Abstract
A critical function of cells is to provide a force-bearing linkage from matrix to matrix, matrix to cells or from cell to cell in a tissue and organ as well as a force generating structure. In fully differentiated skin cells, much of the force is borne by intermediate filaments. In dynamic tissues or isolated cells on matrix, high forces are generated by myosin II pulling on actin; either through stress fibers or through some other trans-cytoplasmic network. In epithelia, myosin II clearly plays a critical role in forming a contractile ring around wounds that provides turgor and restructuring forces. A major mystery is how a dynamic cytoskeleton can create a mechanically coherent cytoplasm. We suggest that the key lies in the continuous assembly of actin and myosin filaments in the cell periphery that has been recently found in isolated fibroblasts.
Introduction
Cells in an organism constantly encounter mechanical loads such as shear force and stretching force. These mechanical loads are critical in the regulation of cell shape, motility, growth, differentiation, survival, and tissue development and maintenance [1,2]. Not only must cells actively generate forces but they must also aid in maintaining the coherence of the tissue — the capacity to resist and transmit forces as well as to maintain mechanical integrity. Although the mechanical load-bearing ability of tissues has been most extensively studied in skin where many informative mutations that compromise the mechanical properties of the tissue have been identified [3,4], similar static forces are present in other tissues and the loss of forces gives rise to a dramatic response. This raises the question of how mechanical coherence, the mechanical continuity that propagates force from one side of the cell to the other, is maintained in cells. We will consider this question first in the context of epithelia and then in light of the recent findings in fibroblasts. In both cases, the predominant way that coherence is maintained appears through the continuous peripheral assembly of actin and myosin filaments.
Cell Mechanics
The mechanical organization of cells has been extensively studied and is a clear function of the cell microenvironment, e.g. cells on flat stiff surfaces have different mechanics than cells in epithelial monolayers on soft basement membranes. It is well accepted that the cytoplasm of animal cells is under pressure; and when the plasma membrane separates from the cytoskeleton, it will bleb outwards [5,6]. The pressure in the cytoplasm is relatively low and could be generated by a gradient of 5 micromolar of solute across semipermeable plasma membrane (cytoplasm side is higher) [6]. Rather than being maintained by ion transport, it seems that the pressure is mainly determined by myosin contraction and increased myosin contraction can lead to blebbing [7]. Since myosin only generates contractile force, this raises the question of what normally resists the contractile force of myosin. Although much has been postulated about the cell as a tensegrity structure that is composed of interacting cytoskeletal rigid struts and elastic cables [1], the internal load bearing elements are very weak at best, with maximally about 13% of the load being borne by microtubules [8]. In the case of epithelial tissues where the microtubules are oriented perpendicular to the plane of the tissue, they would have even a smaller role. Thus, we suggest that the primary load of cell contraction is borne by extracellular contacts connecting to principally matrix or adjacent cells, with the cytoplasm supporting the majority of the remainder through the membrane. When the force applied to the cell exceeds the force of the myosin contraction, what resists cell stretching and fragmentation?
Skin Epithelia
In the relatively tough outer layers of the skin, there is an extensive keratin intermediate filament network that is believed to support most of the applied forces and prevent tissue breakage. Although the keratin network extends from one cell to another to form a continuous network in the outer layers of the skin, the dynamics (constant assembly and disassembly of contacts) in the inner layers suggests that the network can be repaired if links are broken [9]. Further, the application of mechanical force to the tissue leads to proliferation of the tissue through as yet undefined signaling pathways [10].
Dynamic Epithelia
Many other epithelia, however, experience far lower external stresses and most of the forces in the tissue are generated internally. These tissues are typically more dynamic than skin and rely much less upon intermediate filaments. Several recent studies have shown that wound healing in epithelia involves the actin-based motor protein myosin II that forms a subapical band adjacent to the tight junction protein ZO1 staining regions, particularly when small holes form in the monolayers [11-13] (Figure 1). In recent studies, cell-cell adhesions appear dynamic since there is a rapid lateral movement of the components in the plasma membrane around the junctional complexes [14] that belies a role for myosin in the transport of the complexes. The dynamic movements of the complexes junctions are critical for the observed movements of cell boundaries in epithelia in vitro that presumably reflect a normal movement process that occurs in vivo. We understand very little about the control of the boundary movements but they must entail rearrangement of cell-cell adhesions as well as the actin cytoskeleton.
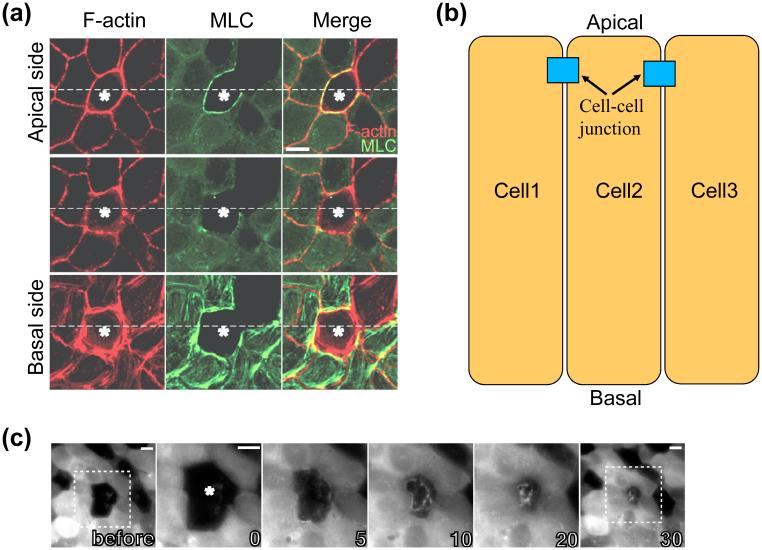
Figure 1.
Myosin II forms a ring at subapical position and contracts to close the wound. (a) 5 min after laser ablation of the cells indicated by the star, a monolayer of MDCK cells were fixed and stained for F-actin. The myosin light chain (MLC)-EGFP in cells neighboring to the ablated cells forms a contractile ring at the subapical position. Note MLC is a component of myosin II; it binds to myosin II heavy chain to constitute the functional myosin II. (b) Illustration of apical-basal polarity of an epithelial cell layer. (c) Dynamics of the contraction of MLC ring in wound closure induced by laser ablation of cells. Time in minutes. Figure modified, with permission, from Tamada M, Perez TD, Nelson WJ, and Sheetz MP (2007) J Cell Biol, 176:27-33.
Movements of the epithelia are driven by myosin as well. Recent studies have found that the activation of apical myosin is critical for cell ingression (the movement of cells from the surface into the internal cavity) in epithelia of the drosophila embryo [15]. Similarly, myosin contraction provides the force for narrowing and lengthening of tissues in models of the process [16].
We suggest that myosin could be important for maintaining the coherence at tissue level and at single cell level as well.
Mechanical Coherence of Single Cell
When cells are spread on extracellular matrix for hours to days, they become well polarized and develop stress fibers that, as the name implies, are involved in generating high forces on substrate contacts. The stress fibers are anchored in focal adhesions that grow in response to contractile force. Recent analyses of the actin dynamics in stress fibers indicates that actin filaments are added to the adhesion sites and they enable the rapid turnover of actin in the stress fibers [17] (Figure 2).
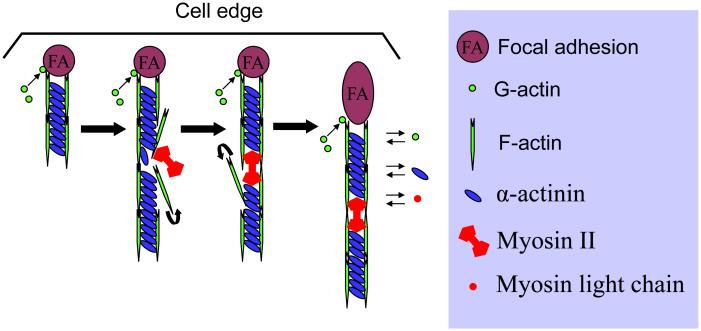
Figure 2.
Addition of actin filament at the adhesion sites and rapid turnover of actin in stress fibers. At focal adhesions, there is active polymerization of actin filaments and crosslinking of actin filaments by α-actinin. Myosin II incorporates into the α-actinin crosslinked actin filament bundles to displace α-actinin and to contract actin bundles, generating contractile force. Consequently, focal adhesions grow in response to the contraction force. The process is extremely dynamic; there are always actin polymerization and depolymerization, myosin II assembly and disassembly, and α-actinin association and disassociation with actin filaments. Figure modified, with permission, from Hotulainen P and Lappalainen P (2006) J Cell Biol 173:383-394.
As cells are spreading or migrating on extracellular matrix coated substrates, they often generate periodic edge contractions along the cell periphery [18]. Actin filaments are constantly assembled at plasma membrane. At the back of lamellipodium, myosin II periodically pulls the lamellipodial actin filaments and condenses them into lamellar actin bundles [19]. Similar observation have also been made recently by Anderson et al. [20]. Importantly, the cell periphery is where high cell traction forces are generated, as supported by the analyses of traction force generated by isolated cells — large traction forces are directed inward and focused at the cell periphery whereas small traction forces are exerted on the substrate in the central regions of the cell [21-25]. Further, inhibition of myosin II activity abolishes periodic edge contractions [19] and high traction force generation [21,22,24] while stimulation of myosin II contraction increases periodic contraction speed [19]. All these observations suggest that the myosin II pulling on actin filaments inward at the cell periphery might be critical for generation of peripheral large traction forces.
The traction forces generated by fibroblast are in the order of several hundred nanonewtons [21,25], far more than what is needed for cell body translocation [26,27] as cells still migrate, even faster in some cases, when traction force generation by cells is suppressed [28]. The forces at the front is only slightly larger than the forces at the rear in migrating cells [29]. Thus, it appears that the bulk of the forces are counterbalanced inside the cell. Why do cells generate so much more forces than what is actually needed? A possibility is that cells use these forces to hold cytoplasm together so it can not be torn apart by pulling forces from actin polymerization and extracellular matrix. Given that there are no large forces generated in the central cell regions and most large forces are directed inward, the peripheral large forces on opposite sides must be counterbalanced in the cell. For this to occur, a coherent cytoskeletal network is necessary. Although there are three cytoskeletal components — actin filaments, microtubules and intermediate filaments and they interact with each other [30], actin filaments appear to be a better candidate because that they are crosslinked into a contractile and dynamic network through myosin II that exist as hexamers in cells by forming bipolar filaments [31] and that the dynamic interaction of actin filaments with myosin II at cell periphery leads to large force generation.
Conclusions
Based upon both the theoretical considerations for the need to have a continuity in tissues and matrix connections and the experimental findings of a dynamic cytoskeleton, there is a need for dynamic coherence that could best be satisfied by actin and myosin assembly at the cell periphery.
Acknowledgements
We thank Pere Roca-Cusachs for critical reading and helpful comments on the manuscript. We apologize for not being able to cite many other relevant contributions from our colleagues owing to space constraints.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Ingber DE. Tensegrity-based mechanosensing from macro to micro. Prog Biophys Mol Biol. 2008;97:163–179. doi: 10.1016/j.pbiomolbio.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 3.Omary MB, Coulombe PA, McLean WH. Intermediate filament proteins and their associated diseases. N Engl J Med. 2004;351:2087–2100. doi: 10.1056/NEJMra040319. [DOI] [PubMed] [Google Scholar]
- 4.Gu LH, Coulombe PA. Keratin function in skin epithelia: a broadening palette with surprising shades. Curr Opin Cell Biol. 2007;19:13–23. doi: 10.1016/j.ceb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Sheetz MP, Sable JE, Dobereiner HG. Continuous membrane-cytoskeleton adhesion requires continuous accommodation to lipid and cytoskeleton dynamics. Annu Rev Biophys Biomol Struct. 2006;35:417–434. doi: 10.1146/annurev.biophys.35.040405.102017. [DOI] [PubMed] [Google Scholar]
- 6.Dai J, Sheetz MP. Membrane tether formation from blebbing cells. Biophys J. 1999;77:3363–3370. doi: 10.1016/S0006-3495(99)77168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagmann J, Burger MM, Dagan D. Regulation of plasma membrane blebbing by the cytoskeleton. J Cell Biochem. 1999;73:488–499. [PubMed] [Google Scholar]
- 8.Stamenovic D, Mijailovich SM, Tolic-Norrelykke IM, Chen J, Wang N. Cell prestress. II. Contribution of microtubules. Am J Physiol Cell Physiol. 2002;282:C617–624. doi: 10.1152/ajpcell.00271.2001. [DOI] [PubMed] [Google Scholar]
- 9.Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J Invest Dermatol. 2007;127:2499–2515. doi: 10.1038/sj.jid.5701015. [DOI] [PubMed] [Google Scholar]
- 10.Reichelt J. Mechanotransduction of keratinocytes in culture and in the epidermis. Eur J Cell Biol. 2007;86:807–816. doi: 10.1016/j.ejcb.2007.06.004. • Discusses some molecules and pathways in the mechanical stress perception and mechnotransduction underlying the proliferation and differentitation of keratincytes. [DOI] [PubMed] [Google Scholar]
- 11.Moyer RA, Wendt MK, Johanesen PA, Turner JR, Dwinell MB. Rho activation regulates CXCL12 chemokine stimulated actin rearrangement and restitution in model intestinal epithelia. Lab Invest. 2007;87:807–817. doi: 10.1038/labinvest.3700595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bement WM. Actomyosin rings: the riddle of the sphincter. Curr Biol. 2002;12:R12–14. doi: 10.1016/s0960-9822(01)00639-x. [DOI] [PubMed] [Google Scholar]
- 13.Tamada M, Perez TD, Nelson WJ, Sheetz MP. Two distinct modes of myosin assembly and dynamics during epithelial wound closure. J Cell Biol. 2007;176:27–33. doi: 10.1083/jcb.200609116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavey M, Rauzi M, Lenne PF, Lecuit T. A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature. 2008;453:751–756. doi: 10.1038/nature06953. [DOI] [PubMed] [Google Scholar]
- 15.Corrigall D, Walther RF, Rodriguez L, Fichelson P, Pichaud F. Hedgehog signaling is a principal inducer of Myosin-II-driven cell ingression in Drosophila epithelia. Dev Cell. 2007;13:730–742. doi: 10.1016/j.devcel.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Skoglund P, Rolo A, Chen X, Gumbiner BM, Keller R. Convergence and extension at gastrulation require a myosin IIB-dependent cortical actin network. Development. 2008;135:2435–2444. doi: 10.1242/dev.014704. •• Provides evidence, using Xenopus laevis as a model system, that myosin IIB-dependent contraction forces are essensial for convergent extension. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol. 2006;173:383–394. doi: 10.1083/jcb.200511093. •• Dynamic studies of stress fibers detail their origin and show contraction-dependent stimulation of actin assembly in stress fibers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giannone G, Dubin-Thaler BJ, Dobereiner HG, Kieffer N, Bresnick AR, Sheetz MP. Periodic lamellipodial contractions correlate with rearward actin waves. Cell. 2004;116:431–443. doi: 10.1016/s0092-8674(04)00058-3. [DOI] [PubMed] [Google Scholar]
- 19.Giannone G, Dubin-Thaler BJ, Rossier O, Cai Y, Chaga O, Jiang G, Beaver W, Dobereiner HG, Freund Y, Borisy G, et al. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell. 2007;128:561–575. doi: 10.1016/j.cell.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson TW, Vaughan AN, Cramer LP. Retrograde flow and myosin II activity within the leading cell edge deliver F-actin to the lamella to seed the formation of graded polarity actomyosin II filament bundles in migrating fibroblasts. Mol Biol Cell. 2008;19:5006–5018. doi: 10.1091/mbc.E08-01-0034. • The authors show that the delivery of a portion of actin filaments from cell leading edge to lamella by myosin II forms lamellar actomyosin II bundles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai Y, Biais N, Giannone G, Tanase M, Jiang G, Hofman JM, Wiggins CH, Silberzan P, Buguin A, Ladoux B, et al. Nonmuscle myosin IIA-dependent force inhibits cell spreading and drives F-actin flow. Biophys J. 2006;91:3907–3920. doi: 10.1529/biophysj.106.084806. •• Demonstrates that in spreading fibroblasts, myosin IIA generates large forces at cell periphery whereas myosin IIB produces a smaller fraction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelham RJ, Jr., Wang Y. High resolution detection of mechanical forces exerted by locomoting fibroblasts on the substrate. Mol Biol Cell. 1999;10:935–945. doi: 10.1091/mbc.10.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J. 1999;76:2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc Natl Acad Sci U S A. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubin-Thaler BJ, Hofman JM, Cai Y, Xenias H, Spielman I, Shneidman AV, David LA, Dobereiner HG, Wiggins CH, Sheetz MP. Quantification of cell edge velocities and traction forces reveals distinct motility modules during cell spreading. PLoS ONE. 2008;3:e3735. doi: 10.1371/journal.pone.0003735. •• Whole-cell analyses of isotropic cell spreading show that fibroblasts generate different levels of traction forces in three distinct spreading phases (early, middle and late) with only large forces being produced in the late phase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 27.Harris AK, Stopak D, Wild P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature. 1981;290:249–251. doi: 10.1038/290249a0. [DOI] [PubMed] [Google Scholar]
- 28.Sandquist JC, Swenson KI, Demali KA, Burridge K, Means AR. Rho kinase differentially regulates phosphorylation of nonmuscle myosin II isoforms A and B during cell rounding and migration. J Biol Chem. 2006;281:35873–35883. doi: 10.1074/jbc.M605343200. • Show that cells with suppressed myosin II activity migrate faster after depletion of myosin IIA but slower after depletion of myosin IIB. [DOI] [PubMed] [Google Scholar]
- 29.Galbraith CG, Sheetz MP. Forces on adhesive contacts affect cell function. Curr Opin Cell Biol. 1998;10:566–571. doi: 10.1016/s0955-0674(98)80030-6. [DOI] [PubMed] [Google Scholar]
- 30.Herrmann H, Bar H, Kreplak L, Strelkov SV, Aebi U. Intermediate filaments: from cell architecture to nanomechanics. Nat Rev Mol Cell Biol. 2007;8:562–573. doi: 10.1038/nrm2197. • The authors discuss the structures of intermediate filament proteins, the assembly, mechanics and dynamics of intermediate filaments as well as the involvement of intermediate filaments in mechanotransduction and diseases. [DOI] [PubMed] [Google Scholar]
- 31.Conti MA, Adelstein RS. Nonmuscle myosin II moves in new directions. J Cell Sci. 2008;121:11–18. doi: 10.1242/jcs.007112. [DOI] [PubMed] [Google Scholar]