.Klaver CC, Wolfs RC, Vingerling JR, Hofman A, de Jong PT. Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Arch Ophthalmol. 1998;116:653–658. doi: 10.1001/archopht.116.5.653. [DOI] [PubMed] [Google Scholar]
- 2.Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995;39:367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- 3.Smith RT, Sohrab MA, Busuioc M, Barile G. Reticular macular disease. Am J Ophthalmol. 2009;148:733–743. doi: 10.1016/j.ajo.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zweifel SA, Spaide RF, Curcio CA, Malek G, Imamura Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010;117:303–312. e301. doi: 10.1016/j.ophtha.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Xu L, Blonska AM, Pumariega NM, Bearelly S, Sohrab MA, Hageman GS, et al. Reticular macular disease is associated with multilobular geographic atrophy in age-related macular degeneration. Retina. 2013;33:1850–1862. doi: 10.1097/IAE.0b013e31828991b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith RT, Chan JK, Busuoic M, Sivagnanavel V, Bird AC, Chong NV. Autofluorescence characteristics of early, atrophic, and high-risk fellow eyes in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47:5495–5504. doi: 10.1167/iovs.05-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zweifel SA, Imamura Y, Spaide TC, Fujiwara T, Spaide RF. Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology. 2010;117:1775–1781. doi: 10.1016/j.ophtha.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Pumariega NM, Smith RT, Sohrab MA, LeTien V, Souied EH. A prospective study of reticular macular disease. Ophthalmology. 2011;118:1619–1625. doi: 10.1016/j.ophtha.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein R, Meuer SM, Knudtson MD, Iyengar SK, Klein BE. The epidemiology of retinal reticular drusen. Am J Ophthalmol. 2008;145:317–326. doi: 10.1016/j.ajo.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz-Valckenberg S, Alten F, Steinberg JS, Jaffe GJ, Fleckenstein M, Mukesh BN, et al. Geographic Atrophy Progression (GAP) Study Group. Reticular drusen associated with geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:5009–5015. doi: 10.1167/iovs.11-7235. [DOI] [PubMed] [Google Scholar]
- 11.Curcio CA, Messinger JD, Sloan KR, McGwin G, Jr, Medeiros NE, Spaide RF. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina. 2013;33:265–276. doi: 10.1097/IAE.0b013e31827e25e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg A, Oll M, Yzer S, Chang S, Barile GR, Merriam JC, et al. Reticular pseudodrusen in early age-related macular degeneration are associated with choroidal thinning. Invest Ophthalmol Vis Sci. 2013;54:7075–7081. doi: 10.1167/iovs.13-12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marsiglia M, Boddu S, Bearelly S, Xu L, Breaux BE, Jr, Freund KB, et al. Association between geographic atrophy progression and reticular pseudodrusen in eyes with dry age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54:7362–7369. doi: 10.1167/iovs.12-11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maguire P, Vine AK. Geographic atrophy of the retinal pigment epithelium. Am J Ophthalmol. 1986;102:621–625. doi: 10.1016/0002-9394(86)90535-0. [DOI] [PubMed] [Google Scholar]
- 15.Fleckenstein M, Schmitz-Valckenberg S, Lindner M, Bezatis A, Becker E, Fimmers R, et al. Fundus Autofluorescence in Age-Related Macular Degeneration Study Group. The “diffuse-trickling” fundus autofluorescence phenotype in geographic atrophy. Invest Ophthalmol Vis Sci. 2014;55:2911–2920. doi: 10.1167/iovs.13-13409. [DOI] [PubMed] [Google Scholar]
- 16.Tan JS, Wang JJ, Liew G, Rochtchina E, Mitchell P. Age-related macular degeneration and mortality from cardiovascular disease or stroke. Br J Ophthalmol. 2008;92:509–512. doi: 10.1136/bjo.2007.131706. [DOI] [PubMed] [Google Scholar]
- 17.Duan Y, Mo J, Klein R, Scott IU, Lin HM, Caulfield J, et al. Age-related macular degeneration is associated with incident myocardial infarction among elderly Americans. Ophthalmology. 2007;114:732–737. doi: 10.1016/j.ophtha.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 18.Alexander SL, Linde-Zwirble WT, Werther W, Depperschmidt EE, Wilson LJ, Palanki R, et al. Annual rates of arterial thromboembolic events in medicare neovascular age-related macular degeneration patients. Ophthalmology. 2007;114:2174–2178. doi: 10.1016/j.ophtha.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen-Khoa BA, Goehring EL, Jr, Werther W, Gower EW, Do DV, Jones JK. Hospitalized cardiovascular diseases in neovascular age-related macular degeneration. Arch Ophthalmol. 2008;126:1280–1286. doi: 10.1001/archopht.126.9.1280. [DOI] [PubMed] [Google Scholar]
- 20.Hyman L, Schachat AP, He Q, Leske MC. Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Arch Ophthalmol. 2000;118:351–358. doi: 10.1001/archopht.118.3.351. [DOI] [PubMed] [Google Scholar]
- 21.Wong TY, Tikellis G, Sun C, Klein R, Couper DJ, Sharrett AR. Age-related macular degeneration and risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Ophthalmology. 2007;114:86–91. doi: 10.1016/j.ophtha.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 22.Wong TY, Kamineni A, Klein R, Sharrett AR, Klein BE, Siscovick DS, et al. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the cardiovascular health study. Arch Intern Med. 2006;166:2388–2394. doi: 10.1001/archinte.166.21.2388. [DOI] [PubMed] [Google Scholar]
- 23.Klein R, Klein BE, Tomany SC, Cruickshanks KJ. The association of cardiovascular disease with the long-term incidence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 2003;110:1273–1280. doi: 10.1016/S0161-6420(03)00599-2. [DOI] [PubMed] [Google Scholar]
- 24.Spaide RF, Curcio CA. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: literature review and model. Retina. 2011;31:1609–1619. doi: 10.1097/IAE.0b013e3182247535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spaide RF. Questioning optical coherence tomography. Ophthalmology. 2012;119:2203–2204. e2201. doi: 10.1016/j.ophtha.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Staurenghi G, Sadda S, Chakravarthy U, Spaide RF International Nomenclature for Optical Coherence Tomography (IN•OCT) Panel. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN•OCT concensus. Ophthalmology. 2014;121:1572–1578. doi: 10.1016/j.ophtha.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 27.Zweifel SA, Imamura Y, Freund KB, Spaide RF. Multimodal fundus imaging of pseudoxanthoma elasticum. Retina. 2011;31:482–491. doi: 10.1097/IAE.0b013e3181f056ce. [DOI] [PubMed] [Google Scholar]
- 28.Genead MA, Fishman GA, Lindeman M. Spectral-domain optical coherence tomography and fundus autofluorescence characteristics in patients with fundus albipunctatus and retinitis punctata albescens. Ophthalmic Genet. 2010;31:566–572. doi: 10.3109/13816810903584971. [DOI] [PubMed] [Google Scholar]
- 29.Aleman TS, Garrity ST, Brucker AJ. Retinal structure in vitamin A deficiency as explored with multimodal imaging. Doc Ophthalmol. 2013;127:239–243. doi: 10.1007/s10633-013-9403-0. [DOI] [PubMed] [Google Scholar]
- 30.Saito Y, Tano Y. Retinal pigment epithelial lesions associated with choroidal ischemia in preeclampsia. Retina. 1998;18:103–108. doi: 10.1097/00006982-199818020-00002. [DOI] [PubMed] [Google Scholar]
- 31.Mrejen S, Sato T, Curcio CA, Spaide RF. Assessing the cone photoreceptor mosaic in eyes with pseudodrusen and soft drusen in vivo using adaptive optics imaging. Ophthalmology. 2014;121:545–551. doi: 10.1016/j.ophtha.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Querques G, Massamba N, Srour M, Boulanger E, Georges A, Souied EH. Impact of reticular pseudodrusen on macular function. Retina. 2014;34:321–329. doi: 10.1097/IAE.0b013e3182993df1. [DOI] [PubMed] [Google Scholar]
- 33.Curcio CA, Presley JB, Malek G, Medeiros NE, Malek G, Avery DV, et al. Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Exp Eye Res. 2005;81:731–741. doi: 10.1016/j.exer.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Oak AS, Messinger JD, Curcio CA. Subretinal drusenoid deposits: further characterization by lipid histochemistry. Retina. 2014;34:825–826. doi: 10.1097/IAE.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 35.Boddu S, Lee MD, Marsiglia M, Marmor M, Freund KB, Smith RT. Risk factors associated with reticular pseudodrusen versus large soft drusen. Am J Ophthalmol. 2014;157:985–993. e982. doi: 10.1016/j.ajo.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Querques G, Canoui-Poitrine F, Coscas F, Massamba N, Querques L, Mimoun G, et al. Analysis of progression of reticular pseudodrusen by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:1264–1270. doi: 10.1167/iovs.11-9063. [DOI] [PubMed] [Google Scholar]
- 37.Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986;111:283–290. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]