Abstract
Antioxidative and cytotoxic effects of chamomile (Matricaria chamomilla) fermented by Lactobacillus plantarum were investigated to improve their biofunctional activities. Total polyphenol (TP) content was measured by the Folin-Denis method, and the antioxidant activities were assessed by the 1,1-diphenyl-2-picrylhydrazyl (DPPH) method and β-carotene bleaching method. AGS, HeLa, LoVo, MCF-7, and MRC-5 (normal) cells were used to examine the cytotoxic effects by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay. The TP content of fermented chamomile reduced from 21.75 to 18.76 mg gallic acid equivalent (mg GAE)/g, but the DPPH radical capturing activity of fermented chamomile was found to be 11.1% higher than that of nonfermented chamomile after 72 h of fermentation. Following the β-carotene bleaching, the antioxidative effect decreased because of a reduction in pH during fermentation. Additionally, chamomile fermented for 72 h showed a cytotoxic effect of about 95% against cancer cells at 12.7 mg solid/ml of broth, but MRC-5 cells were significantly less sensitive against fermented chamomile samples. These results suggest that the fermentation of chamomile could be applied to develop natural antioxidative and anticancer products.
Keywords: Chamomile, Flavonoid, Lactobacillus plantarum, Matricaria chamomilla, Antioxidant, Cytotoxicity
1. Introduction
Matricaria chamomilla L. (German chamomile) is a member of the Asteraceae (Compositae) family and has been known to be one of the most popular medicinal plants worldwide. In particular, it has a long history of use in herbal medicine. The plant is an annual herb with erect branching and finely divided leaves, grows to a height of 50–90 cm, and produces daisy-like flowers. The flower of M. chamomilla is a nontoxic and edible plant, so it is used in various commercial product types such as tea, infusions, liquids, and capsules for the convenience and acceptability of the consumers. The major components of M. chamomilla are known to be phenolics and other bioactive compounds such as α-bisabolol, which is a natural monocyclic sesquiterpene alcohol, and it has been listed as generally recognized as safe (GRAS) by the US Food and Drug Administration (FDA) owing to its safety (Bianco et al., 2008). Many researchers have reported that M. chamomilla has pharmacological properties, including antimicrobial (Batista et al., 2014), anti-inflammatory (Batista et al., 2014), antioxidative (Sebai et al., 2014), antispasmodic (Farideh et al., 2010), antiviral (Koch et al., 2008), and sedative (McKay and Blumberg, 2006) activities owing to the terpenoids, flavonoids (such as apigenin and luteolin), coumarins, and spiroethers in the plant (McKay and Blumberg 2006). Recently, it has been studied as a therapeutic agent against aphthous stomatitis (Tadbir et al., 2015).
Fermentation technology has been used frequently to increase food quality, including the shelf-life, nutritional value, and sensory properties (Zhang et al., 2012). Cvetanović et al. (2015b) concluded that the major phytochemicals in the ligulate florets of chamomile anthodium are apigenin and its glucoside. Furthermore, they studied their bioactivities in the fermentation process using enzymes produced from chamomile to hydrolyze apigenin-7-O-β-glucoside to apigenin. This biotechnology using microorganisms was also studied recently to enhance the production and extraction yields of bioactive compounds in the food and pharmaceutical industries (Torino et al., 2013). For instance, rhamnosidase plays a meaningful role in wine fermentation through the hydrolysis of glycosylated aromatic compounds such as terpenes. Michlmayr et al. (2011) reported that putative two genes of rhamnosidase (ram and ram2) in Pediococcus acidilactici released the monoterpenes linalool and cis-linalool oxide from wine extracts in combination with a bacterial glucosidase under optimum conditions. Santos et al. (2012) also used various lactic acid bacteria (LAB) such as Lactobacillus acidophilus, Lactobacillus casei, and Lactobacillus plantarum as major strains in the bioconversion process of oleuropein-α-polyphenol present in olives. Recently, Jo et al. (2014) presented that a ginseng extract fermented using Aspergillus usamii had higher cytotoxic effect than the nonfermented extract on cancer cell lines such as HepG2 (human liver hepatoblastoma), AGS (human stomach adenocarcinoma), and DLD-1 (human colon adenocarcinoma) cells. Yoon et al. (2015) demonstrated that black rice bran fermented by Bacillus subtilis produced effective antioxidant and cytotoxic activities.
Oxidative stress is defined as the imbalance between free radicals or reactive oxygen species (ROS) produced by metabolic oxidation and the antioxidants present in living cells (Reuter et al., 2010). Particularly, ROS (such as hydrogen peroxide (H2O2), superoxide anion (O2 −), and hydroxyl radical (OH∙)) and various peroxide compounds are produced intracellularly by the respiratory chain system in mitochodria (Poyton et al., 2009). Ultimately, oxidative stress is known to damage important biomolecules and cells, potentially affecting cell viability (Durackova, 2010). In particular, proteins and lipids in cells are known to be very susceptible to oxidative attacks, and the oxidatively modified molecules can increase the risk of cancer (Schraufstätter et al., 1988).
The aims of this work are to ferment extract of M. chamomilla by L. plantarum KCCM 11613P and to evaluate the improved antioxidative and cytotoxic activities of this substance against various cancer cell lines for practical application in the functional food and medicinal industries.
2. Materials and methods
2.1. Strains, plants, and chemicals
L. plantarum KCCM 11613P strain was obtained from the Korean Culture Center of Microorganisms (KCCM), Seoul, Korea. The strain was cultivated in the de Man-Rogosa-Sharpe (MRS, Difco Laboratories, Detroit, USA) broth at 37 °C for 12 h and used for fermentation of samples. The strains were maintained at −80 °C in the MRS broth with glycerol (20%, v/v) and sub-cultured in MRS before being used in experiments.
Chamomile was obtained from the Herb Kingdom Agriculture Corporation (Namwon, Korea). The whole florets of chamomile were dried in an oven (OF12GW, Jeio-Tech Co., Seoul, Korea) at 60 °C for 24 h. The dried sample was pulverized to a particle size of less than 10 mm using a mixer (Blander 7012S, Warning, Torrington, CT, USA) and was stored at 4 °C until used. 1,1-Diphenyl-2-picrylhydrazyl (DPPH), β-carotene, linoleic acid, 2,4,6-tripyridyl-S-triazine (TPTZ), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT), and quercetin as a standard reagent were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
2.2. Cell lines and culture conditions
AGS, HeLa, LoVo, and MCF-7 as human cancer cell lines and MRC-5 as one normal cell line were obtained from the Korean Cell Line Bank (KCLB; Seoul National University, Seoul, Korea). AGS, LoVo, and MCF-7 cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco Laboratories, Grand Island, NY, USA) containing 100 μg/ml streptomycin, 100 U/ml penicillin, and 10% (0.1 g/ml) heat-inactivated fetal bovine serum (FBS; HyClone, Logan, UT, USA). Both HeLa (human cervical adenocarcinoma) and MRC-5 cells were cultured in the minimum essential medium (MEM) containing 100 μg/ml streptomycin, 100 U/ml penicillin, and 10% FBS. All cell lines were grown at 37 °C in a CO2 incubator (MCO-18AIC, SANYO Electric Co., Ltd., Japan). The atmosphere for culturing was controlled to 5% CO2/95% air. At the logarithmic phase, each adherent cell line was harvested with 2.5 g/L trypsin (Invitrogen Corp., Carlsbad, CA, USA) and enumerated by using a hemocytometer (Hausser Scientific, Horsham, PA, USA). Each cell line grown to 80% confluence was inoculated in new dishes and prepared for cytotoxicity assay.
2.3. Extraction and fermentation of chamomile florets
Twenty grams of the powdered chamomile whole florets were blended with 2.5 g of peptone and 10 g of glucose in 0.5 L of distilled water (d-water) and extracted at 120 °C for 30 min in an autoclave. After cooling, 10 ml of L. plantarum KCCM 11613P was inoculated into the extract (initial cell number: approximately 1×106 colony-forming unit (CFU)/ml of the total broth). The broth was cultivated at 30 °C for 72 h. After culturing, the broth was centrifuged at 13 000g, filtered with a 0.45-μm membrane filter, and freeze-dried for 2 d. The powder samples were vacuum-packaged and were stored in a freezer (−20 °C) until used.
2.4. Determination of pH, TA, and viable cell number
Ten grams of each fermented sample and 90 ml of 0.85% (8.5 g/L) NaCl solution were mixed for measuring the pH and total acidity (TA) (Yang et al., 2014). The pH was determined with a pH meter (Model 720, WTW Co., Germany). TA was titrated up to pH 8.2 with 0.1 mol/L NaOH solution. Viable bacterial numbers were determined in the fermented samples by duplicate plating on to MRS agar after incubation at 30 °C for 72 h.
2.5. Determination of total polyphenol and flavonoid contents
The total polyphenol (TP) content was evaluated by adding the resultant mixture (100 μl) to 2 ml of 2% (0.02 g/ml) aqueous sodium carbonate (Na2CO3) solution (Yoon et al., 2015). After 3 min, 100 μl of 50% Folin-Ciocalteau’s reagent was added to the mixture. After 30 min of standing, the absorbance was measured at 750 nm with a spectrophotometer (2120UV, Optizen, Daejon, Korea). The TP content was calculated based on the calibration curve of gallic acid, and the results were expressed as milligrams of gallic acid equivalents per gram of solid (dry weight) (mg GAE/g).
The total flavonoid content of a fermented sample was measured with the aluminum nitrate assay (Moreno et al., 2000). A 100-μl aliquot of the sample, 100 μl of 10% (0.1 g/ml) ammonium nitrate, 100 μl of 1.0 mol/L potassium acetate, and 4.7 ml of 80% ethanol were mixed. After incubation at 25 °C for 40 min, the absorbance of samples was detected spectrophotometrically at 415 nm. The content of total flavonoid was calculated based on a standard plot using quercetin and the results were expressed as milligrams of quercetin equivalents per gram of solids (mg QE/g).
2.6. Antioxidative activity as assessed by the DPPH method
A 200 μl of each sample was mixed with 1.0 ml of 0.1 mmol/L DPPH solution in methanol. The mixture was shaken and was reacted for 15 min at 25 °C. After reaction, the absorbencies of the mixture were measured at 517 nm. DPPH free radical scavenging effects of samples were calculated as follows:
Radical scavenging effect (%)=[1−(A s/A c)]×100%,
where A s and A c are the absorbencies of sample and control, respectively.
2.7. Antioxidative activity as assessed by β-carotene bleaching
Forty-four microliters of linoleic acid, 200 mg of β-carotene, and 200 μl of Tween 80 were mixed in 10 ml of chloroform. A 5-ml aliquot of the mixture was vacuum-dried and diluted in 100 ml of d-water as the β-carotene mixture. In the antioxidative activity assay, 0.5 ml of sample was added to 4.5 ml of a β-carotene mixture and was incubated at 50 °C, and, while standing, sampling was performed at 2 h intervals. The absorbance of each sample was determined at 400 nm. The antioxidative activity was estimated as follows:
Antioxidative activity (%)=(ODs/OD0)×100%,
where ODs is the absorbance (optical density) after reaction and OD0 is the initial absorbance.
2.8. Antioxidative effect as assessed by FRAP assay
The ferric-reducing ability of plasma (FRAP) assay was performed according to the modified method of Benzie and Strain (1996). A 300 mmol/L acetate buffer was prepared by mixing 3.1 g of sodium acetate trihydrate (C2H3NaO2·3H2O) and 16.0 ml of glacial acetic acid and diluted to 1.0 L with d-water. TPTZ solution was prepared by making a solution of 10 mmol/L TPTZ in 40 mmol/L HCl. The working FRAP mixture was produced by mixing 300 mmol/L acetate buffer (pH 3.6), 10 mmol/L TPTZ solution, and 20 mmol/L FeCl3·6H2O in 10:1:1 (v/v/v) ratio and preheated to 37 °C just before use. The lyophilized samples were vortex-mixed with 70% ethanol for 3 min for extraction. The concentrations of the solid extract were controlled to 0.25–2.00 mg/ml. Then, 100 μl of sample was added to 1.9 ml of the working FRAP mixture and was reacted at 25 °C in a dark chamber. Absorbance was determined at 593 nm after 30 min incubation. The analysis was determined quantitatively by using a linear regression plot ranging from 50 μmol/L to 1.5 mmol/L of FeSO4. The unit was expressed as equivalents of Fe2+, FeSO4 equiv. μmol/L.
2.9. In vitro cytotoxicity assay
Cytotoxicity was determined using the tetrazolium-based colorimetric assay (MTT test) (Wang et al., 2006). The cell suspension (200 ml) was transferred to a microwell and incubated for 24 h. Then, a 100-μl sample was poured into the medium and cultured at 37 °C for 44 h. Doxorubicin (Aldrich, Milwaukee, WI, USA) was used as positive control. After incubation, the mixture was discarded and 100 μl of MTT (2.5 mg/ml in phosphate buffered saline (PBS)) was added to the plate. The culture broth was then incubated for an additional 4 h. The supernatant was aspirated, and 0.1 ml of dimethyl sulfoxide (DMSO) was added to each microwell to solubilize the formazan produced from MTT during incubation with cell lines. The absorbencies were determined at 570 nm with a microplate reader (EL311, Bio-Teck Instrument Inc., Seoul, Korea). The cytotoxic activity was estimated as follows:
Cytotoxic activity (%)=[1−(ODs/ODc)]×100%,
where ODc is the absorbance (optical density) of the control after the reaction.
2.10. Statistical analysis
Each experimental test was performed in triplicate, and analysis of variance (ANOVA) was performed using the SPSS 18 package (Chicago, IL, USA). A significant difference was defined as P<0.05.
3. Results
3.1. Determination of total polyphenol content following fermentation
Cvetanović et al. (2015a) reported that superheated water extraction in the temperature range of 100–374 °C was more effective than the other methods used in their study. In this study, superheated water extraction method was adopted, and then the extract was powdered and inoculated with L. plantarum strain.
Under the conditions used in our study, the stationary phase was attained after 8 h of incubation. The cell number was counted approximately to 8.0 log CFU/ml of cultured sample. Fig. 1 shows that the pH decreased rapidly to 3.3±0.2, but changed very little after 24 h. Meanwhile, the TA was increased from 1.15% to (13.3±0.3)% during culturing. From these results, the culturing time for fermentation was established as 24 h.
Fig. 1.
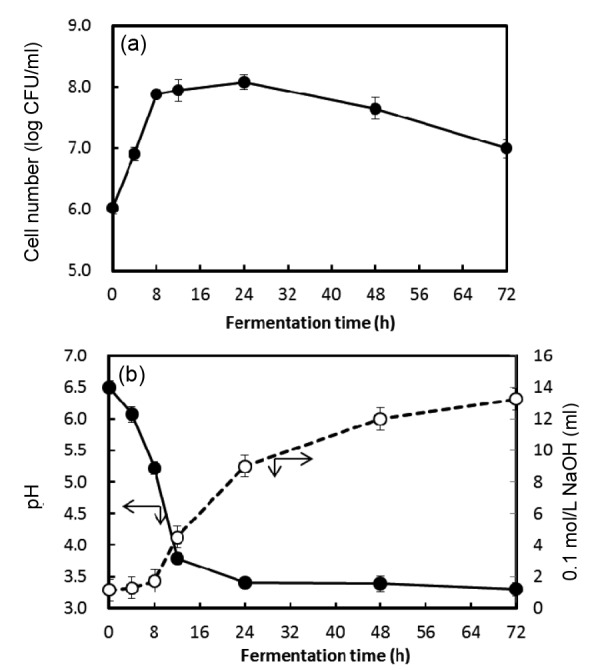
Changes of pH and total acidity during the fermentation of chamomile extract at 30 °C for 72 h
(a) Growth curve;. (b) pH (●) and total acidity (○). Data are represented as mean±standard deviation (SD) in triplicate
TP content is known to be an important factor in evaluating the bioactive properties of plants. From Table 1, it appeared that the solid and TP contents in fermented chamomiles decreased from 36.2 to 25.3 mg/ml and from 718.55 to 559.78 mg GAE/g of solid, respectively, following 72 h of fermentation, but further fermentation did not produce any reduction. Some reports suggest that the change in the TP content depends on the method of fermentation (Torino et al., 2013) and the microbial strains used (Dong et al., 2014). Meanwhile, the total flavonoid content in the solid of extract increased from 266.69 to 363.14 mg QE/g of solid depending on the fermentation time. Dueñas et al. (2005) reported that complex polyphenols could be hydrolyzed to other higher biofunctional active compounds by microorganisms. In this study, it was presumed that L. plantarum KCCM 11613P could discompose the non-flavonic phenol compounds, and the composition of flavonoids in the fermented extract increased relatively.
Table 1.
Contents of solid, total phenolics, and total flavonoids in chamomile broth fermented by L. plantarum KCCM 11613P
| Fermentation time (h) | Solid content (mg/ml) | Total phenolic content (mg GAE/g) | Total flavonoid content (mg QE/g) |
| 0 | 36.2±0.2 | 718.55±0.07 | 266.69±0.41 |
| 12 | 31.1±0.3 | 678.20±0.15 | 292.64±0.22 |
| 24 | 30.0±0.3 | 636.58±0.10 | 315.62±0.33 |
| 48 | 29.4±0.4 | 555.73±0.09 | 342.17±0.27 |
| 72 | 25.3±0.4 | 559.78±0.08 | 363.14±0.12 |
GAE: gallic acid equivalent; QE: quercetin equivalent. Data are represented as mean±standard deviation (SD) in triplicate
3.2. Antioxidative activities
Table 2 shows the antioxidative activities of chamomile fermented by L. plantarum KCCM 11613P using the DPPH method, β-carotene method, and FRAP assay.
Table 2.
Antioxidant activity of chamomile broth fermented by L. plantarum KCCM 11613P using the DPPH and β-carotene methods
| Fermentation time (h) | Antioxidant activity (%) |
|||
| DPPH method |
β-Carotene method |
|||
| NFC | FCLP | NFC | FCLP | |
| 0 | 83.08±2.59 | 83.93±0.33 | 71.84±2.49 | 71.22±1.49 |
| 24 | 90.81±0.48 | 71.79±1.75 | ||
| 48 | 89.85±1.76 | 22.97±16.19 | ||
| 72 | 92.21±0.38 | 15.92±13.91 | ||
NFC: nonfermented chamomile; FCLP: fermented chamomile by L. plantarum KCCM 11613P. Data are represented as mean±standard deviation (SD) in triplicate
DPPH is a stable free radical that has been widely used for studying the free-radical scavenging activities of various antioxidants (Dong et al., 2014). As shown in Table 2, DPPH radical scavenging activities increased from 83.93% to 92.21% depending on the fermentation time, while no significant changes were observed in the nonfermented samples (control). From these results, it appeared that the antioxidative effects were directly proportional to the concentration of the total flavonoids in the extract.
In the β-carotene linoleate bleaching assay, antioxidants inhibit bleaching by neutralizing the hydroperoxides produced by the oxidation of linoleic acid in this system (Othman et al., 2007). Ibrahim et al. (2014) reported that lacto-fermented herbal teas had significantly higher (P<0.05) antioxidative activity compared to freshly prepared herbal teas, which displayed values ranging between 70% and 80%. As shown in Table 2, it appeared that fermented chamomile decreased the bleaching activity to 15.92% after 72 h of fermentation. Hur et al. (2014) reported that the bleaching activity of β-carotene is affected by changes in pH. During fermentation, the pH value decreased from the initial 6.5 to about 3.3.
The FRAP method is also used frequently as an indicator of the phenolic antioxidant activity. The potential of the antioxidants was estimated by their ability to reduce Fe3+-TPTZ to Fe2+-TPTZ (Pulido et al., 2000). The ferric ion-reducing potentials of samples were calculated as FeSO4 equiv. μmol/L Concentrations of 25–36 mg solid/ml did not show any significant differences in the ferric reducing power. However, the antioxidative activities in the FRAP assay were seen to be correlated with the solid concentrations of 0.5–2.0 mg/ml after extraction with 70% ethanol (Table 3). In this case, it is assumed that the antioxidative compounds obtained by 70% ethanol extraction were concentrated separately to a higher value, and a threefold increase in activity was observed compared to that observed using the culture broth.
Table 3.
Ferric-reducing antioxidant power of chamomile extracts fermented by L. plantarum KCCM 11613P
| Concentration (mg/ml) | Antioxidant powera
|
|
| Nonfermented | Fermented | |
| 0.25 | ||
| 0.5 | 32.13±3.52 | 42.85±5.56 |
| 1.0 | 150.70±5.32 | 188.04±6.82 |
| 2.0 | 346.80±1.26 | 374.10±1.90 |
Unit: FeSO4 equiv. in μmol/L. Data are represented as mean±standard deviation (SD) in triplicate
The reason for the stronger antioxidant activity in chamomile fermented by L. plantarum KCCM 11613P compared to the freshly prepared chamomile (control) is probably due to factors such as the bacteria itself. Yang et al. (2014) found that LAB itself may have an antioxidant effect. We performed several different assays (rather than depending on a single assay) to observe the antioxidant activity, because every method works methodologically through a different mechanism and limitation, and each antioxidant result can be different depending on the analytical methods.
3.3. Cytotoxicity in vitro assay
Choi et al. (2006) suggested that the lactobacilli strain might be useful as an antioxidant and anticancer agent. In our study, the cytotoxic activities of fermented chamomile against four different human cancer cell lines were estimated by MTT assay and, to evaluate the cytotoxicity against normal cell line, MRC-5 also was tested. Succinate dehydrogenase, which is a mitochondrial enzyme found in living cells, cleaves the tetrazolium ring of MTT and converts it to insoluble purple formazan. Therefore, the number of surviving cells can be calculated through the amount of formazan produced (Lee et al., 2004).
As shown in Table 4, MTT assay indicated that fermented chamomile reduced the viabilities of AGS, HeLa, LoVo, and MCF-7 cells. Particularly, fermented chamomile was highly cytotoxic to AGS, HeLa, and LoVo cells; furthermore, the AGS cells, among all the cancer cell lines tested, were the most susceptible to the overall extraction treatments. It appeared that cytotoxicity of fermented chamomile after 72-h fermentation was estimated to have about 95% inhibitory effects on the growth of AGS, HeLa, and LoVo cells at 12.7 mg solid/ml in the broth. On the other hand, MRC-5 (human lung cell line) cells were not significantly affected (P>0.05) by fermented chamomile.
Table 4.
Cytotoxic effects of fermented chamomile extract (12.7 mg solid/ml) on various cancer cell lines
| Culture time (h) | Cytotoxic activity (%) |
|||||||||
| AGS |
HeLa |
LoVo |
MCF-7 |
MRC-5 |
||||||
| NFC | FCLP | NFC | FCLP | NFC | FCLP | NFC | FCLP | NFC | FCLP | |
| 0 | 62.92±0.43 | 62.31±1.60 | 76.28±0.47 | 78.12±2.44 | 72.30±8.77 | 74.53±7.13 | 62.50±2.97 | 62.92±1.27 | 70.96±5.08 | 72.33±2.08 |
| 12 | 74.13±0.33 | 78.93±3.26 | 80.67±2.93 | 78.26±1.99 | 71.36±1.06 | |||||
| 24 | 80.72±0.45 | 83.04±0.29 | 80.40±1.15 | 78.39±0.97 | 73.12±3.02 | |||||
| 48 | 82.72±0.16 | 82.77±0.43 | 81.13±0.84 | 79.41±0.24 | 72.24±2.01 | |||||
| 72 | 95.75±0.36 | 95.87±0.19 | 95.01±1.06 | 91.99±0.64 | 84.50±2.19 | |||||
| PC | 92.75±0.63 | 91.78±0.27 | 93.01±2.05 | 93.01±0.46 | 86.42±1.91 | |||||
NFC: nonfermented chamomile; FCLP: fermented chamomile by L. plantarum KCCM 11613P; PC: positive control (1.2 mg/L doxorubicin). Data are represented as mean±standard deviation (SD) in triplicate
4. Discussion
Apigenin and quercetin are known to be major flavonoids in chamomile (Petroianu et al., 2009; Guzelmeric et al., 2014), and have been shown to inhibit strongly the growth of cells and, furthermore, to trigger apoptosis of human cancer cells (Ooi et al., 2015). Therefore, the cytotoxic activities of fermented chamomile may be mediated by apoptosis or attributed to an increase in the osmotic pressure resulting from the high sample concentration, which is one of the most potent defense mechanisms against cancer (Sun et al., 2004). Li et al. (2010) have reported that apoptosis is also related to the ROS levels in cancer cells; specifically, they demonstrated that the ROS levels are negatively correlated with the degree of apoptosis in different cancer cells. Therefore, the antioxidative activity of fermented chamomile can affect the incidence of apoptosis in cancer cells. Many studies have shown that some flavonoids (particularly, luteolin and quercetin) have apoptotic effects on cancer cell lines (Vijayababu et al., 2006; Lim et al., 2007), but Chang et al. (2008) showed that the anticancer mechanisms of flavonoids are diverse and include the induction of cell cycle arrest.
Cvetanović et al. (2015b) studied only the enzymatic hydrolysis of phytochemicals (apigenin) to aglycones in chamomile extract during aging. However, the results in this study suggest that chamomile extract fermented using microorganisms such as L. plantarum KCCM 11613P has significantly greater antioxidative and cytotoxic activity than the unfermented extract and that various compounds as well as apigenin can be bioconversed or hydrolyzed during fermentation. Therefore, the microbial fermentation of phytochemicals can be applied in diverse ways to functional food materials in the food and pharmaceutical industry, e.g., as antioxidants or anticanceric agents. Although apigenin is the major flavonoid in chamomile (Cvetanović et al., 2015b), various other phytochemicals also coexist in chamomile extract, which could be bioconversed and/or hydrolyzed to have higher bioactivities. The mechanism would be complicated and is not known clearly to date. As further research, we plan to study the fermentation mechanisms of each compound and identify the major effective end-products and analyze them quantitatively with high-performance liquid chromatography-mass spectrometry (HPLC-MS); moreover, we also intend to study the specific anticancer mechanisms of the fermented extract of chamomile in more detail.
Footnotes
Project supported by the Ministry of Agriculture, Food and Rural Affairs (No. 314073-03), the Ministry for Food, Agriculture, Forestry and Fisheries, Korea (No. 614102-2), and the National Research Foundation of Korea (No. 2009-0093824)
Compliance with ethics guidelines: Eun-Hye PARK, Won-Young BAE, Su-Jin EOM, Kee-Tae KIM, and Hyun-Dong PAIK declare that they have no conflict of interest.
References
- 1.Batista ALA, Diógenes Alves UchÔa Lins R, de Souza Coelho R, et al. Clinical efficacy analysis of the mouth rinsing with pomegranate and chamomile plant extracts in the gingival bleeding reduction. Complement Ther Clin Pract. 2014;20(1):93–98. doi: 10.1016/j.ctcp.2013.08.002. (Available from: http://dx.doi.org/10.1016/j.ctcp.2013.08.002) [DOI] [PubMed] [Google Scholar]
- 2.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. (Available from: http://dx.doi.org/10.1006/abio.1996.0292) [DOI] [PubMed] [Google Scholar]
- 3.Bianco MI, Lúquez C, de Jong LIT, et al. Presence of Clostridium botulinum spores in Matricaria chamomilla (chamomile) and its relationship with infant botulism. Int J Food Microbiol. 2008;121(3):3357–3360. doi: 10.1016/j.ijfoodmicro.2007.11.008. (Available from: http://dx.doi.org/10.1016/j.ijfoodmicro.2007.11.008) [DOI] [PubMed] [Google Scholar]
- 4.Chang H, Mi M, Ling W, et al. Structurally related cytotoxic effects of flavonoids on human cancer cells in vitro . Arch Pharm Res. 2008;31(9):1137–1144. doi: 10.1007/s12272-001-1280-8. (Available from: http://dx.doi.org/10.1007/s12272-001-1280-8) [DOI] [PubMed] [Google Scholar]
- 5.Choi SS, Kim Y, Han KS, et al. Effects of Lactobacillus strains on cancer cell proliferation and oxidative stress in vitro . Lett Appl Microbiol. 2006;42(5):452–458. doi: 10.1111/j.1472-765X.2006.01913.x. (Available from: http://dx.doi.org/10.1111/j.1472-765X.2006.01913.x) [DOI] [PubMed] [Google Scholar]
- 6.Cvetanović A, Švarc-Gajić J, Mašković P, et al. Antioxidant and biological activity of chamomile extracts obtained by different techniques: perspective of using superheated water for isolation of biologically active compounds. Ind Crop Prod. 2015;65:582–591. (Available from: http://dx.doi.org/10.1016/j.indcrop.2014.09.044) [Google Scholar]
- 7.Cvetanović A, Švarc-Gajić J, Zeković Z, et al. Comparative analysis of antioxidant, antimicrobiological and cytotoxic activities of native and fermented chamomile ligulate flower extracts. Planta. 2015;242(3):721–732. doi: 10.1007/s00425-015-2308-2. (Available from: http://dx.doi.org/10.1007/s00425-015-2308-2) [DOI] [PubMed] [Google Scholar]
- 8.Dong J, Zhao L, Cai L, et al. Antioxidant activities and phenolics of fermented Bletilla formosana with eight plant pathogen fungi. J Biosci Bioeng. 2014;118(4):396–399. doi: 10.1016/j.jbiosc.2014.03.003. (Available from: http://dx.doi.org/10.1016/j.jbiosc.2014.03.003) [DOI] [PubMed] [Google Scholar]
- 9.Dueñas M, Fernandez D, Hernandez T, et al. Bioactive phenolic compounds of cowpeas (Vigna sinensis L.). Modifications by fermentation with natural microflora and with Lactobacillus plantarum ATCC 14917. J Sci Food Agric. 2005;85(2):297–304. (Available from: http://dx.doi.org/10.1002/jsfa.1924) [Google Scholar]
- 10.Durackova Z. Some current insights into oxidative stress. Physiol Res. 2010;59:459–469. doi: 10.33549/physiolres.931844. [DOI] [PubMed] [Google Scholar]
- 11.Farideh ZZ, Bagher M, Ashraf A, et al. Effects of chamomile extract on biochemical and clinical parameters in a rat model of polycystic ovary syndrome. J Reprod Infertil. 2010;11(3):169–174. [PMC free article] [PubMed] [Google Scholar]
- 12.Guzelmeric E, Vovk I, Yesilada E. Development and validation of an HPTLC method for apigenin 7-O-glucoside in chamomile flowers and its application for fingerprint discrimination of chamomile-like materials. J Pharm Biomed Anal. 2014;107(25):108–118. doi: 10.1016/j.jpba.2014.12.021. (Available from: http://dx.doi.org/10.1016/j.jpba.2014.12.021) [DOI] [PubMed] [Google Scholar]
- 13.Hur SJ, Lee SY, Kim YC, et al. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014;160(1):346–356. doi: 10.1016/j.foodchem.2014.03.112. (Available from: http://dx.doi.org/10.1016/j.foodchem.2014.03.112) [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim NA, Mustafa S, Ismail A. Effect of lactic fermentation on the antioxidant capacity of Malaysian herbal teas. Int Food Res J. 2014;21(4):1483–1488. [Google Scholar]
- 15.Jo MN, Jung JE, Lee JH, et al. Cytotoxicity of the white ginseng extract and red ginseng extract treated with partially purified β-glucosidase from Aspergillus usamii KCTC 6954. Food Sci Biotechnol. 2014;23(1):215–219. (Available from: http://dx.doi.org/10.1007/s10068-014-0029-0) [Google Scholar]
- 16.Koch C, Reichling J, Kehm R, et al. Efficacy of anise oil, dwarf-pine oil and chamomile oil against thymidine-kinase-positive and thymidine-kinase-negative herpesviruses. J Pharm Pharmacol. 2008;60(11):1545–1550. doi: 10.1211/jpp/60.11.0017. (Available from: http://dx.doi.org/10.1211/jpp.60.11.0017) [DOI] [PubMed] [Google Scholar]
- 17.Kotnik P, Skerget M, Knez Z. Supercritical fluid extraction of chamomile flower heads: comparison with conventional extraction, kinetics and scale-up. J Supercrit Fluids. 2007;43(2):192–198. (Available from: http://dx.doi.org/10.1016/j.supflu.2007.02.005) [Google Scholar]
- 18.Lee JY, Hwang WI, Lim ST. Antioxidant and anticancer activities of organic extracts from Platycodon grandiflorum A. De Candolle roots. J Ethnopharmacol. 2004;93(2-3):409–415. doi: 10.1016/j.jep.2004.04.017. (Available from: http://dx.doi.org/10.1016/j.jep.2004.04.017) [DOI] [PubMed] [Google Scholar]
- 19.Li B, Wang CZ, He TC, et al. Antioxidants potentiate American ginseng-induced killing of colorectal cancer cells. Cancer Lett. 2010;289(1):62–70. doi: 10.1016/j.canlet.2009.08.002. (Available from: http://dx.doi.org/10.1016/j.canlet.2009.08.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim Y, Jeong T, Tyner AL, et al. Induction of cell cycle arrest and apoptosis in HT-29 human colon cancer cells by dietary compounds luteolin. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):66–75. doi: 10.1152/ajpgi.00248.2006. (Available from: http://dx.doi.org/10.1152/ajpgi.00248.2006) [DOI] [PubMed] [Google Scholar]
- 21.Michlmayr H, Brandes W, Eder R, et al. Characterization of two distinct glycosyl hydrolase family 78 α-L-rhamnosidases from Pediococcus acidilactici . J Appl Environ Microbiol. 2011;77(18):6524–6530. doi: 10.1128/AEM.05317-11. (Available from: http://dx.doi.org/10.1128/AEM.05317-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.) Phytother Res. 2006;20(8):619–633. doi: 10.1002/ptr.1900. (Available from: http://dx.doi.org/10.1002/ptr.1936) [DOI] [PubMed] [Google Scholar]
- 23.Moreno MIN, Isla MI, Sampietro AR, et al. Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J Ethnopharmacol. 2000;71(1-2):109–114. doi: 10.1016/s0378-8741(99)00189-0. (Available from: http://dx.doi.org/10.1016/S0378-8741(99)00189-0) [DOI] [PubMed] [Google Scholar]
- 24.Ooi KL, Loh SI, Tan ML, et al. Growth inhibition of human liver carcinoma HepG2 cells and α-glucosidase inhibitory activity of Murdannia bracteata (C.B. Clarke) Kuntze ex J.K. Morton extracts. J Ethnopharmacol. 2015;162(13):55–60. doi: 10.1016/j.jep.2014.12.030. (Available from: http://dx.doi.org/10.1016/j.jep.2014.12.030) [DOI] [PubMed] [Google Scholar]
- 25.Othman A, Ismail A, Ghani NA, et al. Antioxidant capacity and phenolic content of cocoa beans. Food Chem. 2007;100(4):1523–1530. (Available from: http://dx.doi.org/10.1016/j.foodchem.2005.12.021) [Google Scholar]
- 26.Petroianu G, Szoke E, Kalasz H, et al. Monitoring by HPLC of chamomile flavonoids exposed to rat liver microsomal metabolism. Open J Med Chem. 2009;3:1–7. doi: 10.2174/1874104500903010001. (Available from: http://dx.doi.org/10.2174/1874104500903010001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poyton RO, Ball KA, Castello PR. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol Metab. 2009;20(7):332–340. doi: 10.1016/j.tem.2009.04.001. (Available from: http://dx.doi.org/10.1016/j.tem.2009.04.001) [DOI] [PubMed] [Google Scholar]
- 28.Pulido R, Bravo L, Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem. 2000;48(8):3396–3402. doi: 10.1021/jf9913458. (Available from: http://dx.doi.org/10.1021/jf9913458) [DOI] [PubMed] [Google Scholar]
- 29.Reuter S, Gupta SC, Chaturvedi MM, et al. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. (Available from: http://dx.doi.org/10.1016/j.freeradbiomed.2010.09.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos MM, Piccirillo C, Castro PM, et al. Bioconversion of oleuropein to hydroxytyrosol by lactic acid bacteria. World J Microbiol Biotechnol. 2012;28(6):2435–2440. doi: 10.1007/s11274-012-1036-z. (Available from: http://dx.doi.org/10.1007/s11274-012-1036-z) [DOI] [PubMed] [Google Scholar]
- 31.Schraufstätter I, Hyslop PA, Jackson JH, et al. Oxidant-induced DNA damage of target cells. J Clin Investig (Lond) 1988;82(3):1040–1050. doi: 10.1172/JCI113660. (Available from: http://dx.doi.org/10.1172/JCI113660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sebai H, Jabri MA, Souli A, et al. Antidiarrheal and antioxidant activities of chamomile (Matricaria recutita L.) decoction extract in rats. J Ethnopharmacol. 2014;152(2):327–332. doi: 10.1016/j.jep.2014.01.015. (Available from: http://dx.doi.org/10.1016/j.jep.2014.01.015) [DOI] [PubMed] [Google Scholar]
- 33.Sun SY, Hail NJr, Lotan R. Apoptosis as a novel target for cancer chemoprevention. J Nat Cancer Inst. 2004;96(9):662–672. doi: 10.1093/jnci/djh123. (Available from: http://dx.doi.org/10.1093/jnci/djh123) [DOI] [PubMed] [Google Scholar]
- 34.Tadbir AA, Pourshahidi S, Ebrahimi H, et al. The effect of Matricaria chamomilla (chamomile) extract in Orabase on minor aphthous stomatitis, a randomized clinical trial. J Herb Med. 2015;5(2):71–76. (Available from: http://dx.doi.org/10.1016/j.hermed.2015.05.001) [Google Scholar]
- 35.Torino MI, Limon RI, Martinez-Villaluenga C, et al. Antioxidant and antihypertensive properties of liquid and solid state fermented lentils. Food Chem. 2013;136(2):1030–1037. doi: 10.1016/j.foodchem.2012.09.015. (Available from: http://dx.doi.org/10.1016/j.foodchem.2012.09.015) [DOI] [PubMed] [Google Scholar]
- 36.Vijayababu MR, Kanagaraj P, Arunkumar A, et al. Quercetin induces p53-independent apoptosis in human prostate cancer cells by modulating Bcl-2-related proteins: a possible mediation by IGFBP-3. Oncol Res. 2006;16(2):67–74. doi: 10.3727/000000006783981224. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Wei Y, Yuan S, et al. Potential anticancer activity of litchi fruit pericarp extract against hepatocellular carcinoma in vitro and in vivo . Cancer Lett. 2006;239(1):144–150. doi: 10.1016/j.canlet.2005.08.011. (Available from: http://dx.doi.org/10.1016/j.canlet.2005.08.011) [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Tang H, Holmes E. Metabolomic strategy for the classification and quality control of phytomedicine: a case study of chamomile flower (Matricaria recutita L.) Planta Med. 2004;70(3):250–255. doi: 10.1055/s-2004-815543. (Available from: http://dx.doi.org/10.1055/s-2004-815543) [DOI] [PubMed] [Google Scholar]
- 39.Yang J, Ji Y, Park H, et al. Selection of functional lactic acid bacteria as starter cultures for the fermentation of Korean leek (Allium tuberosum Rottler ex Sprengel) Int J Food Microbiol. 2014;191(17):164–171. doi: 10.1016/j.ijfoodmicro.2014.09.016. (Available from: http://dx.doi.org/10.1016/j.ijfoodmicro.2014.09.016) [DOI] [PubMed] [Google Scholar]
- 40.Yoon HJ, Lee KA, Lee JH, et al. Effect of fermentation by Bacillus subtilis on antioxidant and cytotoxic activities of black rice bran. Int J Food Sci Technol. 2015;50(3):612–618. (Available from: http://dx.doi.org/10.1111/ijfs.12693) [Google Scholar]
- 41.Zhang Z, Lv G, Pan H, et al. Production of powerful antioxidant supplements via solid-state fermentatin of wheat (Triticum aestivum Linn.) by Cordyceps militaris . Food Technol Biotechnol. 2012;50(1):32–39. [Google Scholar]


