Abstract
We studied the effects of hardwood-derived biochar (BC) and the phytohormone-producing endophyte Galactomyces geotrichum WLL1 in soybean (Glycine max (L.) Merr.) with respect to basic, macro-and micronutrient uptakes and assimilations, and their subsequent effects on the regulation of functional amino acids, isoflavones, fatty acid composition, total sugar contents, total phenolic contents, and 1,1-diphenyl-2-picrylhydrazyl (DPPH)-scavenging activity. The assimilation of basic nutrients such as nitrogen was up-regulated, leaving carbon, oxygen, and hydrogen unaffected in BC+G. geotrichum-treated soybean plants. In comparison, the uptakes of macro-and micronutrients fluctuated in the individual or co-application of BC and G. geotrichum in soybean plant organs and rhizospheric substrate. Moreover, the same attribute was recorded for the regulation of functional amino acids, isoflavones, fatty acid composition, total sugar contents, total phenolic contents, and DPPH-scavenging activity. Collectively, these results showed that BC+G. geotrichum-treated soybean yielded better results than did the plants treated with individual applications. It was concluded that BC is an additional nutriment source and that the G. geotrichum acts as a plant biostimulating source and the effects of both are additive towards plant growth promotion. Strategies involving the incorporation of BC and endophytic symbiosis may help achieve eco-friendly agricultural production, thus reducing the excessive use of chemical agents.
Keywords: Phytohormone-producing endophytic fungi, Nutrients uptake, Assimilation, Nutritional quality, Soybean
1. Introduction
The conversion of biomass to a stable soil carbon pool negatively regulates atmospheric carbon (C) concentrations, and this phenomenon underlies the concept of biochar (BC) production (Lehmann, 2007). BC has been used to improve agricultural productivity in traditional systems, with examples of its use being traced in the terra preta of the Amazon Basin (Lehmann, 2007). BC is the product of the slow pyrolysis of biomass, and its properties vary depending on certain factors such as feedstock type, time, and temperature conditions during preparation (de Corato et al., 2015; Gwenzi et al., 2015; Li et al., 2015). However, incorporating BC into soil proved to operate as a conditioner, by improving soil fertility through increasing soil organic C levels and nutrient availability (releasing its own nutrients and cycling existing nutrients in the soil and preventing their leaching), transforming phosphorus (P), enhancing soil field capacity, and decreasing bulk density (Ahmed and Schoenau, 2015; Bayabil et al., 2015; Dong et al., 2015; Gwenzi et al., 2015; Zhang et al., 2015). The large surface area and negative charges of BC immobilize nitrogen (N) and reduce N pollution (Dong et al., 2015). Other important aspects that make BC a promising candidate for environmentally friendly agriculture are its ability to inhibit methane emission (Gwenzi et al., 2015), remediate heavy metal-contaminated soil, neutralize soil toxic compounds, and increase beneficial microbial activities (Waqas et al., 2014; Li et al., 2015). BC has also been found to reduce the incidence of crop disease and mitigate biotic stress (de Corato et al., 2015; Gwenzi et al., 2015; Haider et al., 2015).
Endophytic fungi are ubiquitous with plant species, and live asymptomatically inside healthy plant tissues in mutualistic association. This symbiotic association demonstrates the ecological and evolutionary importance for the establishment of plants in terrestrial ecosystems (Hartley et al., 2015; Zhou et al., 2015). Endophytes colonize all plant organs, and they have been isolated from the roots, stems, and leaves (Zhou et al., 2015). The occurrence of endophytes in specific tissues supports the view that they have a particular role in host plant tissues. For example, endophytes found in plant roots help with water uptake, nutrient acquisition, and plant growth promotion, while those found in the leaves and stems strengthen plant defense mechanisms (Khan et al., 2013; 2014; Waqas et al., 2014; 2015; Hartley et al., 2015; Zhou et al., 2015). Endophytes enhance the performance of host tissue functions by producing a vast array of biologically active secondary metabolites (Khan et al., 2012a; 2013; Waqas et al., 2014; 2015). Phytohormones and similar substances are examples of biologically active secondary metabolites, which are mostly responsible for enhancing plant growth and development under normal, abiotic, and biotic stress conditions. Endophytes (fungi and bacteria) have the ability to produce gibberellins and indole-3-acetic acid (IAA); these hormones have been extensively reported to enhance plant growth attributes (Khan et al., 2012b; 2013; 2014; Waqas et al., 2012).
The combined application of phytohormone-producing fungal endophytes and hardwood-derived BC ameliorates high Zn concentrations and enhances soybean plant growth in the presence and absence of heavy metal stress (Waqas et al., 2014). To move on from the results of the previous experiment, the current study was conducted and we hypothesized that: (1) the endophytic fungus and BC individually or in combination enhance the provisions and uptakes of macro-and micronutrients; (2) as a result, the nutritional and medicinal qualities of soybean are improved.
2. Materials and methods
2.1. Soil preparation and biochar addition
In this study, the substrate TBT (Soil and Fertilizer Technology, Korea) was used instead of common soil. Hence, the focus was to evaluate the roles of endophytes and BC in plant growth promotion. Therefore, other properties, such as bulk density and porosity, were not determined. The nutrient composition of the substrate consisted of coco peat (45%–50%), perlite (35%–40%), peat moss (10%–15%), and zeolite (6%–8%), and it contained P2O5 0.35 mg/g, K2O 0.1 mg/g, NO3 − 0.205 mg/g, and NH4 + 0.09 mg/g. The BC was obtained commercially from Kangwon Grasses Industries Ltd. (Kangwondo, Korea) and the company information provided on bags shows that the pine trees-derived BC via slow pyrolysis is alkaline in nature, has an average particle size of 5 mm or less and a moisture level of 10%, and contains 2%–3% ash. Chemical characterization of BC was performed with an elemental analyzer and inductively coupled plasma mass spectrometry (ICP). The analysis revealed the basic elemental composition of BC as 80% C, 1.62% N, and 1.49% hydrogen (H), and macro-/micronutrient (mg/kg) as 2.58 P, 3.64 potassium (K), 43.58 calcium (Ca), 2.95 sulfur (S), 1.81 manganese (Mn), 20.30 magnesium (Mg), 13.21 molybdenum (Mo), 2.09 aluminum (Al), 6.63 boron (B), 0.35 copper (Cu), 1.13 iron (Fe), and 6.88 sodium (Na). BC was added at 10:90 (w/w) to the substrate, and was mixed well by stirring and rotating end-over-end in sealed plastic bags to incorporate it homogeneously. The substrates with and without BC were moistened in the amount of water of half of its weight, packed in plastic bags, and left for 7 d in dark conditions at room temperature to equilibrate. The substrates with and without BC were then sterilized (at 121 °C for 15 min) three times to create microbe-free conditions. The autoclavable plastic plant growth pots (30 cm×15 cm; sterilized at 121 °C for 15 min) were filled with 1 kg substrate. Plastic bases were placed in the pots to collect any leaching water, which was then added back to the pots to prevent the loss of BC. Culture broth containing mycelia (40 ml, (200±10) mg) of Galactomyces geotrichum WLL1, along with potato dextrose agar (PDA)-inoculated disks, was applied in the center of each substrate-filled pot in the endophyte treatment with and without BC. The substrate in pots with endophytes was then mixed by stirring with a sterile rod to uniformly distribute the mycelia and PDA-inoculated disks in the rhizosphere. Autoclaved 40 ml Czapek broth medium and 20 ml non-inoculated disks were added to the center of control and only BC pots as well for the purpose of balancing the nutrients status of the substrate compared to inoculated pots. The pots were then aseptically incubated for 5 d to be ready for transplantation.
2.2. Biological materials and culture condition
G. geotrichum WLL1 (NCBI GenBank accession number KJ817904) was previously isolated from Trapa japonica inhabiting Nak-Dong river (35°44′47.20′′ N, 128°23′07.79′′ E) with a rainfall catchment area from an abandoned zinc mine (Waqas et al., 2014). The G. geotrichum WLL1 was capable of producing gibberellins (GA1=(7.83±0.40) ng/ml, GA4=(54.11±1.50) ng/ml, GA7=(4.12±0.13) ng/ml), indole acetic acid ((76.89±2.35) μg/ml), and reactive oxygen species (peroxide, superoxide), and found to extensively colonize soybean root in the presence and absence of BC with or without Zn heavy metal stress. For the current experiment, Czapek broth media (40 ml; 1% (0.01 g/ml) glucose, 1% (0.01 g/ml) peptone, 0.05% (0.5 g/L) KCl, 0.05% (0.5 g/L) MgSO4∙7H2O, and 0.001% (0.01 g/L) FeSO4∙7H2O; pH 7.3±0.2) in 50 ml flasks were inoculated with G. geotrichum and grown at 30 °C for 10 d at 120 r/min under dark conditions. At the same time PDA media (20 ml; 0.4% (4 g/L) potato starch, 2.0% (20 g/L) dextrose, and 1.5% (15 g/L) agar; pH 5.6±0.2) in 70 mm Petri dishes were also inoculated with G. geotrichum and kept for 7 d at 25 °C under dark conditions. At the time of each application into every pot, from these G. geotrichum-inoculated broth and agar media, one flask of Czapek culture broth and PDA plate disks divided into small pieces with sterilized blades were used.
2.3. Soybean growth experiment under controlled condition
Soybean seeds (Glycine max L. var. Hwangkeumkong) were obtained from the Soybean Genetic Resource Center (Prof. Jeong Deong LEE, Kyungpook National University, Korea). The seeds were healthy, with 6% moisture content and 95% germination. At the same time as pot preparation, the seeds were germinated (28 °C and relative humidity of 60%) for 5 d to get seedlings of identical size in the sprouting trays. Before germination, the seeds were surface-sterilized in autoclaved pots with 2.5% (25 g/L) sodium hypochlorite for 30 min, and were then rinsed with autoclaved double-distilled water. After germination, seedlings of equal size were randomly selected, screened for the presence of any microbes and transplanted to pots. To ensure that microbial free plants were transplanted, microscopic analysis was performed using an Olympus (BX50; Olympus Optical Co. Ltd., Shinjuku, Tokyo, Japan) light microscope according to Likar and Regvar (2013) and Waqas et al. (2014; 2015) before further experimentation. The roots of randomly collected soybean plants from the sprouting tray fragmented into 1-cm-long pieces were evaluated under a light microscope. The experimental treatments included: (1) soybeans without endophyte and BC, (2) endophyte-inoculated soybeans, (3) soybeans with BC (10%, w/w), and (4) both endophyte and BC (10%, w/w) applied to soybeans. The soybeans were left to grow for 22 d under controlled growth chamber conditions (day/night cycle: 14 h at 28 °C/10 h at 25 °C; relative humidity 60%–70%; light intensity 1000 μE/(m2∙s) from sodium lamps). The soybeans were irrigated daily with 30 ml of distilled water to minimize BC leaching from pots. During the growth period, 40 ml of Czapek broth with fungal mycelia ((200±10) mg) and inoculated PDA disks were applied to the root zone at the beginning of V3 and V4 life stages of soybean for optimal endophytic infection after transplantation. Sterile Czapek and PDA media of the same amount were added to the control/only BC-treated plants to balance the nutrient status of the substrate with that of the endophytic treatment that might have occurred because of its application along with the endophyte. The plants were immediately stored after harvesting in liquid nitrogen and then freeze-dried for one week. Before harvesting, three plants per treatment (endophyte) were selected for the re-isolation and molecular identification of endophytes from the soybean secondary root pieces to check their inside colonization according to the procedure described by Khan et al. (2012b). The re-isolated purified endophytes were compared with those of the original plates and showed 100% morphological similarity. In case of molecular identification, the obtained sequences of the internal transcribed spacer (ITS) and large subunit (LSU) regions from ribosomal DNA (rDNA) were BLAST-searched and confirmed 100% sequence homology with G. geotrichum WLL1.
2.4. Macro-and micronutrient analyses in soil and plant tissues
To determine macro-and micronutrient uptakes, representative fresh plants were randomly selected from each replicated treatment. The plants were carefully harvested and divided into roots and shoots. The roots were carefully washed with double-distilled water to remove all debris and apoplastic contents (Waqas et al., 2014). After washing, the roots were dried with autoclaved Kimtech Science Wipers (Yuhan-Kimberly Inc., Seoul, Korea). The roots and shoots were immediately placed in liquid nitrogen, lyophilized at −50 °C for 3–4 d, and ground to a fine powder using a grinder. To analyze the macro-and micronutrient concentrations of the substrate, three samples were randomly collected from each replicated treatment. The three samples were mixed together, air-dried, sieved to 2 mm, and ground to a fine powder in an agate mortar for further analysis. Macro-and micronutrient levels, including P, K, S, Ca, Mg, Mn, Fe, and Cu, were analyzed in the shoots, roots, and substrates using ICP having 0.1 mg/kg limit of detection (Optima 7300DV, Perkin-Elmer, USA). The samples (0.1 g) were prepared with microwave (ETHOS 1, advanced microwave digestion system) assisted concentrated HNO3, H2O2 and H2SO4 acid digestions. Here, H2O2 and H2SO4 have been used for safe, fast, and reliable sample digestion prior to ICP analysis due to their abilities for complete sample digestion and high boiling point, respectively. Although H2O2 completely decomposes the sample, its combination with HNO3 is the best substitute for perchloric acid because of safety issues as well as the fact that it increases solubility of the sulphate salts, which are not obtained accurately with H2SO4. The digested samples were diluted with double distilled water before analysis. For the external proficiency testing scheme, ICP multi-element standard solution IV (Merck KGaA, 64271 Darmstadt, Germany) was used. The coefficient value was found to be 0.99 for all calibration curves. C, H, O, and N contents were determined by an elemental analyzer (Flash2000, ThermoFisher Scientific Inc., Waltham, MA, USA) in whole plant tissues. The elemental analyzer with 0.3 mg/kg limit of detection was calibrated with the standard (BBOT standard, Fisons Instruments SpA Strada Rivoltana, 20090 Rodano, Milan, Italy) for elemental analysis.
2.5. Functional amino acid analysis
Functional amino acids were analyzed according to the method described by Khan et al. (2013) and Waqas et al. (2015) in freeze-dried plant samples of all treatment types (50 mg). An amino acid standard mixture solution (type H) for an automatic amino acid analyzer (L-8900 Hitachi, Japan) was used for the quantification of endogenous amino acids. Each treatment was replicated three times.
2.6. Flavonoid extraction and quantification
Flavonoids were extracted and analyzed from powdered freeze-dried plant samples (0.2 g) of each treatment as described previously by Khan et al. (2013). After extraction, the samples (10 μl) were injected in a PerkinElmer series 200 high-performance liquid chromatography (HPLC) system (USA) fitted with COL-CHOICE C18 column 4.6 mm×150 mm (5 μm) pack. The solvent flow rate of using gradient solutions, viz. acetonitrile and 0.1% (1 g/L) acetic acid in water, was 1.0 ml/min. The elution was monitored at 260 nm by using series 200 UV/vis detector. Isoflavones were identified on the basis of comparisons with retention time of genuine standards obtained from Sigma Chemical Co., USA and LC Laboratory, USA.
2.7. Fatty acid analysis
For fatty acid extraction, the method described in Khan et al. (2012b) was followed. The processed final extraction from each sample (fresh 0.2 g) of 1 μl was collected and injected for analysis, carried out on gas chromatography (GC) with an Agilent Model 7890A series (Agilent, Dover, DE, USA) equipped with an Agilent 5975C mass spectrometry (MS) detector with MS ChemStation Agilent v. A.03.00 (Table S1). The constituent fatty acids were recognized based on the evaluation of their relative retention time and mass spectra with those of the standards, Wiley7N, the NIST library data of the GC-MS system, and data from the published literature. The numerical data were expressed as percentage area.
2.8. Determination of total sugar content, total phenolic content, and DPPH-scavenging activity
To determine total sugar content, a modified method of DuBois et al. (1956) was followed, as described in Albalasmeh et al. (2013).
Total phenolic content was quantified using Folin-Ciocalteu reagent through an improved version of the assay described by Slinkard and Singleton (1977). The total phenolic values having absorbance at 765 nm for all samples were expressed in terms of gallic acid equivalents (μg/ml). The blank (dimethyl sulphoxide (DMSO)) was treated in the same way as the other samples. However, the dilution factor was taken into account for those samples where dilution was performed.
1,1-Diphenyl-2-picrylhydrazyl (DPPH)-scavenging activity was analyzed by an assay modified from Gulati et al. (2012) and the absorbance was recorded at 490 nm. The results were compared with the control, i.e. 50 μl ethanol instead of culture samples, and their respective antioxidant activity was expressed as percent of inhibition (I): I (%)=(A 490,c−A 490,s)/A 490,c×100%, where A 490,c and A 490,s are the absorbances at 490 nm of control and samples, respectively.
2.9. Statistical analysis
The experiment was conducted in a completely randomized design and repeated three times with each treatment being replicated six times. Analysis of variance (ANOVA) was employed for statistical analysis and the mean values were compared with the Duncan’s multiple range test (DMRT) (P<0.05) using the statistical software program SAS (Version 9.2, Cary, NC, USA).
3. Results
3.1. Basic/essential elemental composition and fresh biomass of soybean individually and in combination with biochar and G. geotrichum
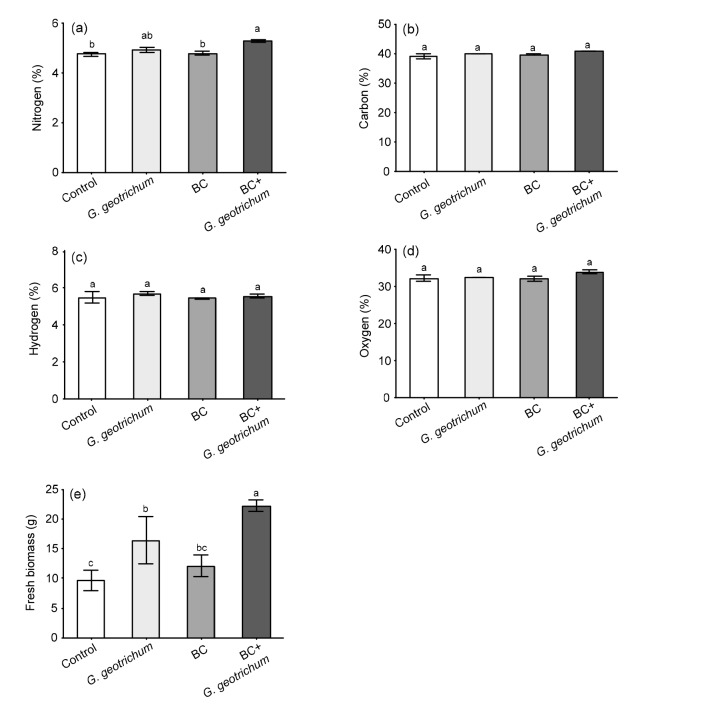
Percentages of N, C, H, and O contents were analyzed in whole plant tissue samples to evaluate the effect of treatments on basic/essential elemental composition or their assimilation in soybean plants (Fig. 1). In comparison to the control ((4.75±0.09)%), N content significantly increased in soybean plants treated with the co-application of BC+G. geotrichum ((5.28±0.05)%), followed by G. geotrichum alone ((4.95±0.02)%), as shown in Fig. 1a. However, C, H and O compositions in treated and control soybean plant tissues were not significantly different (Figs. 1b‒ 1d). Subsequently, the recorded fresh biomass revealed the same behavior and the highest values were documented in BC+G. geotrichum ((22.17±2.95) g) followed by G. geotrichum alone ((16.39±4.00) g) (Fig. 1e).
Fig. 1.
Basic elemental composition/assimilation and fresh biomass of soybean grown in substrates in the presence and absence of biochar (BC) and phytohormone-producing endophytic fungus, G. geotrichum, applied individually or in combination
(a) N content; (b) C content; (c) H content; (d) O content; (e) Fresh biomass. Each column bar with error bar represents the mean±standard deviation (SD) from six replicates of three independent experiments. The data were analyzed by ANOVA using SAS. Different letters show the significant differences (P<0.05) among treatments based on DMRT
3.2. Macro-and micronutrient dynamics in the substrate, root, and shoot of soybean with the individual and combined applications of biochar and G. geotrichum
Macro-and micronutrient analyses of the control and treated substrates show that P, K, S, Ca, Mg, and Mn were significantly higher in individual BC application, followed by control and BC+G. geotrichum application (Table 1). The individual application of G. geotrichum produced the lowest substrate nutrient status for P, K, S, Ca, Mg, and Mn. Fe was significantly higher in the control substrate, followed by the substrate treated with BC alone. The lowest concentration of Fe was found in the substrate treated with G. geotrichum alone and BC+G. geotrichum. Cu was found to be similar in the substrate treated with BC alone and control, but was not detected in the substrate treated with G. geotrichum alone or BC+G. geotrichum.
Table 1.
Macro-and micronutrient dynamics in the substrate after plant growth in the presence and absence of biochar and phytohormone-producing endophytic fungus, G. geotrichum, when applied individually and in combination
| Treatment | P (mg/kg) | K (mg/kg) | S (mg/kg) | Ca (mg/kg) | Mg (mg/kg) | Mn (mg/kg) | Fe (mg/kg) | Cu (mg/kg) |
| Control | 9.45±0.39b | 91.73±0.50b | 44.51±0.76b | 384.30±0.07b | 106.90±0.11b | 2.72±0.81b | 185.90±0.56a | 0.15±0.08a |
| GG | 7.22±0.49c | 42.99±0.46d | 22.36±0.28d | 45.23±0.47d | 44.97±0.28d | 1.01±0.64d | 78.12±0.42c | ND |
| BC | 13.44±0.99a | 122.50±0.67a | 92.97±0.94a | 441.50±0.79a | 217.20±0.64a | 3.41±0.95a | 113.50±0.59b | 0.15±0.01a |
| BC+GG | 6.93±0.73d | 48.00±0.53c | 39.09±0.57c | 148.20±0.95c | 90.57±0.36c | 1.29±0.69c | 72.59±0.19d | ND |
BC: biochar; GG: G. geotrichum; ND: not detected or not determined in the samples. Each value is expressed as mean±SD of six replicates from three independent experiments. The data were analyzed by ANOVA using SAS. Values in a column followed by different letters show the significant differences (P<0.05) among the treatments based on DMRT
The analysis of soybean root tissues (Table 2) revealed that P, K, S, Ca, Mg, and Mn were significantly higher in plants treated with G. geotrichum alone, followed by BC+G. geotrichum. The lowest amounts of these elements were found in plants treated with BC alone and the control. Fe was significantly higher in plants treated with BC alone, followed by G. geotrichum and BC+G. geotrichum (Table 2). Moreover, Cu was not detected in any of the treatments, including the control.
Table 2.
Macro-and micronutrient dynamics of soybean roots grown in the presence and absence of biochar and phytohormone-producing endophytic fungus, G. geotrichum, when applied individually and in combination
| Treatment | P (mg/kg) | K (mg/kg) | S (mg/kg) | Ca (mg/kg) | Mg (mg/kg) | Mn (mg/kg) | Fe (mg/kg) | Cu (mg/kg) |
| Control | 16.15±0.50c | 68.31±0.43c | 12.78±0.38b | 12.10±0.28c | 4.28±0.91c | 0.30±0.57c | 1.32±0.53d | ND |
| GG | 20.71±0.11a | 83.26±0.67a | 17.73±0.67a | 19.69±0.11a | 8.71±0.52a | 0.38±0.09a | 6.89±0.11b | ND |
| BC | 14.99±0.56d | 66.13±0.83d | 12.54±0.24b | 16.28±0.12b | 7.14±0.53b | 0.29±0.02d | 11.68±0.72a | ND |
| BC+GG | 17.40±0.72b | 81.31±0.96b | 17.68±0.57a | 19.60±0.18a | 7.15±0.09b | 0.33±0.04b | 3.36±0.40c | ND |
BC: biochar; GG: G. geotrichum; ND: not detected or not determined in the samples. Each value is expressed as mean±SD of six replicates from three independent experiments. The data were analyzed by ANOVA using SAS. Values in a column followed by different letters show the significant differences (P<0.05) among treatments based on DMRT
Macro-and micronutrients were differentially regulated in the tissues of treated and control soybean shoots (Table 3). Compared to the control, P, K, and S were significantly higher in the BC+G. geotrichum and individual BC treatments. Furthermore, Ca, Mg, Mn, and Fe were significantly higher in plants treated with BC alone, followed by the BC+G. geotrichum treatment and the control. However, Cu was also not detected in any of treatments.
Table 3.
Macro-and micronutrient dynamics in soybean shoots grown in the presence and absence of biochar and phytohormone-producing endophytic fungus, G. geotrichum, when applied individually and in combination
| Treatment | P (mg/kg) | K (mg/kg) | S (mg/kg) | Ca (mg/kg) | Mg (mg/kg) | Mn (mg/kg) | Fe (mg/kg) | Cu (mg/kg) |
| Control | 14.63±0.01c | 63.60±0.96c | 13.77±0.71c | 44.29±0.74b | 16.44±0.11c | 0.37±0.34c | 0.59±0.08d | ND |
| GG | 19.47±0.78b | 90.32±0.37b | 17.90±0.11b | 29.32±0.21c | 13.12±0.55d | 0.25±0.83d | 0.68±0.36c | ND |
| BC | 23.44±0.88a | 105.00±0.44a | 20.32±0.96a | 51.06±0.09a | 22.20±0.86a | 0.44±0.36a | 0.81±0.03a | ND |
| BC+GG | 24.87±0.69a | 107.20±0.62a | 22.60±0.90a | 43.15±0.69b | 19.71±0.81b | 0.41±0.25b | 0.75±0.61b | ND |
BC: biochar; GG: G. geotrichum; ND: not detected or not determined in the samples. Each value is expressed as mean±SD of six replicates from three independent experiments. The data were analyzed by ANOVA using SAS. Values in a column followed by different letters show the significant differences (P<0.05) among treatments based on DMRT
3.3. Functional amino acids of soybean in the presence and absence of biochar and G. geotrichum
The composition of functional amino acids from aliphatic, hydroxyl, aromatic, acidic, basic, and cyclic groups of soybean shoots in response to the individual and combined BC and G. geotrichum was analyzed, and the results are presented in Table 4. Compared to the control, from the analyzed amino acids, the isoleucine, glutamic acid, glycine, phenylalanine, methionine, cysteine, and arginine contents significantly increased in soybean tissues treated with BC+G. geotrichum. Aspartic acid, threonine, and lysine were significantly higher in soybean treated with G. geotrichum alone compared to the control. However, leucine and cysteine were significantly higher in plants treated with BC alone. Furthermore, compared to the control, valine, histidine, and proline levels were found to be statistically the same in treated soybean tissues, and remained significantly higher. In addition, tyrosine was only detected in soybean plants treated with G. geotrichum alone and the control.
Table 4.
Functional amino acid contents of soybean in response to the presence and absence of biochar and G. geotrichum when applied individually and in combination
| Treatment | Asp (µg/g) | Thr (µg/g) | Met (µg/g) | ILE (µg/g) | Ser (µg/g) | Glu (µg/g) | Leu (µg/g) | Tyr (µg/g) | Gly (µg/g) | Phe (µg/g) | Lys (µg/g) | Cys (µg/g) | Val (µg/g) | His (µg/g) | Arg (µg/g) | Pro (µg/g) | Total amino acids (µg/g) | |
| Control | 20.73±2.13d | 7.09±0.37c | 1.52±0.56c | 11.42±0.71b | 4.64±0.84d | 13.96±1.96d | 24.16±2.10b | 5.37±0.22a | 5.70±0.38d | 11.39±1.94ab | 8.62±0.62ab | 2.78±0.63d | 10.34±1.51b | 3.88±0.41b | 10.59±0.92c | 7.86±2.81b | 150.05c | |
| GG | 45.70±5.95a | 11.51±1.15a | 1.79±0.49b | 11.79±1.32ab | 9.03±0.75a | 20.55±1.45b | 26.42±2.64b | 5.79±0.14a | 8.28±0.15b | 8.36±1.64b | 13.00±1.02a | 2.86±0.73c | 14.02±3.85a | 5.30±0.24a | 15.14±1.94b | 10.66±2.26a | 210.05a | |
| BC | 31.99±3.78c | 9.51±0.76b | 1.41±0.07d | 8.28±0.96c | 5.99±0.25c | 18.04±1.15c | 30.49±2.90a | 0.00±0.00b | 6.39±0.95c | 11.03±1.30ab | 7.08±0.29b | 3.57±0.55a | 14.51±2.75a | 5.23±0.34a | 15.42±2.26b | 10.42±1.05a | 179.36b | |
| BC+GG | 36.12±4.71b | 9.62±1.45b | 2.12±0.86a | 12.45±1.24a | 6.26±0.31b | 30.66±1.76a | 22.95±0.00c | 0.00±0.00b | 11.52±1.08a | 12.22±1.17a | 9.80±0.68ab | 3.66±0.35a | 14.98±2.94a | 6.18±0.47a | 17.07±1.16a | 11.32±2.65a | 206.57a | |
BC: biochar; GG: G. geotrichum; Asp: aspartic acid; Thr: threonine; Met: methionine; ILE: isoleucine; Ser: serine; Glu: glutamic acid; Leu: leucine; Tyr: tyrosine; Gly: glycine; Phe: phenylalanine; Lys: lysine; Cys: cysteine; Val: valine; His: histidine; Arg: arginine; Pro: proline. The amino acids were analyzed from freeze-dried soybean plants in the presence and absence of biochar and G. geotrichum applied individually and in combination, to identify the beneficial effects of their interactions. Each value is expressed as mean±SD of six replicates from three independent experiments. The data were analyzed by ANOVA using SAS. Values in a column followed by different letters show the significant differences (P<0.05) among treatments based on DMRT
3.4. Isoflavone regulation in soybean plants in the individual and combined applications of biochar and G. geotrichum
Compared to the control plants and those treated individually with G. geotrichum, total isoflavone content significantly increased in individual BC and BC+G. geotrichum treatments (Table 5). Of the aglycone class of isoflavones, daidzein and glycitein significantly increased in plants treated with BC alone, followed by BC+G. geotrichum, compared to the control. However, genistein was only detected in the control, but not in any of the treated soybean plants.
Table 5.
Isoflavone contents of soybean in response to the presence and absence of biochar and G. geotrichum applied individually and in combination
| Treatment | Isoflavone aglycones (μg/g) |
Malonyl isoflavones (μg/g) |
Acetyl isoflavones (μg/g) |
Isoflavone glucosides (μg/g) |
Total (μg/g) | |||||||
| Daidzein | Glycitein | Genistein | Daidzin | Glycitin | Genistin | Daidzin | Genistin | Daidzin | Glycitin | Genistin | ||
| Control | 95.40±0.03c | 144.07±0.04b | 1.11±0.67a | 4.53±0.08c | 20.07±0.88b | 31.80±0.42c | ND | ND | ND | 0.00±0.00c | 0.60±0.09a | 297.58±55.74b |
| GG | 95.85±0.17c | 148.81±0.22b | ND | 4.45±0.00c | 20.31±1.03b | 34.15±0.24c | ND | ND | ND | 2.61±0.06b | ND | 306.18±58.84b |
| BC | 111.15±0.08a | 183.30±0.26a | ND | 11.99±0.02b | 24.64±0.06a | 42.81±0.10b | ND | ND | ND | 3.86±0.24a | ND | 377.75±70.38a |
| BC+GG | 105.18±0.07b | 148.00±0.31b | ND | 29.37±0.20a | 21.51±0.21b | 77.00±0.35a | ND | ND | ND | 4.16±0.19a | ND | 385.22±55.69a |
BC: biochar; GG: G. geotrichum; ND: not detected or not determined in the samples. The isoflavones were analyzed from freeze-dried soybean plants in the presence and absence of biochar and G. geotrichum applied individually and in combination, to identify the beneficial effect of their interaction. Each value is expressed as mean±SD of six replicates from three independent experiments. The data were analyzed by ANOVA using SAS. Values in a column followed by different letters show the significant differences (P<0.05) among treatments based on DMRT
In the malonyl class of isoflavones, malonyldaidzin and malonylgenistin significantly increased compared to the control in the plants treated with BC+G. geotrichum, while malonylglycitin increased in plants treated with BC alone. However, none of the isoflavones in the acetyl class was detected in any of the treatments, including the control.
In the glucoside class of isoflavones, only glycitin was detected in all of the treatments, and was significantly higher in plants treated with BC alone and BC+G. geotrichum than in the control. Daidzin was not detected in any of the treatments, while genistin was only detected in control plants.
3.5. Saturated and unsaturated fatty acid composition
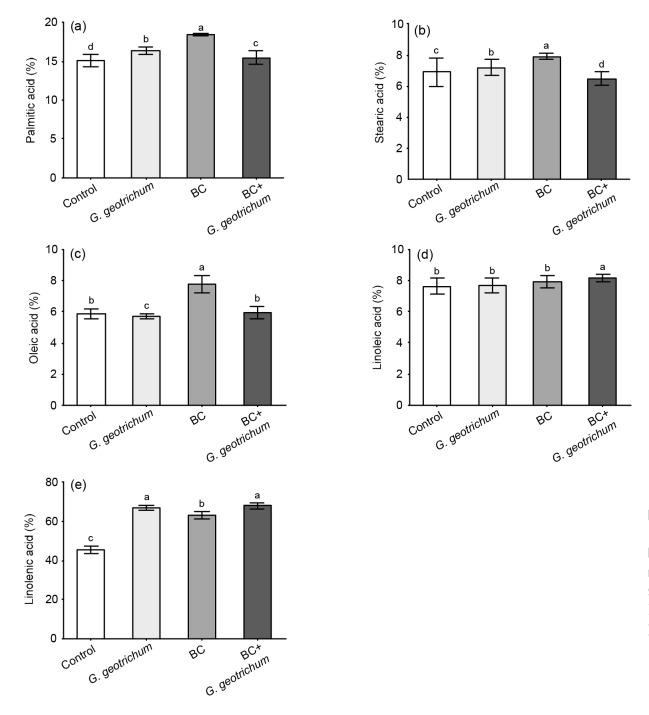
The analysis of free fatty acid composition showed that palmitic acid (C16:0), stearic acid (C18:0), and oleic acid (C18:1) significantly increased in soybean plants treated with BC alone compared to the control (Figs. 2a‒2c). In contrast, these fatty acids (C16:0, C18:0, and C18:1) were significantly reduced in soybean plant tissues treated with BC+G. geotrichum. However, linoleic (C18:2) and linolenic (C18:3) acids were significantly higher in the BC+G. geotrichum treatment compared to the control (Figs. 2d and 2e).
Fig. 2.
Fatty acid contents of soybean grown in substrate in the presence and absence of biochar (BC) and phytohormone-producing endophytic fungus, G. geotrichum, when applied individually and in combination
Each column bar with error bar represents the mean±SD from six replicates of three independent experiments. The data were analyzed by ANOVA using SAS. Different letters show the significant differences (P<0.05) among treatments based on DMRT
3.6. Total sugar and total phenolic contents and DPPH-scavenging activity
Compared to the control ((0.62±0.02) mg/g), total sugar content was significantly higher in soybean plants treated with BC+G. geotrichum ((1.71±0.33) mg/g) and G. geotrichum alone ((1.62±0.32) mg/g) (Table 6). Similarly, compared to the control ((300.71±5.13) mg/g), total phenolic content (Table 6) was significantly higher in soybean plants treated with BC+G. geotrichum ((407.50±12.90) mg/g) and G. geotrichum alone ((389.20±8.30) mg/g).
Table 6.
Biosynthesis of total sugar content, total phenolic content, and DPPH inhibition activity of soybean grown in the presence and absence of biochar and phytohormone-producing endophytic fungus, G. geotrichum, when applied individually and in combination
| Treatment | Total sugar content (mg/g) | Total phenolic content (mg/g) | DPPH inhibition activity (%) |
| Control | 0.62±0.02b | 300.70±5.13b | 51.28 |
| GG | 1.62±0.32a | 389.20±8.30a | 68.92 |
| BC | 0.62±0.15b | 306.90±4.10b | 55.28 |
| BC+GG | 1.71±0.33a | 407.50±12.90a | 73.34 |
|
| |||
| BC: biochar; GG: G. geotrichum. Each value is expressed as mean±SD of six replicates from three independent experiments. The data were analyzed by ANOVA using SAS. Values in a column followed by different letters show the significant differences (P<0.05) among treatments based on DMRT | |||
DPPH-scavenging activity is used to measure the antioxidant properties of plants or their food product and hence the nutraceutical importance of plants is indexed and recommended for human food consumption. In the light of this importance, this parameter was measured. Therefore, compared to the control (51.28%), the highest percentage of DPPH-scavenging activity was obtained for soybean plants treated with BC+G. geotrichum (73.34%) and G. geotrichum (68.92%) alone (Table 6).
4. Discussion
The significant increase in a basic element, namely N (Fig. 1a), in soybean treated with BC+G. geotrichum may be attributed to the additive effect of the plant growth promoting effect of BC and the phytohormone-producing endophytic fungus G. geotrichum (Waqas et al., 2014). Both BC and, particularly, endophytes have been reported to improve basic elemental composition under normal and stressful conditions (Waqas et al., 2012; Khan and Lee, 2013; Zhang et al., 2014). For instance, it was found that the optimization of composted green waste for optimum plant growth of Calathea insignis was best obtained with 20% BC and 0.7% humic acid, which, compared to the control, significantly increased the total N content of C. insignis leaves by 66.4% (Zhang et al., 2014). In addition to this property of BC, the combined application of endophytes in the current study may further enhance the assimilation of N, as also reported in Khan and Lee (2013). This paper reported that endophytic association offered by Penicillium funiculosum increased the N assimilation of the host soybean plants, maintaining high growth under normal and stressed conditions (copper heavy metal). Furthermore, high N levels would enhance plant metabolism and fix more atmospheric C via photosynthesis (Evans, 1989; Newman et al., 2003; Heinonsalo et al., 2015). The high assimilation of CO2 depends on N and its allocation in plant organs. This phenomenon was observed in Scots pine, in which more C was allocated to the roots than to the shoots, which was because of the presence of more N in the roots (Heinonsalo et al., 2015).
Most published studies have shown that the BC contains significant amounts of macro-and micronutrients, with the amount depending on the feedstock source, which leads to variation in the extent to which soil nutrient status is improved (Martinsen et al., 2014; Waqas et al., 2014; Zhao et al., 2014; Butnan et al., 2015; Haider et al., 2015). The high amount of these nutrients in BC-amended substrate (Table 1) may be because they were present in the BC (see materials and methods) applied in this experiment and, therefore, had an advantage over the control and only endophyte-treated soils (Zhao et al., 2014; Butnan et al., 2015). However, the TBT substrate used does not simulate the real situation as the common soil constituents could interact with plant and endophytic fungi. BC also indirectly enhances soil nutrient availability by modifying soil structure, reducing Al3+ solubility, increasing soil pH, and increasing cation exchange capacity (Martinsen et al., 2014; Waqas et al., 2014; Zhao et al., 2014; Butnan et al., 2015). However, the high P availability in BC-amended soil detected in our study supports the findings of Zhai et al. (2015) but contradicts those of Ahmed and Schoenau (2015). The reason for this discrepancy with Ahmed and Schoenau (2015) may be differences in the characteristics of the feedstock used, the pyrolysis process used for BC preparation, or soil temperature conditions. BC derived from various feed stock sources exhibits high variation in nutrient content; thus BC sourced from a different feed stock may show the same or opposite behavior. Therefore, the hardwood-derived BC in the current study showed the same behavior of increasing soil P availability as that of the maize residue BC used by Zhai et al. (2015), but is different from the BC derived from wheat straw, flax straw, and willow stems used by Ahmed and Schoenau (2015). Compared to the control, the lowest amount of nutrients was obtained in the substrate of soybean plants treated with G. geotrichum alone, followed by BC+G. geotrichum, and finally the control. These results demonstrate the nutrient uptake ability of G. geotrichum. As a result, the macro-and micronutrient statuses of endophyte-amended substrate in the presence and absence of BC were diminished, while their concentrations increased in soybean, as shown by the analysis of the root and shoot tissues (Tables 2 and 3). Here, the G. geotrichum may have acted as a biostimulating organism and therefore more macro-and micronutrients were assimilated in the shoot from the additional provided source in the form of BC. These findings support those of Waqas et al. (2012), Khan and Lee (2013), and Hammer et al. (2014), who reported that endophytes increase the ability of host plants to uptake macro-and micro-nutrients, particularly P, K, S, and Ca. For example, Hammer et al. (2014) provided the first evidence of the ability of a plant-associated fungus to enhance nutrient transfer from BC to the host plant.
In the current study, most nutrients were retained in the roots of G. geotrichum and BC+G. geotrichum treatment, as shown in the analysis comparing the amount of nutrients present in the shoots of the same plants. The translocation of nutrients inside the plant organs of endophyte-infected plants supports our previous results regarding the uptake of Zn during heavy metal stress (Waqas et al., 2014). Zn was significantly higher in endophyte-treated roots compared to the roots in the other treatments (Waqas et al., 2014).
Amino acids are the building blocks of proteins and contribute to several important metabolic functions during normal and stress conditions in plants (Khan et al., 2013; Tegeder, 2014; Waqas et al., 2015). Amino acid synthesis depends on photosynthetic activity, which, in turn, depends on other factors, including nutrient availability (Weckopp and Kopriva, 2015). N and S are the two major nutrients that plants use for the synthesis of amino acids (Khan et al., 2013; Tegeder, 2014). Here, compared with the control, higher amounts of amino acids were synthesized (Table 4) in soybean plants treated with BC+G. geotrichum and individually with G. geotrichum and BC, which may be because of the greater uptakes of S and N and other supporting nutrients, like Mg (component of chlorophyll compound structure), due to greater availability. The significant amounts of amino acids in soybean shoots treated with BC+endophyte (G. geotrichum) and G. geotrichum alone corroborate the results of Khan et al. (2013), Khan and Lee (2013), and Waqas et al. (2015). The studies by these authors showed that the application of endophytes extends their beneficial effects by regulating amino acid production under normal and stress conditions. The regulation of amino acid synthesis subsequently mitigates and confers (abiotic and biotic) stress resistance in pepper, soybean, and sunflower, in addition to enhancing their anti-oxidative and hormone signaling activities (Khan et al., 2013; Khan and Lee, 2013; Waqas et al., 2015). In the case of amino acid up-regulation, soybean shoots treated with BC via individual application contributed more nutrients to plants (Elad et al., 2011). An increase in amino acid levels allows soybean plants to carry out secondary metabolite formation efficiently, and synthesize more vitamins (like vitamins B1, B2, B3, B5, B7, B9, and E) and proteins (Tzin and Galili, 2010; Miret and Munné-Bosch, 2014). The up-or down-regulation of both N and S limits the function of other nutrients (McGrath and Zhao, 1996). The application of BC may also serve as an additional balanced source of S and N provisions (Elad et al., 2011; Cheah et al., 2014; Waqas et al., 2014). The role of both nutrients is central and interrelated to amino acid biosynthesis and, hence, proteins (McGrath and Zhao, 1996).
Isoflavones are the main secondary metabolites of legumes, and are synthesized through the phenylpropanoid pathway (Khan et al., 2013; Algar et al., 2014). Isoflavones have several important functions during host plant environmental interactions. For example, isoflavones induce nodulation gene expression to facilitate the association of biological nitrogen fixing bacteria with legume crops, and they produce phytoalexins for defense against insects and pathogens (Algar et al., 2014; Ramos-Solano et al., 2015). Furthermore to reduce stress damaging effects, flavonoids act as antioxidant agents to neutralize reactive oxygen species and maintain the normal functioning of the plant cellular membrane (Khan et al., 2013). The enhanced production of isoflavones (Table 5) in soybean treated with BC and BC+G. geotrichum demonstrates treatment efficacy, and that they may contribute to plant nutritional value and defense requirements. Because they are secondary metabolite products, isoflavonoids are strongly induced by external stimuli to keep plants in prime condition (Hao et al., 2010; Ramos-Solano et al., 2015). Here, the enhanced production of isoflavonoids in the presence of BC alone or in combination with the fungal endophyte (G. geotrichum) demonstrates the priming effects of both treatments, supporting the findings of Hao et al. (2010), Khan et al. (2013), and Waqas et al. (2014). Waqas et al. (2014) reported that the priming effect of BC alone or in combination with fungal endophytes keeps soybean plants in a steady state, and improves growth and development under high Zn heavy metal stress. Moreover, Elad et al. (2011) and Harel et al. (2012) explored the possible involvement of the BC priming effect in eliciting the systemic acquired resistance (SAR) and induced systemic resistance (ISR) pathways. The results of these two studies showed that BC removes biotic stress caused to strawberry plants by necrotrophic, hemi-biotrophic, and biotrophic pathogens by inducing general defense pathways. Thus, in addition to fungal endophytes, BC may contribute to the elicitation of the flavonoid/phenylpropanoid pathway, which may explain the enhanced defense mechanism in strawberry observed by Harel et al. (2012).
Fatty acids have many important roles in maintaining the normal functioning of plant physiological processes during biotic and abiotic stress (Upchurch, 2008; Steindal et al., 2015). During necrotrophic pathogen and pests attack, the membrane lipid releases α-linolenic acid (a precursor molecule of phyto-oxylipin) to initiate the synthesis of jasmonic acids for defense purposes. Similarly, in Arabidopsis, oleic acid in the chloroplast mediates pathogen attack by the normal expression of the defense response (Upchurch, 2008). The results of the current investigation indicate that saturated fatty acids significantly increased in plants treated with BC alone, but showed the opposite trend for BC+G. geotrichum (Fig. 2). However, the unsaturated fatty acids were significantly higher in plants treated with BC+G. geotrichum. These observations show that the fatty acid profile is highly responsive to agronomic practices. Among agronomic practices, optimum nutrient management and the supply of adequate moisture are very important for plant growth and have a profound effect on plant yield and quality (Bellaloui et al., 2011; 2015). Bellaloui et al. (2011) conducted an experiment to evaluate the response of fatty acid composition to different agronomic (irrigated and non-irrigated) conditions along with the individual and combined dosages of S and N. It was concluded that the nutritional composition of soybean seeds was altered due to differences in the treatments, with it possible to tailor the desirable treatment to the specific requirement. The similar application and optimization of BC (i.e. in the presence and absence of endophytes in this study) to field crops could provide another way of improving the nutrient value of agricultural produce. In the current study, the combination of gibberellin and IAA-producing endophyte (e.g. G. geotrichum) with BC increased fatty acid content; however, various studies obtained the same results for the association of endophytic fungi with their host plants (Khan et al., 2012a; 2012b). In these and other studies, the production of secondary metabolites (mainly phytohormones) by endophytes was found to be the main reason for this effect (Khan et al., 2012a; 2012b; Jusoh et al., 2015). Furthermore, GA production by endophytes (Khan et al., 2012a; 2012b) and the exogenous application of IAA (Jusoh et al., 2015) have been reported to modulate fatty acids in plants under normal and stress conditions. Both types of phytohormones were detected in G. geotrichum (Waqas et al., 2014) and may be factors for the same results being reported.
Soybean is one of the most important sources of carbohydrate (sugar) and phenolic compounds with antioxidant activity (Malenčić et al., 2008; Bellaloui et al., 2015). It is important to mention that the modulation in phenolic compounds due to different treatments was measured in term of DPPH-scavenging activity and evaluation of the antioxidative properties. The DPPH free radicals readily accept reactive hydrogen or electrons and become a stable diamagnetic compound. In this manner, the unwanted production of reactive oxygen species is controlled to prevent damage to cellular organelles and the structural integrity of cells under stress conditions (Izuta et al., 2009). The enhancements of total sugar content, phenolic content, and DPPH-scavenging activity of soybean plants (Table 6) in response to BC+G. geotrichum and G. geotrichum alone support the results of Obledo et al. (2003), Huang et al. (2007a; 2007b), Pańka et al. (2013), and Patel et al. (2015). During a five-year experiment, Patel et al. (2015) reported that continuous organic amendments enhanced produce quality through building and sustaining soil health and productivity. The same building and conditioning characteristics have been reported for BC in agricultural soil, along with the long term provision of nutrients through the slow release mechanism (Lehmann, 2007; Elad et al., 2011; Martinsen et al., 2014; Zhang et al., 2014). This phenomenon might explain why BC applied in combination with an endophyte showed a synergistic effect in the current experiment. However, several studies have reported that endophytes alone improve plant primary and secondary metabolites (Obledo et al., 2003; Huang et al., 2007a; 2007b; Khan and Lee, 2013). The increased accumulations of total sugar content, phenolic content, and DPPH-scavenging activity in soybean biomass after treatment with BC+G. geotrichum or G. geotrichum alone demonstrate that both factors have a priming effect on plants, readying them for disturbance by abiotic and biotic stress (Porcel and Ruiz-Lozano, 2004; Huang et al., 2007b).
5. Conclusions
Sequestration of increasing atmospheric carbon (C) is highly desirable for a safe and sustainable environment in the current industrial era. BC and bioactive fungal endophytes have recently been confirmed as a stable source for mitigating C in terrestrial ecosystems (Lehmann, 2007; Iqbal et al., 2012) and stimulating plant growth, but have been previously much less reported for their ability in combination to enhance plant nutrient uptake and the nutritional status. To investigate these issues, we determined from the results of this study that the combined and individual applications of BC (hardwood-derived) and a bioactive endophyte (G. geotrichum WLL1) significantly improved the assimilation and uptakes of basic, macro-and micronutrients in soybean. The ensuing beneficial effect was then observed by the improved biosynthesis of functional amino acids and nutritional characteristics, including isoflavone regulation, saturated and unsaturated fatty acid contents, total sugar and total phenolic contents, and antioxidative properties based on DPPH-scavenging activity. It may be concluded that the symbiotic association of bioactive fungal endophytes with BC generated additive effects, making them more effective in combination than individually. Thus, it is recommended that endophytes should be applied with BC to enhance soybean crop quality by creating a more desirable environment-friendly conditions in a highly enriched carbon concentrated atmosphere. However, to provide recommendations, field-scale studies should be conducted first to ensure that the BC used is compatible (type-wise by origin/substrate and preparation or pyrolysis condition) with the selected endophyte.
List of electronic supplementary materials
Details of GC-MS conditions for the analysis of fatty acids
Footnotes
Project supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agriculture, Food and Rural Affairs Research Center Support Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (716001-7)
Electronic supplementary materials: The online version of this article (http://dx.doi.org/10.1631/jzus.B1500262) contains supplementary materials, which are available to authorized users
Compliance with ethics guidelines: Muhammad WAQAS, Yoon-Ha KIM, Abdul Latif KHAN, Raheem SHAHZAD, Sajjad ASAF, Muhammad HAMAYUN, Sang-Mo KANG, Muhammad Aaqil KHAN, and In-Jung LEE declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Ahmed HP, Schoenau JJ. Effects of biochar on yield, nutrient recovery, and soil properties in a canola (Brassica napus L)-wheat (Triticum aestivum L) rotation grown under controlled environmental conditions. BioEnerg Res. 2015;8(3):1183–1196. (Available from: http://dx.doi.org/10.1007/s12155-014-9574-x) [Google Scholar]
- 2.Albalasmeh AA, Berhe AA, Ghezzehei TA. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr Polym. 2013;97(2):253–261. doi: 10.1016/j.carbpol.2013.04.072. (Available from: http://dx.doi.org/10.1016/j.carbpol.2013.04.072) [DOI] [PubMed] [Google Scholar]
- 3.Algar E, Gutierrez-Mañero FJ, Garcia-Villaraco A, et al. The role of isoflavone metabolism in plant protection depends on the rhizobacterial MAMP that triggers systemic resistance against Xanthomonas axonopodis pv. glycines in Glycine max (L.) Merr. cv. Osumi. Plant Physiol Biochem. 2014;82:9–16. doi: 10.1016/j.plaphy.2014.05.001. (Available from: http://dx.doi.org/10.1016/j.plaphy.2014.05.001) [DOI] [PubMed] [Google Scholar]
- 4.Bayabil HK, Stoof CR, Lehmann JC, et al. Assessing the potential of biochar and charcoal to improve soil hydraulic properties in the humid Ethiopian Highlands: the Anjeni watershed. Geoderma. 2015;243-244:115–123. (Available from: http://dx.doi.org/10.1016/j.geoderma.2014.12.015) [Google Scholar]
- 5.Bellaloui N, Ebelhar MW, Gillen AM, et al. Soybean seed protein, oil, and fatty acids are altered by S and S+N fertilizers under irrigated or non-irrigated environments. Agric Sci. 2011;2(4):465–476. (Available from: http://dx.doi.org/10.4236/as.2011.24060) [Google Scholar]
- 6.Bellaloui N, Bruns H, Abbas HK, et al. Agricultural practices altered soybean seed protein, oil, fatty acids, sugars, and minerals in the Midsouth USA. Front Plant Sci. 2015;6:31. doi: 10.3389/fpls.2015.00031. (Available from: http://dx.doi.org/10.3389/fpls.2015.00031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butnan S, Deenik JL, Toomsan B, et al. Biochar characteristics and application rates affecting corn growth and properties of soils contrasting in texture and mineralogy. Geoderma. 2015;237-238:105–116. (Available from: http://dx.doi.org/10.1016/j.geoderma.2014.08.010) [Google Scholar]
- 8.Cheah S, Malone SC, Feik CJ. Speciation of sulfur in biochar produced from pyrolysis and gasification of oak and corn stover. Environ Sci Technol. 2014;48(15):8474–8480. doi: 10.1021/es500073r. (Available from: http://dx.doi.org/10.1021/es500073r) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Corato U, Pane C, Bruno GL, et al. Co-products from a biofuel production chain in crop disease management: a review. Crop Prot. 2015;68:12–26. (Available from: http://dx.doi.org/10.1016/j.cropro.2014.10.025) [Google Scholar]
- 10.Dong D, Feng Q, McGrouther K, et al. Effects of biochar amendment on rice growth and nitrogen retention in a waterlogged paddy field. J Soils Sediments. 2015;15(1):153–162. (Available from: http://dx.doi.org/10.1007/s11368-014-0984-3) [Google Scholar]
- 11.DuBois M, Gilles KA, Hamilton JK, et al. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350–356. (Available from: http://dx.doi.org/10.1021/ac60111a017) [Google Scholar]
- 12.Elad Y, Cytryn E, Harel YM, et al. The biochar effect: plant resistance to biotic stresses. Phytopathol Mediterr. 2011;50:335–349. [Google Scholar]
- 13.Evans JR. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia. 1989;78(1):9–19. doi: 10.1007/BF00377192. (Available from: http://dx.doi.org/10.1007/BF00377192) [DOI] [PubMed] [Google Scholar]
- 14.Gulati V, Harding IH, Palombo EA. Enzyme inhibitory and antioxidant activities of traditional medicinal plants: potential application in the management of hyperglycemia. BMC Complement Altern Med. 2012;12(1):77. doi: 10.1186/1472-6882-12-77. (Available from: http://dx.doi.org/10.1186/1472-6882-12-77) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gwenzi W, Chaukura N, Mukome FND, et al. Biochar production and applications in sub-Saharan Africa: opportunities, constraints, risks and uncertainties. J Environ Manage. 2015;150:250–261. doi: 10.1016/j.jenvman.2014.11.027. (Available from: http://dx.doi.org/10.1016/j.jenvman.2014.11.027) [DOI] [PubMed] [Google Scholar]
- 16.Haider G, Koyro HW, Azam F, et al. Biochar but not humic acid product amendment affected maize yields via improving plant-soil moisture relations. Plant Soil. 2015;395(1):141–157. (Available from: http://dx.doi.org/10.1007/s11104-014-2294-3) [Google Scholar]
- 17.Hammer EC, Balogh-Brunstad Z, Jakobsen I, et al. A mycorrhizal fungus grows on biochar and captures phosphorus from its surfaces. Soil Biol Biochem. 2014;77:252–260. (Available from: http://dx.doi.org/10.1016/j.soilbio.2014.06.012) [Google Scholar]
- 18.Hao G, Du X, Zhao F, et al. Fungal endophytes-induced abscisic acid is required for flavonoid accumulation in suspension cells of Ginkgo biloba . Biotechnol Lett. 2010;32(2):305–314. doi: 10.1007/s10529-009-0139-6. (Available from: http://dx.doi.org/10.1007/s10529-009-0139-6) [DOI] [PubMed] [Google Scholar]
- 19.Harel YM, Elad Y, Rav-David D, et al. Biochar mediates systemic response of strawberry to foliar fungal pathogens. Plant Soil. 2012;357(1):245–257. (Available from: http://dx.doi.org/10.1007/s11104-012-1129-3) [Google Scholar]
- 20.Hartley SE, Eschen R, Horwood JM, et al. Infection by a foliar endophyte elicits novel arabidopside-based plant defence reactions in its host, Cirsium arvense . New Phytol. 2015;205(2):816–827. doi: 10.1111/nph.13067. (Available from: http://dx.doi.org/10.1111/nph.13067) [DOI] [PubMed] [Google Scholar]
- 21.Heinonsalo J, Juurola E, Linden A, et al. Ectomycorrhizal fungi affect Scots pine photosynthesis through nitrogen and water economy, not only through increased carbon demand. Environ Exp Bot. 2015;109:103–112. (Available from: http://dx.doi.org/10.1016/j.envexpbot.2014.08.008) [Google Scholar]
- 22.Huang WY, Cai YZ, Hyde KD, et al. Endophytic fungi from Nerium oleander L (Apocynaceae): main constituents and antioxidant activity. World J Microbiol Biotechnol. 2007;23(9):1253–1263. (Available from: http://dx.doi.org/10.1007/s11274-007-9357-z) [Google Scholar]
- 23.Huang WY, Cai YZ, Xing J, et al. A potential antioxidant resource: endophytic fungi from medicinal plants. Econ Bot. 2007;61(1):14–30. (Available from: http://dx.doi.org/10.1663/0013-0001(2007)61[14:APAREF]2.0.CO;2) [Google Scholar]
- 24.Iqbal J, Siegrist JA, Nelson JA, et al. Fungal endophyte infection increases carbon sequestration potential of southeastern USA tall fescue stands. Soil Biol Biochem. 2012;44(1):81–92. (Available from: http://dx.doi.org/10.1016/j.soilbio.2011.09.010) [Google Scholar]
- 25.Izuta H, Narahara Y, Shimazawa M, et al. 1,1-Diphenyl-2-picrylhydrazyl radical scavenging activity of bee products and their constituents determined by ESR. Biol Pharm Bull. 2009;32(12):1947–1951. doi: 10.1248/bpb.32.1947. (Available from: http://dx.doi.org/10.1248/bpb.32.1947) [DOI] [PubMed] [Google Scholar]
- 26.Jusoh M, Loh SH, Chuah TS, et al. Indole-3-acetic acid (IAA) induced changes in oil content, fatty acid profiles and expression of four fatty acid biosynthetic genes in Chlorella vulgaris at early stationary growth phase. Phytochemistry. 2015;111:65–71. doi: 10.1016/j.phytochem.2014.12.022. (Available from: http://dx.doi.org/10.1016/j.phytochem.2014.12.022) [DOI] [PubMed] [Google Scholar]
- 27.Khan AL, Lee IJ. Endophytic Penicillium funiculosum LHL06 secretes gibberellin that reprograms Glycine max L. growth during copper stress. BMC Plant Biol. 2013;13(1):86. doi: 10.1186/1471-2229-13-86. (Available from: http://dx.doi.org/10.1186/1471-2229-13-86) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan AL, Hamayun M, Waqas M, et al. Exophiala sp. LHL08 association gives heat stress tolerance by avoiding oxidative damage to cucumber plants. Biol Fertil Soils. 2012;48(5):519–529. (Available from: http://dx.doi.org/10.1007/s00374-011-0649-y) [Google Scholar]
- 29.Khan AL, Hamayun M, Radhakrishnan R, et al. Mutualistic association of Paecilomyces formosus LHL10 offers thermotolerance to Cucumis sativus . Antonie Van Leeuwenhoek. 2012;101(2):267–279. doi: 10.1007/s10482-011-9630-x. (Available from: http://dx.doi.org/10.1007/s10482-011-9630-x) [DOI] [PubMed] [Google Scholar]
- 30.Khan AL, Kang SM, Dhakal KH, et al. Flavonoids and amino acid regulation in Capsicum annuum L. by endophytic fungi under different heat stress regimes. Sci Hortic. 2013;155:1–7. (Available from: http://dx.doi.org/10.1016/j.scienta.2013.02.028) [Google Scholar]
- 31.Khan AL, Waqas M, Hussain J, et al. Fungal endophyte Penicillium janthinellum LK5 can reduce cadmium toxicity in Solanum lycopersicum (Sitiens and Rhe) Biol Fertil Soils. 2014;50(1):75–85. (Available from: http://dx.doi.org/10.1007/s00374-013-0833-3) [Google Scholar]
- 32.Lehmann J. A handful of carbon. Nature. 2007;447(7141):143–144. doi: 10.1038/447143a. (Available from: http://dx.doi.org/10.1038/447143a) [DOI] [PubMed] [Google Scholar]
- 33.Li M, Lou Z, Wang Y, et al. Alkali and alkaline earth metallic (AAEM) species leaching and Cu(II) sorption by biochar. Chemosphere. 2015;119:778–785. doi: 10.1016/j.chemosphere.2014.08.033. (Available from: http://dx.doi.org/10.1016/j.chemosphere.2014.08.033) [DOI] [PubMed] [Google Scholar]
- 34.Likar M, Regvar M. Isolates of dark septate endophytes reduce metal uptake and improve physiology of Salix caprea L. Plant Soil. 2013;370(1):593–604. (Available from: http://dx.doi.org/10.1007/s11104-013-1656-6) [Google Scholar]
- 35.Malenčić D, Maksimović Z, Popović M, et al. Polyphenol contents and antioxidant activity of soybean seed extracts. Bioresource Technol. 2008;99(14):6688–6691. doi: 10.1016/j.biortech.2007.11.040. (Available from: http://dx.doi.org/10.1016/j.biortech.2007.11.040) [DOI] [PubMed] [Google Scholar]
- 36.Martinsen V, Mulder J, Shitumbanuma V, et al. Farmer-led maize biochar trials: effect on crop yield and soil nutrients under conservation farming. J Plant Nutr Soil Sci. 2014;177(5):681–695. (Available from: http://dx.doi.org/10.1002/jpln.201300590) [Google Scholar]
- 37.McGrath SP, Zhao FJ. Sulphur uptake, yield responses and the interactions between nitrogen and sulphur in winter oilseed rape (Brassica napus) J Agric Sci. 1996;126(1):53–62. (Available from: http://dx.doi.org/10.1017/S0021859600088808) [Google Scholar]
- 38.Miret JA, Munné-Bosch S. Plant amino acid-derived vitamins: biosynthesis and function. Amino Acids. 2014;46(4):809–824. doi: 10.1007/s00726-013-1653-3. (Available from: http://dx.doi.org/10.1007/s00726-013-1653-3) [DOI] [PubMed] [Google Scholar]
- 39.Newman JA, Abner ML, Dado RG, et al. Effects of elevated CO2, nitrogen and fungal endophyte-infection on tall fescue: growth, photosynthesis, chemical composition and digestibility. Global Change Biol. 2003;9(3):425–437. (Available from: http://dx.doi.org/10.1046/j.1365-2486.2003.00601.x) [Google Scholar]
- 40.Obledo EN, Barragán-Barragán LB, Gutiérrez-González P, et al. Increased photosyntethic efficiency generated by fungal symbiosis in Agave victoria-reginae . Plant Cell Tissue Organ Cult. 2003;74(3):237–241. (Available from: http://dx.doi.org/10.1023/A:1024046925472) [Google Scholar]
- 41.Pańka D, Piesik D, Jeske M, et al. Production of phenolics and the emission of volatile organic compounds by perennial ryegrass (Lolium perenne L.)/Neotyphodium lolii association as a response to infection by Fusarium poae . J Plant Physiol. 2013;170(11):1010–1019. doi: 10.1016/j.jplph.2013.02.009. (Available from: http://dx.doi.org/10.1016/j.jplph.2013.02.009) [DOI] [PubMed] [Google Scholar]
- 42.Patel DP, Das A, Kumar M, et al. Continuous application of organic amendments enhances soil health, produce quality and system productivity of vegetable-based cropping systems in subtropical eastern Himalayas. Exp Agric. 2015;51(1):85–106. (Available from: http://dx.doi.org/10.1017/S0014479714000167) [Google Scholar]
- 43.Porcel R, Ruiz-Lozano JM. Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. J Exp Bot. 2004;55(403):1743–1750. doi: 10.1093/jxb/erh188. (Available from: http://dx.doi.org/10.1093/jxb/erh188) [DOI] [PubMed] [Google Scholar]
- 44.Ramos-Solano B, Algar E, Gutierrez-Mañero FJ, et al. Bacterial bioeffectors delay postharvest fungal growth and modify total phenolics, flavonoids and anthocyanins in blackberries. LWT Food Sci Technol. 2015;61(2):437–443. (Available from: http://dx.doi.org/10.1016/j.lwt.2014.11.051) [Google Scholar]
- 45.Slinkard K, Singleton VL. Total phenol analysis: automation and comparison with manual methods. Am J Enol Vitic. 1977;28:49–55. [Google Scholar]
- 46.Steindal ALH, Rødven R, Hansen E, et al. Effects of photoperiod, growth temperature and cold acclimatisation on glucosinolates, sugars and fatty acids in kale. Food Chem. 2015;174:44–51. doi: 10.1016/j.foodchem.2014.10.129. (Available from: http://dx.doi.org/10.1016/j.foodchem.2014.10.129) [DOI] [PubMed] [Google Scholar]
- 47.Tegeder M. Transporters involved in source to sink partitioning of amino acids and ureides: opportunities for crop improvement. J Exp Bot. 2014;65(7):1865–1878. doi: 10.1093/jxb/eru012. (Available from: http://dx.doi.org/10.1093/jxb/eru012) [DOI] [PubMed] [Google Scholar]
- 48.Tzin V, Galili G. New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol Plant. 2010;3(6):956–972. doi: 10.1093/mp/ssq048. (Available from: http://dx.doi.org/10.1093/mp/ssq048) [DOI] [PubMed] [Google Scholar]
- 49.Upchurch RG. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol Lett. 2008;30(6):967–977. doi: 10.1007/s10529-008-9639-z. (Available from: http://dx.doi.org/10.1007/s10529-008-9639-z) [DOI] [PubMed] [Google Scholar]
- 50.Waqas M, Khan AL, Kamran M, et al. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules. 2012;17(9):10754–10773. doi: 10.3390/molecules170910754. (Available from: http://dx.doi.org/10.3390/molecules170910754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waqas M, Khan AL, Kang SM, et al. Phytohormone-producing fungal endophytes and hardwood-derived biochar interact to ameliorate heavy metal stress in soybeans. Biol Fert Soils. 2014;50(7):1155–1167. (Available from: http://dx.doi.org/10.1007/s00374-014-0937-4) [Google Scholar]
- 52.Waqas M, Khan AL, Hamayun M, et al. Endophytic infection alleviates biotic stress in sunflower through regulation of defence hormones, antioxidants and functional amino acids. Eur J Plant Pathol. 2015;141(4):803–824. (Available from: http://dx.doi.org/10.1007/s10658-014-0581-8) [Google Scholar]
- 53.Weckopp SC, Kopriva S. Are changes in sulfate assimilation pathway needed for evolution of C4 photosynthesis. Front Plant Sci. 2015;5:773. doi: 10.3389/fpls.2014.00773. (Available from: http://dx.doi.org/10.3389/fpls.2014.00773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhai L, CaiJi Z, Liu J, et al. Short-term effects of maize residue biochar on phosphorus availability in two soils with different phosphorus sorption capacities. Biol Fertil Soils. 2015;51(1):113–122. (Available from: http://dx.doi.org/10.1007/s00374-014-0954-3) [Google Scholar]
- 55.Zhang L, Sun XY, Tian Y, et al. Biochar and humic acid amendments improve the quality of composted green waste as a growth medium for the ornamental plant Calathea insignis . Sci Hortic. 2014;176:70–78. (Available from: http://dx.doi.org/10.1016/j.scienta.2014.06.021) [Google Scholar]
- 56.Zhang Q, Du Z, Lou Y, et al. A one-year short-term biochar application improved carbon accumulation in large macroaggregate fractions. CATENA. 2015;127:26–31. (Available from: http://dx.doi.org/10.1016/j.catena.2014.12.009) [Google Scholar]
- 57.Zhao X, Wang JW, Xu HJ, et al. Effects of crop-straw biochar on crop growth and soil fertility over a wheat-millet rotation in soils of China. Soil Use Manage. 2014;30(3):311–319. (Available from: http://dx.doi.org/10.1111/sum.12124) [Google Scholar]
- 58.Zhou SL, Yan SZ, Liu QS, et al. Diversity of endophytic fungi associated with the foliar tissue of a hemi-parasitic plant Macrosolen cochinchinensis . Curr Microbiol. 2015;70(1):58–66. doi: 10.1007/s00284-014-0680-y. (Available from: http://dx.doi.org/10.1007/s00284-014-0680-y) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of GC-MS conditions for the analysis of fatty acids