Summary
The Aux/IAA proteins are auxin-sensitive repressors that mediate diverse physiological and developmental processes in plants [1, 2]. There are 29 Aux/IAA genes in Arabidopsis that exhibit unique but partially overlapping patterns of expression [3] (Figure S1A). Although some studies have suggested that individual Aux/IAA genes have specialized function, genetic analyses of the family have been limited by the scarcity of loss-of-function phenotypes [4]. Further, with a few exceptions, our knowledge of the factors that regulate Aux/IAA expression is limited [1, 5]. We hypothesize that transcriptional control of Aux/IAA genes plays a central role in the establishment of the auxin-signaling pathways that regulate organogenesis, growth, and environmental response. Here we describe a screen for transcription factors (TFs) that regulate the Aux/IAA genes. We identify TFs from 38 families including 26 members of the DREB/CBF family. Several DREB/CBF TFs directly promote transcription of the IAA5 and IAA19 genes in response to abiotic stress. Recessive mutations in these IAA genes result in decreased tolerance to stress conditions demonstrating a role for auxin in abiotic stress. Our results demonstrate that stress pathways interact with the auxin gene regulatory network (GRN) through transcription of the Aux/IAA genes. We propose that the Aux/IAA genes function as hubs that integrate genetic and environmental information to achieve the appropriate developmental or physiological outcome.
Keywords: auxin, Aux/IAA, plant hormone, abiotic stress, repressor
eToc
The Aux/IAA repressors are key regulators of auxin response in plants but the factors that regulate their synthesis are largely unknown. Shani et al. identify transcription factors that regulate these genes. The DREB proteins directly regulate expression of IAA5 and IAA19. Further they show that these Aux/IAAs are required for stress tolerance.
Results
A screen for regulators of the Aux/IAA genes identifies diverse transcription factors
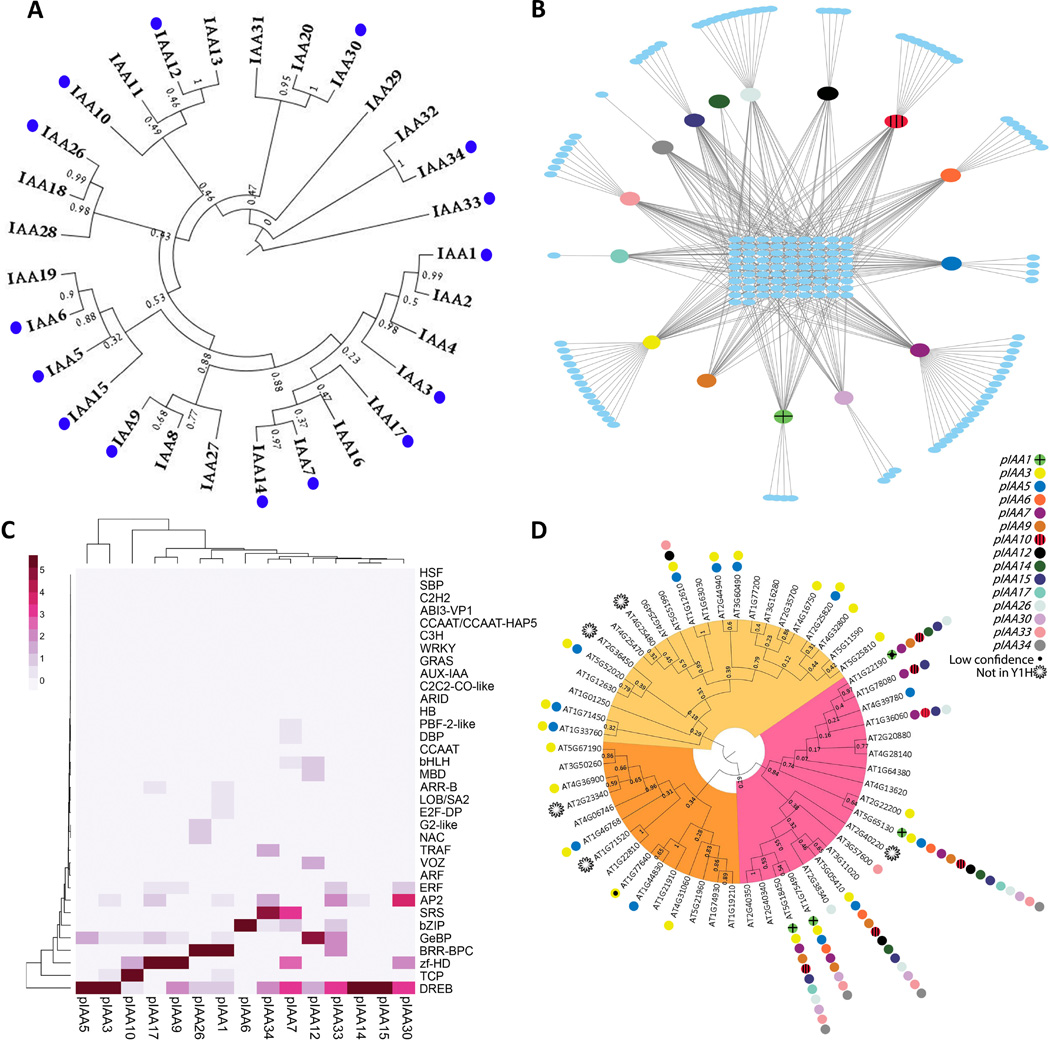
Auxin regulates diverse aspects of growth and development in response to both genetic and environmental inputs [1, 2, 6]. Since the Aux/IAA genes have a central role in auxin response, we reasoned that transcriptional regulation of these genes might act to integrate environmental inputs into the auxin GRN. To identify novel factors that function in the auxin GRN we implemented a yeast-1-hybrid (Y1H) approach. We cloned the first ~500 bp of 15 different Aux/IAA promoters (Table S1) with at least one promoter from each of the major subclades (Figure 1A) and screened them against 1,956 Arabidopsis TFs [7] (see Supplemental Experimental Procedures). The screen identified 433 different interactions, with an average reproducibility of 41% between two screens (Figure 1B, dataset S1) in line with previous genome-scale yeast screens [8–10]. The 433 interactions are a sum of 173 different TFs that fall into 38 TF families. 83 TFs bound to several pIAAs while 90 TFs interacted with a single pIAA (Figure 1B, Table S2). The ARF proteins, which are expected to bind some of IAA promoters directly [11], were not recovered, possibly because full length ARFs are not expressed well in yeast [7]. Most IAA promoters displayed specificity for particular TF families (Figure 1C, Table S2). For example, 13 TCP proteins bound to pIAA10, consistent with earlier in vivo data [12].
Figure 1. Y1H screen for regulators of Aux/IAA genes.
(A) Phylogenetic tree of Arabidopsis Aux/IAA proteins. Blue dots mark Aux/IAA promoters cloned for the Y1H screen. (B) IAA promoter - TF interaction network. The IAA promoters are indicated by colored coded ovals; interacting TFs are blue ovals. 83 TFs interacted with several pIAAs while 90 TFs interacted with a single pIAA. (C) Heatmap showing enrichment of TF families for each promoter fragment. The enrichment score for each TF family on the promoter fragments was calculated by Fisher’s exact test. The −log10 p-value of this enrichment score is shown above with darker colors indicating a greater enrichment score. (D) Phylogenetic tree of the Arabidopsis DREB/CBF proteins. Color-coding sub-families A1 and A4 (yellow); sub-families A2, A3 and A6 (pink); sub-family A5 (orange). Interactions between DREB/CBF proteins and IAA promoters (color-coded circles) are plotted next to gene number. See also Figure S1, and Tables S1 and S2.
Interestingly, the DREB/CBF family had high enrichment scores across most of the IAA promoters (Figure 1D, Figure S1B). Because a connection between the DREB/CBFs and auxin signaling has not been described, we elected to focus on these TFs. The DREB/CBF gene family consist of 56 members in Arabidopsis, divided into subfamilies A1-6 [13, 14]. DREB/CBF proteins from subfamilies A2, A3 and A6 displayed a robust interaction with most of the pIAAs, while proteins from the subfamilies A1, A4 and A5 clades showed specific interaction to pIAA3 and pIAA5 (Figure 1D).
Stress conditions inhibit auxin response
The DREB/CBF proteins are key factors in stress response [15]. Members of these families regulate the expression of large numbers of genes in response to stresses such as drought, cold, and high salinity, and when overexpressed confer increased drought tolerance in Arabidopsis and other species [16–18]. Although some DREB/CBF target genes are known to function in stress tolerance, our knowledge of the greater stress regulatory network remains quite limited [19].
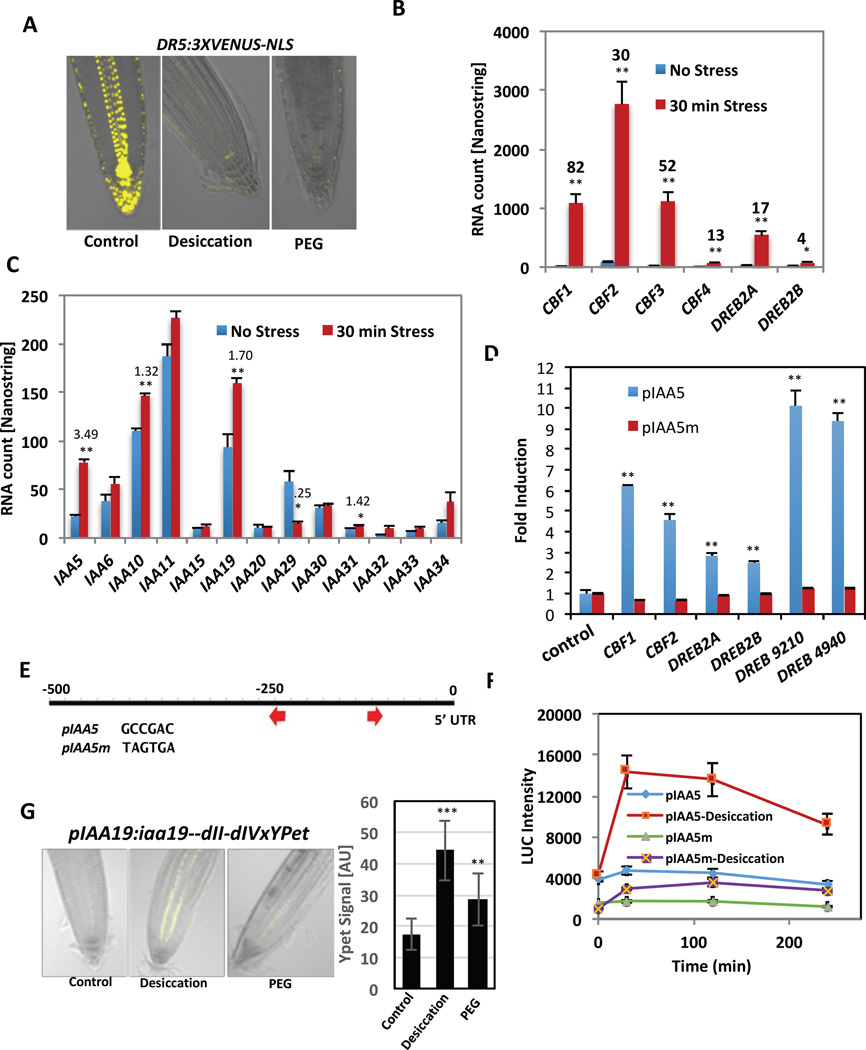
To investigate whether DREB/CBF binding to the pIAAs is physiologically relevant we first asked if abiotic stresses affect auxin response using the DR5:3XVENUS-NLS auxin reporter. The results in Figure 2A and Figure S2A show that desiccation of seedlings or growth on medium containing PEG dramatically suppressed auxin response. In addition, we found that PEG strongly inhibited growth of the hypocotyl in response to auxin (Figure S2A). To explore this effect further we used Nanostring technology to test the transcriptional response of 57 auxin-related genes to a 30 min desiccation stress. The results show that desiccation strongly induced the 6 DREB/CBF genes that showed strong binding to pIAA5 (Fig 2B). Expression of the auxin co-receptors TIR1, AFB2 and AFB4 was not affected while expression of AFB3 and AFB1 was reduced (Figure S3B). Several auxin biosynthetic genes were also affected (Figure S2C). Expression of many of Aux/IAA genes was affected by the treatment (Figure 2C and S2D).
Fig 2. The DRE element is required for IAA5 and IAA19 expression.
(A) DR5:3XVENUS-NLS seedlings after 30 min of desiccation. (B–C) Expression level in response to a 30 min desiccation period. n = 3 biological replicates. (B) Selected DREBs. The numbers above the bars represent fold change; (C) Selected IAAs. (D) Y1H interaction assay for selected DREB/CBF proteins with pIAA5 and pIAA5m. Bar graph is the mean of 4 biological replicates. (E) Representation of the IAA5 promoter (first 500 bp). Arrows indicate the position of DRE/CRT motifs. In pIAA5m the two DRE/CRT motifs are mutated as indicated. (F). Expression of the pIAA5:LUC and pIAA5m:LUC reporters in response to desiccation. Shown are averages (+/− SE) for three independent pIAA5 and pIAA5m lines (n = 56 seedlings). Results are representative of at least three independent experiments. (G) Roots of iaa19-dII-dIV-3xYPet seedlings in response to desiccation (1h) and PEG (3h). YPet florescence shown in yellow. The graph at right is the quantification of YPet florescence in Arbitrary Units (AU). For all panels, differences are significant at p<0.05 (*) and p<0.01 (**) Student’s t-test. See also Figures S2, S3, S4, and S5.
Because the largest fold-induction was observed for IAA5 (3.5-fold change) we elected to focus on this gene and its close relatives IAA6 and IAA19. The IAA19 gene was induced 1.7 fold by desiccation (Figure 2C). First, we retested the interaction between pIAA5 and CBF2 and DREB2A by Y1H. We also included the well-characterized CBF1 and DREB2B as well as two additional DREBs, At1G19210 and At2G44940 in this experiment. All six proteins interacted with pIAA5 (Figure 2D). Since IAA19 was not included in the original screen, we tested this promoter against the 6 DREB/CBFs. Similar to pIAA5, each DREB/CBF protein showed strong binding to pIAA19 (Figure S3A).
The DREB/CBF proteins bind the conserved DRE/CRT element [15, 17]. At least one DRE element is present within 500 bps upstream of IAA2, IAA5, IAA6, IAA9, and IAA19 (Figure S3B). To characterize the DRE elements adjacent to IAA5, we mutated both elements and repeated the Y1H assay with selected TFs (Figure 2D and E). The DREB/CBFs displayed dramatically reduced binding to the mutated pIAA5 (pIAA5m) (Figure 2D) compared to the wild-type promoter, indicating that the DRE elements are essential for DREB/CBF binding to pIAA5 in yeast.
To further investigate the activity of the IAA5 promoter, we generated plants that express LUC under control of the IAA5 promoter (pIAA5:LUC) as well as a variant promoter in which both DRE/CRT elements were mutated as described above (pIAA5m:LUC). Plants with the wild-type construct displayed a ~3-fold induction after a 30 min desiccation period (Figure 2F). Interestingly, the pIAA5m:LUC line had a significantly reduced basal level of LUC signal in 3 independent lines, suggesting that the DRE elements are important even in the absence of stress (Figure 2F). Further, the response of the mutant construct to desiccation was dramatically reduced compared to the wild type confirming that the DREs are required for stress induction of IAA5. Finally, plants expressing iaa19-dII-dIV-3xYPet under control of the IAA19 promoter (pIAA19:iaa19--dII-dIVxYPet) [20] showed a significant increase in YPet florescence in the stele following a 1 hour desiccation treatment (Figure 2G) [20].
The IAA19 gene (also known as MASSUGU2) has been previously characterized [21]. To learn more about the IAA5 gene, we generated pIAA5:GUS, pIAA5:IAA5-GUS, and pIAA5:IAA5dII-GUS lines. The dII mutation is an amino acid substitution in the degron sequence that is expected to stabilize the protein. The results in Figure S4 show that IAA5 transcription is induced by auxin and that the IAA5dII protein accumulates to a greater extent than the wild-type protein. In addition, expression of the mutant protein confers a phenotype that is very characteristic of a gain-of-function aux/iaa mutant[22]. Thus, IAA5 is a typical member of the Aux/IAA family.
CBF1 and DREB2A directly regulate IAA5 and IAA19 expression
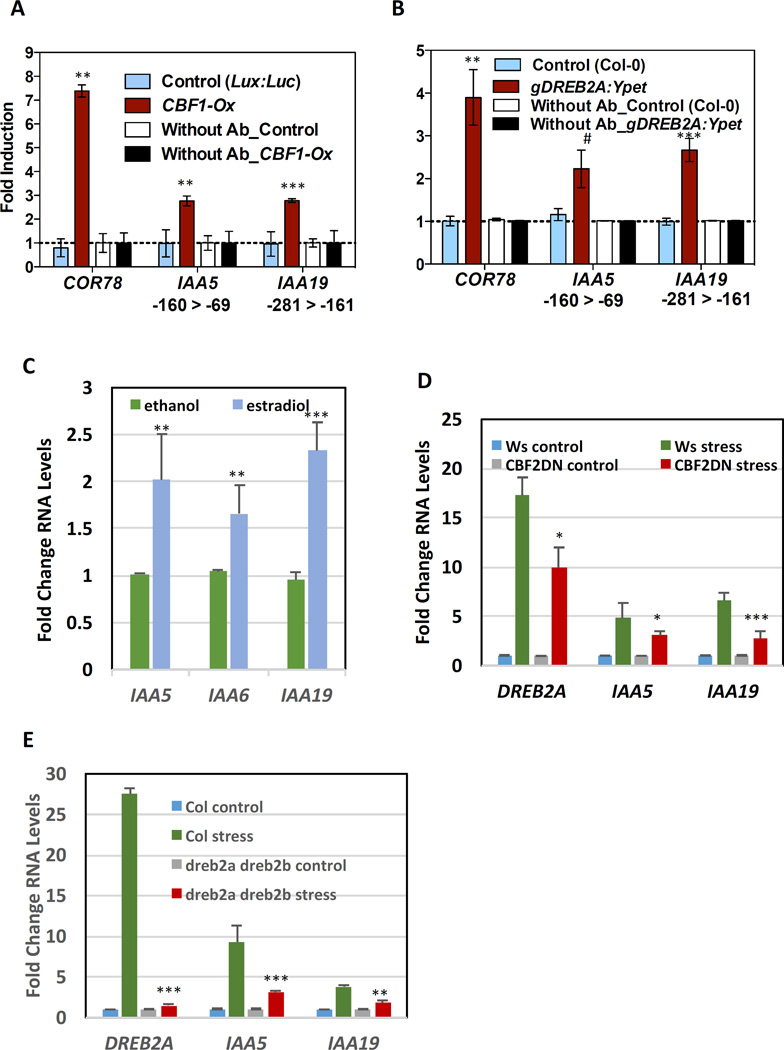
At this point we elected to focus on regulation of the Aux/IAA genes by CBF1 and DREB2A. To determine if these two TFs bind the Aux/IAA promoters in vivo, we performed chromatin immunoprecipitation (ChIP) experiments with 35S:CBF1-YFP and gDREB2A:DREB2A-Ypet-His-FLAG lines [23]. For CBF1 ChIP, chromatin was isolated from light grown whole seedlings while for DREB2A ChIP, chromatin was isolated from roots dissected from light-grown whole seedlings that had been subjected to a 1 h desiccation treatment to increase DREB2A protein. The IAA5 promoter has two DRE motifs in the −160 to – 69 bp interval while pIAA19 has a single DRE element within the −281 to −161 bp fragment. Both of these sequences were enriched after ChIP with either CBF1-YFP or DREB2A-Ypet (Figure 3A, B). We used a COR78 (also known as RD29) promoter fragment containing a DRE element as a positive control, which showed enrichment in both ChIPs, consistent with a previous report [23]. Taken together the data from Y1H and ChIP experiments confirm that DREBs/CBFs bind to DRE elements in the IAA5 and IAA19 promoters in yeast and in plants.
Fig 3. DREB/CBFs regulate transcription of IAA5, IAA6, and IAA19.
(A–B) CBF1 and DREB2A bind to the promoter of COR78, IAA5 and IAA19. (A) CBF1 binds to the promoter of COR78, IAA5 and IAA19 in 10-day-old light-grown whole seedlings. (B) DREB2A binds to the promoter of COR78, IAA5 and IAA19 in the roots of 10-day-old light-grown seedlings that were desiccated for 1h. Sonicated chromatin was immunoprecipitated either with or without anti-GFP antibody. Fold induction was normalized to no antibody and wild-type controls. Data represents mean with standard error from two independent biological replicates with two technical replicates for CBF1 and three independent biological replicates for DREB2A. Differences are significant at p<0.01(**), p<0.005 (***), and p<0.08 (#). (C, D, E) CBF1 and DREB2A directly regulate IAA5 and IAA19 transcription. (C) Estradiol regulated DREB2A overexpression (Est.≫DREB2A) induces IAA5, IAA6 and IAA19 expression. Data represent means with standard error from three independent biological replicates. Significant at p<0.005 (***) and p<0.05 (**). (D) Expression of DREB2A, IAA5 and IAA19 in CBF2DN line after 1 h desiccation. Differences are significant at p<0.05 (*) and p<0.005 (***). (E) IAA5 and IAA19 levels in dreb2a-dreb2b double mutant after 1 h desiccation. Differences are significant at p<0.005 (***) and p<0.01 (**) Student’s t-test.
Next, we asked if the DREBs regulate IAA5 and IAA19 in accordance with the ChIP data. We generated a β-Estradiol inducible DREB2A line in which the G1090 promoter in the pER-GW promoter was fused to the DREB2A CDS and treated seedlings with β-Estradiol for 4 h (Figure 3C). This treatment resulted in a substantial increase in IAA5 and IAA19 RNA levels confirming that these genes are targets of DREB2A. Interestingly we also found that IAA6 was slightly induced by DREB2A overexpression suggesting that this gene may also be a DREB/CBF target. To further examine the transcriptional regulation of IAA5 and IAA19 by DREBs we utilized the CBF2-DN line that overexpresses a C-terminal truncated and inactive version of CBF2 [24]. This truncated protein acts to repress DREB-mediated transcription. We found that basal expression of DREB2A, IAA5, and IAA19 was similar in the CBF2-DN and control lines. However, as reported previously, stress induction of DREB2A was compromised in the CBF2-DN line (Figure 3D) [24]. Similarly, we found that induction of both IAA5 and IAA19 was reduced in the transgenic lines. Finally, induction of IAA19-YPet in pIAA19:IAA19-3xYPet after a 70 m desiccation period was reduced in CBF2-DN (Figure S5).
As a cbf1 dreb2a mutant was not available, we used a dreb2a dreb2b double mutant to further examine the role of these proteins in stress-induction of the Aux/IAA genes. qPCR analysis showed that accumulation of IAA5 and IAA9 transcript was reduced after desiccation in the dreb2a dreb2b double mutant (Figure 3E). These results confirm that the DREB/CBF proteins are required for stress-induced expression of these two Aux/IAA genes. The effects of both the CBF2-DN transgene and dreb2a dreb2b double mutant on IAA6 levels were minimal and variable and therefore not included here.
The IAA5 and IAA19 genes are required for stress tolerance
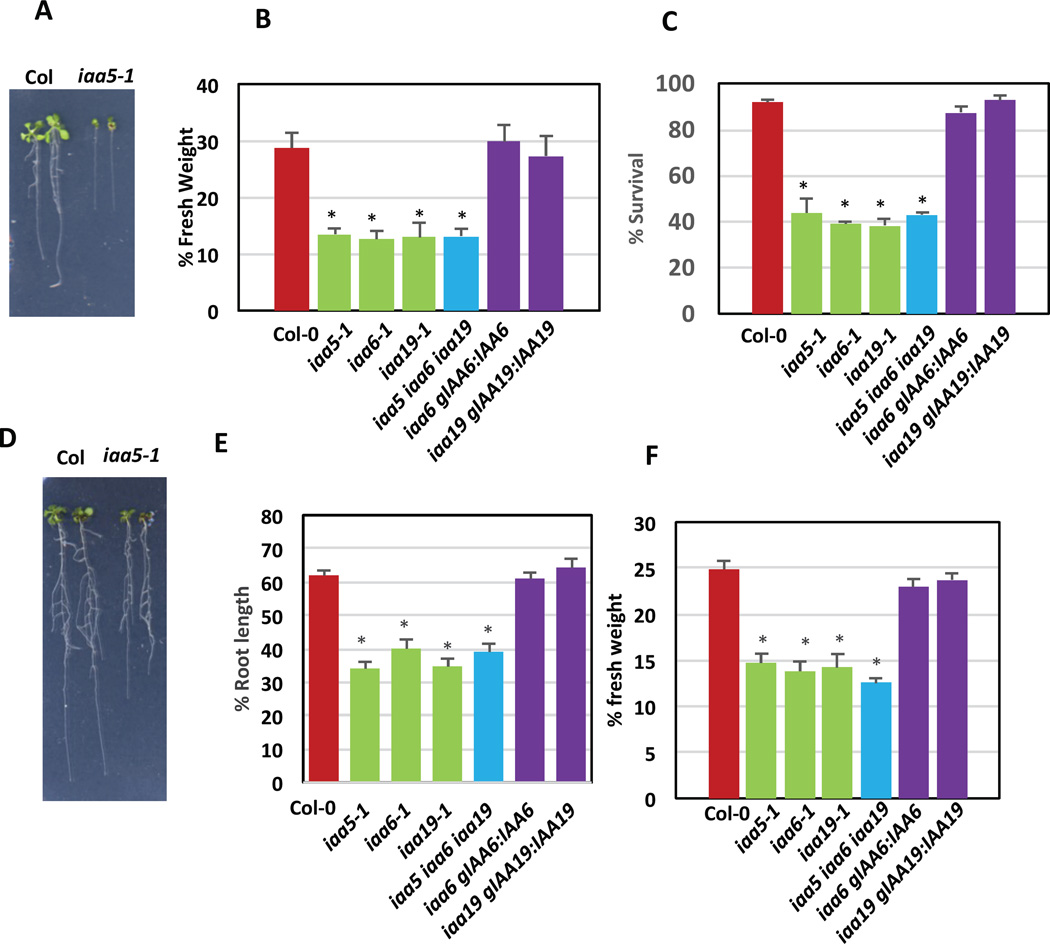
Because the DREB/CBF and auxin GRNs are directly linked through IAA5 and IAA19 we wondered if these genes might contribute to stress response. We also examined the role of the closely related IAA6 gene. The recessive iaa5-1, iaa6-1, and iaa19-1 mutants do not have an obvious phenotype when grown under optimal conditions [4]. However, when we applied a 75-min desiccation treatment to 7-day-old seedlings, followed by rescue on MS plates, we found that all three single mutants, as well as the iaa5 iaa6 iaa19 triple mutant, were hypersensitive compared to wild type with a pronounced effect on survival and subsequent growth (Figure 4A, B, and C). To determine if the genes were also required for tolerance to other stresses, we grew the mutants on medium supplemented with polyethylene glycol-8000 (PEG-8000, Fisher Scientific) (−1.0 Mpa) for 9 days. All four lines displayed PEG hypersensitivity with a 45 to 50% reduction in primary root length after transfer to PEG (Figure 4D, E), and ~ 50% reduction in fresh weight compared to unstressed plants (Figure 4F). To confirm that these defects are due to mutations in the IAA genes, we introduced the wild-type sequence into the mutant line under control of the native promoter. Both IAA6 and IAA19 restored the wild-type level of stress tolerance confirming that the mutations are causative.
Fig 4. The IAA5, IAA6, and IAA19 genes are required for stress tolerance.
(A–C) Response of wild-type and mutant seedlings to desiccation. 7 days-old seedlings were placed on parafilm for 75 min and transferred to ½ MS plates for 10 days. (A) Representative seventeen-day-old Col-0 and iaa5-1 seedlings after recovery from desiccation stress. (B) Fresh weight after desiccation stress relative to respective control plants. (C) Survival rate after desiccation. Data represents mean +/− standard error from one of the three independent biological replicates. n=12–15 seedlings for each genotype. Differences are significant at p<0.001 (*) Student’s t-test for all mutants compared to Col-0. (D–F) Response of wild-type and mutant seedlings to growth on medium containing PEG-8000 (−1.0 Mpa). Seedlings were grown on ½ MS for 5 days and transferred to PEG-infused plates for a further 10 days (D) Representative fifteen-day-old Col-0 and iaa5-1 seedlings after PEG stress. (E) Primary root growth of seedlings after PEG stress compared to the respective control plants. (F) Fresh weight of seedlings after PEG stress relative to controls. Data represents mean +/− standard error from one of the three independent biological replicates. n=12–15 seedlings for each genotype. Differences are significant at p<0.001 (*) Student’s t-test for all mutant genotypes compared to Col-0. See also Figure S6.
Discussion
Genetic studies of the Aux/IAA genes have long been hampered by the lack of loss-of-function phenotypes [4]. There have been a few reports describing the effects of recessive aux/iaa mutations on growth and development [5, 25–29], but in general investigators have relied on gain-of-function stabilizing mutations to discern the function of individual members of the family. Our discovery that recessive mutations in IAA5, IAA6, and IAA19 confer a stress phenotype should encourage the careful examination of loss-of-function mutations in other IAA genes. The identification of novel conditional phenotypes will increase our knowledge of the function of these genes.
The Aux/IAA proteins are known to function as transcriptional repressors. This implies that stress tolerance requires repression of a subset of auxin-regulated genes. The identity of these genes is presently unknown. One possibility is suggested by studies which show that stress tolerance is associated with growth inhibition [30, 31]. Gibberellic acid has been implicated in this inhibition but it is possible that auxin is also involved[32]. If stress-induced growth inhibition involves repression of auxin-responsive genes, loss of the IAAs may reduce this response. Since the ultimate consequence of reduced tolerance is decreased growth, the initial lack of growth inhibition may not be apparent. Alternatively, it is possible that Aux/IAA target genes that are not directly associated with growth are involved in stress tolerance, since many auxin responsive genes are not obviously related to growth [33]. In fact, a recent study in the moss Physcomitrella patens demonstrates that over a third of the genes in genome are regulated by the Aux/IAA proteins [34].
It is interesting that the phenotype of the triple mutant is similar to that of each of the single mutants. This may be because the three genes have distinct patterns of expression in the root. Indeed, examination of publically available expression data shows that the three genes exhibit distinct expression patterns in the root (http://bar.utoronto.ca) [35](Figure S6). IAA5 is expressed primarily in the epidermis. Both IAA6 and IAA19 are expressed in the stele, but the IAA6 expression zone extends further upwards towards the base of the root. Alternatively, it has been suggested that Aux/IAAs function in higher order complexes that could include many Aux/IAA proteins [36, 37]. It is possible that several different Aux/IAA proteins must be present in these complexes for activity. It is also interesting to note that some of the Aux/IAA genes are expressed at a higher level in whole seedlings than IAA5, IAA6, and IAA19 (Fig. S2D). However, at cellular resolution the situation can be quite different. For example, IAA7 is expressed at a much higher level than IAA6 or IAA19 in seedlings, but at a lower level than in the stele [35].
It is clear that plant responses to abiotic stress are complex and involve multiple signaling pathways that regulate many aspects of growth and physiology. Here we show that an auxin GRN is directly integrated into the DREB/CBF stress pathway through regulation of Aux/IAA genes. Although we have focused on IAA5, IAA6, and IAA19, it is possible that other members of the family are also regulated by DREB/CBF proteins. Indeed, in our analysis 11 Aux/IAAs are affected by desiccation treatment in addition to IAA5 and IAA19. Further, mining of transcriptome data reveals that other Aux/IAA genes respond to abiotic stress (For examples see Fig S1). In the future, it will be vital to determine if these genes also contribute to stress tolerance and to identify the Aux/IAA targets that contribute to this stress response.
Supplementary Material
Highlights.
The Aux/IAA genes of Arabidopsis are regulated by diverse TFs.
The Aux/IAAs function as hubs that integrate signals from diverse pathways.
The CBF1 and DREB2A TFs directly regulate two Aux/IAA genes, IAA5 and IAA19.
The IAA5, IAA6, and IAA19 are required for stress tolerance.
Acknowledgments
MS would like to thank Estelle lab members for helpful discussions and Paul Chen for assistance. Thanks to Jose Alonso and Anna Stepanova for sharing the IAA19:iaa19-dII-dIV-3xYPet line. This work was supported by a grant by the NIH (GM43644 to M E., R01GM067837 and R01GM56006 to S.A.K.), the NSF (MCB1024999 to J.R.E.), the Gordon and Betty Moore Foundation (GBMF3038 to ME., GBMF3034 to J.R.E.), the Vaadia–BARD Postdoctoral Fellowship (FI-431-10 to ES) and the Israel Science Foundation (1832/14 and 2158/14 to ES). M.E. and J.R.E. are investigators of the Howard Hughes Medical Institute..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
E.S., S.M., S.E.S., C.D., M.E. designed the experiments
E.S., S.M., Y.Z., S.E.S., C.D., I.T., O.P. conducted the experiments
E.S., S.M., S.E.S., C.D., M.E. analyzed and interpreted the data
E.S., S.M., L.S., J.R.E., M.E., drafted or revised the article
C.C.M, R.W., L.S., J.R.E., S.A.K., J.P-P., contributed unpublished essential reagents
References
- 1.Salehin M, Bagchi R, Estelle M. SCFTIR1/AFB-Based Auxin Perception: Mechanism and Role in Plant Growth and Development. The Plant cell. 2015;27:9–19. doi: 10.1105/tpc.114.133744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weijers D, Wagner D. Transcriptional Responses to the Auxin Hormone. Annual review of plant biology. 2016;67:539. doi: 10.1146/annurev-arplant-043015-112122. [DOI] [PubMed] [Google Scholar]
- 3.Bargmann BO, Vanneste S, Krouk G, Nawy T, Efroni I, Shani E, Choe G, Friml J, Bergmann DC, Estelle M, et al. A map of cell type-specific auxin responses. Mol Syst Biol. 2013;9:688. doi: 10.1038/msb.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Overvoorde PJ, Okushima Y, Alonso JM, Chan A, Chang C, Ecker JR, Hughes B, Liu A, Onodera C, Quach H, et al. Functional genomic analysis of the AUXIN/INDOLE-3-ACETIC ACID gene family members in Arabidopsis thaliana. The Plant cell. 2005;17:3282–3300. doi: 10.1105/tpc.105.036723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dello Ioio R, Nakamura K, Moubayidin L, Perilli S, Taniguchi M, Morita MT, Aoyama T, Costantino P, Sabatini S. A genetic framework for the control of cell division and differentiation in the root meristem. Science. 2008;322:1380–1384. doi: 10.1126/science.1164147. [DOI] [PubMed] [Google Scholar]
- 6.Salehin M, Estelle M. Ethylene prunes translation. Cell. 2015;163:543–544. doi: 10.1016/j.cell.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 7.Pruneda-Paz JL, Breton G, Nagel DH, Kang SE, Bonaldi K, Doherty CJ, Ravelo S, Galli M, Ecker JR, Kay SA. A genome-scale resource for the functional characterization of Arabidopsis transcription factors. Cell reports. 2014;8:622–632. doi: 10.1016/j.celrep.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaudinier A, Zhang L, Reece-Hoyes JS, Taylor-Teeples M, Pu L, Liu Z, Breton G, Pruneda-Paz JL, Kim D, Kay SA, et al. Enhanced Y1H assays for Arabidopsis. Nat Methods. 2011;8:1053–1055. doi: 10.1038/nmeth.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vermeirssen V, Deplancke B, Barrasa MI, Reece-Hoyes JS, Arda HE, Grove CA, Martinez NJ, Sequerra R, Doucette-Stamm L, Brent MR, et al. Matrix and Steiner-triple-system smart pooling assays for high-performance transcription regulatory network mapping. Nat Methods. 2007;4:659–664. doi: 10.1038/nmeth1063. [DOI] [PubMed] [Google Scholar]
- 10.Reece-Hoyes JS, Diallo A, Lajoie B, Kent A, Shrestha S, Kadreppa S, Pesyna C, Dekker J, Myers CL, Walhout AJ. Enhanced yeast one-hybrid assays for high-throughput gene-centered regulatory network mapping. Nat Methods. 2011;8:1059–1064. doi: 10.1038/nmeth.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau S, De Smet I, Kolb M, Meinhardt H, Jürgens G. Auxin triggers a genetic switch. Nature Cell Biology. 2011;13:611–615. doi: 10.1038/ncb2212. [DOI] [PubMed] [Google Scholar]
- 12.Koyama T, Mitsuda N, Seki M, Shinozaki K, Ohme-Takagi M. TCP transcription factors regulate the activities of ASYMMETRIC LEAVES1 and miR164, as well as the auxin response, during differentiation of leaves in Arabidopsis. The Plant cell. 2010;22:3574–3588. doi: 10.1105/tpc.110.075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakano T, Suzuki K, Fujimura T, Shinshi H. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant physiology. 2006;140:411–432. doi: 10.1104/pp.105.073783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration-and cold-inducible gene expression. Biochemical and biophysical research communications. 2002;290:998–1009. doi: 10.1006/bbrc.2001.6299. [DOI] [PubMed] [Google Scholar]
- 15.Shinozaki K, Yamaguchi-Shinozaki K, Seki M. Regulatory network of gene expression in the drought and cold stress responses. Current opinion in plant biology. 2003;6:410–417. doi: 10.1016/s1369-5266(03)00092-x. [DOI] [PubMed] [Google Scholar]
- 16.Mallikarjuna G, Mallikarjuna K, Reddy MK, Kaul T. Expression of OsDREB2A transcription factor confers enhanced dehydration and salt stress tolerance in rice (Oryza sativa L.) Biotechnol Lett. 2011;33:1689–1697. doi: 10.1007/s10529-011-0620-x. [DOI] [PubMed] [Google Scholar]
- 17.Sakuma Y, Maruyama K, Osakabe Y, Qin F, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. The Plant cell. 2006;18:1292–1309. doi: 10.1105/tpc.105.035881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Wang H, Shao H, Tang X. Recent Advances in Utilizing Transcription Factors to Improve Plant Abiotic Stress Tolerance by Transgenic Technology. Front Plant Sci. 2016;7:67. doi: 10.3389/fpls.2016.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida T, Mogami J, Yamaguchi-Shinozaki K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Current opinion in plant biology. 2014;21:133–139. doi: 10.1016/j.pbi.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Zhou R, Benavente LM, Stepanova AN, Alonso JM. A recombineering-based gene tagging system for Arabidopsis. The Plant journal : for cell and molecular biology. 2011;66:712–723. doi: 10.1111/j.1365-313X.2011.04524.x. [DOI] [PubMed] [Google Scholar]
- 21.Tatematsu K, Kumagai S, Muto H, Sato A, Watahiki MK, Harper RM, Liscum E, Yamamoto KT. MASSUGU2 encodes Aux/IAA19, an auxin-regulated protein that functions together with the transcriptional activator NPH4/ARF7 to regulate differential growth responses of hypocotyl and formation of lateral roots in Arabidopsis thaliana. The Plant cell. 2004;16:379–393. doi: 10.1105/tpc.018630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liscum E, Reed JW. Genetics of Aux/IAA and ARF action in plant growth and development. Plant molecular biology. 2002;49:387–400. [PubMed] [Google Scholar]
- 23.Chow BY, Sanchez SE, Breton G, Pruneda-Paz JL, Krogan NT, Kay SA. Transcriptional regulation of LUX by CBF1 mediates cold input to the circadian clock in Arabidopsis. Curr Biol. 2014;24:1518–1524. doi: 10.1016/j.cub.2014.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park S, Lee CM, Doherty CJ, Gilmour SJ, Kim Y, Thomashow MF. Regulation of the Arabidopsis CBF regulon by a complex low-temperature regulatory network. The Plant journal : for cell and molecular biology. 2015;82:193–207. doi: 10.1111/tpj.12796. [DOI] [PubMed] [Google Scholar]
- 25.Koenig D, Bayer E, Kang J, Kuhlemeier C, Sinha N. Auxin patterns Solanum lycopersicum leaf morphogenesis. Development. 2009;136:2997–3006. doi: 10.1242/dev.033811. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Gera H, Shwartz I, Shao MR, Shani E, Estelle M, Ori N. ENTIRE and GOBLET promote leaflet development in tomato by modulating auxin response. The Plant journal : for cell and molecular biology. 2012;70:903–915. doi: 10.1111/j.1365-313X.2012.04939.x. [DOI] [PubMed] [Google Scholar]
- 27.Tian Q, Reed JW. Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development. 1999;126:711–721. doi: 10.1242/dev.126.4.711. [DOI] [PubMed] [Google Scholar]
- 28.Swarup K, Benkova E, Swarup R, Casimiro I, Peret B, Yang Y, Parry G, Nielsen E, De Smet I, Vanneste S, et al. The auxin influx carrier LAX3 promotes lateral root emergence. Nat Cell Biol. 2008;10:946–954. doi: 10.1038/ncb1754. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Jones B, Li Z, Frasse P, Delalande C, Regad F, Chaabouni S, Latche A, Pech JC, Bouzayen M. The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. The Plant cell. 2005;17:2676–2692. doi: 10.1105/tpc.105.033415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naser V, Shani E. Auxin response under osmotic stress. Plant molecular biology. 2016:1–12. doi: 10.1007/s11103-016-0476-5. [DOI] [PubMed] [Google Scholar]
- 31.Skirycz A, Inze D. More from less: plant growth under limited water. Curr Opin Biotechnol. 2010;21:197–203. doi: 10.1016/j.copbio.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Achard P, Gong F, Cheminant S, Alioua M, Hedden P, Genschik P. The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. The Plant cell. 2008;20:2117–2129. doi: 10.1105/tpc.108.058941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chapman EJ, Greenham K, Castillejo C, Sartor R, Bialy A, Sun TP, Estelle M. Hypocotyl transcriptome reveals auxin regulation of growth-promoting genes through GA-dependent and -independent pathways. PloS one. 2012;7:e36210. doi: 10.1371/journal.pone.0036210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavy M, Prigge MJ, Tao S, Shain S, Kuo A, Kirchsteiger K, Estelle M. Constitutive auxin response in Physcomitrella reveals complex interactions between Aux/IAA and ARF proteins. Elife. 2016;5 doi: 10.7554/eLife.13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brady SM, Orlando DA, Lee JY, Wang JY, Koch J, Dinneny JR, Mace D, Ohler U, Benfey PN. A high-resolution root spatiotemporal map reveals dominant expression patterns. Science. 2007;318:801–806. doi: 10.1126/science.1146265. [DOI] [PubMed] [Google Scholar]
- 36.Korasick DA, Jez JM, Strader LC. Refining the nuclear auxin response pathway through structural biology. Current opinion in plant biology. 2015;27:22–28. doi: 10.1016/j.pbi.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korasick DA, Westfall CS, Lee SG, Nanao MH, Dumas R, Hagen G, Guilfoyle TJ, Jez JM, Strader LC. Molecular basis for AUXIN RESPONSE FACTOR protein interaction and the control of auxin response repression. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:5427–5432. doi: 10.1073/pnas.1400074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.