Abstract
AIM
To investigated the feasibility of using sinusoidal endotheliitis (SE) as a histological marker for liver allograft rejection.
METHODS
We compared the histological features of 88 liver allograft biopsies with acute cellular rejection (ACR) and 59 cases with no evidence of ACR. SE was scored as: (1) focal linear lifting up of the endothelial cells by lymphocytes with no obvious damage to adjacent hepatocytes; (2) focal disruption of the endothelial lining by a cluster of subendothelial lymphocytes (a group of > 3 lymphocytes); and (3) severe confluent endotheliitis with hemorrhage and adjacent hepatocyte loss.
RESULTS
The sensitivity and specificity of SE was 81% and 85%, respectively. Using SE as the only parameter, the positive predictive value for ACR (PPV) was 0.89, whereas the negative predictive value for ACR (NPV) was 0.75. The correlation between RAI and SE was moderate (R = 0.44, P < 0.001) (Figure 3A), whereas it became strong (R = 0.65, P < 0.001) when correlating SE with the venous endotheliitis activity index only.
CONCLUSION
Our data suggest that SE scoring could be a reliable and reproducible supplemental parameter to the existing Banff schema for diagnosing acute liver allograft rejection.
Keywords: Liver transplantation, Acute cellular rejection, Sinusoidal endotheliitis
Core tip: In this clinico-pathological study, we have found that scoring of the sinusoidal endotheliitis could be a reliable and practically reproducible supplemental parameter to the existing Banff schema for diagnosing early acute cellular rejection in liver allograft as well as predicting the occurrence of acute cellular rejection in appropriate clinical setting.
INTRODUCTION
Liver transplantation (LT) has become a viable option for the treatment of end stage liver diseases due to the establishment of the concept of brain death in donors, and the availability of effective immunsuppressive agents, including calcineurin inhibitors for allograft recipients. The liver allograft is unique in that Kupffer cells are capable of sequestering cytotoxic antibodies formed against the graft, and the venous vascular endothelium could be gradually replaced by host hematopoietic cells over time[1]. Therefore, although HLA cross matching is not routinely performed in LT, antibody-mediated hyperacute rejection rarely occurs in liver allografts[2]. Acute cellular rejection (ACR) in the liver allograft often occurs between 5 and 30 d after LT[3]. The overall frequency of ACR varies with the baseline immunosuppression regimen used, ranging from 30% to 70%[4,5]. The diagnosis of ACR is usually suspected based on clinical manifestations and abnormal liver function tests, and confirmed by the examination of a core needle biopsy. The Banff schema is currently the standard system for diagnosing and grading the severity of ACR[3,6]. In the Banff schema, the severity of three morphological parameters, i.e., portal inflammation, bile duct inflammation, and venous endotheliitis, are assigned individual scores from 0 to 3, and the sum of these scores is called the rejection activity index (RAI). However, experienced transplant pathologists often pay more attention to the presence of, and the degree of, vascular endothelial damage in portal veins and in central veins.
The sinusoidal endothelium, with its relatively low hydrostatic pressure and large surface area, forms a unique interface between the graft and the recipient’s immune system. Sinusoidal endothelial damage has been recognized as a histological parameter for diagnosing graft-vs-host disease in the setting of hematopoietic stem cell transplantation[7,8]. Sinusoidal inflammation was recently incorporated as part of antibody-mediated liver allograft rejection in the updated Banff criteria[9]. However, the association between sinusoidal endothelial damage and ACR has not been examined systematically. In our assessment of liver allograft biopsies from patients with concurrent or subsequent ACR, we frequently observed sinusoidal subendothelial lymphocytic infiltration, with a range of severity that increases from lifting of the endothelium by a linear arrangement or clustering of lymphocytes, to disruption of the intact endothelial lining, to hemorrhage and damage to adjacent hepatocytes. In the present study, we investigated whether sinusoidal endotheliitis (SE) could be a reliable and reproducible supplemental parameter to the existing Banff schema for diagnosing ACR in liver allograft biopsies.
MATERIALS AND METHODS
Patients
After obtaining the approval of the institutional review board (IRB), all biopsies from 2010 to 2015 at the McGill University Health Center (MUHC) were studied. Most liver transplant cases were performed for hepatitis C-related cirrhosis, the leading indication for liver transplantation in Canada[10]. Cases with detailed clinical history and a definite histopathologic diagnosis of ACR were recruited into our study. Liver allograft biopsies were divided into two groups: cases with or without histological evidence of ACR according to the Banff schema. Biopsies were performed between 6 and 180 d post-transplant either for clinical indication or protocol biopsy. There is no skewed distribution in terms of biopsy time or clinical indication/protocol biopsy between the two groups. All patients received baseline immunosuppressive therapy or antiviral treatment if the primary liver disease was hepatitis C. A total of 88 cases with a definitive histological diagnosis of ACR were obtained and were designated as the ACR-positive group. The primary liver diseases of this group included 82 cases of hepatitis C, two cases of postpartum liver failure, one case of primary sclerosing cholangitis, one case of non-alcoholic steatohepatitis (NASH), one case of hepatitis B, and one case of cholestasis of uncertain etiology. Cases with recurrent hepatitis C were excluded from the ACR-positive group if serum HCV RNA levels were high (> 8.00 log IU/mL) or if there was histologic evidence of hepatitis C infection. The ACR-negative group was comprised of 59 cases, including 45 cases of recurrent hepatitis C, seven cases of NASH, six cases of cholestasis of uncertain etiology, and one case of primary biliary cholangitis.
Biopsy preparation for light microscopy
Tissue was fixed in 10% neutral buffered formalin, embedded in paraffin, processed and cut into 3 µm-thick sections. The slides were stained with hematoxylin-eosin, Masson trichrome, reticulin, PAS, PAS + diastase, and Prussian blue iron stains.
Grading of acute liver allograft rejection
The liver biopsies were evaluated by three pathologists to reach a consensus on the diagnosis of ACR. Following the guidelines of the 1997 Banff schema for grading liver allograft rejection, the rejection activity index (RAI) was determined by summing the individual scores of the parameters (on a 0 to 3 scale), i.e., portal inflammation, bile duct inflammation and, and venous endotheliitis.
Definition and grading of sinusoidal endotheliitis
SE was defined as subendothelial lymphocytic infiltration with lifting and/or damage to the sinusoidal endothelial cells. Sinusoidal lymphocytes were counted on HE slides in five high-power fields (HPF). Greater than 100 lymphocytes/HPF was considered an increase. Increase of intrasinusoidal lymphocytes and adhesion of lymphocytes to the endothelium were not considered to be SE. Grading of SE was as follows: (1) focal linear lifting up of the endothelial cells by lymphocytes with no obvious damage to adjacent hepatocytes; (2) focal disruption of the endothelial lining with a cluster of subendothelial lymphocytes (a group of > 3 lymphocytes); and (3) severe confluent endotheliitis with hemorrhage, adjacent hepatocyte loss, with or without fibrosis.
Statistical analysis
The linear correlation coefficient (r) was calculated to evaluate the correlation between SE and RAI and between SE and the score of portal venous endotheliitis using Excel software (Pearson correlation coefficient test). The sensitivity, specificity, positive, and negative predictive value of SE for ACR were calculated using the total Banff RAI scores.
RESULTS
Histologic findings of acute cellular rejection
Among the 88 cases with ACR, 82 cases had ACR with average RAI scores of 5. Six cases had a RAI below 3, but with definitive evidence of endotheliitis in the portal or central veins. The 59 cases without evidence of ACR had no or very minimal inflammation in biopsies or showed histologic features and clinical presentation (e.g., elevated hepatitis C viral load, or morbid obesity, etc.) pointing to another etiology.
When using the Banff criteria to diagnose ACR, the participating pathologists felt that portal vein or central vein endotheliitis was more reliable and reproducible than portal inflammation or ductulitis. The spectrum of portal and central venous pathology is illustrated in Figure 1. The non-inflamed portal tract has little or no inflammatory cells (Figure 1A). Mild portal inflammation without venular endothelial damage was considered negative (Figure 1B). Portal vein endotheliitis was unequivocal when the endothelium was lifted up by subendothelial lymphocytic infiltration (Figure 1C). A severe case of endotheliitis is characterized by perivenular liver cell necrosis in addition to endothelial damage (Figure 1D).
Figure 1.
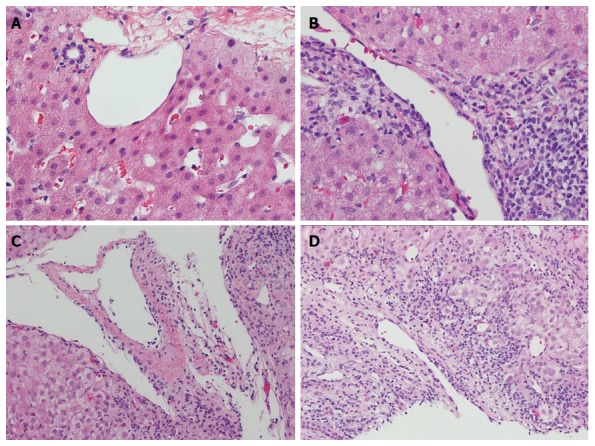
Spectrum of portal and central venous pathologic findings. A: Intact portal triad; B: Portal inflammation with intact venule; C: Portal vein with subendothelial lymphocytic infiltration (endotheliitis); D: Severe endotheliitis with perivenular hepatocyte necrosis (hematoxylin-eosin staining, magnification × 400).
Increase of intrasinusoidal lymphocytic infiltration without sinusoidal damage is a universal finding (100%) in ACR-positive cases (Figure 2A), and is also frequently seen in ACR-negative cases (specificity 57%). These lymphocytes may appear to attach to the sinusoid wall, or float in the lumen at different levels of the section. Therefore, an increase in lymphocytes in the sinusoids was not considered as reliable evidence of ACR due to poor reproducibility and lack of specificity. Sometimes, diffuse lymphocytic infiltration, Kupffer cell hypertrophy and hyperplasia can occur with unknown significance. True SE is characterized by lymphocytic infiltration underneath the sinusoidal endothelium, typically lifting up and detaching the overlying endothelium from the basement membrane (Figure 2B and C). There were total 80 cases with SE, including 35 cases of grade 1, 32 cases of grade 2, and 13 cases of grade 3. In grade 1, we observed focal linear lymphocytic infiltration with the formation of a “pearl band” along the sinusoidal subendothelial space (Figure 2B). Focal clustering (> 3 lymphocytes per cluster) of lymphocytic infiltration between the endothelium and the basement membrane that interrupts the integrity of the endothelium represents grade 2 SE (Figure 2C). In the grade 1 and grade 2 SE case scenarios, SE was associated with sinusoidal dilatation. Grade 3 endotheliitis is characterized by further damage causing hemorrhage and adjacent hepatocyte loss with mixed lymphohistiocytic infiltration (Figure 2D). The findings of a collapsed reticulin framework (Figure 2E) and deposition of collagen bands (Figure 2F) are consistent with the loss of hepatocytes. The presence of hemorrhage and adjacent SE can help to distinguish it from lobular hepatitis-related hepatocyte loss.
Figure 2.
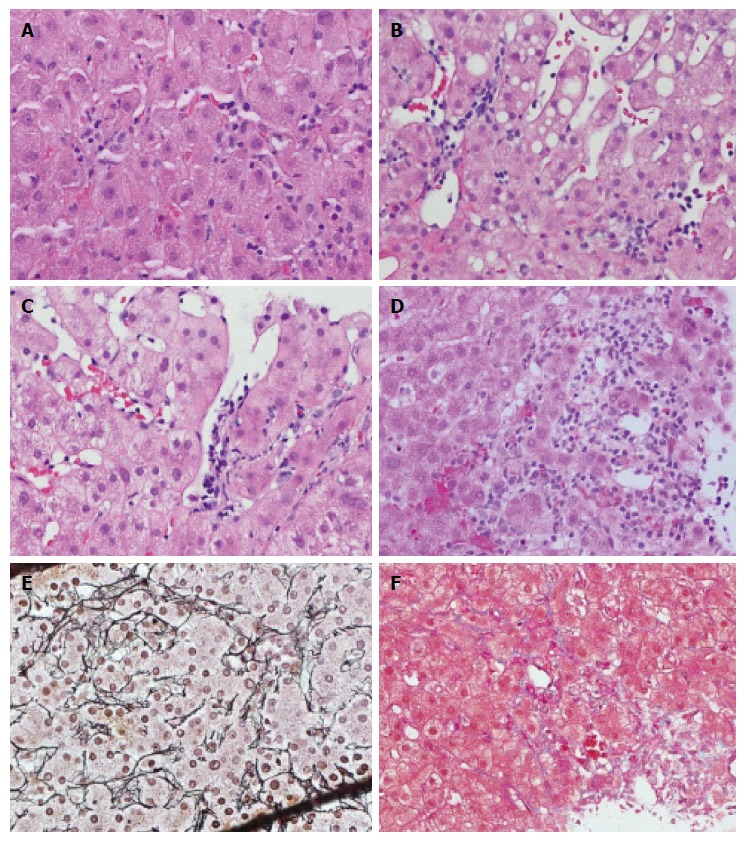
Spectrum of sinusoidal pathology. A: lymphocytes in sinusoidal spaces, with adhesion to endothelium but without lifting of endothelial cells; B: Grade 1 sinusoidal endotheliitis with subendothelial linear lymphocytic infiltration; C: Grade 2 sinusoidal endotheliitis with subendothelial lymphocyte clusters and partially disrupted endothelium; D: Grade 3 sinusoidal endotheliitis with endothelial damage, fresh hemorrhage, lymphohistiocytic infiltration and adjacent liver cell necrosis; E: Grade 3 sinusoidal endotheliitis with collapsed liver cell plates on reticulin staining; F: Grade 3 sinusoidal endotheliitis with collagen deposition on Masson trichrome staining (hematoxylin-eosin staining, magnification × 400).
Evaluation of sinusoidal endotheliitis as a new parameter for diagnosing acute cellular rejection
As shown in Table 1, in the 88 ACR-positive cases and 59 ACR-negative cases, as diagnosed by the Banff schema, the sensitivity of SE was 81% and the specificity was 85%.
Table 1.
Association between sinusoidal endotheliitis and acute cellular rejection
| ACR positive | ACR negative | Total | |
| SE positive | 71 | 9 | 80 |
| SE negative | 17 | 50 | 67 |
| Total | 88 | 59 | 147 |
ACR: Acute cellular rejection; SE: Sinusoidal endotheliitis.
Using SE as the only parameter, the positive predictive value for ACR (PPV) was 0.89, whereas the negative predictive value for ACR (NPV) was 0.75. The correlation between RAI and SE was moderate (R = 0.44, P < 0.001) (Figure 3A), whereas it became strong (R = 0.65, P < 0.001) when correlating SE with the venous endotheliitis activity index only (Figure 3B).
Figure 3.
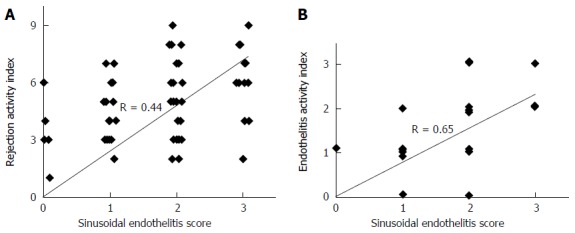
Correlation between scores of sinusoidal endotheliitis and the rejection activity index of the Banff schema. A: The sinusoidal endotheliitis score (SES) moderately correlates with the overall rejection activity index; B: The SES strongly correlates with the portal and central vein endotheliitis activity score (hematoxylin-eosin staining, magnification × 400).
DISCUSSION
The Banff schema is currently the gold standard for the diagnosis of ACR and for the assessment of its severity. However, this seemingly uncomplicated practice is not without challenges. For instance, the number of portal tracts in each liver biopsy varies and portal changes are often patchy, resulting in false negative biopsies. Additionally, portal inflammation and bile duct injury are features which are often shared by other liver diseases, in particular recurrent hepatitis C[11] or drug toxicity[3]. In this study, we demonstrate that SE is a useful supplemental parameter for diagnosing ACR in the liver with high sensitivity, specificity, and positive and negative predictive value. The SE score showed a strong correlation with the portal venous endotheliitis index score of the Banff criteria. In 6 cases, SE was an early sign that predicted the development of subsequent ACR.
Sinusoids are low-pressure vascular channels lined by a specialized endothelium with slit-like spaces, which lie between plates of hepatocytes, providing these cells with a large interface for the exchange of various substances with the circulating blood[12]. In the normal human liver, a small number of functional T lymphocytes can be seen in the portal tracts and scattered throughout the liver parenchyma. Lymphocytic infiltration of the liver could be the result of an immune response to many insults. Lymphocyte recruitment to the human liver is mediated by distinct combinations of molecules depending on whether recruitment occurs via the portal vascular endothelium or the hepatic sinusoids[13]. Intravital microscopy has revealed that leucocyte recruitment to the hepatic parenchyma can occur through the sinusoids in a process that involves direct adhesive interactions with the sinusoidal endothelium[14]. An animal model of liver injury in rat has demonstrated that most lymphocytes are recruited to the liver via the hepatic sinusoids with subsequent redistribution to the hepatic parenchyma in lobular hepatitis or to the portal tracts in portal and interface hepatitis[15]. Given the much larger surface area, the low pressure and relatively slow blood flow, sinusoidal endothelium should, theoretically, bear more immunological damage than either portal or central venous endothelium in patients with ACR. Sinusoidal lymphocyte infiltration has been recognized as a common histological finding in the liver in experimental and clinical graft-vs-host disease[7,8]. Previous studies have demonstrated that infiltration of lymphocytes in the sinusoidal space, and particularly adherence of lymphocytes to the endothelium are associated with various liver diseases[16-18]. In reality, it is often difficult to determine whether sinusoidal lymphocytes are attached to the endothelium or simply the result of tangential cuts. Furthermore there is no consensus regarding the upper limit of the number of lymphocytes in the sinusoids[19]. In the present study, presence of > 100 lymphocytes/HPF was considered an increase in intrasinusoidal lymphocytes. However, we found that the increase in sinusoidal lymphocytes was not specific to ACR because it was observed not only in all ACR-positive cases but also in some ACR-negative cases. Therefore, to ensure reproducibility, the definition of SE in the current study is identical to endotheliitis that occurs in the portal or central vein, as characterized by linear or clustered subendothelial lymphocytic infiltration that lifts the endothelial cells or disrupts the integrity of the sinusoidal endothelium with or without peripheral hepatocyte necrosis.
Recurrent hepatitis C in post-transplant biopsy is problematic, because portal inflammation, bile duct damage, and lobular hepatitis can mimic ACR. The combination of clinical presentation, the pattern of elevated liver enzymes, the viral titer, and careful examination of the histological pattern are often required to distinguish it from bona fide ACR[11]. To complicate the matter even further, cases of mixed ACR and recurrent HCV do exist. In this study, cases with elevated HCV RNA levels or histologic evidence of hepatitis C infection were excluded from the ACR-positive group so that we could focus exclusively on the latter.
The 3-tier grading of SE is a measure of the severity of the rejection process, but also somewhat reflects the evolving process of the disease: starting from subendothelial linear lymphocytes that lift up the intact sinusoidal endothelium, to the formation of clusters of lymphocytes that interrupt the intact endothelial lining, to causing hemorrhage and adjacent hepatocyte necrosis and subsequent collagen deposition. Not surprisingly, the scores of SE were more strongly correlated with the portal vein or central vein endotheliitis activity index of the Banff schema than they were with the overall RAI. Both SE and portal or central vein endotheliitis are more specific for ACR than other parameters, and they should be given more weight in scoring the severity of ACR.
In the six cases that were negative for ACR by the Banff criteria, but positive for SE, a follow up repeat biopsy revealed the subsequent development of ACR. This could be due to patchiness of portal vein endotheliitis with limited available portal numbers in a biopsy. However, it is more likely that SE was a precursor, presenting earlier than portal or central vein endotheliitis because of the larger surface area of sinusoids and easier access to lymphocytes.
The limitations of our study include the study population and the sample size. Our study population was composed predominantly of patients with hepatitis C as primary disease, so that these findings may not necessarily hold true for patients with other primary disease leading to liver transplantation. Hepatitis C virus (HCV)-caused cirrhosis is the most common indication for liver transplantation (LT) in Canada.[10] Despite advances in antiviral therapy, reinfection of HCV in liver allografts is almost universal[20,21]. The recurrence of HCV as defined by elevation of HCV RNA in serum, and histologic evidence of HCV can be demonstrated in 70%-90% of recipients after 1 year and in 90%-95% after 5 years[22,23]. Most post-transplant liver biopsies in our institute were cases with or without serum HCV RNA to rule out acute cellular rejection (ACR). Since recurrent HCV shares some histology features with ACR, cases of ACR with high HCV RNA were excluded from ACR group to simplify the comparison. In ACR negative group, cases with high HCV RNA were included because there weren’t enough cases of HCV RNA negative patients in this group. Post-liver transplant patients with neither ACR nor HCV RNA were rarely indicated for biopsy. Exceptions are the cases with other etiology liver diseases, such as non-alcoholic steatohepatitis (NASH), or cholestatic disease. Therefore, there were more non-HCV cases in ACR negative group than those in ACR group. Secondly, despite our study including a significant number of cases, confirmation in larger numbers of biopsies and with follow up repeat biopsies should be carried out to provide further support of SE as an early and reliable histological marker of ACR.
In summary, we demonstrated that SE scoring could be a reliable, practical supplemental parameter to the existing Banff schema for diagnosing ACR of liver allograft as well as for predicting the occurrence of ACR in an appropriate clinical setting.
COMMENTS
Background
In the last two decades, Banff schema has been the standard system for diagnosing and grading the severity of acute cellular rejection in liver allografts. The Banff schema evaluates portal inflammation; bile duct damage; and venous endotheliitis. Each component is scored on a scale of 0-3 and added together to report a final rejection activity index (RAI). In practice, experienced transplant pathologists often pay more attention to the presence of, and the degree of, vascular endothelial damage in portal veins and in central veins. One of the limitations of this system is the variation in portal tract number in each liver biopsy and the patchiness of portal changes, causing false negative biopsies. Additionally, portal inflammation and bile duct injury are features which are often shared by other liver diseases.
Research frontiers
The sinusoidal endothelium, with its relatively low hydrostatic pressure and large surface area, form a unique interface between the graft and the recipient’s immune system. Sinusoidal endothelial damage has been recognized as a histological parameter for diagnosing graft-vs-host disease in the setting of hematopoietic stem cell transplantation. Sinusoidal inflammation was recently incorporated as part of antibody-mediated liver allograft rejection in the updated Banff criteria. However, the association between sinusoidal endothelial damage and ACR has not been examined systematically.
Innovations and breakthroughs
In this study, the authors investigated the feasibility of using sinusoidal endotheliitis (SE) as an early diagnostic marker for liver allograft rejection by comparing the histological features of 82 liver transplant (LT) biopsies with acute rejection (AR) and 65 cases with no evidence of AR. The sensitivity and specificity of SE was 81% and 85%, respectively. Using SE as the only parameter, the positive predictive value for ACR (PPV) was 0.89, whereas the negative predictive value for ACR (NPV) was 0.75. The correlation between RAI and SE was moderate (R = 0.44, P < 0.001) (Figure 3A), whereas it became strong (R = 0.65, P < 0.001) when correlating SE with the venous endotheliitis activity index only. This is the first study to propose the concept of SE and its diagnostic value in ACR. It represents a significant contribution to understanding ACR in routine pathology practice and potential improvement in diagnosing ACR.
Applications
The authors’ data suggest that SE scoring was a sensitive and specific parameter for diagnosing ACR. These results could be useful to pathologist in daily practice, especially when liver biopsy with limited portal tract number or showing the patchiness of portal changes.
Terminology
SE was defined as subendothelial lymphocytic infiltration with lifting and/or damage to the sinusoidal endothelial cells. Sinusoidal lymphocytes were counted on HE slides in five high-power fields (HPF). Greater than 100 lymphocytes/HPF was considered an increase. Increase of intrasinusoidal lymphocytes and adhesion of lymphocytes to the endothelium were not considered to be SE. Grading of SE was as follows: (1) focal linear lifting up of the endothelial cells by lymphocytes with no obvious damage to adjacent hepatocytes; (2) focal disruption of the endothelial lining with a cluster of subendothelial lymphocytes (a group of > 3 lymphocytes); and (3) severe confluent endotheliitis with hemorrhage, adjacent hepatocyte loss, with or without fibrosis.
Peer-review
The work of Shi and co-workers investigates the impact of sinusoidal endotheliitis for qualification of liver graft rejection. This parameter is an additional parameter to the qualification categories of the RAI Score currently used in clinical routine to express the degree of rejection activity after liver transplantation. Since quantification of sinusoidal endotheliitis reached a sensitivity of 81% and a specificity of 85% it may reflect a more sensitive parameter than the currently used categories (lymphocyte infiltration around portal veins, centrilobular veins and bile ducts). Alternatively, this new category might reflect an additional parameter, which would improve the accurateness of the RAI score.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by institutional review board (IRB) at McGill University. All routine liver biopsy specimens from the patients were taken after informed consent and ethical permission was obtained for this retrospective study.
Conflict-of-interest statement: The authors have no potential conflicts of interest to disclose.
Data sharing statement: Dataset available from the corresponding author at Zu-hua.gao@mcgill.ca.
Peer-review started: October 18, 2016
First decision: November 21, 2016
Article in press: January 11, 2017
P- Reviewer: Rentsch M, Salvadori M S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Gao Z, McAlister VC, Williams GM. Repopulation of liver endothelium by bone-marrow-derived cells. Lancet. 2001;357:932–933. doi: 10.1016/s0140-6736(00)04217-3. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi BH. Consensus and Controversies on HLA Matching and Crossmatching in Transplantation. Saudi J Kidney Dis Transpl. 1997;8:138–144. [PubMed] [Google Scholar]
- 3.Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658–663. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 4.Watson CJ, Dark JH. Organ transplantation: historical perspective and current practice. Br J Anaesth. 2012;108 Suppl 1:i29–i42. doi: 10.1093/bja/aer384. [DOI] [PubMed] [Google Scholar]
- 5.Manzarbeitia CY, Ortiz JA, Jeon H, Rothstein KD, Martinez O, Araya VR, Munoz SJ, Reich DJ. Long-term outcome of controlled, non-heart-beating donor liver transplantation. Transplantation. 2004;78:211–215. doi: 10.1097/01.tp.0000128327.95311.e3. [DOI] [PubMed] [Google Scholar]
- 6.Ormonde DG, de Boer WB, Kierath A, Bell R, Shilkin KB, House AK, Jeffrey GP, Reed WD. Banff schema for grading liver allograft rejection: utility in clinical practice. Liver Transpl Surg. 1999;5:261–268. doi: 10.1002/lt.500050418. [DOI] [PubMed] [Google Scholar]
- 7.McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, Hardin BJ, Shulman HM, Clift RA. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255–267. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 8.Nonomura A, Kono N, Yoshida K, Mizukami Y, Nakanuma Y, Matsubara F. Histological changes of the liver in experimental graft-versus-host disease across minor histocompatibility barriers. V. A light and electron microscopic study of the intralobular changes. Liver. 1991;11:149–157. doi: 10.1111/j.1600-0676.1991.tb00508.x. [DOI] [PubMed] [Google Scholar]
- 9.Demetris AJ, Bellamy C, Hübscher SG, O’Leary J, Randhawa PS, Feng S, Neil D, Colvin RB, McCaughan G, Fung JJ, et al. 2016 Comprehensive Update of the Banff Working Group on Liver Allograft Pathology: Introduction of Antibody-Mediated Rejection. Am J Transplant. 2016 doi: 10.1111/ajt.13909. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Myers RP, Krajden M, Bilodeau M, Kaita K, Marotta P, Peltekian K, Ramji A, Estes C, Razavi H, Sherman M. Burden of disease and cost of chronic hepatitis C infection in Canada. Can J Gastroenterol Hepatol. 2014;28:243–250. doi: 10.1155/2014/317623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrovic LM, Villamil FG, Vierling JM, Makowka L, Geller SA. Comparison of histopathology in acute allograft rejection and recurrent hepatitis C infection after liver transplantation. Liver Transpl Surg. 1997;3:398–406. doi: 10.1002/lt.500030407. [DOI] [PubMed] [Google Scholar]
- 12.Saxena R, Theise ND, Crawford JM. Microanatomy of the human liver-exploring the hidden interfaces. Hepatology. 1999;30:1339–1346. doi: 10.1002/hep.510300607. [DOI] [PubMed] [Google Scholar]
- 13.Lalor PF, Shields P, Grant A, Adams DH. Recruitment of lymphocytes to the human liver. Immunol Cell Biol. 2002;80:52–64. doi: 10.1046/j.1440-1711.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 14.Jaeschke H, Smith CW. Cell adhesion and migration. III. Leukocyte adhesion and transmigration in the liver vasculature. Am J Physiol. 1997;273:G1169–G1173. doi: 10.1152/ajpgi.1997.273.6.G1169. [DOI] [PubMed] [Google Scholar]
- 15.Xu XD, Ueta H, Zhou S, Shi C, Koga D, Ushiki T, Matsuno K. Trafficking of recirculating lymphocytes in the rat liver: rapid transmigration into the portal area and then to the hepatic lymph. Liver Int. 2008;28:319–330. doi: 10.1111/j.1478-3231.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- 16.Nonomura A, Mizukami Y, Matsubara F, Kobayashi K. Clinicopathological study of lymphocyte attachment to endothelial cells (endothelialitis) in various liver diseases. Liver. 1991;11:78–88. doi: 10.1111/j.1600-0676.1991.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 17.Lory J, Zimmermann A. Endotheliitis-like changes in chronic hepatitis C. Histol Histopathol. 1997;12:359–366. [PubMed] [Google Scholar]
- 18.Siddiqui I, Selzner N, Hafezi-Bakhtiari S, Marquez MA, Adeyi OA. Infiltrative (sinusoidal) and hepatitic patterns of injury in acute cellular rejection in liver allograft with clinical implications. Mod Pathol. 2015;28:1275–1281. doi: 10.1038/modpathol.2015.84. [DOI] [PubMed] [Google Scholar]
- 19.Carmack S, Taddei T, Robert ME, Mistry P, Jain D. Increased T-cell sinusoidal lymphocytosis in liver biopsies in patients with chronic hepatitis C and mixed cryoglobulinemia. Am J Gastroenterol. 2008;103:705–711. doi: 10.1111/j.1572-0241.2007.01603.x. [DOI] [PubMed] [Google Scholar]
- 20.Wright TL, Donegan E, Hsu HH, Ferrell L, Lake JR, Kim M, Combs C, Fennessy S, Roberts JP, Ascher NL. Recurrent and acquired hepatitis C viral infection in liver transplant recipients. Gastroenterology. 1992;103:317–322. doi: 10.1016/0016-5085(92)91129-r. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Retortillo M, Forns X, Feliu A, Moitinho E, Costa J, Navasa M, Rimola A, Rodes J. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology. 2002;35:680–687. doi: 10.1053/jhep.2002.31773. [DOI] [PubMed] [Google Scholar]
- 22.Berenguer M, López-Labrador FX, Wright TL. Hepatitis C and liver transplantation. J Hepatol. 2001;35:666–678. doi: 10.1016/s0168-8278(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 23.Gane E. The natural history and outcome of liver transplantation in hepatitis C virus-infected recipients. Liver Transpl. 2003;9:S28–S34. doi: 10.1053/jlts.2003.50248. [DOI] [PubMed] [Google Scholar]


