Abstract
Polyphenols are one of the most important secondary metabolites, and affect the decomposition of litter and soil organic matter. This study aims to monitor the mass loss rate of tea leaf litter and nutrient release pattern, and investigate the role of tea polyphenols played in this process. High-performance liquid chromatography (HPLC) and classical litter bag method were used to simulate the decomposition process of tea leaf litter and track the changes occurring in major polyphenols over eight months. The release patterns of nitrogen, potassium, calcium, and magnesium were also determined. The decomposition pattern of tea leaf litter could be described by a two-phase decomposition model, and the polyphenol/N ratio effectively regulated the degradation process. Most of the catechins decreased dramatically within two months; gallic acid (GA), catechin gallate (CG), and gallocatechin (GC) were faintly detected, while others were outside the detection limits by the end of the experiment. These results demonstrated that tea polyphenols transformed quickly and catechins had an effect on the individual conversion rate. The nutrient release pattern was different from other plants which might be due to the existence of tea polyphenols.
Keywords: Tea polyphenol, Catechin, Decomposition, Nutrient release, Polyphenol/N ratio
1. Introduction
In terrestrial ecosystems, apart from fertilization, the decomposition of plant litter is regarded as the main pathway for soil organic matter amendment. Litter decomposition strongly impacts nutrient cycling and the global carbon budget. Many studies have focused on it since the 1960s (Swift et al., 1979; McClaugherty et al., 1985; Aerts, 1997). Climate and litter quality are generally considered as the main factors that regulate the litter decomposition rate (Meentemeyer, 1978; CouˆTeaux and Bottner, 1995; Trofymow et al., 2002). Although there exists an interaction between climate and litter quality, climate dominates the decomposition process on a large scale, while on a regional level, litter quality is more important (Chadwick et al., 1998; Preston et al., 2000; Berg, 2014).
Parameters such as the ratios of carbon/nitrogen (C/N), lignin/N, lignocellulose/N, or the contents of N and phosphorous (P) are generally used to describe litter qualities (Taylor et al., 1989; Palm and Rowland, 1997; Parton et al., 2007). Commonly a litter with a higher C/N or lignin/N ratio decomposes more slowly (Melillo et al., 1982), because low N content limits the growth of soil microbes leading to a fall in the litter decomposition rate. Much of the literature on the effect of litter quality on its decomposition has focused on lignin, one of the most recalcitrant ingredients, because it only can be utilized by certain unique microbes, and it apparently explains the changes of decomposition rate in a majority of plant species, but it is not suitable in all cases. Some scientists have noticed that polyphenols are strongly correlated with litter decomposition under special conditions (Mesquita et al., 1998; Kurokawa and Nakashizuka, 2008). According to Palm and Sanchez (1990), polyphenol-rich plant litters decompose slowly and the release of nitrogen from their tissues is restricted, even if the degrading materials have a high nitrogen concentration, e.g. poplar trees (Findlay et al., 1996). Moreover, the entrance of a polyphenol, as potential carbon resource or toxicant, may regulate soil microbial activity, and then inversely affects litter decomposition rate. Apart from the close correlation with litter decomposition, polyphenols also play a vital role in nutrient cycling especially of nitrogen, because it can bind with proteins, thereby immobilizing nitrogen (Valus and Jones, 1973; Tharayil et al., 2013). Thus the nutrient release pattern during litter degradation is also worth studying.
Tea is widely cultivated in Southeast Asia as a commercial crop, especially in China (Chen and Lin, 2015), and it uniquely contains a lot of phenolic compounds. Tea polyphenols are distributed in almost every part of the plant, but are particularly prevalent in the leaves accounting for approximately 18%–34% of the dry leaf weight (Wan, 2008). In conventional tea plantations, a large number of trimmed branches return to the soil surface, putting a large amount of polyphenols into the soil, and consequently affect a series of soil processes such as the formation of humus, and nitrogen mineralization. Up to now, there has been little information about the decomposition of tea leaf or pruning branches in situ, and the role of tea polyphenols is unclear. Thus a field experiment was designed to determine the decomposition of tea leaves in terms of mass loss rate, changes in polyphenols, and the nutrient release pattern. We hypothesized that (1) tea polyphenols would stimulate the decomposition rate of tea leaves in the short term; (2) the conversion rate of the tea polyphenol monomers would be different due to their differences in chemical structure; (3) simultaneously the nutrient release pattern might be inconsistent with other plant litters/leaves because of the existence of polyphenols.
2. Materials and methods
2.1. Site description
The research was carried out at the experimental station of the Tea Science Department (30°268′ N, 120°199′ E), Zhejiang University, located in the north of Hangzhou City, China, which was established for germplasm protection with conventional management in the late 1980s. This area has a subtropical monsoon climate. The mean annual rainfall in this area is 1490 mm, and the mean annual temperature is 18 °C. The soil pH is 5.0, and the soil (0–20 cm depth) comprises 20.22% clay and 44.34% silt.
2.2. Experimental design
The classical litter bag method (Bocock and Gilbert, 1957; Fujii and Takeda, 2010) was used in this study. We found a plot of 12 m×9 m and divided it into three subplots of 12 m×3 m within the area. On the 22nd January, 2013, mature leaves still attached to the trees were collected equally and randomly from the crowns and immediately transported back to the laboratory. In the laboratory, the tea leaves were air-dried at ambient temperature (approximately 20 °C) and stored until determination.
Approximately 4.00 g of air-dried leaves (W s) were enclosed in 10 cm×20 cm nylon nets (1-mm mesh), all the bags having been weighed (W 1) and labeled previously. In March 2013, after the removal of newly fallen litter, we placed these litter bags on the surface of tea plantation soil immediately beneath the crowns, using metal pins to secure them. Each subsequent month, three replicates from every subplot were sampled until the end of yearly production. From March to November 2013, a total of 27 bags (three replicates×9) were collected and taken back to the laboratory for analysis. We removed soil particles and living plant parts adhering to the bag surface, and the bags were then dried at 40 °C to constant weight. The dry mass of the bags and leaves (W 2) was then determined. The mass loss of each sample was calculated using the following formula: mass remaining (%)=(W 1−W 2)/W s×100%; monthly mass loss was equal to the difference between the two remaining adjacent mass values.
2.3. Chemical analysis
Samples were oven-dried at 70 °C and finely ground through a 20-mesh sieve for analysis. Total polyphenol content was estimated using the Folin-Ciocalteu method (Heimler et al., 2006) and was calculated by comparison with the standard curve obtained with gallic acid. The results were expressed as gallic acid equivalent (GAE) (mg/100 g of dry weight). To determine the changes in the composition of tea polyphenols (specifically catechins) during the decomposition process, high-performance liquid chromatography (HPLC) analysis was adopted with the modified method proposed by Liang et al. (1990). Ground samples (0.20 g) were extracted in a conical flask containing 5 ml 50% (v/v) ethanol in a 70 °C water bath for 10 min, and were stirred in the middle of the process. The extracts were centrifuged at 3500 r/min for 10 min after the flask had cooled to air temperature. The supernatant was transferred to a 10-ml flask and the residues were re-extracted as previously described. The extracts were combined to produce a constant volume, and then filtered through a 0.45-μm filter. The filtrate (10 μl) was subjected to HPLC analysis using a C18 column (Agilent Technologies Inc., Santa Clara, CA, USA). The mobile phase A was purified water (MiniQ, Aquapro International Company LLC, USA)/acetonitrile/acetic acid (96.5/3.0/0.5; v/v/v), and the mobile phase B was purified water/acetonitrile/acetic acid (69.5/30.0/0.5; v/v/v). The gradient program was as follows: the mobile phase B was linearly increased from 20% to 65% during the first 35 min, then instantaneously decreased to 20%, and maintained for 5 min. The samples were eluted at 35 °C at a flow rate of 1 ml/min and monitored at 280 nm. The peaks were identified through a comparison of the retention time for the samples with those of the authentic standards.
Total carbon and nitrogen content were determined by dry combustion using a CN LECO-1000 autoanalyzer (LECO Corporation, St. Joseph, MI, USA). Total water-soluble carbohydrate (WSC) was measured by the modified sulphuric-anthrone acid method (Grandy et al., 2000). To the extracted solution derived from the boiling water, 0.5 ml anthrone ethyl acetate solution and 5.0 ml sulphuric acid (H2SO4) were added. The optical density of the solutions was then measured at 630 nm using a spectrophotometer and compared with known concentrations of D-glucose as standards. The phosphorus (P), potassium (K), aluminum (Al), calcium (Ca), and magnesium (Mg) contents were analyzed using an inductive coupled plasma emission (ICP) spectrometer (iCAP 6000 Series, Thermo Scientific, USA) after microwave digestion (ETHOS One, Milestone, Italy) following pretreatment with acid wet oxidation in a mixed solution of HNO3 (65% (v/v), 6 ml) and H2O2 (30% (v/v), 2 ml). The phosphorus content was determined using the molybdate-ascorbic acid method (Olsen et al., 1982). Acid-dissolved fiber and lignin were determined by the sulfuric acid method using a Foss FibreCap (AOAC, 2000).
2.4. Statistical analyses
One-way analysis of variance (ANOVA) followed by Bonferroni multiple comparisons at P<0.01 was performed to test the variance of mass loss and nutrient mass remaining. The Pearson test was used to detect the statistical correlation between the polyphenol/N ratio and mass loss. All data were analyzed using SPSS statistics software (Version 16.0, SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Initial chemical properties and climatic conditions
To determine the influence of litter quality on the decomposition process, initial litter chemical parameters were investigated. The total polyphenol content was 49.20 mg/g, which accounted for approximately 32.5% of the total mass. The initial epigallocatechin gallate (EGCG) concentration was 19.35 mg/g, making it the most abundant catechin monomer among the tea polyphenols. Holocellulose (hemicellulose and cellulose), lignin, and WSC separately shared approximately 19.7%, 3.8%, and 0.1% of the total dry mass; apparently WSC in tea leaves was relatively lower than other organic components. The initial percentages of the main nutrient elements C, N, K, P, Mg, and Ca were 45.6%, 2.6%, 1.3%, 0.2%, 0.3%, and 1.1% of the total mass, respectively. The initial value of the C/N ratio was 17.62 and its dynamic was showed in Fig. 1. Fig. 2 presents the basic climatic conditions for 2013 including the average monthly temperatures and relative humidity levels. The air temperature was higher in July and August than in other months; the relative humidity was less in April, July, and August than in other months.
Fig. 1.
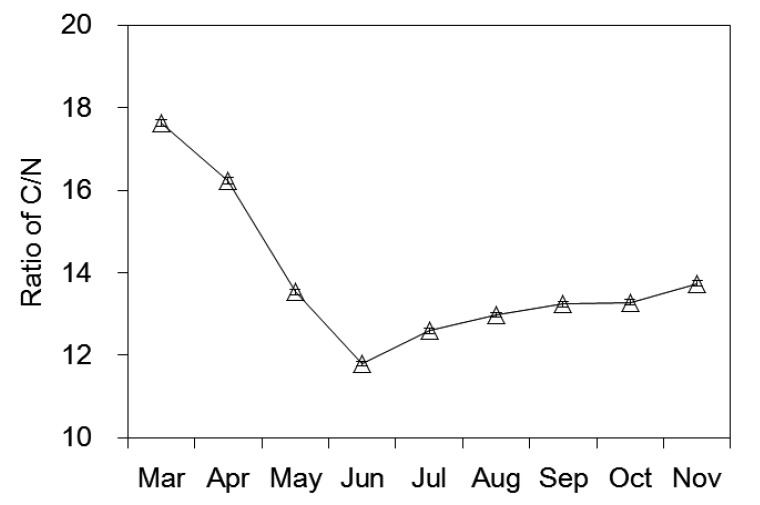
Variation of C/N ratio of tea leaf litter through the experiment
Error bars represent standard errors
Fig. 2.
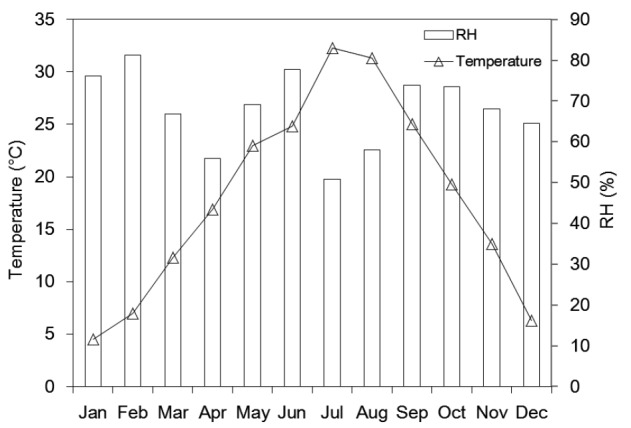
Average monthly temperature and relative humidity in 2013
Recorded by the Hangzhou Bureau of Meteorology
3.2. Mass loss and monthly mass loss
The mass remaining (%) and the monthly mass loss (%) over the experiment were monitored, and they both changed notably (Fig. 3, P<0.01). From the beginning of the study to July, the mass remaining dropped by approximately 45.0%, whereas only 7.2% mass loss occurred from July to November. After experiment, 56.0% of the total mass disappeared, which was relatively rapid compared with the results of a study on the Japanese cypress (Chamaecyparis obtusa) (Fujii and Takeda, 2010). Higher monthly mass loss occurred in July, April, and May (14.6%, 13.4%, and 11.1%, respectively); a lower monthly mass loss was observed in August, September, and October; and negative values were recorded in August and September. At the end of the experiment, all the litter samples had broken down into tiny particles and the leaf structure could barely be recognized, which indicated the comprehensive effects of chemical transformation and physical degradation on the tea leaf litter.
Fig. 3.
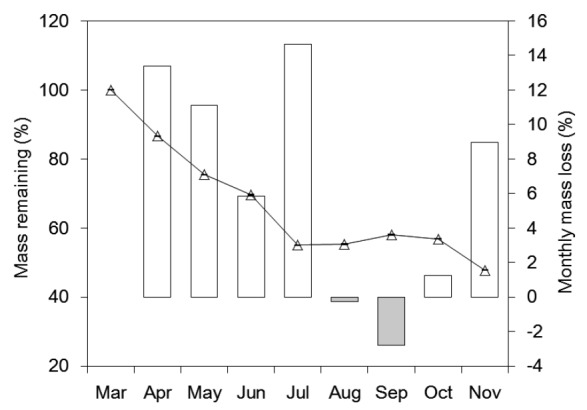
Mass remaining and monthly mass loss during the experimental period
Line with hollow triangles represents the mass remaining (percentage of initial), and error bars indicate standard errors; columns stand for the monthly mass loss, columns in white and light gray represent positive and negative values, respectively
3.3. Variation of organic components
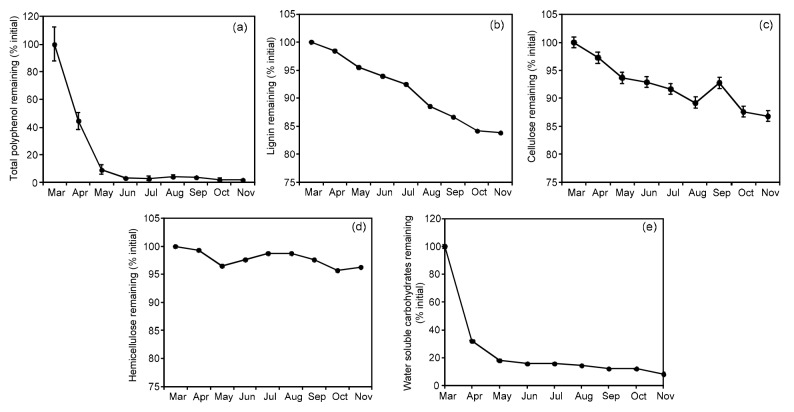
The total polyphenols decreased dramatically in the first two months, and less than 10% remained; the monomer catechins also fell sharply during the same period. From May to November, the total polyphenol content remained at 2.0%–3.0%, and only gallic acid (GA), catechin gallate (CG), and gallocatechin (GC) were detected at very low levels (Fig. 4a, Table 1). The mass remaining of WSC reduced greatly in the first two months from 100.0% to 18.2% (Fig. 4e). Hemicellulose barely changed during the experiment; however, cellulose and lignin decreased steadily, and were eventually reduced to 13.2% and 16.2%, respectively (Figs. 4b–4d).
Fig. 4.
Mass remaining of chemical components in tea leaf litters through the experimental period
(a) Polyphenol; (b) Lignin; (c) Cellulose; (d) Hemicellulose; (e) Water soluble carbohydrates. Error bars indicate standard errors
Table 1.
Mean concentration of catechins determined by HPLC at different sampling time
| Sampling time | Catechins (mg/g) |
||||||||
| GA | GC | EGC | C | EC | EGCG | GCG | ECG | CG | |
| Mar. | 0.50±0.00a | 3.75±0.01a | 12.34±0.01a | 0.54±0.05a | 4.85±0.03a | 19.35±0.01a | 0.56±0.01a | 0.92±0.10a | 0.74±0.02a |
| Apr. | 0.07±0.01b | 2.57±0.01b | 0.67±0.01b | 0.01±0.00b | 0.04±0.00b | 0.03±0.00b | 0.15±0.00b | 0.46±0.05b | 0.58±0.01b |
| May | 0.05±0.01c | 0.51±0.01d | 0 | 0 | 0 | 0 | 0.09±0.00c | 0.01±0.00c | 0.09±0.03d |
| Jun. | 0.03±0.00d | 0.04±0.01f | 0 | 0 | 0 | 0 | 0.02±0.00d | 0.01±0.00c | 0.03±0.04d |
| Jul. | 0.03±0.01cd | 0.15±0.01e | 0 | 0 | 0 | 0 | 0 | 0 | 0.04±0.00d |
| Aug. | 0.03±0.01d | 0.70±0.01c | 0 | 0 | 0 | 0 | 0 | 0 | 0.26±0.01c |
| Sep. | 0.03±0.02d | 0.67±0.01c | 0 | 0 | 0 | 0 | 0 | 0 | 0.25±0.12c |
| Oct. | 0.03±0.01d | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nov. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
“0” means “not found” or values less than 0.01. Data are expressed as mean±SD (n=3). Values with lowercases were significantly different (P˂0.01). GA: gallic acid; GC: gallocatechin; EGC: epigallocatechin; C: catechin; EC: epicatechin; EGCG: epigallocatechin gallate; GCG: gallocatechin gallate; ECG: epicatechin gallate; CG: catechin gallate
3.4. Nutrient release
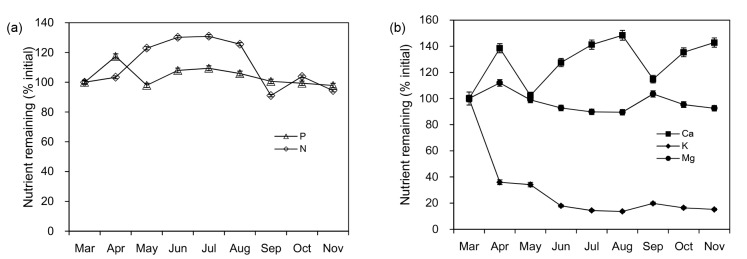
Potassium was released most rapidly from 100% in March to 17.9% in June, and remained constant thereafter (Fig. 5b). Phosphorous remaining (%) was enhanced in the first month (117.0%), and thereafter stayed in the range of 97.8%–109.0%, which means that the phosphorous content barely changed during decomposition (Fig. 5a). For nitrogen, there was a steady accumulation period from March to September, the mass remaining even increased to 130.0% in June and July, followed by a tiny decrease in the later stage (Fig. 5a). Magnesium gradually declined after a short period of increase from March to April, while a modest increase occurred in September (Fig. 5b). The mass remaining of calcium fluctuated through the decomposition process but was always higher than the original value (Fig. 5b).
Fig. 5.
Dynamics of nutrient remaining during the experiment
(a) Hollow triangles and diamonds represent the dynamics of phosphorus (P) and nitrogen (N), respectively; (b) Solid squares, diamonds, and circles represent the dynamics of calcium (Ca), potassium (K), and magnesium (Mg), respectively. Error bars indicate standard errors
4. Discussion
4.1. Mass loss pattern and organic matter dynamics
Based on the above results, the short-term decomposition of tea leaves can be described as a two-part process: an initial stage of rapid and constant mass loss and a stagnant stage of very slow mass loss. This pattern is similar to the two-phase decomposition model reported by Melillo et al. (1989) for red pine litter decomposition. The reason for the fast degradation during the early stage is generally attributed to the swift leaching of soluble components and the rapid decomposition of carbohydrates (Mesquita et al., 1998; Trofymow et al., 2002; Peng et al., 2014). As the decomposition continues, the proportion of recalcitrant constituents such as lignin, polyphenolics, and cellulose gradually increases, resulting in a slow decomposition stage.
Phenolic compounds and lignin have been reported to influence decomposition rates (Melillo et al., 1982; Northup et al., 1998; Hättenschwiler and Vitousek, 2000). In this study, the proportion of lignin was relatively small compared with polyphenols and its mass loss rate was comparatively stable; thus it could not explain the rapid mass loss. In contrast, the rapid decomposition of polyphenols exactly matched the mass loss pattern. From March to May, polyphenols notably decreased by 67.5%, indicating the highest decomposition rate compared to other organic components (Fig. 4a). The reduction of tea polyphenols is largely due to their water solubility and strong oxidizing capacity; polyphenols have multiple reactive groups and active sites facilitating their involvement in a variety of chemical reactions (Wan, 2008). However, the transition of tea polyphenols was too rapid to be detected, as suggested by Mesquita et al. (1998), mainly owing to the extremely high concentration of polyphenols in tea leaves and their chemical properties.
The stagnation stage from June to November mostly related to holocellulose rather than lignin in this study (Figs. 4c and 4d). The lignin degradation rate is about twice that of cellulose, according to previous reports that lignin can physically inhibit microbial attacks on the holocellulose fraction or compound the recalcitrant matrix by the encrustation of cellulose (Sinsabaugh and Linkins, 1989; Berg and McClaugherty, 2003; Adair et al., 2008). Additionally, the degradation of both lignin and cellulose is mainly dominated by microorganisms. Given the rich polyphenols in tea plantations’ ecosystems, we speculated that the diversity and community structure of decomposers in tea plantations might be affected by phenolic compounds, then consequently changing the decomposition rate of lignocellulose. Instead of the decreasing mass loss rate, monthly mass loss was relatively high in July (Fig. 4b). At the same time, we observed the highest average temperature of 32.3 °C and the lowest relative humidity in July, so the biggest monthly mass loss might result from the increasing photo-thermal effect during that period as suggested by Wang et al. (2015) where ultraviolet (UV) exposure could increase litter biodegradability by increasing the microbial accessibility of lignin, as well as the labile carbon supply to microbes.
4.2. Role of polyphenol in the short-term decomposition of tea leaf litter
To verify the hypothesis of the role of polyphenols in tea leaf litter decomposition, we examined a few common indicators according to prior studies. The C/N ratio is a classical predictor of litter decomposition rate: a higher value represents a higher proportion of organic components in the litter, ultimately resulting in slower decomposition. In the present study, the initial C/N ratio was 17.62 in tea leaf litter samples. It declined sharply in the first three months, and then rose gradually in the latter five months (Fig. 1). The loss of total polyphenol explained the reduction in the C/N ratio during the first two months, with the constant disappearance of these easily decomposed substances in the first stage. Lignocellulose components finally took control of the rest of the process, leading to an increasing C/N ratio. The changes in the C/N ratio perfectly reflected the monthly mass loss from March to June (Fig. 3), but with the slight augmentation of the C/N ratio after June, and the monthly mass loss did not decrease as predicted. Apparently, the C/N ratio can explain a part of the decomposition rate of tea leaf litter but not the whole process.
In addition to the C/N ratio, many studies have also treated the recalcitrant components such as lignin and holocellulose (cellulose and hemicellulose) as important factors for stimulating the litter decomposition rate, especially in the latter stages of the process. Commonly, the higher these recalcitrant components, the slower the decomposition (Cooke and Whipps, 1993; Moore et al., 1999; Silver and Miya, 2001). The initial value of lignin/N was 1.46 in the present study, with little change throughout the experiment. Using the Pearson correlation test, we did not find significant correlations either between the lignin/N ratio and mass loss, or between the lignin/N ratio and monthly loss. Similarly, no correlation existed between cellulose/holocellulose and mass loss/monthly mass loss. Thus, lignin and cellulose cannot explain the short-term tea leaf litter decomposition rate. This is mainly because of the relatively small proportion of lignin in tea leaf litter.
Therefore, we turned to tea polyphenols, which accounted for about 32.5% of the total dry mass in this study. The variation of tea polyphenol content is presented in Fig. 4a. The initial polyphenol/N ratio of tea leaf litter was 12.9. In the first three months of decomposition, the polyphenol/N ratio significantly and rapidly decreased, and remained in the range of 0.2–0.5 thereafter. A significantly negative correlation (r=0.88, P<0.01) between the polyphenol/N ratio and mass loss was recognized, indicating the significant depression of polyphenols on tea leaf decomposition. A similar inhibition effect of polyphenols on litter decomposition has been found in pigmy forests (Northup et al., 1998).
The reason why polyphenols affect litter decomposition is still unclear, although a great many hypotheses have attempted to explain the phenomenon. Polyphenols regulate the mobility of nutrients by a series of mechanisms. Adsorption to clay minerals and/or complexation with sesquioxides protects them from microbial attack, reduces the toxic effects of aluminum in acid soils, and competes effectively with other negatively charged compounds for sorption sites. Polyphenols binding to sesquioxides can not only depress phosphate sorption, but also desorb immobile phosphate; thus, high polyphenol concentrations might contribute to the maintenance of phosphorus availability in highly weathered and acidic soils with high levels of iron and aluminum sesquioxides. The property of accumulating iron and aluminum in tea and tea plantation soils (Ruan et al., 2003), and the higher concentration of phosphorus in tea leaf litter (2.04–2.09 mg/g) than in forest leaf litter, indirectly confirmed the combination of polyphenols and phosphorus. In addition, the stimulation of polyphenols on microbial activity is not well known (Schmidt et al., 2013; Winder et al., 2013). Further investigation is required to determine the genuine function of polyphenols in tea leaf decomposition and the interaction between them and other factors.
4.3. Nutrient release pattern
The release of nutrients during litter decomposition is a dynamic rather than a direct process. It is associated with the litter quality and the decomposition period, and is also associated with the characteristics of the nutrient itself. Generally, there are three release patterns for nutrients during litter decomposition: (1) the leaching-release pattern; (2) the leaching-enrichment-release pattern; and (3) the enrichment-release pattern.
Nitrogen and phosphorus are important for decomposer growth (Melillo et al., 1982; Dziadowiec, 1987) and are not mobilized nor fixed by microorganisms to any great extent. In the present study, both nitrogen and phosphorus accumulated in the early stage then decreased slowly, representing an enrichment-release pattern. The short period of phosphorus increase from March to May might be related to the polyphenol dynamics mentioned above. The accumulation of nitrogen mainly appeared in May to August, during which time the average nitrogen remaining was about 127.3% (Fig. 5a). This enhancement of nitrogen was consistent with previous studies (Johansson, 1993; Berg and Cortina, 1995; Fujii and Takeda, 2010). Low-quality litter with limited nutrients often needs to adsorb nutrients from the environment at the beginning of the decomposition, and it always takes a long time to reach the threshold for release. With a relatively low nitrogen concentration in tea leaf litter, nitrogen accumulated for five months before release, and even the final nitrogen remaining was still as high as 94.5%. The periodical accumulation and release of nitrogen during decomposition are usually attributed to a higher initial C/N ratio or a higher lignin concentration, which could retain nitrogen in the substrate (Osono and Takeda, 2004a; 2004b). Berg et al. (1993) assumed that nitrogen fixation occurred when litter had approximately 0.3%–1.4% nitrogen, while nitrogen release appeared when the nitrogen content was in the range 0.6%–2.8%. Obviously this hypothesis cannot explain nitrogen accumulation during the decomposition for tea leaf litter with initial nitrogen content of 2.63%. However, some other studies have found that the impact of litter polyphenol concentration or the polyphenol/N ratio on the nitrogen release rate is stronger than that of the C/N or lignin/N ratio (Fox et al., 1990; Palm and Sanchez, 1991; Oglesby and Fownes, 1992), especially in acidic and infertile soils. This observation agrees well with the results from this experiment.
The fast release of potassium was similar to many previous studies of leaf decomposition (Fahey et al., 1988; Laskowski et al., 1995b; Osono and Takeda, 2004b), mostly due to its mobility. Potassium is hard to incorporate into organic structures (Marschner and Rimmington, 1988). Magnesium was also highly mobile, but it decreased quite slowly (Fig. 5b), unlike in other studies (Fahey et al., 1988; Laskowski et al., 1995a). This phenomenon might contribute to the ability of polyphenols to absorb exchangeable inorganic cations (Ca, Mg, and K) by providing sorption sites (Wan, 2008). Calcium fluctuated dramatically and ultimately increased to 127.0% (Fig. 5b), which was similar to the result from an experiment carried out by Fujii and Takeda (2010), in which the calcium in Japanese cypress leaves was released slowly, and might be due to the incorporation into structural organic compounds; thus it is mostly released by microbial activity (Dziadowiec, 1987).
5. Conclusions and prospects
During the short-term decomposition of tea leaves, the conversion of tea polyphenols was rapid. Simultaneously it regulated the mass loss rate as well as affected the process of nutrient release indirectly. Our study highlighted that a large number of polyphenols in tea plantation ecosystems had a very close relationship with nutrient cycling, but their long-term effects need further investigation. Management in conventional tea plantation is quite intensive, and a large number of pruned branches and leaves can enter the soil by tillage, so the decomposition process of tea plant litter below ground is worth future study.
When a large quantity of polyphenols enter tea plantation soils, soil microbes could use them as a carbon resource for growth, but it is possible that they might sometimes inhibit the growth of microorganisms. Microorganisms are involved in a series of metabolic processes such as soil respiration and nutrient cycling. Therefore, it is very important to study the soil microbial activity and community structure after polyphenol amendment in tea plantations because this could provide information on the huge potential for carbon sequestration in tea plantation soils.
Acknowledgments
The authors would like to thank Prof. Yao-ping LUO and Hai-rong XU (both from the Institute of Tea Science, Zhejiang University, Hangzhou, China) for assistance with the experimental design and other members in the Xiao-chang WANG’s lab.
Footnotes
Project supported by the Open Foundation of the State Key Laboratory of Soil and Sustainable Agriculture (No. 0812201215) and the New Cultivation Model for Ecological Tea Plantation Development (No. H20151653), China
Compliance with ethics guidelines: Dong-mei FAN, Kai FAN, Cui-ping YU, Ya-ting LU, and Xiao-chang WANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Adair EC, Parton WJ, del Grosso SJ, et al. Simple three-pool model accurately describes patterns of long-term litter decomposition in diverse climates. Global Change Biol. 2008;14(11):2636–2660. [Google Scholar]
- 2.Aerts R. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos. 1997;79(3):439–449. (Available from: http://dx.doi.org/10.2307/3546886) [Google Scholar]
- 3.AOAC (Association of Official Analytical Chemists) Official Methods of Analysis of AOAC, 17th Ed. Gaitherburg, MD, USA: AOAC International; 2000. [Google Scholar]
- 4.Berg B. Decomposition patterns for foliar litter–a theory for influencing factors. Soil Biol Biochem. 2014;78:222–232. (Available from: http://dx.doi.org/10.1016/j.soilbio.2014.08.005) [Google Scholar]
- 5.Berg B, Cortina J. Nutrient dynamics in some decomposing leaf and needle litter types in a Pinus sylvestris forest. Scand J Forest Res. 1995;10(1-4):1–11. (Available from: http://dx.doi.org/10.1080/02827589509382860) [Google Scholar]
- 6.Berg B, McClaugherty C. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration. Berlin, Germany: Springer-Verlag Heidelberg; 2003. [Google Scholar]
- 7.Berg B, McClaugherty C, Johansson MB. Litter mass-loss rates in late stages of decomposition at some climatically and nutritionally different pine sites. Long-term decomposition in a Scots pine forest. VIII. Can J Bot. 1993;71(5):680–692. (Available from: http://dx.doi.org/10.1139/b93-078) [Google Scholar]
- 8.Bocock KL, Gilbert OJW. The disappearance of leaf litter under different woodland conditions. Plant Soil. 1957;9(2):179–185. (Available from: http://dx.doi.org/10.1007/BF01398924) [Google Scholar]
- 9.Chadwick DR, Ineson P, Woods C, et al. Decomposition of Pinus sylvestris litter in litter bags: influence of underlying native litter layer. Soil Biol Biochem. 1998;30(1):47–55. (Available from: http://dx.doi.org/10.1016/S0038-0717(97)00090-4) [Google Scholar]
- 10.Chen ZM, Lin Z. Tea and human health: biomedical functions of tea active components and current issues. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2015;16(1):87–102. doi: 10.1631/jzus.B1500001. (Available from: http://dx.doi.org/10.1631/jzus.B1500001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooke RC, Whipps JM. Ecophysiology of Fungi. Blackwell Scientific Publications; 1993. [Google Scholar]
- 12.CouˆTeaux MM, Bottner P, et al. Litter decomposition, climate and liter quality. Trends Ecol Evol. 1995;10(2):63–66. doi: 10.1016/S0169-5347(00)88978-8. (Available from: http://dx.doi.org/10.1016/S0169-5347(00)88978-8) [DOI] [PubMed] [Google Scholar]
- 13.Dziadowiec H. The decomposition of plant litter fall in an oak-linden-hornbeam forest and an oak-pine mixed forest of the Białowieża National Park. Acta Soc Bot Pol. 1987;56(1):169. (Available from: http://dx.doi.org/10.5586/asbp.1987.019) [Google Scholar]
- 14.Fahey TJ, Hughes JW, Pu M, et al. Root decomposition and nutrient flux following whole-tree harvest of northern hardwood forest. Forest Sci. 1988;34(3):744–768. [Google Scholar]
- 15.Findlay S, Carreiro M, Krischik V, et al. Effects of damage to living plants on leaf litter quality. Ecol Appl. 1996;6(1):269–275. (Available from: http://dx.doi.org/10.2307/2269570) [Google Scholar]
- 16.Fox RH, Myers RJK, Vallis I. The nitrogen mineralization rate of legume residues in soil as influenced by their polyphenol, lignin, and nitrogen contents. Plant Soil. 1990;129(2):251–259. (Available from: http://dx.doi.org/10.1007/BF00032420) [Google Scholar]
- 17.Fujii S, Takeda H. Dominant effects of litter substrate quality on the difference between leaf and root decomposition process above and belowground. Soil Biol Biochem. 2010;42(12):2224–2230. (Available from: http://dx.doi.org/10.1016/j.soilbio.2010.08.022) [Google Scholar]
- 18.Grandy AS, Erich MS, Porter GA. Suitability of the anthrone–sulfuric acid reagent for determining water soluble carbohydrates in soil water extracts. Soil Biol Biochem. 2000;32(5):725–727. (Available from: http://dx.doi.org/10.1016/S0038-0717(99)00203-5) [Google Scholar]
- 19.Hättenschwiler S, Vitousek PM. The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol Evol. 2000;15(6):238–243. doi: 10.1016/s0169-5347(00)01861-9. (Available from: http://dx.doi.org/10.1016/S0169-5347(00)01861-9) [DOI] [PubMed] [Google Scholar]
- 20.Heimler D, Vignolini P, Dini MG, et al. Antiradical activity and polyphenol composition of local Brassicaceae edible varieties. Food Chem. 2006;99(3):464–469. (Available from: http://dx.doi.org/10.1016/j.foodchem.2005.07.057) [Google Scholar]
- 21.Johansson MB. Biomass, decomposition and nutrient release of vaccinium myrtillus leaf litter in four forest stands. Scand J Forest Res. 1993;8(1-4):466–479. (Available from: http://dx.doi.org/10.1080/02827589309382793) [Google Scholar]
- 22.Kurokawa H, Nakashizuka T. Leaf herbivory and decomposability in a Malaysian tropical rain forest. Ecology. 2008;89(9):2645–2656. doi: 10.1890/07-1352.1. (Available from: http://dx.doi.org/10.1890/07-1352.1) [DOI] [PubMed] [Google Scholar]
- 23.Laskowski R, Maryanski M, Niklinska M. Changes in the chemical composition of water percolating through the soil profile in a moderately polluted catchment. Water Air Soil Poll. 1995;85(3):1759–1764. (Available from: http://dx.doi.org/10.1007/BF00477234) [Google Scholar]
- 24.Laskowski R, Niklinska M, Maryanski M. The dynamics of chemical-elements in forest litter. Ecology. 1995;76(5):1393–1406. (Available from: http://dx.doi.org/10.2307/1938143) [Google Scholar]
- 25.Liang YR, Liu Z, Xu YR, et al. A study on chemical composition of two special green teas (Camellia sinensis) J Sci Food Agric. 1990;53(4):541–548. (Available from: http://dx.doi.org/10.1002/jsfa.2740530411) [Google Scholar]
- 26.Marschner H, Rimmington G. Mineral nutrition of higher plants. Plant Cell Environ. 1988;11(2):147–148. (Available from: http://dx.doi.org/10.1111/j.1365-3040.1988.tb01130.x) [Google Scholar]
- 27.McClaugherty CA, Pastor J, Aber JD, et al. Forest litter decomposition in relation to soil nitrogen dynamics and litter quality. Ecology. 1985;66(1):266–275. (Available from: http://dx.doi.org/10.2307/1941327) [Google Scholar]
- 28.Meentemeyer V. Macroclimate and lignin control of hardwood leaf litter decomposition dynamics. Ecology. 1978;59(3):465–472. (Available from: http://dx.doi.org/10.2307/1936576) [Google Scholar]
- 29.Melillo J, Aber J, Linkins A, et al. Carbon and nitrogen dynamics along the decay continuum: plant litter to soil organic matter. Plant Soil. 1989;115(2):189–198. (Available from: http://dx.doi.org/10.1007/BF02202587) [Google Scholar]
- 30.Melillo JM, Aber JD, Muratore JF. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology. 1982;63(3):621–626. (Available from: http://dx.doi.org/10.2307/1936780) [Google Scholar]
- 31.Mesquita RD, Workman SW, Neely CL. Slow litter decomposition in a cecropia-dominated secondary forest of central Amazonia. Soil Biol Biochem. 1998;30(2):167–175. (Available from: http://dx.doi.org/10.1016/S0038-0717(97)00105-3) [Google Scholar]
- 32.Moore TR, Trofymow JA, Taylor B, et al. Litter decomposition rates in Canadian forests. Global Change Biol. 1999;5(1):75–82. (Available from: http://dx.doi.org/10.1046/j.1365-2486.1998.00224.x) [Google Scholar]
- 33.Northup RR, Dahlgren RA, McColl JG. Polyphenols as regulators of plant-litter-soil interactions in northern California’s pygmy forest: a positive feedback? Biogeochemistry. 1998;42(1-2):189–220. (Available from: http://dx.doi.org/10.1023/A:1005991908504) [Google Scholar]
- 34.Oglesby KA, Fownes JH. Effects of chemical-composition on nitrogen mineralization from green manures of 7 tropical leguminous trees. Plant Soil. 1992;143(1):127–132. (Available from: http://dx.doi.org/10.1007/BF00009137) [Google Scholar]
- 35.Olsen SR, Cole CV, Watanabe FS, et al. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. Washington: United States Department of Agriculture Circular 939; 1954. [Google Scholar]
- 36.Osono T, Takeda H. Accumulation and release of nitrogen and phosphorus in relation to lignin decomposition in leaf litter of 14 tree species. Ecol Res. 2004;19(6):593–602. (Available from: http://dx.doi.org/10.1111/j.1440-1703.2004.00675.x) [Google Scholar]
- 37.Osono T, Takeda H. Potassium, calcium, and magnesium dynamics during litter decomposition in a cool temperate forest. J Forest Res. 2004;9(1):23–31. (Available from: http://dx.doi.org/10.1007/s10310-003-0047-x) [Google Scholar]
- 38.Palm CA, Sanchez PA. Decomposition and nutrient release patterns of the leaves of three tropical legumes. Biotropica. 1990;22(4):330–338. (Available from: http://dx.doi.org/10.2307/2388550) [Google Scholar]
- 39.Palm CA, Sanchez PA. Nitrogen release from the leaves of some tropical legumes as affected by their lignin and polyphenolic contents. Soil Biol Biochem. 1991;23(1):83–88. (Available from: http://dx.doi.org/10.1016/0038-0717(91)90166-H) [Google Scholar]
- 40.Palm CA, Rowland AP. A minimum dataset for characterization of plant quality for decomposition. In: Cadisch G, Giller KE, editors. Driven by Nature: Plant Litter Quality and Decomposition. Wallingford: CAB International; 1997. pp. 379–393. [Google Scholar]
- 41.Parton W, Silver WL, Burke IC, et al. Global-scale similarities in nitrogen release patterns during long-term decomposition. Science. 2007;315(5810):361–364. doi: 10.1126/science.1134853. (Available from: http://dx.doi.org/10.1126/science.1134853) [DOI] [PubMed] [Google Scholar]
- 42.Peng Q, Qi Y, Dong Y, et al. Decomposing litter and the C and N dynamics as affected by N additions in a semi-arid temperate steppe, Inner Mongolia of China. J Arid Land. 2014;6(4):432–444. (Available from: http://dx.doi.org/10.1007/s40333-014-0002-z) [Google Scholar]
- 43.Preston, C.M., Trofymow, J.A., The Canadian Intersite Decomposition Experiment Working Group. Variability in litter quality and its relationship to litter decay in Canadian forests. Can J Bot. 2000;78(10):1269–1287. (Available from: http://dx.doi.org/10.1139/b00-101) [Google Scholar]
- 44.Ruan JY, Wang GQ, Shi YZ, et al. Aluminium in tea soils, rhizosphere soil and the characteristics of Al uptake by tea plant. J Tea Sci. 2003;23(z1):16-20(in Chinese):16–20 (in Chinese). (Available from: http://dx.doi.org/10.3969/j.issn.1000-369X.2003.z1.003) [Google Scholar]
- 45.Schmidt MA, Kreinberg AJ, Gonzalez JM, et al. Soil microbial communities respond differently to three chemically defined polyphenols. Plant Physiol Biochem. 2013;72:190–197. doi: 10.1016/j.plaphy.2013.03.003. (Available from: http://dx.doi.org/10.1016/j.plaphy.2013.03.003) [DOI] [PubMed] [Google Scholar]
- 46.Silver WL, Miya RK. Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia. 2001;129(3):407–419. doi: 10.1007/s004420100740. (Available from: http://dx.doi.org/10.1007/s004420100740) [DOI] [PubMed] [Google Scholar]
- 47.Sinsabaugh RL, Linkins A. Ellulase mobility in decomposing leaf litter. Soil Biol Biochem. 1989;21(2):205–209. (Available from: http://dx.doi.org/10.1016/0038-0717(89)90096-5) [Google Scholar]
- 48.Swift MJ, Heal OW, Anderson JM. Decomposition in Terrestrial Ecosystems. Oxford, London: Blackwell Scientific Publications; 1979. [Google Scholar]
- 49.Taylor BR, Parkinson D, Parsons WFJ. Nitrogen and lignin content as predictors of litter decay-rates–a microcosm test. Ecology. 1989;70(1):97–104. (Available from: http://dx.doi.org/10.2307/1938416) [Google Scholar]
- 50.Tharayil N, Alpert P, Bhowmik P, et al. Phenolic inputs by invasive species could impart seasonal variations in nitrogen pools in the introduced soils: a case study with polygonum cuspidatum. Soil Biol Biochem. 2013;57:858–867. (Available from: http://dx.doi.org/10.1016/j.soilbio.2012.09.016) [Google Scholar]
- 51.Trofymow JA, Moore TR, Titus B, et al. Rates of litter decomposition over 6 years in Canadian forests: influence of litter quality and climate. Can J Forest Res. 2002;32(5):789–804. (Available from: http://dx.doi.org/10.1139/x01-117) [Google Scholar]
- 52.Valus L, Jones RJ. Net mineralization of nitrogen in leaves and leaf litter of desmodium intortum and phaseolus atropurpureus mixed with soil. Soil Biol Biochem. 1973;5(4):391–398. (Available from: http://dx.doi.org/10.1016/0038-0717(73)90065-5) [Google Scholar]
- 53.Wan X. Tea Biochemistry. Beijing: China Agriculture Press; 2008. pp. 9–15. (in Chinese) [Google Scholar]
- 54.Wang J, Liu L, Wang X, et al. The interaction between abiotic photodegradation and microbial decomposition under ultraviolet radiation. Global Change Biol. 2015;21(5):2095–2104. doi: 10.1111/gcb.12812. (Available from: http://dx.doi.org/10.1111/gcb.12812) [DOI] [PubMed] [Google Scholar]
- 55.Winder RS, Lamarche J, Constabel CP, et al. The effects of high-tannin leaf litter from transgenic poplars on microbial communities in microcosm soils. Front Microbiol. 2013;4:1–10. doi: 10.3389/fmicb.2013.00290. (Available from: http://dx.doi.org/10.3389/fmicb.2013.00290) [DOI] [PMC free article] [PubMed] [Google Scholar]