Abstract
Background
Recent guidelines recommend chest computed tomography (CT) for smokers based on studies of individuals aged 55–80 years with 30-pack year smoking to detect lung cancers. Some have postulated that a prior diagnosis of head and neck cancer (HNC) should also warrant lung cancer screening with CT, but no studies have demonstrated benefit in this population. Our goal was to compare survival of HNC patients with second primary lung cancers (SPLCs) with survival of lung cancer-only patients to determine if detecting early stage lung cancer in those with prior HNC could lead to similar survival benefits.
Methods
Survival estimates for patients with early stage SPLC diagnosed between ages 55–74 at least 1 year after HNC diagnosis were compared with from patients with early stage lung cancer and no other cancers.
Results
Median survival of patients with lung cancer only was 38 months. Median survival after SPLC was 22 months. History of head and neck cancer predicted poorer survival after lung cancer diagnosis, p<.0001.
Conclusions
Survival outcomes after early lung cancer are worse after HNC. This finding diminish the effectiveness of chest CT as a screening modality for HNC survivors, and further study should be undertaken prior to its routine use.
Keywords: Lung neoplasm, head and neck neoplasm, second primary neoplasms, early detection of cancer
Introduction
Head and neck cancer (HNC) survival, although having improved over the past several years,1 remains dismal in the presence of second primary malignancies.2 Lung cancer is the most commonly identified second primary malignancy. There is a 5–19% risk of second primary lung cancer (SPLC) associated with HNC, which suggests that patients with prior HNC could be characterized as ‘high-risk’ for having subsequent SPLC.3–7
We sought in particular to assess the survival of HNC patients who develop subsequent early-stage lung cancers. Our purpose was to obtain a population measure of the survival after SPLC diagnosis in HNC patients and thereby better understand the potential benefits of a broad implementation of screening CT in the growing population of HNC survivors. No studies have directly assessed whether the benefits of screening CT are the same for patients with prior HNC as for those included in recent studies of that modality8 which have led to its increasing use. We therefore compared survival from early stage lung cancers among those with prior HNC and those with no cancer prior to diagnosis of lung cancer to determine if early detection is associated with comparable survival times in both groups.
Methods
Data was obtained from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database public use files.9 This database includes comprehensive cancer incidence data from 9 geographic locations (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco/Oakland, Seattle/Puget Sound, and Utah) from 1973 through 2010, from 4 more locations (Los Angeles, San Jose/Monterey, Alaska Natives, and rural Georgia) from 1992 through 2010, and in an additional 5 locations (Kentucky, New Jersey, Louisiana, greater Georgia, and greater California) from 2000 through 2011. The 18 geographic areas contain approximately 27.8% of the US population. The SEER database is a public-use dataset with de-identified data; this study was not considered human subject research by the University of Iowa Institutional Review Board.
We defined early-stage lung cancers as those identified at AJCC 7th Ed. stage 1a, 1b, or 2a.10 Stage information in SEER represents “best stage,” using pathologic information where available and clinical data where pathologic data is unavailable. Using extent-of-disease data, we assembled a cohort of patients diagnosed with a single early-stage lung cancer (LC cohort) and a cohort of patients first diagnosed with a head and neck cancer and subsequently diagnosed with an early-stage lung cancer (HNC-LC cohort). Lung cancers were identified using the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) primary site codes 34.0 – 34.9. Head and neck cancers were defined by primary site codes in the oral cavity (excluding the lip), oropharynx, hypopharynx, nasopharynx, and larynx with in situ or invasive histology. We included only patients diagnosed with lung cancer between the ages of 55–74 years to match the cohort defined by Aberle et al.8 We included patients in the LC cohort only if they had no other cancers reported in SEER; similarly, patients in the HNC-LC cohort had only those two cancers reported in SEER. To minimize bias from patients with synchronous primary cancers, the HNC-LC cohort was limited to patients having at least a 12 month interval between the diagnosis of HNC and SPLC. Note that in patients with prior head and neck cancer, the SEER registry distinguishes between SPLCs, which are reported to the registry, and subsequent lung metastases, which are not reported. The clinical and pathologic documentation forms the only basis for this distinction.11
Statistical analysis was performed using SAS 9.3. Survival estimates were computed using the Kaplan-Meier method, and estimates were compared by log-rank tests. Chi-square was used to assess associations between categorical variables. T-tests were used to measure association between continuous variables.
Results
We identified 903,634 patients in the SEER database with a lung cancer diagnosed between 1983–2011 and no additional second primary cancers. Of this population, 72,074 patients had stage 1a, 1b, or 2a and were between the ages of 55–74 at diagnosis. This was defined as the LC cohort. Characteristics of this group are shown in Table 1.
Table 1.
Comparison of patients in the HNC-LC and LC cohorts.
| Cohort | |||
|---|---|---|---|
| Characteristic | HNC-LC | LC | P value |
| Total | 915 | 72074 | |
| Male, % | 694 (75.9%) | 38539 (53.5) | <0.0001 |
| Mean age, HNC dx (s.d.) | 60.4 (6.0) | N/A | |
| Mean age, lung dx (s.d.) | 65.9(5.3) | 66.0 (5.4) | 0.80 |
| Stage, lung cancer | |||
| 1a | 420 (45.9%) | 27448 (38.1%) | |
| 1b | 452 (49.4) | 41199 (57.2) | <0.0001 |
| 2b | 43 (4.7) | 3427 (4.8) | |
| Lung cancer histology | |||
| Squamous | 479 (52.4%) | 20727 (28.8%) | |
| Adenocarcinoma | 247 (27.0) | 32175 (44.6) | <0.0001 |
| Epithelial neoplasm NOS | 145 (15.9) | 14787(20.5) | |
| Other | 44 (4.8) | 4385 (6.1) | |
HNC-LC cohort: head and neck cancer followed at least 12 months later by an early stage lung cancer. LC cohort: single lung cancer. In both cohorts, lung cancers were diagnosed between ages 55 and 74 years.
NOS, not otherwise specified.
The SEER database identified 8208 patients diagnosed with HNC, one subsequent lung cancer, and no other cancers. When exclusion criteria were applied, there were 915 patients with exactly one HNC and exactly one early stage SPLC that was diagnosed between ages 55–74 at least one year after their HNC diagnosis. This was defined as the HNC-LC cohort. Table 1 shows characteristics of this cohort. There were significant differences between the lung cancers in the LC and the HNC-LC cohorts. There was a significantly higher proportion of men in the HNC-LC cohort. In addition, histologic types were distributed differently in the two cohorts, with significantly more squamous cell carcinomas in the HNC-LC group.
The majority of HNCs (95.7%)were squamous cell carcinomas. The distribution and SEER historic stage of HNC primary sites are described in Table 2.
Table 2.
Characteristics of head and neck primary sites in HNC-LC cohort
| Primary site | Larynx | 429 (46.9%) |
| Oral Cavity | 189 (20.7) | |
| Oropharynx | 190 (20.8) | |
| Hypopharynx | 79 (8.6) | |
| Nasopharynx | 21 (3.1) | |
| Stage at diagnosis | In situ | 23 (2.5%) |
| Localized | 302 (33.0) | |
| Regional | 396 (43.3) | |
| Distant | 46 (5.0) | |
| Unknown | 148 (16.2) | |
Kaplan-Meier survival estimates are shown in Figure 1. The survival estimates were statistically significantly different (p<0.0001). Median survival for the LC cohort was 38 months, and 40% survived to 5 years. Median survival after lung cancer diagnosis for the HNC-LC cohort was 22 months, with 26% survival at 5 years.
Figure 1.
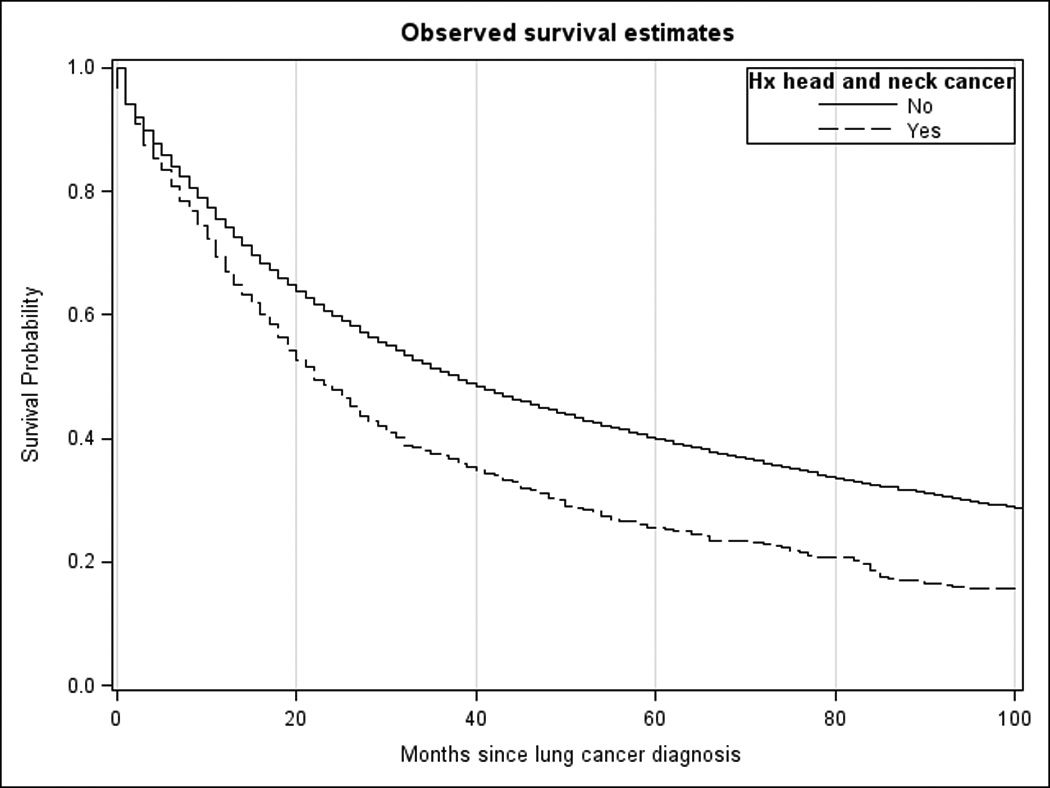
Kaplan-Meier survival estimates for lung cancer cohorts with and without history of head and neck cancer.
To minimize the chance that lung metastases mistaken for SPLC could bias the results, we performed two additional analyses. First, we restricted both HNC-LC and LC cohorts to patients whose lung cancer was an adenocarcinoma. Median survival in the modified HNC-LC and LC cohorts were 25 and 33 months, respectively (p<.0001). Second, we repeated the survival analysis with two different definitions of the HNC-LC cohort: one with patients whose lung cancers were diagnosed at least 2 years after their HNC diagnosis and the other with lung cancers diagnosed at least 6 months after HNC diagnosis. The survival estimates for those modified HNC-LC cohorts remained significantly different from the LC cohort, with median survival 22 months in both cases (data not shown).
Discussion
Our findings suggest that survival after a diagnosis of early stage lung cancer is significantly worse in patients with a history of HNC than in patients without any prior cancer diagnosis. Our results support those of Jayaprakash, et al.,2 who evaluated survival in patients with HNC followed by SPLC. That study included lung cancers of all stages and patients of all ages, and determined median survival to be 7 months after diagnosis of lung cancer.
The types of lung cancers identified in the HNC-LC and LC cohorts were different in ways that can explain the differences in survival outcomes. SPLCs were more likely to have squamous histology, and there was a male predominance. Both male gender,12–15 and squamous histology16 have been shown to be associated with decreased lung cancer survival. However, these differences may well reflect differences in lung cancer behavior between the two cohorts. Differences in survival may also relate to patients’ ability to receive aggressive curative therapy after a lung cancer diagnosis.
These results provide important context to a broad implementation of screening CT to this group of patients. Current practice guidelines from multiple sources including the US Preventive Services Task Force and National Comprehensive Cancer Network now recommend low-dose screening chest CT for current or former smokers to assess for lung cancer.17,18 The latter further advocated implementation of screening chest CT in head and neck cancer patients due to their increased risk of lung cancer. The measure of a screening test is the magnitude of improvement in survival of the screened population,19 related to identification of previously unrecognized cancers, improvement in survival as a result of earlier identification, and manageable risks associated with false positive findings. Our results demonstrate that HNC patients may not stand to derive the same benefit as participants in the National Lung Screening Trial (NLST),8 which found a relative reduction in lung cancer mortality in selected patients who underwent annual screening chest CT when compared to annual chest radiography. The study population was defined as individuals ages 55–74 years who were deemed at ‘high-risk’ for lung cancer due to their current or recent 30-pack year smoking history.
The NLST study described the characteristics of the cancers identified by screening CT in comparison to those identified by plain radiographs.8 As would be expected in a screened population, lung cancers at stages 1a, 1b, and 2a were far more prevalent in the population who received screening CTs, representing 67.1% of cancers identified by screening CT and 34.5% of cancers identified in the group assigned to plain radiography. The theoretical advantage of screening is that incident cancers are identified at an earlier stage rather than at later stage. Our conclusions raise the prospect that, in the population of HNC survivors, simply identifying cancers at earlier stage may not result in the same survival magnitude of survival benefit as seen in those without a history of HNC.
The diminished survival benefit is important as the full range of downstream effects of screening tests is considered. NLST reported that just 3.6% of positive screening tests ultimately led to diagnosis of cancer, at the cost of a very large number of follow up imaging studies, procedures, and complications. The benefits of large-scale screening must be weighed against these drawbacks.
Our study’s use of the SEER database offers strengths related to its sample size and low risk of ascertainment bias. A limitation of the study is that there is no information about how lung cancers were identified or whether patients were symptomatic. In addition, although the SEER program attempts to distinguish between lung metastases and second primary lung cancers, these two entities may be difficult to differentiate. We explored this issue by investigating the dependence of survival results on minimum latency period (the interval between the reported HNC and SPLC). Distant metastases are progressively less likely over time after HNC diagnosis.20 If some SPLCs in our study were truly distantly metastatic HNCs, survival estimates for patients with at least 24 months between HNC and SPLC would be better than those obtained if patients with lung tumors diagnosed between 12 and 24 months later were included. Since we did not observe this effect, the likelihood that our results are confounded by a large number of lung metastases is small. Furthermore, the survival difference persisted when the analysis was limited only to patients with lung adenocarcinoma, largely eliminating the possibility of misclassification.
Conclusion
The utility of a cancer screening test depends on prevalence and test characteristics but also on the magnitude of the improvement in outcomes with early diagnosis. Survival outcomes after diagnosis of the types of lung cancer identified with screening chest CT are not as favorable in HNC survivors, calling into question the benefits of screening CT. Until further investigation is undertaken, screening chest CT should be applied on a case-by-case basis in this population.
Acknowledgments
The authors acknowledge Charles Lynch, MD, PhD for critical review of the manuscript.
Funding source: none
Footnotes
This work was presented at the 2014 Annual Meeting of the American Head and Neck Society, July 28, 2014, New York, NY.
The authors report no conflicts of interest.
References
- 1.Pulte D, Brenner H. Changes in survival in head and neck cancer cancers in the late 20th and early 21st century: a period analysis. Oncologist. 2010;15:994–1001. doi: 10.1634/theoncologist.2009-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jayaprakash V, Cheng C, Reid M, et al. Previous head and neck cancers portend poor prognoses in lung cancer patients. Ann Thorac Surg. 2011;92:1056–1061. doi: 10.1016/j.athoracsur.2011.03.146. [DOI] [PubMed] [Google Scholar]
- 3.Dikshit RP, Boffetta P, Bouchardy C, et al. Risk factors for the development of second primary tumors among men after laryngeal and hypopharyngeal carcinoma. Cancer. 2005;103:2326–2333. doi: 10.1002/cncr.21051. [DOI] [PubMed] [Google Scholar]
- 4.Holland JM, Arsanjani A, Liem BJ, Hoffelt SC, Cohen JI, Stevens KR., Jr Second malignancies in early stage laryngeal carcinoma patients treated with radiotherapy. J Laryngol Otol. 2002;116:190–193. doi: 10.1258/0022215021910500. [DOI] [PubMed] [Google Scholar]
- 5.Gao X, Fisher SG, Mohideen N, Emami B. Second primary cancers in patients with laryngeal cancer: a population-based study. Int J RadiatOncolBiol Phys. 2003;56:427–435. doi: 10.1016/s0360-3016(02)04613-8. [DOI] [PubMed] [Google Scholar]
- 6.Sjogren EV, Snijder S, van Beekum J, Baatenburg de Jong RJ. Second malignant neoplasia in early (TIS-T1) glottic carcinoma. Head Neck. 2006;28:501–507. doi: 10.1002/hed.20453. [DOI] [PubMed] [Google Scholar]
- 7.Hsu YB, Chang SY, Lan MC, Huang JL, Tai SK, Chu PY. Second primary malignancies in squamous cell carcinomas of the tongue and larynx: an analysis of incidence, pattern, and outcome. J Chin Med Assoc. 2008;71:86–91. doi: 10.1016/S1726-4901(08)70080-7. [DOI] [PubMed] [Google Scholar]
- 8.Aberle DR, Adams AM, Berg CD, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;3655:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) Research Data (1973–2011), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch. [released April 2014]; based on the November 2013 submission.
- 10.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. American Joint Committee on Cancer Cancer Staging Manual. 7th. New York: Springer-Verlag; 2010. [Google Scholar]
- 11.Curtis RE, Ries LA. Methods. In: Curtis RE, Friedman DM, Ron E, et al., editors. New Malignancies Among Cancer Survivors: SEER Cancer Registries 1973–2000. Bethesda: National Cancer Institute; 2006. pp. 9–14. [Google Scholar]
- 12.Wisnivesky JP, Halm EA. Sex differences in lung cancer survival: Do tumors behave differently in elderly women? J Clin Oncol. 2007;25(13):1705–1712. doi: 10.1200/JCO.2006.08.1455. [DOI] [PubMed] [Google Scholar]
- 13.Cerfolio RJ, Bryant AS, Scott E, et al. Women with pathologic stage I, II, and III non-small cell lung cancer have better survival than men. Chest. 2006;130(6):1796–1802. doi: 10.1378/chest.130.6.1796. [DOI] [PubMed] [Google Scholar]
- 14.Visbal AL, Williams BA, Nichols FC, 3rd, et al. Gender differences in non-small-cell lung cancer survival: An analysis of 4,618 patients diagnosed between 1997 and 2002. Ann Thorac Surg. 2004;78(1):209–215. doi: 10.1016/j.athoracsur.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 15.de Perrot M, Licker M, Bouchardy C, Usel M, Robert J, Spiliopoulos A. Sex differences in presentation, management, and prognosis of patients with non-small cell lung carcinoma. J Thorac Cardiovasc Surg. 2000;119(1):21–26. doi: 10.1016/s0022-5223(00)70213-3. [DOI] [PubMed] [Google Scholar]
- 16.Subramanian J, Morgensztern D, Goodgame B, et al. Distinctive characteristics of non-small cell lung cancer (NSCLC) in the young: A surveillance, epidemiology, and end results (SEER) analysis. J ThoracOncol. 2010;5(1):23–28. doi: 10.1097/JTO.0b013e3181c41e8d. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Preventive Services Task Force. Lung Cancer Screening, Topic Page. [accessed February 18, 2015]; Available at http://www.uspreventiveservicestaskforce.org/uspstf/uspslung.htm. [Google Scholar]
- 18.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Lung cancer screening, Version 1.2015. [Accessed February 18, 2015]; Available at http://www.nccn.org/professionals/physician_gls/pdf/lung_screening.pdf. [Google Scholar]
- 19.Harris R, Sawaya GF, Moyer VA, Calonge N. Reconsidering the criteria for evaluating proposed screening programs: reflections from 4 current and former members of the U.S. Preventive services task force. Epidemiol Rev. 2011;33(1):20–35. doi: 10.1093/epirev/mxr005. [DOI] [PubMed] [Google Scholar]
- 20.Geurts TW, Nederlof PM, van den Brekel MW, et al. Pulmonary squamous cell carcinoma following head and neck squamous cell carcinoma: Metastasis or second primary? Clin Cancer Res. 2005;11(18):6608–6614. doi: 10.1158/1078-0432.CCR-05-0257. [DOI] [PubMed] [Google Scholar]


