Abstract
The common marmoset (Callithrix jacchus) is a highly vocal New World primate species that has emerged in recent years as a promising model system for studies of auditory and vocal processing. Our recent studies have examined perceptual mechanisms related to the pitch of harmonic complex tones in this species. However, no previous psychoacoustic work has measured marmosets’ frequency discrimination abilities for pure tones across a broad frequency range. Here we systematically examined frequency difference limens (FDLs), which measure the minimum discriminable frequency difference between two pure tones, in marmosets across most of their hearing range. Results show that marmosets’ FDLs are comparable to other New World primates, with lowest values in the frequency range of ~3.5–14 kHz. This region of lowest FDLs corresponds with the region of lowest hearing thresholds in this species measured in our previous study and also with the greatest concentration of spectral energy in the major types of marmoset vocalizations. These data suggest that frequency discrimination in the common marmoset may have evolved to match the hearing sensitivity and spectral characteristics of this species’ vocalizations.
Keywords: marmoset, primate, hearing, FDL, frequency discrimination
INTRODUCTION
The ability to discriminate changes in acoustic frequency is an essential element of auditory behavior, critical for the perception of both simple and complex sounds and crucially important for distinguishing among behaviorally relevant acoustic signals in a complex, noisy environment. Frequency discrimination is an important mechanism underlying many of the sound segregation and grouping processes necessary for auditory scene analysis and essentially sets limits on the perception of musical melodies and species-specific vocalizations such as human speech.
The common marmoset (Callithrix jacchus) is a small-bodied New World primate species that has emerged in recent years as a promising model system for studies of auditory and vocal processing (Wang, 2000, 2007, Miller et al. 2016), including several recent studies examining the behavioral and neural mechanisms of pitch perception for complex sounds (Bendor and Wang, 2005; Bendor et al., 2012; Osmanski et al., 2013; Song et al., 2016). However, no previous psychoacoustic studies have examined frequency discrimination for pure tones across a broad frequency range in the common marmoset, and such data are critical for future work exploring the underlying mechanisms of complex sound processing, including vocal perception, in this species.
The minimum discriminable frequency difference between two pure tones, the frequency difference limen (FDL), is one of the most important measures of hearing ability for a species and provides an indication of the resolving power of that species’ auditory system. FDLs can be presented as either an absolute frequency difference (in Hz) or a relative frequency difference (as a percentage change from a reference frequency; for example, a ~6% change in frequency is approximately a one semitone difference on the musical scale and a 100% change is a one octave difference). To a large extent, human perception of frequency roughly correlates more closely with relative changes than absolute changes in frequency. Human psychoacoustic studies have shown that the FDL for pure tones varies with three main parameters: frequency, duration, and sound level (Moore, 1973; Wier et al., 1977). For example, in humans, relative FDLs generally decrease with increasing frequency up to ~1000 Hz before increasing at higher frequencies. Furthermore, relative FDLs tend to decrease for a particular range of frequencies (up to ~4 kHz) with increasing duration and/or sound level (at least up to ~20–30 dB sensation level).
FDLs have been measured in a number of primate species, including humans (Wier et al., 1977; Sinnott et al., 1985; Sinnott et al., 1987; Sinnott et al., 1992), chimpanzees (Kojima, 1990), Old World monkeys (Stebbins, 1973; Sinnot 1985, 1987, 1992; Prosen et al., 1990), and New World monkeys (Capps and Ades, 1968; Recanzone et al., 1991, Wienicke et al., 2001). In general, relative FDLs are lowest in humans (~0.2% change in frequency), larger for chimpanzees and Old World monkeys (~1–2%), and highest in New World monkeys (~2–5%). Across species, the frequencies associated with lowest FDLs almost always overlap with the range of best hearing sensitivity (e.g., Stebbins, 1973; Wier et al., 1977; Kojima, 1990; Wienicke et al., 2001). There also appears to be a close correspondence between a species’ lowest FDLs and the concentration of spectral energy in that species’ vocalizations (see, for example, Winter et al., 1966; Newman, 2003). Such a relationship may reflect the need to reliably detect and discriminate among species-specific communication signals, a process that has likely played an important role in the evolution of the primate auditory system.
In the present study, we systematically examined pure tone FDLs in marmosets across most of their hearing range and compare their frequency discrimination with that of other primates. In addition, marmosets are a highly vocal non-human species, with a vocal repertoire comprising more than 20 different call types (Pistorio et al. 2006, Agamaite et al., 2015) that are produced in a variety of social and behavioral contexts (e.g., Epple 1968; Rylands 1993; Norcross and Newman, 1993, 1997; Norcross et al., 1994; Norcross et al., 1999; Bezerra et al., 2009; Miller and Thomas, 2012). Our previous study has suggested a close correspondence between the spectral characteristics of their most common vocalizations and their lowest hearing thresholds (Osmanski and Wang, 2011). A secondary objective of this study was thus to determine whether there is also a relationship between the region of best FDL sensitivity in marmosets and the spectral characteristics of their vocal repertoire.
MATERIAL AND METHODS
All experimental procedures were approved by the Johns Hopkins University Animal Care and Use Committee and were in compliance with the guidelines of the National Institutes of Health.
Subjects
The subjects in this experiment were four male marmosets between 2–6 years of age. Each had at least one-year experience in discrimination tasks. Two of the subjects were implanted with a headcap designed for neurophysiological experiments (for a description of procedures, see Lu et al., 2001). One of these implanted subjects (M4Y) was head-fixed throughout all testing sessions while the other (M13W) was head fixed only in the first half of testing sessions. The other two subjects (M59A, M55Y) were not head-fixed during the testing sessions. Subjects were housed in individual cages in a large colony at the Johns Hopkins University School of Medicine and maintained at approximately 90% of their free-feeding weight on a diet consisting of monkey chow, fruit, and yogurt with ad libitum access to water. Subjects were tested five or six days per week between the hours of 0900 and 1800. Each experimental session lasted approximately one hour.
Apparatus
The basic operant task and apparatus were described in detail previously (Osmanski and Wang, 2011; Remington et al., 2012). Briefly, marmosets were seated in a custom restraint chair mounted in the center of a single-walled sound isolation chamber (Industrial Acoustic Company, Model 400A [101(W) ×183(D) × 198(H) cm interior dimensions]) with the inside wall of the chamber lined with 3-inch acoustic absorption foam (Pinta Acoustics, model PROSPEC). Sound stimuli were played through a loudspeaker (Tannoy, model Arena) mounted 40~50 cm away in front of the animal’s head. Liquid reward (a mixture of Gerber single-grain rice cereal, strawberry and/or banana-flavored Nesquik, and a protein powder supplement) was delivered through a food delivery tube attached to the top of the restraint chair and connected to a syringe pump (New Era Pump Systems, Inc., Farmingdale, NY, model NE-500) mounted to the base of the chair. Subject responses were recorded by monitoring when an infrared photobeam, positioned on a custom bracket at the end of the feeding tube, was broken by the subject licking at the feeding tube. Testing sessions were computer-controlled and monitored via webcam video (Logitech, C905 camera).
Stimuli and Behavioral Task
Acoustic stimuli were generated offline using Matlab software (Mathworks, Natick, MA) and delivered at a nominal sampling rate of 100 kHz through a multiprocesser DSP unit (Tucker-Davis Technologies, Alachua, FL, RX6), followed by a programmable attenuator (Tucker-Davis Technologies, PA5), and an audio amplifier (Crown Audio, Model D-75A).
Subjects were tested at eight different tone frequencies at one octave intervals chosen from the musical scale (ISO 16), ranging from 220 Hz (A3 on the musical scale, same notation throughout) to 28160 Hz (A10). All stimuli had a duration of 200 ms with a 10-ms linear ramp (rise/fall time). The inter-stimulus interval during the task was fixed at 300 ms.
Each trial contained a repeating series of reference tones at a fixed frequency during a random waiting period (~5–12 s). After this waiting period, a “target” sound at a higher tone frequency was alternated with the reference sound for 4.8 s. There were seven targets presented across a given session, one target per trial, according to the method of constant stimuli. All targets were equally spaced in the semitone scale (1 octave = 12 semitones, one semitone equals ~6% increase in frequency) and chosen to bracket the presumed threshold. A behavioral response during the reference/target alternation period resulted in reward delivery while responses outside of this period resulted in the chamber light being extinguished for 2–5 seconds and a resetting of the trial. No behavioral response automatically led to the start of the next trial. Targets were presented on 70% of all trials while the remaining 30% were catch trials in which no target was presented.
Subjects were tested on all eight tone conditions in pseudorandom order with the exception of A10 (28160 Hz) which was tested last in all animals (Table 1). A10 was the final frequency tested because this frequency is near the upper limit of marmoset hearing and it was unclear whether the animals would be able to adequately perform the task in this frequency range. A subject was tested on a particular tone frequency until it reached criterion performance (see Data Analysis below), after which it was moved on to the next tone condition.
Table 1.
Threshold values (relative FDL, in semitones and % frequency change, and absolute FDL) for each subject is shown at each reference frequency, along with mean and standard deviation (SD). Testing order and sensation level for each reference frequency are also shown.
| Musical scale | A3 | A4 | A5 | A6 | A7 | A8 | A9 | A10 | |
|---|---|---|---|---|---|---|---|---|---|
| Frequency (Hz) | 220 | 440 | 880 | 1760 | 3520 | 7040 | 14080 | 28160 | |
| FDL (semitones) | M4Y | 3.11 | 2.84 | 1.72 | 1.10 | 0.47 | 0.43 | 0.42 | 0.55 |
| M13W | 2.78 | 2.49 | 2.37 | 1.92 | 0.35 | 0.45 | 0.66 | 1.30 | |
| M55Y | 2.63 | 2.05 | 1.26 | 1.01 | 0.42 | 0.42 | 0.66 | 1.44 | |
| M59A | 3.65 | 3.19 | 2.31 | 1.53 | 0.25 | 0.48 | 0.57 | 1.18 | |
| mean | 3.04 | 2.65 | 1.92 | 1.39 | 0.37 | 0.45 | 0.58 | 1.12 | |
| SD | 0.45 | 0.49 | 0.53 | 0.42 | 0.09 | 0.03 | 0.11 | 0.39 | |
| FDL (%) | M4Y | 19.7 | 17.9 | 10.5 | 6.6 | 2.8 | 2.5 | 2.5 | 3.2 |
| M13W | 17.4 | 15.5 | 14.7 | 11.7 | 2.0 | 2.6 | 3.9 | 7.8 | |
| M55Y | 16.4 | 12.6 | 7.6 | 6.0 | 2.4 | 2.5 | 3.9 | 8.7 | |
| M59A | 23.5 | 20.2 | 14.3 | 9.2 | 1.5 | 2.8 | 3.4 | 7.1 | |
| mean | 19.2 | 16.5 | 11.8 | 8.4 | 2.2 | 2.6 | 3.4 | 6.7 | |
| SD | 3.1 | 3.3 | 3.4 | 2.6 | 0.6 | 0.2 | 0.7 | 2.4 | |
| FDL (Hz) | M4Y | 43.2 | 78.6 | 92.0 | 115.5 | 97.0 | 178.9 | 346.8 | 913.4 |
| M13W | 38.3 | 68.1 | 129.3 | 206.4 | 71.9 | 184.7 | 548.4 | 2202.5 | |
| M55Y | 36.1 | 55.5 | 66.6 | 105.8 | 85.8 | 172.5 | 548.1 | 2439.1 | |
| M59A | 51.6 | 89.0 | 125.7 | 162.1 | 51.7 | 198.3 | 473.2 | 1987.0 | |
| mean | 42.3 | 72.8 | 103.4 | 147.4 | 76.6 | 183.6 | 479.1 | 1885.5 | |
| SD | 6.9 | 14.4 | 29.7 | 46.4 | 19.5 | 11.0 | 95.1 | 673.8 | |
| Testing order | M4Y | 3 | 1 | 2 | 4 | 5 | 6 | 7 | 8 |
| M13W | 4 | 1 | 2 | 3 | 6 | 5 | 7 | 8 | |
| M55Y | 3 | 2 | 1 | 7 | 6 | 4 | 5 | 8 | |
| M59A | 6 | 5 | 2 | 3 | 1 | 4 | 7 | 8 | |
| SL (dB) | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 50 |
Stimulus Calibration
Stimuli were calibrated using a 1/2-inch free-field microphone (Brüel & Kjaer, Type 4191) positioned in the chamber at the same location as the animal’s head. The output of the microphone was amplified using a custom preamplifier, sent directly into a digital signal processor (Tucker-Davis Technologies, RX6), and analyzed using a custom Matlab calibration program written specifically for this hardware configuration. In order to remove sound level as a potential discriminative cue during task performance, all stimuli were played at an average sensation level (SL) of ~40dB, except for the highest testing frequency 28160 Hz (A10) which was played at 50dB SL. SL was calculated based on the average audiogram of marmosets previously measured in the same experimental setup (Osmanski and Wang, 2011). To further ensure that all subjects were attending only to sound frequency in order to correctly perform the task, the sound level of each successive reference tone presentation was randomized within a ±3 dB range. The level of each target sound (which alternated with the reference sounds) was always fixed.
Data Analysis
Discrimination thresholds were calculated across a minimum of three consecutive sessions for each animal according to the following criteria: 1) At least 100 trials were completed for each session, 2) A minimum of 520 trials was completed across sessions (i.e., 52 presentations of each target), 3) False alarm rates in each session were less than 30%, 4) Thresholds across multiple sessions did not deviate by an amount greater than the spacing between targets. All hit rates within a session were corrected (HRcorr) from the raw values (HRraw) based on the false alarm rate (FA) according to the following equation: HRcorr = (HRraw − FA)/(1 − FA) (Geschieder, 1985). Using the above criteria, we defined the FDL for each animal as the frequency difference correctly identified 50% of the time (using linear interpolation of the HRcorr curve) across all sessions in each condition.
Relative FDL (FDLrel) was measured in units of semitones, then converted to absolute FDL (FDLabs) in Hz by the following equation: FDLabs = Fref × (2FDLrel/12 − 1) where Fref is the reference frequency tested in that session. Relative FDL in percentage was calculated by (FDLabs/Fref) × 100%.
Vocalization Analysis
In order to compare our FDL data with the spectral characteristics of marmoset vocalizations, we measured probability density functions for the dominant frequency of each of the four most common marmoset call types. Probability densities were calculated from several thousand marmoset vocalizations (phee: 12841, trill: 2287, trillphee: 2372, twitter: 7662) collected from a captive colony that we had previously recorded and quantitatively analyzed (for a description of procedures, see Agamaite et al., 2015).
RESULTS
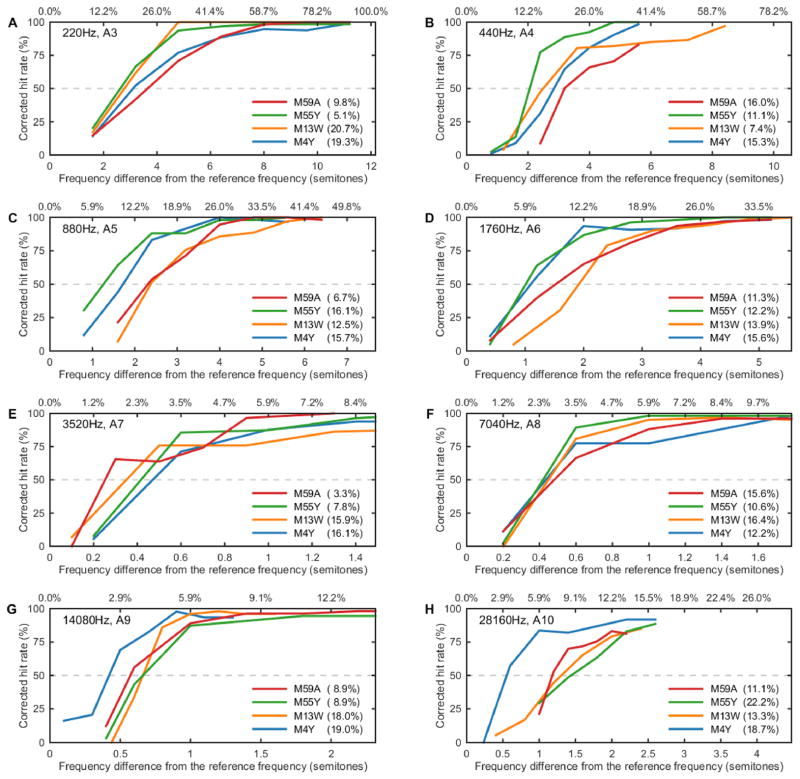
Individual psychometric functions from all four marmosets are shown in Figure 1. Each panel corresponds to a different reference frequency, between 220 Hz (A3) and 28160 Hz (A10). Corrected hit rates are plotted as a function of increasing difference between target and reference frequencies, in units of semitones. Threshold (i.e., 50% correct) is denoted by a horizontal dashed line. Overall, hit rates were consistently high at the largest frequency differences, with animals performing at or near 100% correct. Hit rates were lower at smaller frequency differences, reflecting the increasing difficulty of discriminating the target, and were equivalent to the false alarm rate at the lowest frequency differences. Average false alarm rate across all reference frequencies for all subjects was 13.2 +/− 5.8%.
Figure 1.
Representative psychometric functions from all four subjects at each reference frequency tested (220 Hz [A3] – 28160 Hz [A10]). Changes in corrected hit rate are shown as a function of the difference between reference and target frequencies (in both units of semitones and as a percentage change from the reference frequency). Dashed lines show 50% correct threshold. False alarm rates (measured as a percentage) on each frequency are displayed in the legend next to each subject.
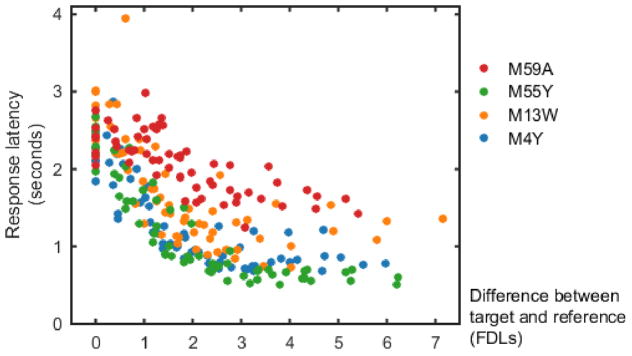
Response latencies measured in the present experiment are shown in Figure 2. In general, latencies increased as the difference between the target and reference tones decreased. At the smallest frequency differences, response latencies were indistinguishable from those measured in catch trials (i.e., false alarm responses), underscoring the increasing difficulty of discriminating the frequency change during target presentation. Figure 2 also shows that subjects show consistent individual differences in their latency to respond to the target sounds. Subject M59A (red circles), for example, is typically slow to respond to target presentations while M55Y (green circles) responds rapidly when the difference between the target and reference tone is large.
Figure 2.
Response latencies measured in the present experiment. Target latencies for each subject have been collapsed across all tone conditions by normalizing the difference between the target and reference tones (in units of FDL). False alarm response latencies are shown at the far left of the panel (represented by a target/reference difference of 0 FDLs).
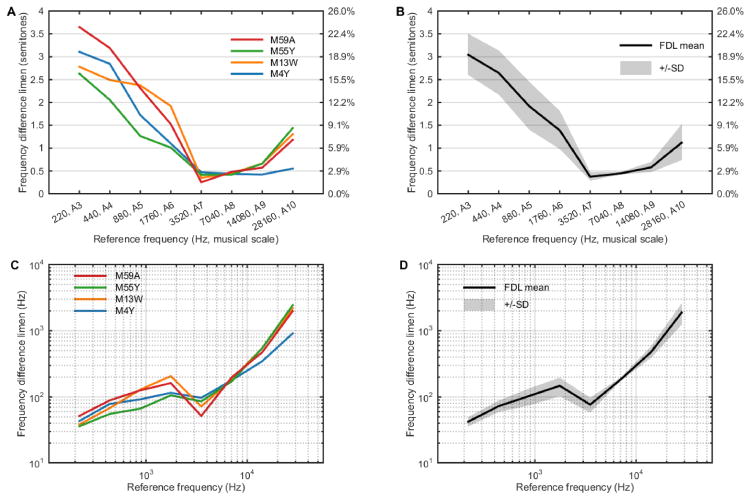
Individual threshold values (absolute FDLs in Hz, relative FDLs in semitones and in % frequency change) for each subject at each reference frequency are listed in Table 1. Relative FDLs for each animal at each reference frequency are also shown in Figure 3A and averaged data are shown in Figure 3B. Overall, there was general agreement in thresholds across all four subjects (Figure 3A and Table 1). The relative FDLs deviate from a flat response across frequencies (Kruskal-Wallis test, p=0.0002). Average relative FDLs decreased from 3.04 semitones at 220 Hz (A3) until reaching a minimum of 0.37 semitones at 3520 Hz (A7) and then increasing again to 1.12 semitones at 28160 Hz (A10) (Figure 3B and Table 1). The largest sensitivity range was 3.4 semitones, measured in subject M59A, with a high threshold of 3.65 semitones (220 Hz, A3) and a low threshold of 0.25 semitones (3520 Hz, A7) (Figure 3A and Table 1). Inter-subject variability in measured thresholds was highest (standard deviation, SD = 0.53 semitones) at low frequencies below 3520 Hz (A7) and reached a minimum (SD = 0.03 semitones) in the frequency range with lowest FDL thresholds (approximately 3520 Hz – 14080 Hz, A7 – A9), likely reflecting a floor effect for discrimination at those frequencies of greatest sensitivity. There was also agreement in threshold measures at the highest frequency tested (28160 Hz, A10), although one subject (M4Y, Figure 3A [blue line]) showed a lower threshold compared to the other three animals (see Discussion below). Absolute FDL values are shown in Figures 3C (individual data) and 3D (averaged data). Average absolute FDL values show an increasing trend across reference frequencies, from 42.3 Hz at 220 Hz (A3) to 1885.5 Hz at 28160 Hz (A10) (Figure 3D and Table 1). This increasing trend is not strictly linear, however, and there is a decrease in absolute FDL values from 147.4 Hz at 1760 Hz (A6) to 76.6 Hz at 3520 Hz (A7) before increasing again. This effect was most prominent in two of the subjects (M59A and M13W, Figure 3C [red and yellow lines]).
Figure 3.
Relative FDLs (in both units of semitones and as a percentage change from the reference frequency) are shown in panels A (individual data) and in panel B (averaged data, shaded area denotes one standard deviation). Absolute FDL values are shown in panels C (individual data) and D (averaged data, shaded area denotes one standard deviation).
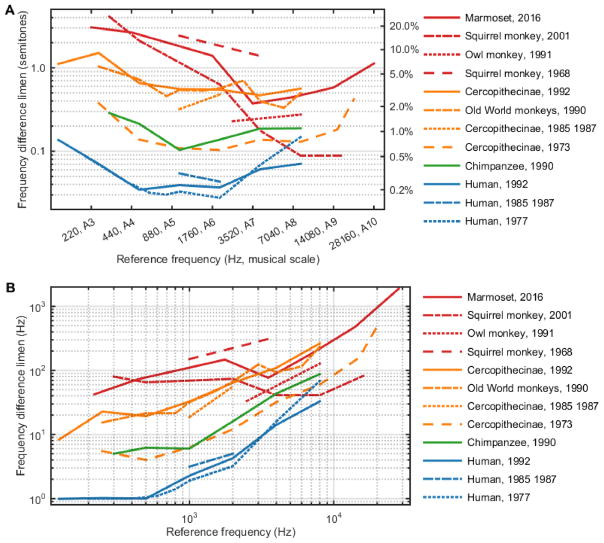
Figure 4 compares data from the current study with previous FDL measurements in other primate species (i.e., human, non-human ape, Old World monkeys, New World monkeys). Despite major methodological differences, almost all primates tested show generally constant relative FDLs across frequency (Figure 4A) and a characteristic increase in absolute FDLs with increases in reference frequency (Figure 4B). In general, humans (blue lines) show the lowest FDLs, followed by non-human apes (green) and Old World monkeys (orange). New World monkeys (red) show the largest FDLs compared to other primates. Current data from this study are shown in a solid red line.
Figure 4.
Comparison of FDLs obtained from the current study (“Marmoset, 2016”, solid red line) with FDLs of other primate species measured by previous studies (squirrel monkey [Wienicke et al., 2001; Capps and Ades, 1968], owl monkey [Recanzone et al., 1991], Old World monkeys [Prosen et al., 1990], Cercopithecinae [Sinnott et al., 1992; Sinnott et al., 1987; Sinnott et al., 1985; Stebbins, 1973], Chimpanzee [Kojima, 1990], and human [Sinnott et al., 1992; Sinnott et al., 1987; Sinnott et al., 1985; Wier et al., 1977]). In general, humans show the lowest FDLs, followed by non-human apes and Old World monkeys. New World monkeys show the largest FDLs compared to other primates.
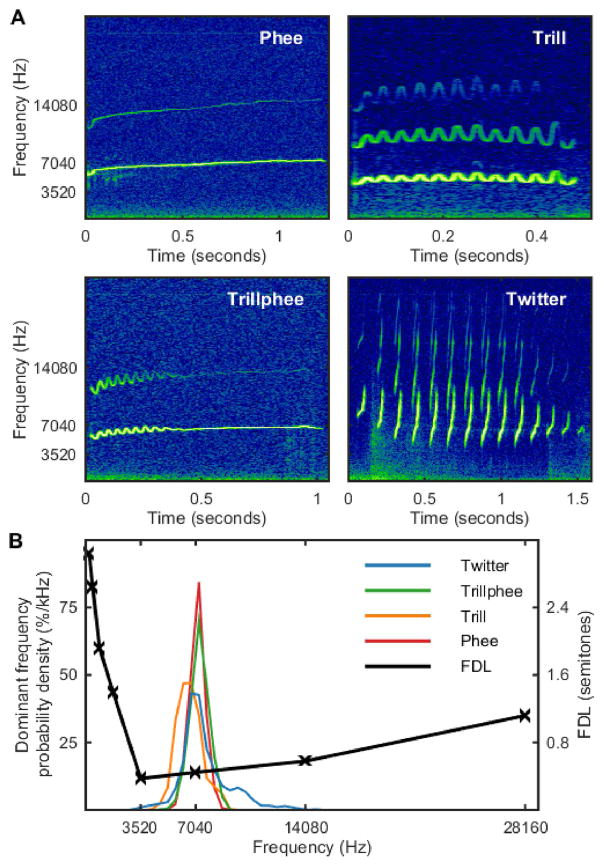
In order to evaluate whether marmosets show a correspondence between their vocal communication signals and the sensitivities of their auditory system, we compared the FDL data from the current study with the spectral characteristics of marmoset vocalizations previously recorded in our colony (Agamaite et al., 2015). The top panels of Figure 5 show representative spectrograms of the four major marmoset call types (i.e., phee, trill, trillphee, and twitter calls), which together account for the majority of vocalizations produced by captive marmosets. Also shown in the bottom panel is the current FDL data plotted against probability density functions for the dominant frequency of each of the major vocalization types. The region of greatest spectral energy (measured as dominant frequency) contained in these marmoset vocalizations is concentrated within the ~5–10 kHz range, which agrees well with the region of lowest FDLs (~3.5–14 kHz) in the current study.
Figure 5.
Representative spectrograms of the four most common marmoset vocalizations (phee, trill, trillphee, and twitter calls) are shown in panel A (from Agamaite et al., 2015). Panel B shows the FDL data from the current study aligned with probability density functions for the dominant frequency of each of the major call types. Most of the spectral energy contained in these marmoset vocalizations is concentrated within the ~5–10 kHz range, which agrees well with the lowest FDLs (~3.5–14 kHz) measured in the current study.
DISCUSSION
The present study showed that pure tone FDLs in the common marmoset were lowest in the frequency range of ~3–14 kHz (3520 Hz [A7]: 0.37 semitones, 2.2% change in frequency; 7040 Hz [A8]: 0.45 semitones, 2.6% change in frequency; 14080 Hz [A9]: 0.58 semitones, 3.4% change in frequency), and increased at higher and lower reference frequencies (Table 1 and Figure 3). Highest thresholds were seen at the lowest frequency tested (220 Hz: 3.04 semitones, 19.2% change in frequency). In general, marmoset FDLs are higher than those measured in humans and Old World monkeys (Stebbins, 1973; Sinnot et al., 1985; Sinnott et al., 1987; Sinnott et al., 1992; Prosen et al., 1990), but agree well with previous FDL measurements in other New World primates (Capps and Ades, 1968; Recanzone et al., 1991, Wienicke et al., 2001). Marmosets showed poorer frequency discrimination abilities at low frequencies below ~1000 Hz, which is also the frequency region of lowest hearing sensitivity in this species (Osmanski and Wang, 2011). Marmosets’ lowest FDLs (0.37–0.58 semitones [2.2–3.4% change in frequency] in the range of 3520 – 14080 Hz [A7 – A9]), however, were adequate to discriminate frequency differences of one semitone, which is the smallest change in Western musical melodies.
Importantly, although the present study examined marmoset FDLs using only male subjects, it is unlikely that females would have shown results different from those of males. Our previous marmoset behavioral audiogram results included both male and female marmosets (Osmanski and Wang, 2011), and we observed no sex difference in frequency sensitivity. Furthermore, both male and female marmosets are highly vocal and produce the same basic call types, with similar spectral and temporal characteristics (Agamaite et al. 2015).
Influence of HRTF at high frequencies
One caveat of the present data concerns the reliability of the measured discrimination thresholds at the highest frequencies tested (i.e., 14080–28160 Hz, A9–A10). Head-related transfer function (HRTF) data in marmosets from a previous study show a relatively stable HRTF profile at frequencies below approximately 18–20 kHz, but high inter-subject variability at higher frequencies (Slee & Young, 2010). Furthermore, there is a large notch in the marmoset HRTF profile at approximately 20 kHz (pronounced at 0° azimuth) which is believed to be an important cue for sound localization. Thus, sensitivity to high frequencies above ~18 kHz is likely to be influenced by head position. In the extreme case, in which the reference frequency falls into the HRTF notch, a 1 semitone difference in frequency could generate up to a ~20 dB difference in sensation level (instead of the predicted change of less than ~2 dB). Although sound level was randomized across a ±3 dB range in the present study, we cannot completely eliminate the possibility that the marmosets were using sound level as a cue to perform the discrimination task at the highest frequencies tested, especially at 28160 Hz. Such a large intensity difference could have facilitated performance, particularly for the two animals that were head-fixed. In fact, the only head-fixed subject (M4Y) in the 28160 Hz testing sessions indeed showed the lowest FDL at this frequency (e.g., Figure 1).
Comparison with previous studies
Data from the current study agree with previous studies in other primate species (Figure 4). Primates generally show constant relative FDLs across frequency and a characteristic increase in absolute FDLs with increases in reference frequency. The lowest FDLs have been measured in humans, followed by non-human apes and Old World monkeys. New World monkeys show the largest FDLs measured in primates.
Frequency discrimination has been examined in only two other New World primates, the owl monkey (Aotus) (Recanzone et al., 1991) and the squirrel monkey (Samiri sciureus) (Capps and Ades, 1968; Wienicke et al., 2001). Of these, the only systematic examination of FDL sensitivity testing more than two frequencies was conducted in the squirrel monkey (Wienicke et al., 2001). As with marmosets, squirrel monkeys also show decreased frequency discrimination performance at low frequencies (<1000 Hz), although they appear to show enhanced sensitivity relative to marmosets at frequencies above ~4 kHz (Figure 4A).
Weber’s law predicts that the ratio between FDL and frequency should be a constant value. Furthermore, Weir et al. (1977) estimated that, for humans, the logarithm of the absolute FDL is linearly related to the square root of frequency. The present data show a deviation from these expected patterns, however, where relative FDL values decrease between 1760Hz (A6) and 3520 Hz (A7), but are relatively constant at higher or lower frequencies (Figure 4A). Deviations from Weber’s law have been reported previously in other primates, including humans at frequencies below 500 Hz (e.g., Sinnott et al., 1992). In the squirrel monkey, the ratio between FDL and frequency is constant only above 8 kHz and increases with decreasing test frequency below 8 kHz (Wienicke et al., 2001).
Relevance to vocal communication
The hearing abilities of a particular species may be predicted to show a correspondence between the acoustic characteristics of its vocal communication signals and the sensitivities of its auditory system in order to enhance information transfer in those signals. Indeed, the match between behaviorally relevant acoustic signals and auditory tuning has been extensively studied over the last 50 years, and has been well established across a wide range of taxa, including insects and frogs (e.g., Capranica and Moffat, 1975; Brzoska et al., 1977; Ryan and Wilczynski, 1988; Gerhardt and Schwartz, 2001; Gerhardt and Huber, 2002; Sueur et al., 2010; Schrode and Bee, 2015), birds (e.g., Dooing and Saunders, 1975; Dooling et al., 1978, 1979, 2000; Farabaugh et al., 1998; Wright et al., 2003; Gall et al., 2012), and bats (e.g., Bohn et al., 2004, 2006). A similar correspondence also exists in at least one New World primate species, the squirrel monkey, where lowest FDLs occur in the frequency range of this species’ vocal repertoire (Winter et al., 1966; Wienicke et al., 2001; Newman, 2003). Similarly, human FDLs are lowest in the spectral region of speech (Wier et al., 1977).
In order to evaluate whether certain auditory sensitivities in the common marmoset have evolved to match the acoustic features of this species’ vocalizations, we compared the FDL data from the current study with the spectral characteristics of marmoset vocalizations recorded in our colony (Figure 5). We found that the region of greatest spectral energy contained in marmoset vocalizations is in the ~5–10 kHz range, which agrees well with the region of lowest FDLs (~3.5–14 kHz) in the current study. Previous work has also shown that the region of greatest hearing sensitivity in marmosets, in terms of the absolute auditory threshold, also falls within this range (Osmanski and Wang, 2011). These results are consistent with data derived from many other vertebrate species, including primates, showing a correspondence between auditory perceptual sensitivities and the acoustic characteristics of vocal communication signals. Future studies examining the vocalizations and hearing abilities of a greater variety of primate species will serve to further our understanding of the extent of this relationship between signal production and reception abilities in primate vocal communication.
Highlights.
Frequency difference limens (FDLs), which measure the minimum discriminable frequency difference between two pure tones, were measured in common marmosets across most of their hearing range.
Marmosets’ FDLs are comparable to other New World primates, with lowest values in the frequency range of ~3.5–14 kHz.
Lowest FDLs found in marmosets correspond with the region of lowest hearing thresholds and the greatest concentration of spectral energy of major vocalization types in this species.
These data suggest that frequency discrimination in the common marmoset may have evolved to match the hearing sensitivity and spectral characteristics of its species-specific vocalizations.
Acknowledgments
We thank S. Smith for help with animal care and J. Lu for generous assistance in performing some of the experiments. This work was supported by NIH grants DC013150 (MSO) and DC003180, DC005808 (XW).
Abbreviations
- FDL
frequency difference limen
- SL
sensation level
- HRTF
head-related transfer function
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agamaite JA, Chang CJ, Osmanski MS, Wang X. A quantitative acoustic analysis of the vocal repertoire of the common marmoset (Callithrix jacchus) Journal of the Acoustical Society of America. 2015;138:2906–2928. doi: 10.1121/1.4934268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendor DA, Wang X. The neuronal representation of pitch in primate auditory cortex. Nature. 2005;436:1161–1165. doi: 10.1038/nature03867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendor DA, Osmanski MS, Wang X. Dual-pitch processing mechanisms in primate auditory cortex. Journal of Neuroscience. 2012;32:16149–16161. doi: 10.1523/JNEUROSCI.2563-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn KM, Boughman JW, Wilkinson GS, Moss CF. Auditory sensitivity and frequency selectivity in greater spear-nosed bats suggest specializations for acoustic communication. J Comp Physiol A. 2004;190:185–192. doi: 10.1007/s00359-003-0485-0. [DOI] [PubMed] [Google Scholar]
- Bohn KM, Moss CF, Wilkinson GS. Correlated evolution between hearing sensitivity and social calls in bats. Biol Lett. 2006;2:561–564. doi: 10.1098/rsbl.2006.0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzoska J, Walkowiak W, Schneider H. Acoustic communication in the grass frog (Rana t. temporaria L.): calls, auditory thresholds and behavioral responses. J Comp Physiol A. 1977;118:173–186. [Google Scholar]
- Bezerra BM, Souto AS, de Oliveira MAB, Halsey LG. Vocalisations of wild common marmosets are influenced by diurnal and ontogenetic factors. Primates. 2009;50:231–237. doi: 10.1007/s10329-009-0132-7. [DOI] [PubMed] [Google Scholar]
- Capps MJ, Ades HW. Auditory frequency discrimination after transfection of the olivocochlear bundle in squirrel monkey. Experimental Neurology. 1968;21:147–158. doi: 10.1016/0014-4886(68)90133-7. [DOI] [PubMed] [Google Scholar]
- Capranica RR, Moffat AJM. Selectivity of the peripheral auditory system of spadefoot toads (Scaphiopus couchi) for sounds of biological significance. J Comp Physiol A. 1975;100:231–249. [Google Scholar]
- Dooling RJ, Lohr B, Dent M. Hearing in Birds and Reptiles. In: Dooling R, Fay R, Popper A, editors. Comparative Hearing: Birds and Reptiles. Springer Handbook of Auditory Research. Springer; NY: 2000. pp. 308–359. [Google Scholar]
- Dooling RJ, Peters SS, Searcy MH. Auditory sensitivity and vocalizations of the field sparrow (Spizella pusilla) Bull Psych Soc. 1979;14:106–108. [Google Scholar]
- Dooling RJ, Saunders JC. Hearing in the parakeet (Melopsittacus undulatus): Absolute thresholds, critical ratios, frequency difference limens, and vocalizations. J Comp Physiol Psychol. 1975;88:1–20. doi: 10.1037/h0076226. [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Zoloth SR, Baylis JR. Auditory sensitivity, equal loudness, temporal resolving power, and vocalizations in the house finch (Carpocadus mexicanus) J Comp Physiol Psychol. 1978;92:867–876. doi: 10.1037/h0077529. [DOI] [PubMed] [Google Scholar]
- Epple G. Comparative studies on vocalization in marmoset monkeys (hapalidae) Folia Primatol. 1968;8:1–40. doi: 10.1159/000155129. [DOI] [PubMed] [Google Scholar]
- Farabaugh SM, Dent ML, Dooling RJ. Hearing and vocalizations of wild-caught Australian budgerigars (Melopsittacus undulatus) J Comp Psychol. 1998;112:74–81. doi: 10.1037/0735-7036.112.1.74. [DOI] [PubMed] [Google Scholar]
- Gall MD, Brierley LE, Lucas JR. The sender–receiver matching hypothesis: support from the peripheral coding of acoustic features in songbirds. J Exp Biol. 2012;215:3742–3751. doi: 10.1242/jeb.072959. [DOI] [PubMed] [Google Scholar]
- Geschieder GA. Psychophysics: Method, Theory, and Application. Lawrence Erlbaum & Assoc; New York: 1985. [Google Scholar]
- Gerhardt HC, Huber F. Acoustic communication in insects and anurans: common problems and diverse solutions. Chicago, IL: University of Chicago Press; 2002. [Google Scholar]
- Gerhardt HC, Schwartz JJ. Auditory tuning and frequency preferences in anurans. In: Ryan JM, editor. Anuran communication. Washington, DC: Smithsonian Institution Press; 2001. pp. 73–85. [Google Scholar]
- ISO. ISO16:1975 - Acoustics - Standard Tuning Frequency. International Organization for Standardization; Geneva: 1975. (Standard Musical Pitch) [Google Scholar]
- Kojima S. Comparison of auditory functions in the chimpanzee and human. Folia Primatol. 1990;55:62–72. doi: 10.1159/000156501. [DOI] [PubMed] [Google Scholar]
- Lu T, Liang L, Wang X. Neural representation of temporally asymmetric stimuli in the auditory cortex of awake primates. Journal of Neurophysiology. 2001;85:2364–2380. doi: 10.1152/jn.2001.85.6.2364. [DOI] [PubMed] [Google Scholar]
- Miller CT, Freiwald WA, Leopold DA, Mitchell JF, Silva AC, Wang X. Marmosets: A Neuroscientific Model of Human Social Behavior. Neuron. 2016;90:219–233. doi: 10.1016/j.neuron.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT, Thomas AW. Individual recognition during bouts of antiphonal calling in common marmosets. J Comp Physiol A. 2012;198:337–346. doi: 10.1007/s00359-012-0712-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BCJ. Frequency difference limens for short-duration tones. Journal of the Acoustical Society of America. 1973;54:610–619. doi: 10.1121/1.1913640. [DOI] [PubMed] [Google Scholar]
- Newman JD. Auditory communication and central auditory mechanisms in the squirrel monkey: past and present. In: Ghazanfar AA, editor. Primate Audition: Ethology and Neurobiology. Vol. 13. 2003. pp. 198–203. [Google Scholar]
- Norcross JL, Newman JD. Context and gender specific differences in the acoustic structure of common marmoset (Callithrix jacchus) phee calls. Am J Primatol. 1993;30:37–54. doi: 10.1002/ajp.1350300104. [DOI] [PubMed] [Google Scholar]
- Norcross JL, Newman JD. Social context affects phee call production by nonreproductive common marmosets (Callithrix jacchus) Am J Primatol. 1997;43:135–146. doi: 10.1002/(SICI)1098-2345(1997)43:2<135::AID-AJP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Norcross JL, Newman JD, CoFrancesco LM. Context and sex differences exist in the acoustic structure of phee calls by newly-paired common marmosets (Callithrix jacchus) Am J Primatol. 1999;49:165–181. doi: 10.1002/(SICI)1098-2345(199910)49:2<165::AID-AJP7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Norcross JL, Newman JD, Fitch WT. Responses to natural and synthetic phee calls by common marmosets. Am J Primatol. 1994;33:15–29. doi: 10.1002/ajp.1350330103. [DOI] [PubMed] [Google Scholar]
- Osmanski MS, Wang X. Measurement of absolute auditory thresholds in the common marmoset (Callithrix jacchus) Hearing Research. 2011;277:127–133. doi: 10.1016/j.heares.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmanski MS, Song X, Wang X. The role of harmonic resolvability in pitch perception in a vocal nonhuman primate, the common marmoset (Callithrix jacchus) Journal of Neuroscience. 2013;33:9161–9168. doi: 10.1523/JNEUROSCI.0066-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistorio AL, Vintch B, Wang X. Acoustical analysis of vocal development in a New World primate, the common marmoset (Callithrix jacchus) J Acoust Soc Am. 2006;120:1655–1670. doi: 10.1121/1.2225899. [DOI] [PubMed] [Google Scholar]
- Prosen CA, Moody DB, Sommers MS, Stebbins WC. Frequency discrimination in the monkey. Journal of the Acoustical Society of America. 1990;88:2152–2158. doi: 10.1121/1.400112. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Jenkins WM, Hradek GT, Merzenich MM. A behavioral frequency discrimination paradigm for use in adult primates. Behavioral Research Methods, Instruments, and Computers. 1991;23:357–369. [Google Scholar]
- Remington ED, Osmanski MS, Wang X. An operant conditioning method for studying auditory behaviors in marmoset monkeys. PLoS One. 2012;7:e47895. doi: 10.1371/journal.pone.0047895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MJ, Wilczynski W. Coevolution of sender and receiver: effect on local mate preference in cricket frogs. Science. 1988;240:1786–1788. doi: 10.1126/science.240.4860.1786. [DOI] [PubMed] [Google Scholar]
- Rylands AB, editor. Marmosets and Tamarins: Systematics, behavior, and ecology. Oxford: Oxford University Press; 1993. p. 396. [Google Scholar]
- Schrode KM, Bee MA. Evolutionary adaptations for the temporal processing of natural sounds by the anuran peripheral auditory system. J Exp Biol. 2015;218:837–848. doi: 10.1242/jeb.115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee SJ, Young ED. Sound localization cues in the marmoset monkey. Hearing Research. 2010;260:96–108. doi: 10.1016/j.heares.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott JM, Petersen MR, Hopp SL. Frequency and intensity discrimination in humans and monkeys. Journal of the Acoustical Society of America. 1985;78:1977–1985. doi: 10.1121/1.392654. [DOI] [PubMed] [Google Scholar]
- Sinnott JM, Owren MJ, Petersen MR. Auditory frequency discrimination in primates: Species differences (Cercopithecus, Macaca, Homo) Journal of Comparative Psychology. 1987;101:126–131. [Google Scholar]
- Sinnott JM, Brown CH, Brown FE. Frequency and intensity discrimination in Mongolian gerbils, African monkeys and humans. Hearing Research. 1992;59:205–212. doi: 10.1016/0378-5955(92)90117-6. [DOI] [PubMed] [Google Scholar]
- Song X, Osmanski MS, Guo Y, Wang X. Complex pitch perception mechanisms are shared by humans and a New World monkey. Proceedings of the National Academy of Sciences. 2016;113:781–786. doi: 10.1073/pnas.1516120113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins WC. Hearing in old world monkeys. American Journal of Physical Anthropology. 1973;38:357–364. doi: 10.1002/ajpa.1330380233. [DOI] [PubMed] [Google Scholar]
- Sueur J, Windmill JFC, Robert D. Sound emission and reception tuning in three cicada species sharing the same habitat. J Acoust Soc Am. 2010;127:1681–1688. doi: 10.1121/1.3291036. [DOI] [PubMed] [Google Scholar]
- Wang X. On cortical coding of vocal communication sounds in primates. Proceedings of the National Academy of Sciences. 2000;97:11843–11849. doi: 10.1073/pnas.97.22.11843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Neural coding strategies in auditory cortex. Hearing Research. 2007;229:81–93. doi: 10.1016/j.heares.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Wienicke A, Haüsler U, Jürgens U. Auditory frequency discrimination in the squirrel monkey. Journal of Comparative Physiology A. 2001;187:189–195. doi: 10.1007/s003590100189. [DOI] [PubMed] [Google Scholar]
- Wier C, Jesteadt W, Green D. Frequency discrimination as a function of frequency and sensation level. Journal of the Acoustical Society of America. 1977;61:178–184. doi: 10.1121/1.381251. [DOI] [PubMed] [Google Scholar]
- Winter P, Ploog D, Latta J. Vocal repertoire of the squirrel monkey, its analysis and significance. Experimental Brain Research. 1966;1:359–384. doi: 10.1007/BF00237707. [DOI] [PubMed] [Google Scholar]
- Wright TF, Cortopassi KA, Bradbury JW, Dooling RJ. Hearing and vocalizations in the orange-fronted conure (Aratinga canicularis) J Comp Psychol. 2003;117:87–95. doi: 10.1037/0735-7036.117.1.87. [DOI] [PubMed] [Google Scholar]