Abstract
Despite substantial efforts at early diagnosis, accurate staging and advanced treatments, esophageal cancer (EC) continues to be an ominous disease worldwide. Risk factors for esophageal carcinomas include obesity, gastroesophageal reflux disease, hard-alcohol use and tobacco smoking. Five-year survival rates have improved from 5% to 20% since the 1970s, the result of advances in diagnostic staging and treatment. As the most sensitive test for locoregional staging of EC, endoscopic ultrasound (EUS) influences the development of an optimal oncologic treatment plan for a significant minority of patients with early cancers, which appropriately balances the risks and benefits of surgery, chemotherapy and radiation. EUS is costly, and may not be available at all centers. Thus, the yield of EUS needs to be thoughtfully considered for each patient. Localized intramucosal cancers occasionally require endoscopic resection (ER) for histologic staging or treatment; EUS evaluation may detect suspicious lymph nodes prior to exposing the patient to the risks of ER. Although positron emission tomography (PET) has been increasingly utilized in staging EC, it may be unnecessary for clinical staging of early, localized EC and carries the risk of false-positive metastasis (over staging). In EC patients with evidence of advanced disease, EUS or PET may be used to define the radiotherapy field. Multimodality staging with EUS, cross-sectional imaging and histopathologic analysis of ER, remains the standard-of-care in the evaluation of early esophageal cancers. Herein, published data regarding use of EUS for intramucosal, local, regional and metastatic esophageal cancers are reviewed. An algorithm to illustrate the current use of EUS at The University of Texas MD Anderson Cancer Center is presented.
Keywords: Esophageal squamous cell carcinoma, Endosonography, Echoendoscope, Esophagus cancer, Esophageal adenocarcinoma
Core tip: Endoscopic ultrasound (EUS) is not necessary or adds little in management of many cases, such as, in patients with distant metastases or following pre-operative (neoadjuvant) chemoradiotherapy. EUS is the most sensitive test to exclude local tumor invasion and regional nodal disease that would make endoscopic resection (ER) unsafe or unnecessary. Thus, for early esophageal cancer staging, EUS followed by ER and histopathologic analysis, remains the standard-of-care. For a minority of locally advanced cancers, EUS-fine-needle aspiration can define the radiotherapy field by providing tissue samples of suspicious lymph nodes that are remote from the primary tumor.
INTRODUCTION
Dysphagia to solid food is the most common presenting symptom of patients with advanced esophageal cancer (EC). As the sixth most lethal cancer diagnosed worldwide, there are more than 450000 cases of EC diagnosed annually[1,2]. The American Cancer Society estimates 16910 cases of EC will be diagnosed in the United States in 2016[2-4]. The incidence of esophageal adenocarcinoma (EAC) has increased six-fold from 1975 to 2000, making it the most rapidly increasing cancer incidence in America[5,6]. Obesity, defined as body mass index > 30 kg/m2, has been strongly linked to EAC, with an odds ratio of 16.2 (95%CI: 6.3-41.4) compared with the leanest persons with body mass index < 22 kg/m2[7]. Meanwhile, the incidence of squamous cell carcinoma (SCC) in the US is declining[8].
Men are more commonly effected by EC; the median age at diagnosis is 67 and lifetime incidence is 1 in 125 (a rate 3 to 4 times higher than for women)[3,9]. Fifteen percent of EC are diagnosed in people younger than 55 years old. Additional risk factors for EC depend upon histologic subtype and include: European ancestry, gastroesophageal reflux disease, sleep apnea, and intestinal metaplasia (Barrett’s esophagus) for EAC; vs African ancestry, tobacco smoking, distilled alcohol consumption, palmoplantar keratosis (tylosis), and Plummer-Vinson syndrome for SCC[2,4,10-13]. Less common EC (such as sarcoma, melanoma, and lymphoma) may occur, although data regarding use of endoscopic ultrasound (EUS) in these cancers are limited.
The majority of patients (about 60%) have advanced cancer when diagnosed, as early EC are frequently asymptomatic[14,15]. Five-year relative survival rates for localized, regional, and distant stages of all types of esophageal cancers are currently estimated at 40%, 21%, and 4%, respectively[3]. Overall five-year survival rates for patients with EC have improved four-fold over the past four to five decades (Figure 1)[3,9]. This substantial improvement in life expectancy likely represents advances in accurate staging and treatment by dedicated professionals with research support from cancer societies, patient groups, industry, and local and national agencies. Per the National Institutes of Health (NIH)/National Cancer Institute, resource utilization and expenditures in 2010 for EC topped $1.3 billion, which is projected to increase to $1.8 billion by 2020[16].
Figure 1.
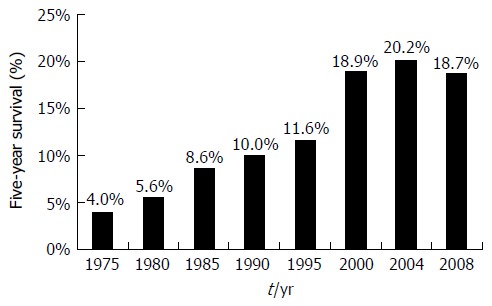
Five-year survival trends in esophageal cancer. Data from Surveillance, Epidemiology, and End Results Cancer Statistics Factsheets: Esophageal Cancer. National Cancer Institute. Bethesda, MD[9].
Since the mid-1980s, EUS has evolved to occupy an important niche in EC staging, particularly in evaluating tumor invasion and surrounding lymph nodes. According to NIH/Surveillance, Epidemiology, and End Results program data, local and regional esophageal carcinomas, which are most amenable to EUS evaluation, are found in half of the patients (Figure 2)[9]. With radial and linear endoechoscopes, the five major layers of the esophagus are visible (Figure 3) and represent: (1) the innermost superficial mucosa or squamous epithelium; (2) the deep mucosa or lamina propria; (3) the submucosa, which contains an innumerable number of lymphatics, blood vessels, nerves and mucous glands, and is the most common route of extra-esophageal cancer spread; (4) the hypoechoic muscularis propria; and (5) the hyperechoic adventitia. Cytology specimens may be obtained from suspicious nodes using fine-needle aspiration (FNA).
Figure 2.
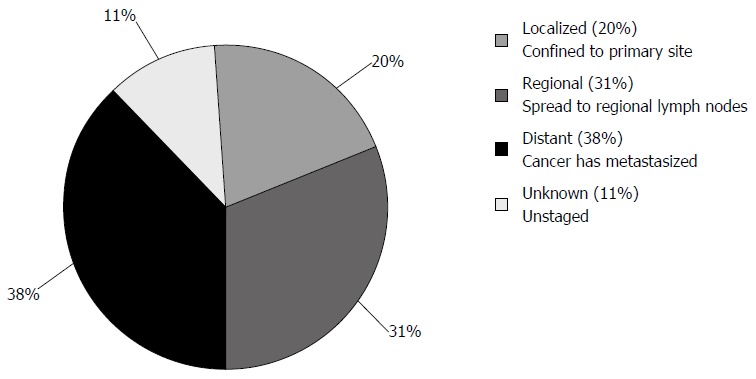
Esophageal cancer stages at diagnosis. Surveillance, Epidemiology, and End Results Cancer Statistics Factsheets: Esophageal Cancer. National Cancer Institute. Bethesda, MD[9].
Figure 3.
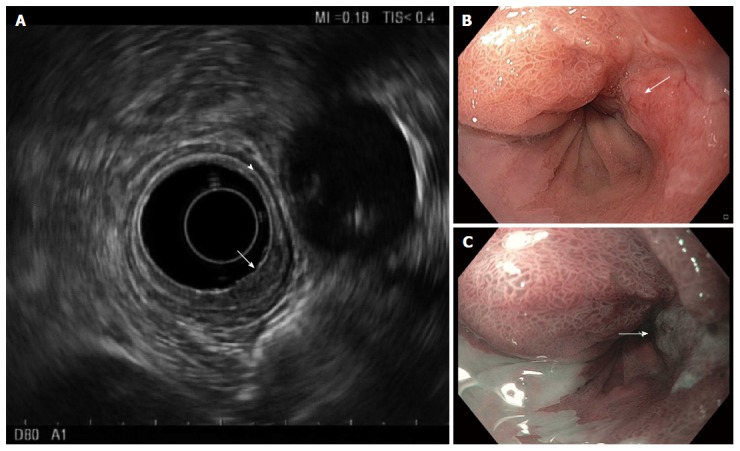
Endosonography of distal esophageal adenocarcinoma. A: Five layers of the esophagus are visible with standard frequency (7.5 MHz) endoscopic ultrasound. From innermost to outermost: the hyperechoic (bright) superficial mucosa, hypoechoic (dark) deep mucosa, the submucosa (arrowhead), followed by the muscularis propria (hypoechoic, very dark), and adventitia (outer echogenic layer). The T1b adenocarcinoma (arrow) causes thickening and distortion of the mucosal layers and submucosa, without invasion of the muscularis propria; B: White-light; and C: Narrow band images are presented for comparison, with arrows to mark the cancer.
EC JARGON
The seventh edition of the tumor-node-metastasis (TNM) staging system, developed by the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control, is the most commonly used staging system[17-19].
In general, localized disease refers to esophageal carcinoma, including intra-esophageal (T1-2) and penetrating cancers (T3-4, also known as, locally advanced cancers). Regional disease describes surrounding lymph node involvement (N-stages), such as celiac and thoracic lymph nodes. Together locoregional cancers fall into the AJCC anatomic stage/prognostic group I-III (so called stage I-III cancers; Figure 4). Distant/metastatic disease (M1) is identified by cancer spread to adjacent organs, distant lymph nodes (i.e., lungs or supraclavicular lymph nodes) or below the diaphragm (i.e., liver or mesenteric lymph nodes); stage IV is the anatomic stage/prognostic group[20]. While the TNM components for staging EAC vs SCC are identical, the AJCC anatomic stage/prognostic groups differ depending on histologic type because of differing mortality rates between EAC and SCC stages.
Figure 4.
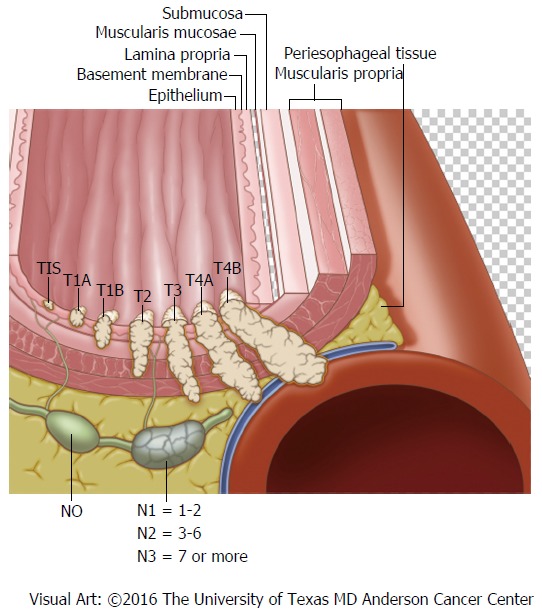
Locoregional esophageal cancer staging.
An understanding of evolving TNM sub-stages is necessary, such as, EUS stage (i.e., uT4), vs clinical stage [i.e., cT4; based upon pre-surgical evaluation, including endoscopic resection (ER)], vs postoperative stage (i.e., pT4; based upon pathologic examination of surgical specimen), vs neoadjuvant postoperative stage (i.e., ypT4)[14,21]. Cancers involving the submucosa (T1b) are further divided into sm1 to sm3 stages based upon the depth of invasion[22].
LITERATURE SEARCH
A literature search was completed using Google, PubMed and Cochrane Library for combinations of “EUS” and “EC”. Study titles and abstracts were screened for relevance. Then, full text publications in English were selected for in-depth review and the references were further scrutinized to identify pertinent studies.
DISCUSSION
In 1980, a group of investigators from SRI International (formerly of Stanford University) and Mayo Clinic developed the “Ultrasonic Endoscope” prototype. It was felt with planned improvements in size and design that this device “should improve the investigation of cardiac, gastrointestinal, and genitourinary diseases”[23]. In 1986, EUS was used for evaluation of lesions of the upper gastrointestinal tract by Gordon, Rifkin and Goldberg, who described the endosonographic anatomy of the upper gastrointestinal tract in 25 patients[24]. Since then, a median of 50 manuscripts per year have been indexed for PubMed on the topics of “EC” and “EUS” (total 1286, range 1-83).
Radial EUS scopes provide a circumferential view of the visceral wall and surrounding tissues, similar to axial images obtained by computed tomography (CT). Often considered easier to interpret by early users, radial EUS images are more similar to transverse/cross-sectional imaging displays. The linear array echoendoscope is commonly used for tissue acquisition via fine needle aspiration (FNA) or biopsy, as it allows for direct needle visualization during passes into the target abnormality[25,26].
Higher frequency EUS devices yield increased superficial anatomic resolution, but lack deeper sonographic tissue penetration, limiting regional assessment. For example, most radial and curvilinear array echoendoscopes operate at frequencies of 7.5-12 megahertz (MHz), and penetrate 3-4 cm of surrounding tissue with good resolution. Very high frequency, through the scope, EUS miniature probes (mini-probes) can readily distinguish seven layers of the esophagus with a frequency of 20-30 MHz. However, useful sound wave breadth and depth with EUS mini-probes are substantially reduced and inadequate for cancer staging.
If malignant lesions extend to the fundus or gastric cardia, or if intra-esophageal cancers are small; conventional radial or linear EUS may not accurately evaluate the depth of the lesion due to the technical difficulty in reaching or locating the lesion by the echoendoscopes. In those cases, a high frequency EUS mini-probe may be employed under endoscopic guidance to most accurately stage the tumor. For example, in distinguishing T1a vs T1b intramucosal lesions, high frequency mini-probes have been shown to more accurately assess depth of invasion in comparison to radial or linear EUS. The disadvantage of using an EUS mini-probe is the limited sonographic width and depth, which precludes a comprehensive survey of regional lymph nodes. Furthermore, if the lesion is large (i.e., 5 cm), EUS mini-probes cannot expediently assess penetration depth of the entire lesion.
EUS FOR EC STAGING
When assessed by EUS, malignant lymph nodes classically originate near the intraluminal cancer, and appear as round, hypoechoic nodes with smooth borders that may be enlarged (> 10 mm)[27]. Per 2016 guidelines published by the National Comprehensive Cancer Network, once distant metastases from EC have been excluded, EUS should be employed for evaluation with possible FNA cytologic sampling[28]. At the time of diagnosis, a contrast-enhanced CT scan of the chest and abdomen is recommended to assess for distant metastases (i.e., to liver, lung, bone or adrenals), thereby distinguishing M0 vs M1 stages. Following EUS, the optimal treatment regimen changes significantly based on the presence of tumor invasion into the submucosa, detection of regional lymph node malignant spread or distant malignancy. EUS is the most sensitive test for locoregional staging of EC, and maintains a critical role in developing an accurate therapy plan[27,29-35]. EUS influences the treatment of a significant, although small portion of patients with early disease, as particular attention may be given to the depth of esophageal invasion and celiac lymph node axis, which is thought to act as a gateway for distant metastatic spread[36-38]. Current data confirm the number of malignancy-involved lymph nodes is more important for prognosis than regional anatomic location, which further substantiates EUS-FNA use[39-42]. The results of meta-analyses focused on EUS are summarized in Tables 1 and 2.
Table 1.
Baseline characteristics of meta-analyses on endoscopic ultrasound in esophageal carcinoma
| Ref. | Timeframe | Patients (No. studies; P/R) | EUS types (MHz) | Study criteria |
| Puli et al[52], 2008 | 1986-2005 | 2020 (25; 10/15) | NR | EUS accuracy confirmed by surgery in distal and celiac axis lymph node metastasis |
| van Vliet et al[29], 2008 | 1985-2005 | 4713 (84; NA1) | NR | Comparison of diagnostic staging performance of EUS, CT and PET |
| Puli et al[32], 2008 | 1986-2005 | 2558 (49; 16/33) | NR | EUS studies on T and N staging confirmed by surgery |
| Thosani et al[30], 2012 | 1988-2008 | 1019 (19; 12/7) | Radial and/or mini-probe (7.5-30) | EUS in T1a vs T1b lesions compared to histology by EMR or surgery/excluded studies on < 15 patients, or with suspicious lymph nodes (> 1 cm) |
| Sun et al[76], 2015 | 1992-2013 | 724 (16; 10/6) | Radial, linear and/or mini-probe (5-20) | EUS staging accuracy after neoadjuvant chemotherapy. Surgery was confirmatory test in all included studies. |
| Qumseya et al[36], 2015 | 1994-2012 | 656 (11; 4/7) | Radial, linear and/or mini-probe (NR) | EUS in BE and HGD, or esophageal adenocarcinoma (EAC)/excluded studies on advanced esophageal cancer |
Did not report retrospective or prospective nature of studies. References[29,30,32,36,52,76]. P/R: Prospective to retrospective ratio; NR: Not reported; BE: Barrett’s esophagus; HGD: High-grade dysplasia; EAC: Esophageal adenocarcinoma; EUS: Endoscopic ultrasonography; CT: Computed tomography; PET: Positron emission tomography; NA: Not applicable.
Table 2.
Outcomes of meta-analyses on endoscopic ultrasound in esophageal carcinoma
| Ref. | Sensitivity (95%CI) | Specificity (95%CI) | Heterogeneity | Conclusion/interpretation |
| Puli et al[52], 2008 | Celiac N = 66% (62-71); M = 67% (63-72) | Celiac N = 98% (97-99); M = 98% (97-99) | Insignificant: P > 0.10 for all estimates | EUS has low sensitivity and utility for staging metastases to celiac lymph nodes and distant sites. |
| van Vliet et al[29], 2008 | N staging: EUS = 80% (75-84); CT = 50% (41-60); PET 57% (43-70) | N staging: EUS = 70% (65-75); CT = 83% (77-89); PET = 85% (76-95) | NR | EUS, CT, and PET have distinctive roles in staging. For distant metastases, PET probably has higher sensitivity than CT. No evidence of publication bias in CT vs EUS studies; other analyses too small to test. |
| Puli et al[32], 2008 | T1 = 82% (78-85); T4 = 92% (89-95); w/o FNA N = 85% (83-86); w/ FNA N = 97% (92-99) | T1 = 99.4% (99-100); T4 = 97% (97-98); w/o FNA N = 85% (83-86); w/ FNA N = 96% (91-98) | Insignificant: P > 0.10 for all estimates | EUS has excellent accuracy, with better performance in T4 over T1 disease (AUC 0.94-0.98). N staging is improved with FNA use (AUC 0.99 vs 0.89). |
| Thosani et al[30], 2012 | T1a = 85% (82-88); T1b = 86% (82-89) | T1a = 87% (84-90); T1b = 86% (83-89) | Significant; P < 0.05 by χ2 | EUS has good accuracy for T1a and T1b lesions; AUC ≥ 0.93. Technical factors can affect the diagnostic accuracy of EUS. |
| Sun et al[76], 2015 | T1 = 23% (16-32); T2 = 29% (19-41); T3 = 81% (72-88); T4 = 43% (31-56); N = 69% (58-79) | T1 = 95% (93-97); T2 = 84% (77-88); T3 = 42% (33-52); T4 = 96% (94-97) N = 52% (42-62) | Significant; I2 = 0%-75% depending on stage (table presented in article) | EUS has modest accuracy after neoadjuvant therapy; AUC for T staging ranges from 0.64 to 0.84, while AUC for N-staging was 0.64. |
| Qumseya et al[36], 2015 | ≥ T1sm = 56% (47-65) | >/-T1sm = 89% (85-92) | Significant; I2 = 82%; Q = 56, P < 0.0001 | Advanced disease detected in 14% (95%CI: 8%-22%; P < 0.0001). The NNT (performing EUS) to identify 1 case of advanced disease was 7 (95%CI: 5-13). EUS significantly changes therapeutic approach. |
NR: Not reported; EUS: Endoscopic ultrasonography; CT: Computed tomography; PET: Positron emission tomography; AUC: Area under the curve.
DISTANT METASTATIC EC STAGING
Detection of distant metastases is improved with the use of positron emission tomography (PET), when compared to CT and EUS[29,43,44]. Use of PET and/or CT may spare the need of performing EUS when distant metastases are detected, as evaluation of the regional lymph nodes is not necessary prior to initiation of palliative chemotherapy or chemoradiotherapy. When indicated, EUS may be used to confirm the presence distant metastases and exclude benign findings. Confirmation or exclusion of nodal involvement by EUS will help calculate the exact radiation field, especially when the lymph node is away from the primary tumor, thus minimizing radiation induced complications.
LOCOREGIONAL EC STAGING
Use of CT or PET is considered inadequate for staging celiac and mediastinal lymphadenopathy[26,29,31]. PET may not be necessary for clinical staging if distant metastatic disease is detected on CT scan. Conversely, in patients with superficial EC (T1 disease) use of PET carries risk of over-staging due to false-positive regional/distant enhancement[21].
For evaluation of regional lymph nodes, the combination of EUS and CT (EUS-CT) has been shown to be more accurate than either modality alone, and EUS-CT outperformed PET, 69% vs 48%, respectively. The sensitivity of combined EUS-CT was 83% vs 22% for PET[45]. Some data support PET scan consideration for: (1) patients with locally advanced (T2 or greater) cancers following EUS (with or without ER); (2) those with positive regional lymph nodes (N1 or greater) detected by EUS-FNA; and (3) patients in whom complete EUS examination was not possible (i.e., due to severe malignant stenoses)[29,46].
When PET scan is performed before EUS, it can provide a road map to potentially positive lymph nodes and decrease or obviate the need for stricture dilation, thus lessening the risk of esophageal perforation. One in three malignant stenoses may initially be too narrow for the EUS scope to traverse[47,48]. Incremental dilation of severe malignant strictures often is not necessary, as completion of EUS may not change treatment[49].
SUPERFICIAL EC STAGING
Intramucosal cancers (T1a) have a 6%-10% risk of metastasis, while invasion into the submucosa (T1b) increases the risk of metastasis to 19%-23%[50]. In a meta-analysis including 1019 patients with T1 (superficial) esophageal cancers, Thosani et al[30] evaluated the diagnostic accuracy of EUS in differentiating mucosal (T1a) vs submucosal invasion (T1b) by EC. Nineteen international studies (12 prospective, 7 retrospective) conducted between 1988 and 2008 were included. Studies using mini-probe EUS dominated (14 mini-probe, 9 radial scopes; five studies used both) in comparing findings to the gold-standard, surgical resections of SCC and/or EAC (with or without endoscopic mucosal resection). The area under the curve for pooled sensitivity and specificity was at least 0.93 for both T1a mucosal and T1b submucosal lesions. The pooled sensitivity, specificity of EUS for T1a staging were 0.85 (95%CI: 0.82-0.88), 0.87 (95%CI: 0.84-0.90); and for T1b staging a sensitivity 0.86 (95%CI: 0.82-0.89) and specificity of 0.86 (95%CI: 0.83-0.89) were estimated. Heterogeneity was present among the studies, as the χ2 P value for heterogeneity was < 0.05 for all pooled estimates.
MULTIMODAL STAGING OF LOCAL ESOPHAGEAL CANCERS
ER should be considered with EUS for staging superficial EC (T1 lesions, generally < 2 cm), which provides locoregional staging and histologic assessment of primary tumor depth and lymphovascular invasion. Due to lack of a singular near perfect test, combining EUS with ER functions as a “double check” to prevent staging errors by sonographic or histologic evaluation[51,52]. Superficial tumor invasion, which may be difficult to visualize by standard radial EUS (7.5-12 MHz) due to lower resolution, can be more accurately assessed by histology of ER specimens[51-53]. The addition of EUS to ER confers the benefit of nodal assessment with possible FNA sampling. Furthermore, EUS excludes deeper invasive cancer (T2 or deeper lesions) that would make ER unsafe and unnecessary[32,54].
For confirmed T1a cancers, ER followed by ablation of high-risk residual tissue via radiofrequency ablation or photodynamic therapy, offers survival rates similar to surgery[55-63]. EUS prior to ER is especially important in patients with large intraluminal tumors[64]. When EUS is combined with cross-sectional imaging, patients are considered to have completed clinical staging, thereby identifying stage T2 or T3 patients who may benefit from radical esophagectomy with extended lymphadenectomy[58,61,65,66].
ENDOSONOGRAPHY FOR ESOPHAGOGASTRIC JUNCTION CANCERS
Data regarding the utility of EUS in cancers of the EGJ is limited, and liberal use of ER has been suggested[53]. In a study by Dhupar et al[53] 181 patients with EGJ cancers (98% adenocarcinomas) were included that underwent EUS staging and resection (surgical or endoscopic) without neoadjuvant therapy from 1995 to 2014. The authors found that EUS accuracy at the EGJ was inferior to that of other regions of the esophagus when compared to resected specimens; with 23% under-staged and 29% over-staged by EUS. The negative effect was particularly pronounced with smaller, early EGJ cancers being more frequently over-staged.
NEOADJUVANT THERAPY PRIOR TO SURGERY
Neoadjuvant (induction) therapy may be given pre-operatively to patients with locally advanced or locoregional disease, due to improvement in survival compared to surgery alone for cancers of the esophagus and EGJ[67-73]. Data suggest the accuracy of EUS after neoadjuvant chemotherapy for locoregional cancers is subpar[35,74-76]. The reasons for lower accuracy of EUS after induction therapy are due to regional changes in response to healing and inflammation.
A meta-analysis by Sun et al[76] evaluated the staging accuracy of EUS for EC after preoperative chemotherapy. The authors included 724 patients (69% with adenocarcinoma) from sixteen studies (ten prospective, six retrospective) conducted between 1992 and 2013. Most procedures were performed with 7.5 and 12 MHz echoendoscopes. Pooled estimates of EUS test characteristics were used in either fixed-effects or a random-effects model, depending on study heterogeneity. EUS was most sensitive in localized staging of T3 lesions at 81% (95%CI: 72%-88%) with 42% specificity (95%CI: 33%-52%). EUS sensitivity in stages T1, T2, and T4 was poor, with T1 lesions estimated at 23% (95%CI: 16%-32%) and 95% specificity (95%CI: 93%-97%); T2 lesions at 29% (95%CI: 19%-41%) and specificity 84% (95%CI: 77%-88%), and finally T4 lesions at 43% (95%CI: 31%-56%) with specificity 96% (95%CI: 94%-97%). When assessing for regional lymph node spread, EUS had sensitivity 69% (95%CI: 58%-79%) and specificity 52% (95%CI: 42%-62%). Overall, EUS was found to be moderately accurate after neoadjuvant therapy; AUC for T staging ranged from 0.64 to 0.84, while the AUC for N-staging was 0.64. EUS accuracy did not improve with time following neoadjuvant chemotherapy in a subgroup analysis. Therefore, EUS should only be performed in specific cases after neoadjuvant therapy, such as FNA of a suspicious lymph node that would change management.
ADJUVANT THERAPY AFTER SURGERY
Postoperative (adjuvant) therapy has been shown to improve survival and reduce the risk of local recurrence, in patients with positive resection margins, or with positive lymph nodes in cancers of the esophagus or EGJ[77-81]. However, an intensified adjuvant chemoradiation regimen found no improvement in disease-free or overall survival in patients with EGJ and gastric adenocarcinomas[82]. A recent review concluded there are no validated adjuvant treatment strategies for SCC[82]. PET may be used to evaluate for cancer response and recurrence after multimodal therapy[83]. Data regarding the utility of EUS following surgery and adjuvant chemoradiation are limited.
RADIATION THERAPY FIELD DELINEATION
Precise EC tumor measurements are important for accurate radiation targeting and treatment. PET has been found to be accurate for evaluation of tumor length in esophageal cancers[84-86]. In a retrospective study of 53 patients by Rollins et al[84] PET and EUS were compared to surgical pathology for measurement of tumor length. Both PET and EUS correlated significantly with resection specimen tumor length; PET (Pearson R = 0.5977, 95%CI: 0.390-0.747, P < 0.0001) vs EUS (Pearson R = 0.5365, 95%CI: 0.311-0.705, P < 0.0001). In a subgroup analysis, after excluding tumors with significant response to neoadjuvant chemotherapy, both PET and EUS again correlated significantly with tumor length; PET (R = 0.5651, P = 0.0005) vs EUS (R = 0.4637, P = 0.0057). These data suggest EUS or PET may reliably be used in evaluation of tumor length for radiotherapy field definition, and the addition of EUS to PET imaging in these cases is low-yield. However, in patients with suspicious (but not-diagnostic) lymphadenopathy EUS-FNA may further define the radiation field (Figure 5).
Figure 5.
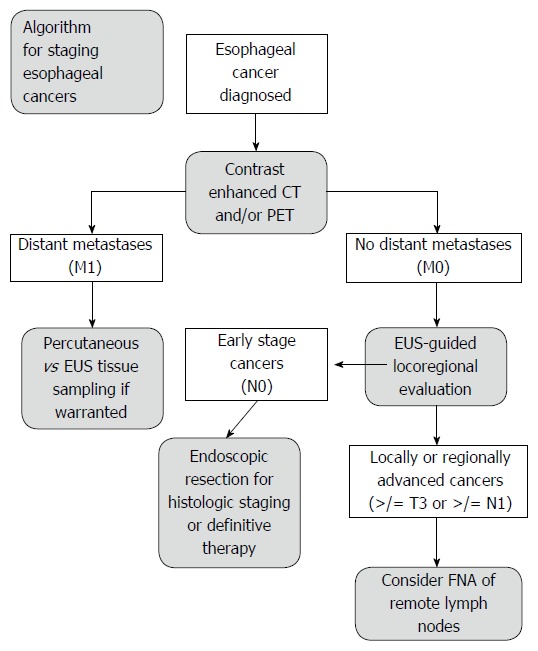
Algorithm for staging esophageal cancers proposed by DaVee and Lee. Esophagogastric junction cancers excluded. EUS: Endoscopic ultrasound with selective fine-needle aspiration; T, N, M: Tumor, node, and metastasis stages; CT: Computed tomography; PET: Positron emission tomography; FNA: Fine-needle aspiration.
COST ANALYSIS
EUS has been shown to be economical in multiple studies. For initial staging, EUS was found to be the least costly strategy by Hadzijahic et al[87] as EUS found T4 and/or M1 disease more frequently than CT (44% vs 13%, P < 0.0001). Furthermore, in patients without metastatic disease, EUS was found to be the most cost effective EC staging modality at $13811, vs CT-guided FNA $14350 and surgery $13992[88]. Pretreatment EC staging by EUS was found to save an average of $3443 per patient, by identification of stage I and stage IV tumors, which prevented unnecessary neoadjuvant chemoradiotherapy or surgery, respectively[89]. Furthermore, selective use of FNA for suspicious lymph nodes during EUS, resulted in reduced costs compared to routine FNA[34], however the effect on patient-outcomes remains to be determined.
EMERGING ADJUNCTS TO SONOMORPHOLOGIC EVALUATION
Generally, healthy tissue is softer and more elastic than cancerous tissues. Elastography, or elasticity imaging, may be combined with ultrasound or magnetic resonance modalities and is a non-invasive method to measure the flexibility of tissues. There are many elastography techniques under investigation, such as quasistatic/strain imaging and shear wave elasticity imaging; however, all techniques rely on measuring the degree of distortion within the tissue. Much like Doppler ultrasound, which uses color to highlight flow in vessels, EUS elastography provides the operator with a colorized image displaying the variation of elasticity of tissues. Typically, when using EUS elastography, firm tissues appear blue to violet, while softer tissues appear red, yellow or green. Elastography-enhanced EUS has been shown in small studies to improve the diagnostic accuracy of regional lymph node staging in EC patients when compared to standard EUS sonomorphologic evaluation[90-92]. Currently, the role and clinical efficacy are undefined for EUS elastography in EC, although the we speculate the technique could replace FNA cytology, as it is noninvasive and possibly lower risk for the patient.
When unique contrast agents are parenterally administered, contrast-enhanced harmonic EUS (CEH-EUS) may be used to further characterize the microvascular pattern of lesions identified by standard imaging modalities[93]. In 2016, the United States Food and Drug Administration approved the use of sulfur hexafluoride lipid-type A microspheres (Lumason®) for ultrasonographic characterization of focal liver lesions. CEH-EUS has not been rigorously studied in esophageal carcinomas, but preliminary data suggest contrast-enhanced images are of limited value due to the relative avascularity of common esophageal malignancies[93,94].
Tridimensional (3D) EUS may be used alone, or with ultrasonographic contrast, to evaluate the invasion depth of tumors. The 3D images are thought to more accurately convey the relationship of cancers to nearby organs and vessels, and may reduce the operator-dependent error that is inherent to standard EUS[95].
LIMITATIONS
Studies on EUS techniques are often limited by several factors, such as changes in practice patterns, radiographic or pathologic techniques, and sonography equipment; which has considerably evolved from 1980 to the current era. Testing characteristics for EUS vary widely depending on the type of equipment used (frequency of ultrasound probe, FNA vs fine needle biopsy, gauge of needles, and expertise of the endosonographer, cytotechnician, and/or pathologist).
Squamous cell esophageal cancers are more common in Japan, which may contribute to variation in EUS diagnostic accuracy and practice patterns in comparison to the United States[96]. Japan Esophageal Society guidelines suggest sm1 lesions (T1b cancers with less than 200 micrometers invasion into submucosa) may be resected endoscopically, in contrast to EC invading the middle or deep submucosa (sm2 or sm3 lesions)[22,96].
In interpreting meta-analyses, the biostatistical model chosen (fixed-effects vs random effects models) and heterogeneity (variation) among studies may confound analysis and interpretation[97]. Higher levels of heterogeneity in meta-analyses decrease confidence in drawing conclusions about the studied relationship[98,99]. Cochran’s Q test and the χ2 heterogeneity statistic may be used to assess for the presence of heterogeneity within a meta-analysis[100], however the I2 quantitatively describes the degree of heterogeneity[98,99]. In example, an I2 index of 25%, 50%, or 75% express a numerical value that may be interpreted as low, moderate, or high levels of heterogeneity among selected studies, respectively[97].
CONCLUSION
Despite modern improvements in diagnosis and treatment, EC continues to carry a high risk of morbidity and mortality, as most cases are diagnosed at advanced stages. EUS, the most sensitive test for locoregional assessment of EC, should be considered in patients without distant metastases prior to neoadjuvant (induction) chemoradiotherapy. EUS may not add additional information in some cases of locally advanced esophageal cancers, and is not routinely recommended. When suspicious lymph nodes are identified remote to the primary tumor, EUS-FNA can obtain cytology specimens to more accurately define the radiotherapy field. Aggressive efforts at early diagnosis and innovative treatments for EC are desperately needed.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No potential competing interests.
Peer-review started: September 20, 2016
First decision: October 10, 2016
Article in press: December 21, 2016
P- Reviewer: Chen CH, Fusaroli P S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society: Cancer Facts and Figures 2016. Atlanta, Ga. Available from: http://www.cancer.org/cancer/esophaguscancer/detailedguide/esophagus-cancer-survival-rates.
- 4.Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19:5598–5606. doi: 10.3748/wjg.v19.i34.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–1187. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demeester SR. Epidemiology and biology of esophageal cancer. Gastrointest Cancer Res. 2009;3:S2–S5. [PMC free article] [PubMed] [Google Scholar]
- 7.Lagergren J, Bergström R, Nyrén O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med. 1999;130:883–890. doi: 10.7326/0003-4819-130-11-199906010-00003. [DOI] [PubMed] [Google Scholar]
- 8.Devesa SS, Blot WJ, Fraumeni JF. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 9.Surveillence, Epidemiology, and End Results (SEER) Program. Cancer Statistics Factsheets: Esophageal Cancer. National Cancer Institute. Bethesda, MD. Last revised: 2016-04. Available from: http://seer.cancer.gov/statfacts/html/esoph.html.
- 10.Kuang JJ, Jiang ZM, Chen YX, Ye WP, Yang Q, Wang HZ, Xie DR. Smoking Exposure and Survival of Patients with Esophagus Cancer: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract. 2016;2016:7682387. doi: 10.1155/2016/7682387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leggett CL, Gorospe EC, Calvin AD, Harmsen WS, Zinsmeister AR, Caples S, Somers VK, Dunagan K, Lutzke L, Wang KK, et al. Obstructive sleep apnea is a risk factor for Barrett’s esophagus. Clin Gastroenterol Hepatol. 2014;12:583–588.e1. doi: 10.1016/j.cgh.2013.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leggett CL, Nelsen EM, Tian J, Schleck CB, Zinsmeister AR, Dunagan KT, Locke GR, Wang KK, Talley NJ, Iyer PG. Metabolic syndrome as a risk factor for Barrett esophagus: a population-based case-control study. Mayo Clin Proc. 2013;88:157–165. doi: 10.1016/j.mayocp.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambert R, Hainaut P. The multidisciplinary management of gastrointestinal cancer. Epidemiology of oesophagogastric cancer. Best Pract Res Clin Gastroenterol. 2007;21:921–945. doi: 10.1016/j.bpg.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 14.UpToDate. Diagnosis and staging of esophageal cancer. Wolters Kluwer Health. The Netherlands. Last revised: 2016-06. Available from: http://www.uptodate.com/contents/diagnosis-and-staging-of-esophageal-cancer. [Google Scholar]
- 15.Ripley RT, Sarkaria IS, Grosser R, Sima CS, Bains MS, Jones DR, Adusumilli PS, Huang J, Finley DJ, Rusch VW, et al. Pretreatment Dysphagia in Esophageal Cancer Patients May Eliminate the Need for Staging by Endoscopic Ultrasonography. Ann Thorac Surg. 2016;101:226–230. doi: 10.1016/j.athoracsur.2015.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Cancer Institute at the National Institutes of Health. Cancer Prevalence and Cost of Care Projections. National Expenditures for Cancer Care. Bethesda, MD. Last accessed: 2014-06. Available from: https://costprojections.cancer.gov/expenditures.html.
- 17.The University of Texas MD Anderson Cancer Center. Esophageal Cancer Diagnosis. Houston, TX. Last accessed: 2016-06. Available from: https://www.mdanderson.org/cancer-types/esophageal-cancer/esophageal-cancer-diagnosis.html.
- 18.Medscape. Esophageal Cancer Staging. TNM Classification for Esophageal Cancer. WebMD, LLC. Last revised: 2015-12-16. Available from: http://emedicine.medscape.com/article/2003224-overview.
- 19.Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721–1724. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 20.Tachibana M, Dhar DK, Kinugasa S, Kotoh T, Shibakita M, Ohno S, Masunaga R, Kubota H, Nagasue N. Esophageal cancer with distant lymph node metastasis: prognostic significance of metastatic lymph node ratio. J Clin Gastroenterol. 2000;31:318–322. doi: 10.1097/00004836-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Cuellar SL, Carter BW, Macapinlac HA, Ajani JA, Komaki R, Welsh JW, Lee JH, Swisher SG, Correa AM, Erasmus JJ, et al. Clinical staging of patients with early esophageal adenocarcinoma: does FDG-PET/CT have a role? J Thorac Oncol. 2014;9:1202–1206. doi: 10.1097/JTO.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 22.Kuwano H, Nishimura Y, Oyama T, Kato H, Kitagawa Y, Kusano M, Shimada H, Takiuchi H, Toh Y, Doki Y, et al. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus April 2012 edited by the Japan Esophageal Society. Esophagus. 2015;12:1–30. doi: 10.1007/s10388-014-0465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiMagno EP, Buxton JL, Regan PT, Hattery RR, Wilson DA, Suarez JR, Green PS. Ultrasonic endoscope. Lancet. 1980;1:629–631. doi: 10.1016/s0140-6736(80)91122-8. [DOI] [PubMed] [Google Scholar]
- 24.Gordon SJ, Rifkin MD, Goldberg BB. Endosonographic evaluation of mural abnormalities of the upper gastrointestinal tract. Gastrointest Endosc. 1986;32:193–198. doi: 10.1016/s0016-5107(86)71803-8. [DOI] [PubMed] [Google Scholar]
- 25.Early DS, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Evans JA, Fanelli RD, Fisher DA, Fonkalsrud L, Hwang JH, et al. Adverse events associated with EUS and EUS with FNA. Gastrointest Endosc. 2013;77:839–843. doi: 10.1016/j.gie.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Jue TL, Sharaf RN, Appalaneni V, Anderson MA, Ben-Menachem T, Decker GA, Fanelli RD, Fukami N, Ikenberry SO, Jain R, et al. Role of EUS for the evaluation of mediastinal adenopathy. Gastrointest Endosc. 2011;74:239–245. doi: 10.1016/j.gie.2011.03.1255. [DOI] [PubMed] [Google Scholar]
- 27.Evans JA, Early DS, Chandraskhara V, Chathadi KV, Fanelli RD, Fisher DA, Foley KQ, Hwang JH, Jue TL, Pasha SF, et al. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc. 2013;77:328–334. doi: 10.1016/j.gie.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 28.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Esophageal and Esophagogastric Junction Cancers, Version 1.2016. Last Revised: 04/22/2016 [Google Scholar]
- 29.van Vliet EP, Heijenbrok-Kal MH, Hunink MG, Kuipers EJ, Siersema PD. Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer. 2008;98:547–557. doi: 10.1038/sj.bjc.6604200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thosani N, Singh H, Kapadia A, Ochi N, Lee JH, Ajani J, Swisher SG, Hofstetter WL, Guha S, Bhutani MS. Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: a systematic review and meta-analysis. Gastrointest Endosc. 2012;75:242–253. doi: 10.1016/j.gie.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Keswani RN, Early DS, Edmundowicz SA, Meyers BF, Sharma A, Govindan R, Chen J, Kohlmeier C, Azar RR. Routine positron emission tomography does not alter nodal staging in patients undergoing EUS-guided FNA for esophageal cancer. Gastrointest Endosc. 2009;69:1210–1217. doi: 10.1016/j.gie.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Puli SR, Reddy JB, Bechtold ML, Antillon D, Ibdah JA, Antillon MR. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol. 2008;14:1479–1490. doi: 10.3748/wjg.14.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chandawarkar RY, Kakegawa T, Fujita H, Yamana H, Toh Y, Fujitoh H. Endosonography for preoperative staging of specific nodal groups associated with esophageal cancer. World J Surg. 1996;20:700–702. doi: 10.1007/s002689900106. [DOI] [PubMed] [Google Scholar]
- 34.Vazquez-Sequeiros E, Levy MJ, Clain JE, Schwartz DA, Harewood GC, Salomao D, Wiersema MJ. Routine vs. selective EUS-guided FNA approach for preoperative nodal staging of esophageal carcinoma. Gastrointest Endosc. 2006;63:204–211. doi: 10.1016/j.gie.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 35.Lightdale CJ, Kulkarni KG. Role of endoscopic ultrasonography in the staging and follow-up of esophageal cancer. J Clin Oncol. 2005;23:4483–4489. doi: 10.1200/JCO.2005.20.644. [DOI] [PubMed] [Google Scholar]
- 36.Qumseya BJ, Brown J, Abraham M, White D, Wolfsen H, Gupta N, Vennalaganti P, Sharma P, Wallace MB. Diagnostic performance of EUS in predicting advanced cancer among patients with Barrett’s esophagus and high-grade dysplasia/early adenocarcinoma: systematic review and meta-analysis. Gastrointest Endosc. 2015;81:865–874.e2. doi: 10.1016/j.gie.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 37.Amini A, Xiao L, Allen PK, Suzuki A, Hayashi Y, Liao Z, Hofstetter W, Crane C, Komaki R, Bhutani MS, et al. Celiac node failure patterns after definitive chemoradiation for esophageal cancer in the modern era. Int J Radiat Oncol Biol Phys. 2012;83:e231–e239. doi: 10.1016/j.ijrobp.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reed CE, Mishra G, Sahai AV, Hoffman BJ, Hawes RH. Esophageal cancer staging: improved accuracy by endoscopic ultrasound of celiac lymph nodes. Ann Thorac Surg. 1999;67:319–321; discussion 322. doi: 10.1016/s0003-4975(99)00031-4. [DOI] [PubMed] [Google Scholar]
- 39.Chirieac LR, Swisher SG, Ajani JA, Komaki RR, Correa AM, Morris JS, Roth JA, Rashid A, Hamilton SR, Wu TT. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103:1347–1355. doi: 10.1002/cncr.20916. [DOI] [PubMed] [Google Scholar]
- 40.Rohatgi P, Swisher SG, Correa AM, Wu TT, Liao Z, Komaki R, Walsh GL, Vaporciyan AA, Rice DC, Roth JA, et al. Characterization of pathologic complete response after preoperative chemoradiotherapy in carcinoma of the esophagus and outcome after pathologic complete response. Cancer. 2005;104:2365–2372. doi: 10.1002/cncr.21439. [DOI] [PubMed] [Google Scholar]
- 41.Rizk N, Venkatraman E, Park B, Flores R, Bains MS, Rusch V. The prognostic importance of the number of involved lymph nodes in esophageal cancer: implications for revisions of the American Joint Committee on Cancer staging system. J Thorac Cardiovasc Surg. 2006;132:1374–1381. doi: 10.1016/j.jtcvs.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 42.Mariette C, Piessen G, Briez N, Triboulet JP. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg. 2008;247:365–371. doi: 10.1097/SLA.0b013e31815aaadf. [DOI] [PubMed] [Google Scholar]
- 43.Flamen P, Lerut A, Van Cutsem E, De Wever W, Peeters M, Stroobants S, Dupont P, Bormans G, Hiele M, De Leyn P, et al. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol. 2000;18:3202–3210. doi: 10.1200/JCO.2000.18.18.3202. [DOI] [PubMed] [Google Scholar]
- 44.Rice TW. Clinical staging of esophageal carcinoma. CT, EUS, and PET. Chest Surg Clin N Am. 2000;10:471–485. [PubMed] [Google Scholar]
- 45.Lerut T, Flamen P, Ectors N, Van Cutsem E, Peeters M, Hiele M, De Wever W, Coosemans W, Decker G, De Leyn P, et al. Histopathologic validation of lymph node staging with FDG-PET scan in cancer of the esophagus and gastroesophageal junction: A prospective study based on primary surgery with extensive lymphadenectomy. Ann Surg. 2000;232:743–752. doi: 10.1097/00000658-200012000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romagnuolo J, Scott J, Hawes RH, Hoffman BJ, Reed CE, Aithal GP, Breslin NP, Chen RY, Gumustop B, Hennessey W, et al. Helical CT versus EUS with fine needle aspiration for celiac nodal assessment in patients with esophageal cancer. Gastrointest Endosc. 2002;55:648–654. doi: 10.1067/mge.2002.122650. [DOI] [PubMed] [Google Scholar]
- 47.Dittler HJ, Bollschweiler E, Siewert JR. [What is the value of endosonography in the preoperative staging of esophageal carcinoma?] Dtsch Med Wochenschr. 1991;116:561–566. doi: 10.1055/s-2008-1063649. [DOI] [PubMed] [Google Scholar]
- 48.Grimm H, Binmoeller KF, Hamper K, Koch J, Henne-Bruns D, Soehendra N. Endosonography for preoperative locoregional staging of esophageal and gastric cancer. Endoscopy. 1993;25:224–230. doi: 10.1055/s-2007-1010297. [DOI] [PubMed] [Google Scholar]
- 49.Worrell SG, Oh DS, Greene CL, Demeester SR, Hagen JA. Endoscopic ultrasound staging of stenotic esophageal cancers may be unnecessary to determine the need for neoadjuvant therapy. J Gastrointest Surg. 2014;18:318–320. doi: 10.1007/s11605-013-2398-8. [DOI] [PubMed] [Google Scholar]
- 50.Dubecz A, Kern M, Solymosi N, Schweigert M, Stein HJ. Predictors of Lymph Node Metastasis in Surgically Resected T1 Esophageal Cancer. Ann Thorac Surg. 2015;99:1879–1885; discussion 1886. doi: 10.1016/j.athoracsur.2015.02.112. [DOI] [PubMed] [Google Scholar]
- 51.Young PE, Gentry AB, Acosta RD, Greenwald BD, Riddle M. Endoscopic ultrasound does not accurately stage early adenocarcinoma or high-grade dysplasia of the esophagus. Clin Gastroenterol Hepatol. 2010;8:1037–1041. doi: 10.1016/j.cgh.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 52.Puli SR, Reddy JB, Bechtold ML, Antillon MR, Ibdah JA. Accuracy of endoscopic ultrasound in the diagnosis of distal and celiac axis lymph node metastasis in esophageal cancer: a meta-analysis and systematic review. Dig Dis Sci. 2008;53:2405–2414. doi: 10.1007/s10620-007-0152-3. [DOI] [PubMed] [Google Scholar]
- 53.Dhupar R, Rice RD, Correa AM, Weston BR, Bhutani MS, Maru DM, Betancourt SL, Rice DC, Swisher SG, Hofstetter WL. Endoscopic Ultrasound Estimates for Tumor Depth at the Gastroesophageal Junction Are Inaccurate: Implications for the Liberal Use of Endoscopic Resection. Ann Thorac Surg. 2015;100:1812–1816. doi: 10.1016/j.athoracsur.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 54.Gerke H. Endoscopic mucosal resection for early esophageal cancer: skip EUS and cut to the chase. Gastrointest Endosc. 2011;73:669–672. doi: 10.1016/j.gie.2010.11.051. [DOI] [PubMed] [Google Scholar]
- 55.Cho JW, Choi SC, Jang JY, Shin SK, Choi KD, Lee JH, Kim SG, Sung JK, Jeon SW, Choi IJ, et al. Lymph Node Metastases in Esophageal Carcinoma: An Endoscopist’s View. Clin Endosc. 2014;47:523–529. doi: 10.5946/ce.2014.47.6.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park CH, Kim EH, Kim HY, Roh YH, Lee YC. Clinical outcomes of endoscopic submucosal dissection for early stage esophagogastric junction cancer: a systematic review and meta-analysis. Dig Liver Dis. 2015;47:37–44. doi: 10.1016/j.dld.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 57.Patel V, Burbridge RA. Endoscopic approaches for early-stage esophageal cancer: current options. Curr Oncol Rep. 2015;17:421. doi: 10.1007/s11912-014-0421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leggett CL, Lewis JT, Wu TT, Schleck CD, Zinsmeister AR, Dunagan KT, Lutzke LS, Wang KK, Iyer PG. Clinical and histologic determinants of mortality for patients with Barrett’s esophagus-related T1 esophageal adenocarcinoma. Clin Gastroenterol Hepatol. 2015;13:658–664.e1-3. doi: 10.1016/j.cgh.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leggett CL, Prasad GA. High-grade dysplasia and intramucosal adenocarcinoma in Barrett’s esophagus: the role of endoscopic eradication therapy. Curr Opin Gastroenterol. 2012;28:354–361. doi: 10.1097/MOG.0b013e328352b78a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pech O, May A, Manner H, Behrens A, Pohl J, Weferling M, Hartmann U, Manner N, Huijsmans J, Gossner L, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology. 2014;146:652–660.e1. doi: 10.1053/j.gastro.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 61.Pech O, Bollschweiler E, Manner H, Leers J, Ell C, Hölscher AH. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett’s esophagus at two high-volume centers. Ann Surg. 2011;254:67–72. doi: 10.1097/SLA.0b013e31821d4bf6. [DOI] [PubMed] [Google Scholar]
- 62.Hermansson M, DeMeester SR. Management of stage 1 esophageal cancer. Surg Clin North Am. 2012;92:1155–1167. doi: 10.1016/j.suc.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 63.Ell C, May A, Pech O, Gossner L, Guenter E, Behrens A, Nachbar L, Huijsmans J, Vieth M, Stolte M. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer) Gastrointest Endosc. 2007;65:3–10. doi: 10.1016/j.gie.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 64.Fang TC, Oh YS, Szabo A, Khan A, Dua KS. Utility of dysphagia grade in predicting endoscopic ultrasound T-stage of non-metastatic esophageal cancer. Dis Esophagus. 2016;29:642–648. doi: 10.1111/dote.12394. [DOI] [PubMed] [Google Scholar]
- 65.Pennathur A, Farkas A, Krasinskas AM, Ferson PF, Gooding WE, Gibson MK, Schuchert MJ, Landreneau RJ, Luketich JD. Esophagectomy for T1 esophageal cancer: outcomes in 100 patients and implications for endoscopic therapy. Ann Thorac Surg. 2009;87:1048–1054; discussion 1054-1055. doi: 10.1016/j.athoracsur.2008.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ell C, May A, Gossner L, Pech O, Günter E, Mayer G, Henrich R, Vieth M, Müller H, Seitz G, et al. Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett’s esophagus. Gastroenterology. 2000;118:670–677. doi: 10.1016/s0016-5085(00)70136-3. [DOI] [PubMed] [Google Scholar]
- 67.Campbell NP, Villaflor VM. Neoadjuvant treatment of esophageal cancer. World J Gastroenterol. 2010;16:3793–3803. doi: 10.3748/wjg.v16.i30.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062–5067. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 69.Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007;8:226–234. doi: 10.1016/S1470-2045(07)70039-6. [DOI] [PubMed] [Google Scholar]
- 70.Samalin E, Ychou M. Neoadjuvant therapy for gastroesophageal adenocarcinoma. World J Clin Oncol. 2016;7:284–292. doi: 10.5306/wjco.v7.i3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng Y, Li Y, Liu X, Sun H, Wang Z, Zhang R. Reevaluation of Neoadjuvant Chemotherapy for Esophageal Squamous Cell Carcinoma: A Meta-Analysis of Randomized Controlled Trials Over the Past 20 Years. Medicine (Baltimore) 2015;94:e1102. doi: 10.1097/MD.0000000000001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fu T, Bu ZD, Li ZY, Zhang LH, Wu XJ, Wu AW, Shan F, Ji X, Dong QS, Ji JF. Neoadjuvant chemoradiation therapy for resectable esophago-gastric adenocarcinoma: a meta-analysis of randomized clinical trials. BMC Cancer. 2015;15:322. doi: 10.1186/s12885-015-1341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fiorica F, Di Bona D, Schepis F, Licata A, Shahied L, Venturi A, Falchi AM, Craxì A, Cammà C. Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta-analysis. Gut. 2004;53:925–930. doi: 10.1136/gut.2003.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Isenberg G, Chak A, Canto MI, Levitan N, Clayman J, Pollack BJ, Sivak MV. Endoscopic ultrasound in restaging of esophageal cancer after neoadjuvant chemoradiation. Gastrointest Endosc. 1998;48:158–163. doi: 10.1016/s0016-5107(98)70157-9. [DOI] [PubMed] [Google Scholar]
- 75.Ribeiro A, Franceschi D, Parra J, Livingstone A, Lima M, Hamilton-Nelson K, Ardalan B. Endoscopic ultrasound restaging after neoadjuvant chemotherapy in esophageal cancer. Am J Gastroenterol. 2006;101:1216–1221. doi: 10.1111/j.1572-0241.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 76.Sun F, Chen T, Han J, Ye P, Hu J. Staging accuracy of endoscopic ultrasound for esophageal cancer after neoadjuvant chemotherapy: a meta-analysis and systematic review. Dis Esophagus. 2015;28:757–771. doi: 10.1111/dote.12274. [DOI] [PubMed] [Google Scholar]
- 77.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 78.Wong AT, Shao M, Rineer J, Lee A, Schwartz D, Schreiber D. The Impact of Adjuvant Postoperative Radiation Therapy and Chemotherapy on Survival After Esophagectomy for Esophageal Carcinoma. Ann Surg. 2016 doi: 10.1097/SLA.0000000000001825. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 79.Berry MF. Esophageal cancer: staging system and guidelines for staging and treatment. J Thorac Dis. 2014;6 Suppl 3:S289–S297. doi: 10.3978/j.issn.2072-1439.2014.03.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 81.Zheng B, Zheng W, Zhu Y, Lin XY, Xu BH, Chen C. Role of adjuvant chemoradiotherapy in treatment of resectable esophageal carcinoma: a meta-analysis. Chin Med J (Engl) 2013;126:1178–1182. [PubMed] [Google Scholar]
- 82.Ku GY, Ilson DH. Adjuvant (postoperative) therapy for esophageal cancer. Thorac Surg Clin. 2013;23:525–533. doi: 10.1016/j.thorsurg.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 83.Yang GY, Wagner TD, Jobe BA, Thomas CR. The role of positron emission tomography in esophageal cancer. Gastrointest Cancer Res. 2008;2:3–9. [PMC free article] [PubMed] [Google Scholar]
- 84.Rollins KE, Lucas E, Tewari N, James E, Hughes S, Catton JA. PET-CT offers accurate assessment of tumour length in oesophageal malignancy. Eur J Radiol. 2015;84:195–200. doi: 10.1016/j.ejrad.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 85.Jeganathan R, McGuigan J, Campbell F, Lynch T. Does pre-operative estimation of oesophageal tumour metabolic length using 18F-fluorodeoxyglucose PET/CT images compare with surgical pathology length? Eur J Nucl Med Mol Imaging. 2011;38:656–662. doi: 10.1007/s00259-010-1670-3. [DOI] [PubMed] [Google Scholar]
- 86.Roedl JB, Sahani DV, Colen RR, Fischman AJ, Mueller PR, Blake MA. Tumour length measured on PET-CT predicts the most appropriate stage-dependent therapeutic approach in oesophageal cancer. Eur Radiol. 2008;18:2833–2840. doi: 10.1007/s00330-008-1078-7. [DOI] [PubMed] [Google Scholar]
- 87.Hadzijahic N, Wallace MB, Hawes RH, VanVelse A, LeVeen M, Marsi V, Hoffman BJ, Sahai AV. CT or EUS for the initial staging of esophageal cancer? A cost minimization analysis. Gastrointest Endosc. 2000;52:715–720. doi: 10.1067/mge.2000.108481. [DOI] [PubMed] [Google Scholar]
- 88.Harewood GC, Wiersema MJ. A cost analysis of endoscopic ultrasound in the evaluation of esophageal cancer. Am J Gastroenterol. 2002;97:452–458. doi: 10.1111/j.1572-0241.2002.05499.x. [DOI] [PubMed] [Google Scholar]
- 89.Shumaker DA, de Garmo P, Faigel DO. Potential impact of preoperative EUS on esophageal cancer management and cost. Gastrointest Endosc. 2002;56:391–396. doi: 10.1016/s0016-5107(02)70044-8. [DOI] [PubMed] [Google Scholar]
- 90.Sazuka T, Akai T, Uesato M, Horibe D, Kuboshima M, Kitabayashi H, Matsunaga A, Kagaya A, Muto Y, Takeshita N, et al. Assessment for diagnosis of lymph node metastasis in esophageal cancer using endoscopic ultrasound elastography. Esophagus. 2016;13:254–263. doi: 10.1007/s10388-016-0521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paterson S, Duthie F, Stanley AJ. Endoscopic ultrasound-guided elastography in the nodal staging of oesophageal cancer. World J Gastroenterol. 2012;18:889–895. doi: 10.3748/wjg.v18.i9.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Knabe M, Günter E, Ell C, Pech O. Can EUS elastography improve lymph node staging in esophageal cancer? Surg Endosc. 2013;27:1196–1202. doi: 10.1007/s00464-012-2575-y. [DOI] [PubMed] [Google Scholar]
- 93.Reddy NK, Ioncică AM, Săftoiu A, Vilmann P, Bhutani MS. Contrast-enhanced endoscopic ultrasonography. World J Gastroenterol. 2011;17:42–48. doi: 10.3748/wjg.v17.i1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nomura N, Goto H, Niwa Y, Arisawa T, Hirooka Y, Hayakawa T. Usefulness of contrast-enhanced EUS in the diagnosis of upper GI tract diseases. Gastrointest Endosc. 1999;50:555–560. doi: 10.1016/s0016-5107(99)70083-0. [DOI] [PubMed] [Google Scholar]
- 95.Saftoiu A, Gheonea DI. Tridimensional (3D) endoscopic ultrasound - a pictorial review. J Gastrointestin Liver Dis. 2009;18:501–505. [PubMed] [Google Scholar]
- 96.Shimizu Y, Takahashi M, Yoshida T, Ono S, Mabe K, Kato M, Asaka M. Diagnosis of depth of invasion for patients with superficial esophageal cancer: differentiating upper submucosal versus middle or deep submucosal invasion is important for deciding treatment strategy. Gastrointest Endosc. 2012;76:1073; author reply 1073–1075. doi: 10.1016/j.gie.2012.04.471. [DOI] [PubMed] [Google Scholar]
- 97.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 99.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 100.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]


