Abstract
Bone mesenchymal stem cells (BMSCs) differentiated into neurons have been widely proposed for use in cell therapy of many neurological disorders. It is therefore important to understand the molecular mechanisms underlying this differentiation. We screened differentially expressed genes between immature neural tissues and untreated BMSCs to identify the genes responsible for neuronal differentiation from BMSCs. GSE68243 gene microarray data of rat BMSCs and GSE18860 gene microarray data of rat neurons were received from the Gene Expression Omnibus database. Transcriptome Analysis Console software showed that 1248 genes were up-regulated and 1273 were down-regulated in neurons compared with BMSCs. Gene Ontology functional enrichment, protein-protein interaction networks, functional modules, and hub genes were analyzed using DAVID, STRING 10, BiNGO tool, and Network Analyzer software, revealing that nine hub genes, Nrcam, Sema3a, Mapk8, Dlg4, Slit1, Creb1, Ntrk2, Cntn2, and Pax6, may play a pivotal role in neuronal differentiation from BMSCs. Seven genes, Dcx, Nrcam, Sema3a, Cntn2, Slit1, Ephb1, and Pax6, were shown to be hub nodes within the neuronal development network, while six genes, Fgf2, Tgfβ1, Vegfa, Serpine1, Il6, and Stat1, appeared to play an important role in suppressing neuronal differentiation. However, additional studies are required to confirm these results.
Keywords: Neuronal differentiation, Bone mesenchymal stem cells (BMSCs), Protein-protein interaction network, Differentially expressed genes
1. Introduction
Neurological disorders are problems of the nervous system, which may be possible to treat by the transplantation and differentiation of stem cells to enhance nerve regeneration. Several studies have shown that bone mesenchymal stem cells (BMSCs) can be induced and differentiated into neural cells under specific, controlled microenvironments (Jiang et al., 2005; Çapkın et al., 2012). Thus, BMSCs are known as “seed cells” in the cell therapy of many neurological disorders and have the advantage of being easy to isolate.
Many factors have been reported to induce neuronal cell differentiation from BMSCs, including basic fibroblast growth factor, epidermal growth factor, neurotrophic factors, vascular endothelial growth factor, hepatocyte growth factor, β-mercaptoethanol, dimethylsulfoxide, and butylated hydroxyanisole (Bae et al., 2011; Edamura et al., 2014; Parivar et al., 2015). Bertani et al. (2005) previously showed that gene expression was altered in BMSCs exposed to butylated hydroxyanisole and dimethylsulfoxide neural induction medium, but did not match the set of genes differentially expressed in immature neural tissues compared with untreated BMSCs. However, because of the many factors involved with neuronal cell differentiation, including risk, survival time, and instability, the use of BMSCs has limited application in the clinic. In particular, the process of BMSC differentiation into nerve cells is unstable, and so it is important to define more stable differentiation conditions.
In this study, microarray data were examined to identify genes expressed differentially between immature neural tissues and BMSCs. Bioinformatic methods were used to analyze Gene Ontology (GO) functional enrichment and to construct protein-protein interaction (PPI) networks. We also identified high connectivity (hub genes) associated with neuronal differentiation and neuronal development.
2. Materials and methods
2.1. Materials
The gene expression profiles of GSE68243 and GSE18860 were obtained from the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/ geo). Dataset GSE68243 includes three untreated Sprague-Dawley rat BMSC samples (GSM1666210, GSM1666211, and GSM1666212), while dataset GSE18860 includes three cortical neuron samples from d18 Sprague-Dawley rat embryos (GSM4674922, GSM4674923, and GSM4674924). Both datasets are based on GPL6247 platform information.
2.2. Data preprocessing
The data downloaded as CEL files were preprocessed using Expression Console™ software and underwent background correction and quartile data normalization by the robust multiarray average algorithm (Irizarry et al., 2003) with default parameters. Non-annotated probes were filtered out.
2.3. Screening of differentially expressed genes and hierarchical cluster analysis
Differentially expressed genes (DEGs) between neurons and BMSCs were identified by one-way between-subjects analysis of variance based on Transcriptome Analysis Console software (Torres et al., 2015). Genes with fold-changes |log2|>4 were selected as DEGs, and P of <0.05 and false discovery rate (FDR) of <0.05 were used as thresholds.
2.4. Functional enrichment analysis
GO is widely used in functional studies and the enrichment analysis of gene sets. The DAVID (Sherman et al., 2007) gene annotation tool was used to analyze GO enrichment in DEG functions. Values of P<0.05 and FDR<0.05 were again used as cutoff criteria. GO terms were displayed as a significant network using the BiNGO Plug-in of Cytoscape v. 3.2.1 software (Shannon et al., 2003). DEGs associated with neural differentiation were clustered in neuronal differentiation and regulation of cell proliferation modules.
2.5. Construction of GO:0030182, GO:0048666, and GO:0042127 PPI networks, and the identification of hub genes
The STRING 10 database (Snel et al., 2000) provides known and predicted interactions based on confidence. The interactions contain direct (physical) and indirect (functional) associations, which are derived from genomic context, experiments, coexpression, and previous knowledge. Genes in the neuronal differentiation and regulation of cell proliferation modules were considered to be seed nodes and were mapped onto the STRING database to construct extended PPI networks with medium confidence scores of 0.4. All PPI networks were visualized by Cytoscape v. 3.2.1 software and calculated using the Network Analyzer tool (Assenov et al., 2008) based on parameters of betweenness centrality (BC) and degree. BC reflects the number of shortest paths that pass one node, and is essential in the analysis of nodal importance. The degree indicates the number of interactions of a particular protein. In the final networks, nodes with a high degree were displayed within a large circle while shades of green and yellow represented high to low BC values for the node (Assenov et al., 2008). In this study, nodes with thresholds of BC>0.05 and degrees above the average value of each network were considered to be hub genes.
3. Results
3.1. DEG screening
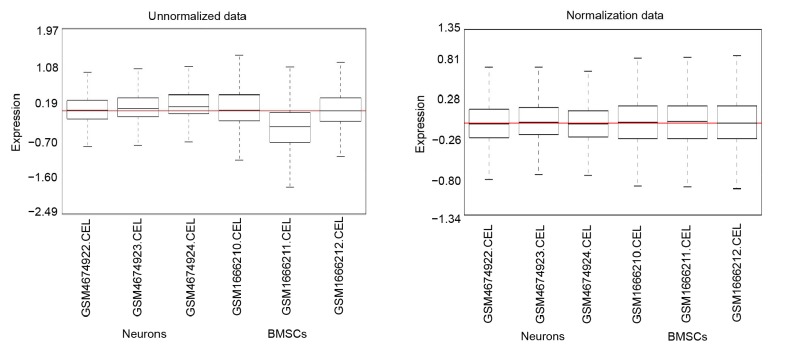
The standardization of expression data revealed a good degree based on all the black lines in Fig. 1 on the same straight line. A total of 2521 genes were identified as being differentially expressed between neurons and BMSCs, 1248 of which were up-regulated and 1273 down-regulated in neurons compared with BMSCs (Table S1). Hierarchical cluster analysis showed that the three neuronal samples were distributed within the neuronal sample cluster and that the three BMSC samples were within the BMSC sample cluster (Fig. 2). This showed that grouping was rational and that the data could be used directly for further analysis.
Fig. 1.
Cassette figures of the expression data before and after standardization
The horizontal axis represents sample names while the vertical axis means the expression value
Fig. 2.
Hierarchical cluster analysis of DEGs
The horizontal axis shows sample names. GSM1666210, GSM1666211, and GSM1666212 are BMSC samples. GSM4674922, GSM4674923, and GSM4674924 are neuron samples. The right vertical axis represents clusters of DEGs, and the above horizontal axis means clusters of samples. Red means up-regulated genes and green signifies down-regulated genes
3.2. Functional enrichment analysis
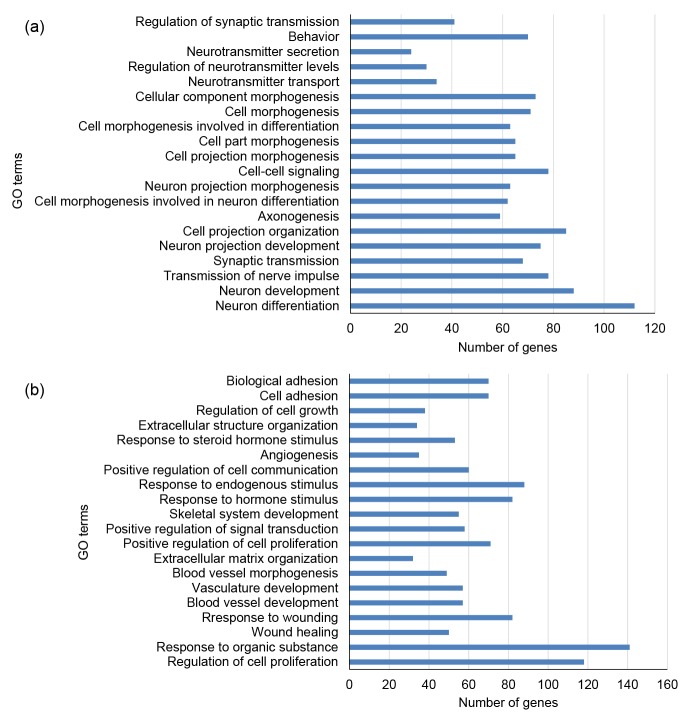
GO enrichment analyzed using DAVID software revealed that the up-regulated genes were significantly enriched in the biological processes of neuronal differentiation (GO:0030182), neuronal development (GO:0048666), transmission of nerve impulses (GO:0019226), synaptic transmission (GO: 0007268), and neuron projection development (GO: 0031175) (Table S2, Fig. 3a), while down-regulated genes were significantly enriched in regulation of cell proliferation (GO:0042127), response to organic substances (GO:0010033), wound healing (GO: 0042060), response to wounding (GO:0009611), and blood vessel development (GO:0001568) (Table S2, Fig. 3b). GO terms for up-regulated and down-regulated genes were calculated using the BiNGO Plug-in of Cytoscape software, and significant terms shown using yellow nodes (Fig. 4).
Fig. 3.
GO enrichment analyses
(a) The top 20 GO enrichment terms of up-regulated genes of neurons compared with BMSCs; (b) The top 20 GO enrichment terms of down-regulated genes of neurons compared with BMSCs. The horizontal axis represents the number of DEGs. The vertical axis represents the enriched GO terms. P values increase from bottom to top
Fig. 4.
GO terms displayed as a network using BiNGO
(a) Up-regulated genes GO terms; (b) Down-regulated genes GO terms. Yellow nodes: nodes with P-value<0.05 and FDR<0.05
3.3. PPI network analysis of GO:0030182, GO:0048666, and GO:0042127
To investigate the molecular mechanism of neural differentiation from BMSCs, we selected the up-regulated genes involved in neuronal differentiation (GO:0030182) and neuronal development (GO:0048666), and the down-regulated genes involved in regulation of cell proliferation (GO: 0042127) because these are closely associated with neuronal cell differentiation. Genes of the three GO terms (Table 1) were used to construct PPI networks with the STRING database. Networks were visualized and analyzed using Cytoscape v. 3.2.1 software. Analysis of nodal properties in each network revealed average degrees of 6.39 for GO:0030182 nodes, 4.88 for GO:0048666 nodes, and 11.94 for GO:0042127 nodes. A total of nine genes were identified as hub genes for neuronal differentiation: neuronal cell adhesion molecule (Nrcam), semaphoring-3A (Sema3a), mitogen-activated protein kinase 8 (Mapk8), discs large MAGUK scaffold protein 4 (Dlg4), slit homolog 1 (Slit1), cyclic adenosine monophosphate (cAMP)-responsive element binding protein 1 (Creb1), neurotrophic tyrosine kinase receptor 2 (Ntrk2), contactin 2 (Cntn2), and paired box gene 6 (Pax6); seven genes were identified as hub genes for neuronal development: doublecortin (Dcx), Nrcam, Sema3a, Cntn2, Slit1, ephrin type-B receptor 1 (Ephb1), and Pax6; and six genes were identified as hub genes for the regulation of cell proliferation: basic fibroblast growth factor 2 (Fgf2), transforming growth factor β-1 (Tgfβ1), vascular endothelial growth factor A (Vegfa), plasminogen activator inhibitor-1 (Serpine1), interleukin 6 (Il6), and signal transducer and activator of transcription (Stat1) (Fig. 5 and Table 2).
Table 1.
Genes in GO terms of neuron differentiation and regulation of cell proliferation
| GO terms | Biological process | Genes |
| GO:0030182 | Neuron differentiation | Gprin1, Plxna3, Efna2, Rorb, I1cam, Cspg5, Klhl1, Nrcam, Atp2b2, Ank3, Unc5a, Robo1, Ss18l1, Dscam, Stmn3, Emx1, Efnb3, Mdga1, Mdga2, Kif5c, Dll1, Tbr1, Ctnna2, Slitrk2, Ascl1, Slitrk1, Ncam2, Slitrk3, Foxg1, Reln, Mapk8, Stmn1, Igsf9, Slitrk5, Cdk5r1, Lppr4, Sox2, Sox5, Rgd1311558, Ephb1, Ephb2, Arx, Crmp1, Lhx2, Lhx6, Olfm3, Bhlhe22, Celsr3, Evl, Celsr2, Gas7, Dlx2, Fezf2, Epha4, Epha7, Nrep, Dlx1, Sema6c, Ntrk2, Map2, Chrnb2, Syngap1, Atl1, Agtpbp1, Uchl1, Pax6, Vgf, Gpc2, Casp3, Dynll2, Mapt, Dlg4, Sema3a, Dlg2, Tubb3, Kcnma1, Slit1, Psd, Sema4f, Cntn2, Cntn4, Gap43, Dcc, Rab3a, Cck, Ndn, Nnat, Brsk1, Bcl11b, Pou3f4, Pou3f2, Cd24, Dcx, Snap25, Nefl, Dclk1, Dfna5, Gnao1, Ptprz1, Creb1, Nfasc, Dpysl5, Nlgn1, Ntng2, Dpysl4, Isl1, Wnt7b, Ppp1r9a, Neurod2,Wnt7a, Apbb1, Fez1 |
| GO:0048666 | Neuron development | Gprin1, Plxna3, Atl1, Efna2, Uchl1, Pax6, I1cam, Rorb, Cspg5, Klhl1, Nrcam, Atp2b2, Ank3, Unc5a, Dynll2, Robo1, Dlg4, Sema3a, Ss18l1, Dlg2, Dscam, Stmn3, Efnb3, Kif5c, Tbr1, Slit1, Ctnna2, Slitrk2, Ncam2, Ascl1, Slitrk1, Slitrk3, Sema4f, Foxg1, Cntn2, Cntn4, Mapk8, Reln, Stmn1, Igsf9, Slitrk5, Gap43, Dcc, Rab3a, Cdk5r1, Lppr4, Cck, Ndn, Rgd1311558, Ephb1, Ephb2, Arx, Crmp1, Bcl11b, Lhx2, Lhx6, Cd24, Dcx, Olfm3, Snap25, Nefl, Dclk1, Gnao1, Ptprz1, Creb1, Nfasc, Nlgn1, Ntng2, Celsr3, Dpysl4, Celsr2, Evl, Isl1, Gas7, Fezf2, Epha4, Epha7, Nrep, Wnt7b, Ppp1r9a, Sema6c, Ntrk2, Map2, Neurod2, Chrnb2, Syngap1, Apbb1, Fez1 |
| GO:0042127 | Regulation of cell proliferation | Dlc1, Apobec1, Thrb, Ptgs2, Il6st, Osmr, Pgf, Pdgfa, Ptgs1, Fgfrl1, Tgfβ3, Fgf10, Tlr4, Jag1, Pmaip1, Il15, Cxcl12, Tgfβ1, Tgfβ2, H19, Pgr, Myd88, S1pr1, Cdkn2b, Serpine1, Pdgfc, Rarg, Mfge8, Thy1, Vegfc, Cd38, Serpinf1, Ptprv, F3, Vegfa, Pdgfra, Wfdc1, Tgif1, Ripk2, Pdgfrb, Pmp22, Ngf, Cav2, Cav1, Ccl2, Ifitm3, Ifi30, Itgb1, Irak4, Lif, Vdr, Itgav, Runx2, Runx3, Bmp4, B4galt1, Bmp2, Sphk1, Tgfbr2, Hgf, Shox2, Notch2, Pla2g4a, Cdkn1a, Atf3, Cd274, Avpr1a, Kctd11, Klf4, Plau, Sat1, Ppard, Fgf7, Acvrl1, Pparg, Prrx1, Gja1, Pawr, Prrx2, Tenc1, Ddr2, Wisp2, Ang, Hmox1, Hey2, Shc1, Fgf1, Il13ra1, Fgf2, Fosl1, Lyn, Cdk6, Mbd2, Mmp12, Pura, Cth, Ccnd1, Adam17, Tbx18, Eif5a2, Csf1, Nr3c1, Stat6, Camk2d, Plcd1, Cd28, Nox4, Ptprc, Il6, Tbx3, Tbx2, Anxa1, Sparc, Stat1, Clec11a, Cyba, Nupr1, Id3 |
Fig. 5.
PPI networks of neuron differentiation (GO:0030182, a), neuron development (GO:0048666, b), and regulation of cell proliferation (GO:0042127, c) biological process
Node color: shades of green to yellow color depict node with highest to lowest values of betweenness centrality (BC); Node size: sizes from biggest to smallest circle mean the node degrees. Bigger and dark colored nodes represent genes with more links
Table 2.
Key genes selected based on visualize parameters like BC and degree
| Network name | Gene | Degree | BC | AD |
| Neuron differentiation | Nrcam | 11 | 0.052 742 72 | 6.39 |
| Sema3a | 15 | 0.053 825 15 | ||
| Mapk8 | 12 | 0.090 814 13 | ||
| Dlg4 | 16 | 0.103 825 38 | ||
| Slit1 | 19 | 0.108 426 54 | ||
| Cntn2 | 12 | 0.110 418 82 | ||
| Creb1 | 10 | 0.113 116 98 | ||
| Ntrk2 | 13 | 0.122 851 31 | ||
| Pax6 | 24 | 0.163 991 00 | ||
| Neuron development | Dcx | 6 | 0.058 705 83 | 4.88 |
| Nrcam | 7 | 0.066 951 81 | ||
| Sema3a | 10 | 0.145 349 80 | ||
| Cntn2 | 10 | 0.268 125 51 | ||
| Slit1 | 11 | 0.101 701 35 | ||
| Ephb1 | 11 | 0.138 484 58 | ||
| Pax6 | 13 | 0.181 375 54 | ||
| Regulation of cell proliferation | Fgf2 | 38 | 0.059 946 33 | 11.94 |
| Tgfβ1 | 34 | 0.063 818 16 | ||
| Vegfa | 40 | 0.074 373 06 | ||
| Serpine1 | 26 | 0.074 690 11 | ||
| Il6 | 39 | 0.116 884 44 | ||
| Stat1 | 40 | 0.126 546 91 |
BC: betweenness centrality; AD: average degree of all nodes
4. Discussion
Neuronal differentiation from BMSCs offers a number of advantages for cell therapy of neurological disorders, but an understanding of the underlying cellular and genetic mechanisms is required. Through the comparative analyses of gene expression data in neuronal cells and BMSCs, we identified a total of 2521 DEGs between the two cell types, of which 1248 were up-regulated and 1273 were down-regulated in neuronal cells compared with BMSCs (Table S1). GO functional enrichment analysis showed that of the up-regulated genes, 10.46% were associated with the neuronal differentiation GO term, 8.22% with the neuronal development GO term, and 7.28% with the transmission of nerve impulses. Among the down-regulated genes, GO functions were associated with the regulation of cell proliferation, which is related to cell differentiation, response to organic substances, and wound healing (Table S2). Neuronal differentiation, neuronal development, and cell proliferation are closely associated with neuronal cell differentiation, indicating that the findings could be viewed with high confidence.
In the present study, specific DEGs were up-regulated in neural tissue compared with untreated BMSCs. These include Sema3a, Mapk8, Nrcam, Dlg4, Slit1, Creb1, Ntrk2, Cntn2, and Pax6, which play important roles in neuronal differentiation, while Dcx, Nrcam, Sema3a, Cntn2, Slit1, Ephb1, and Pax6 may have a critical role in neuronal development. Fgf2, Tgfβ1, Vegfa, Serpine1, Il6, and Stat1 were selected as key genes in the regulation of cell proliferation network, and were down-regulated in neural tissue compared with untreated BMSCs; this implies a role in suppressing neuronal differentiation. Slit1 has previously been reported to be involved in the regeneration and functional recovery of the trigeminal ganglion and inferior alveolar nerve (Ceber et al., 2015), while a role for Slit1 was identified in the development of dopaminergic neurons, which is mediated by NCK adaptor protein 2 to trigger changes in cortical neuron morphology (Round and Sun, 2011). PAX6 is a highly conserved multifunctional transcription factor, which has been demonstrated to be a key protein in neurogenesis and neuronal plasticity by influencing cascades of gene expression (Mishra et al., 2015). In retinoic acid-induced neuronal differentiation, PAX6 was found to be repressed by the recruitment of histone modifier lysine-specific demethylase 1 (Wu et al., 2016). Our results showed that Pax6 is a key gene in neuronal differentiation and neuronal development.
CNTN2 was previously suggested to control axonal growth both in vitro and in vivo. Ratie et al. (2014) showed that Cntn2 expression was restricted to the early axon scaffold populations, cranial ganglia, and spinal motor neurons, as well as mature neurons. However, we observed a likely role for Cntn2 in neuronal differentiation and development. NRCAM, a neuronal cell adhesion molecule, is important in aspects of neural development including cell proliferation and differentiation, axonal growth and guidance, synapse formation, and formation of the myelinated nerve structure (Sakurai, 2012), which is involved in neuron-neuron and neuron-glial adhesions. NRCAM also modulates axonal growth and guidance by a mechanism involving the neuronal receptor axonin-1 (Lustig et al., 1999). NRCAM deletion in mice previously led to elevated spine densities on the apical dendrites of star pyramidal cells during both postnatal and adult stages. NRCAM was also found to form a complex with semaphorin 3F (Sema3F) in the brain to regulate the spine density of star pyramidal neurons (Demyanenko et al., 2014). Dcx expression was followed in migrating and differentiating neurons in the brains of embryonic mouse (Francis et al., 1999). In postnatal mice, reducing the levels of DCX caused abnormal neuronal migration, which affected neuronal development in the postnatal forebrain (Belvindrah et al., 2011). Within the two main adult neurogenic regions, Dcx was shown to be transiently expressed in dividing neural progenitor cells until neuronal maturation (Brown et al., 2003). We observed a likely role for DCX and NRCAM in neuronal development.
Sema3a is a member of the class 3 secreted semaphorin and has been shown to induce growth cone collapse or repel sensory axons of the dorsal root ganglia in vitro (Wu et al., 2014) and regulate adult neurogenesis by controlling stem cells proliferation and differentiation in the subventricular zone (Sun et al., 2016). A recent study has shown that distinct cytoplasmic domains in Plexin-A4 mediated diverse responses to Sema3a in developing mammalian neurons (Mlechkovich et al., 2014). EPHB1 has been indicated to play important roles in synaptic plasticity of the nervous system (Liu et al., 2009), with Ephb1 null mice exhibiting neuronal loss in the substantia nigra pars reticulata and spontaneous locomotor hyperactivity (Richards et al., 2007). Little is known about the role of Dlg4, Ntrk2, Creb1, or Mapk8 in neuronal differentiation and development.
In the present study, Stat1 was represented as the hub gene of regulation of the cell proliferation network, showing the highest degree and BC. The knockdown of Stat1 previously demonstrated that interferon γ (IFN-γ) inhibits the differentiation of neuronal precursor cells by negatively regulating the expression of Neurog2 via the Janus kinase/Stat pathway (Ahn et al., 2015). Pereira et al. (2015) reported that IFN-γ induced neuronal differentiation and acted as an antiproliferative factor via STAT1 in the adult subventricular zone, thereby regulating neurogenesis in normal adult brains. IL-6 has been shown to suppress neurogenesis within human retinal cell suspensions in the presence of N2 culture medium containing FGF2, which enhances neurosphere generation (Balasubramaniam et al., 2009). FGF2 was reported to be a potent neurotrophic factor that promotes the differentiation of BMSCs into dopaminergic neurons and enhances their survival both in vitro and in vivo (Nandy et al., 2014). FGF2 has also been widely used to induce neuronal differentiation in mice and primates, including humans, but was shown to promote self-renewal in a macaque embryonic stem cell line; its effects on the differentiation of stem cells appear to be influenced by the presence or absence of supplemental retinoic acid (Hatori et al., 2014). Additionally, FGF2 has been found to delay neurogenesis in embryonic chicks (McGowan et al., 2013). Moreover, low concentrations of FGF2 increased neurogenesis in human neural progenitor cells while high levels maintained progenitor cell proliferation and blocked neurogenesis (Nelson and Svendsen, 2006). This result is consistent with our own findings.
VEGF is known to modulate learning and memory, the plasticity of mature neurons, synaptic transmission, and neurogenesis (Yang et al., 2016). However, evidence for its role in the regulation of excitatory synaptic activity is conflicting (Yang et al., 2016). In our analysis, Vegfa appeared to be an inhibitory factor in neuronal differentiation. A previous study suggested that TGF-β1 enhances neuronal excitability (Zhang et al., 2016) and protects midbrain dopaminergic neurons from IFN-γ-induced neurotoxicity (Zhou et al., 2015), but there is no evidence to indicate that TGF-β1 suppresses neuronal differentiation. Finally, the role of Serpine1 in neuronal cells is currently unclear.
5. Conclusions
According to the centrality-lethality rule, hubs are more likely to be functionally relevant than other genes (Nair et al., 2014). In this study, we identified that a number of hub genes, including Sema3a, Nrcam, Slit1, Cntn2, and Pax6, have positive roles in neuronal differentiation and neuronal development, that Mapk8, Dlg4, Creb1, and Ntrk2 play pivotal roles in neuronal differentiation, and that Nrcam and Ephb1 are important in neuronal development. Previous studies have shown that these genes have important functions in neuronal differentiation and neuronal development, with the exception of Dlg4, Ntrk2, Creb1, and Mapk8. Fgf2, Tgfβ1, Vegfa, Serpine1, Il6, and Stat1 were shown to suppress neuronal differentiation, while Vegfa and Tgfβ1 were documented as having positive roles in neurogenesis. Further studies are required to confirm these findings.
List of electronic supplementary materials
Information of differentially expressed genes between neurons and BMSCs
Gene ontology enrichment analysis of biological process
Footnotes
Project supported by the Key Project of Hebei North University (No. 120177) and the Science and Technology Research Project of Hebei Province Department Institutions of Higher Learning (No. Z2015047), China
Electronic supplementary materials: The online version of this article (http://dx.doi.org/10.1631/jzus.B1600109) contains supplementary materials, which are available to authorized users
Compliance with ethics guidelines: Li-ning SU, Xiao-qing SONG, Hui-ping WEI, and Hai-feng YIN declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Ahn J, Lee J, Kim S. Interferon-gamma inhibits the neuronal differentiation of neural progenitor cells by inhibiting the expression of Neurogenin2 via the JAK/STAT1 pathway. Biochem Biophys Res Commun. 2015;466(1):52–59. doi: 10.1016/j.bbrc.2015.08.104. (Available from: http://dx.doi.org/10.1016/j.bbrc.2015.08.104) [DOI] [PubMed] [Google Scholar]
- 2.Assenov Y, Ramirez F, Schelhorn SE, et al. Computing topological parameters of biological networks. Bioinformatics. 2008;24(2):282–284. doi: 10.1093/bioinformatics/btm554. (Available from: http://dx.doi.org/10.1093/bioinformatics/btm554) [DOI] [PubMed] [Google Scholar]
- 3.Bae KS, Park JB, Kim HS, et al. Neuron-like differentiation of bone marrow-derived mesenchymal stem cells. Yonsei Med J. 2011;52(3):401–412. doi: 10.3349/ymj.2011.52.3.401. (Available from: http://dx.doi.org/10.3349/ymj.2011.52.3.401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balasubramaniam B, Carter DA, Mayer EJ, et al. Microglia derived IL-6 suppresses neurosphere generation from adult human retinal cell suspensions. Exp Eye Res. 2009;89(5):757–766. doi: 10.1016/j.exer.2009.06.019. (Available from: http://dx.doi.org/10.1016/j.exer.2009.06.019) [DOI] [PubMed] [Google Scholar]
- 5.Belvindrah R, Nissant A, Lledo PM. Abnormal neuronal migration changes the fate of developing neurons in the postnatal olfactory bulb. J Neurosci. 2011;31(20):7551–7562. doi: 10.1523/JNEUROSCI.6716-10.2011. (Available from: http://dx.doi.org/10.1523/JNEUROSCI.6716-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertani N, Malatesta P, Volpi G, et al. Neurogenic potential of human mesenchymal stem cells revisited: analysis by immunostaining, time-lapse video and microarray. J Cell Sci. 2005;118(17):3925–3936. doi: 10.1242/jcs.02511. (Available from: http://dx.doi.org/10.1242/jcs.02511) [DOI] [PubMed] [Google Scholar]
- 7.Brown JP, Couillard-Despres S, Cooper-Kuhn CM, et al. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467(1):1–10. doi: 10.1002/cne.10874. (Available from: http://dx.doi.org/10.1002/cne.10874) [DOI] [PubMed] [Google Scholar]
- 8.Çapkın M, Cakmak S, Kurt FO, et al. Random/aligned electrospun PCL/PCL-collagen nanofibrous membranes: comparison of neural differentiation of rat AdMSCs and BMSCs. Biomed Mater. 2012;7(4):045013. doi: 10.1088/1748-6041/7/4/045013. (Available from: http://dx.doi.org/10.1088/1748-6041/7/4/045013) [DOI] [PubMed] [Google Scholar]
- 9.Ceber M, Mihmanli A, Kilic U, et al. Changes in expression of Slit1 and its receptor Robo2 in trigeminal ganglion and inferior alveolar nerve following inferior alveolar nerve axotomy in adult rats: a pilot study. Int J Oral Maxillofac Surg. 2015;44(4):518–527. doi: 10.1016/j.ijom.2014.09.022. (Available from: http://dx.doi.org/10.1016/j.ijom.2014.09.022) [DOI] [PubMed] [Google Scholar]
- 10.Demyanenko GP, Mohan V, Zhang X, et al. Neural cell adhesion molecule NrCAM regulates Semaphorin 3F-induced dendritic spine remodeling. J Neurosci. 2014;34(34):11274–11287. doi: 10.1523/JNEUROSCI.1774-14.2014. (Available from: http://dx.doi.org/10.1523/JNEUROSCI.1774-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edamura K, Nakano R, Fujimoto K, et al. Effects of cryopreservation on the cell viability, proliferative capacity and neuronal differentiation potential of canine bone marrow stromal cells. J Vet Med Sci. 2014;76(4):573–577. doi: 10.1292/jvms.13-0296. (Available from: http://dx.doi.org/10.1292/jvms.13-0296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis F, Koulakoff A, Boucher D, et al. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23(2):247–256. doi: 10.1016/s0896-6273(00)80777-1. (Available from: http://dx.doi.org/10.1016/S0896-6273(00)80777-1) [DOI] [PubMed] [Google Scholar]
- 13.Hatori M, Shimozawa N, Yasmin L, et al. Role of retinoic acid and fibroblast growth factor 2 in neural differentiation from cynomolgus monkey (Macaca fascicularis) embryonic stem cells. Comp Med. 2014;64(2):140–147. [PMC free article] [PubMed] [Google Scholar]
- 14.Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4(2):249–264. doi: 10.1093/biostatistics/4.2.249. (Available from: http://dx.doi.org/10.1093/biostatistics/4.2.249) [DOI] [PubMed] [Google Scholar]
- 15.Jiang X, Xu R, Guo Z, et al. Neurobiochemistry and neuroelectrophysiology of neuron-like cells differentiated from neural BMSCs-D-NSCs. 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai; 2005. pp. 5215–5218. (Available from: http://dx.doi.org/10.1109/IEMBS.2005.1615654) [DOI] [PubMed] [Google Scholar]
- 16.Liu WT, Han Y, Li HC, et al. An in vivo mouse model of long-term potentiation at synapses between primary afferent C-fibers and spinal dorsal horn neurons: essential role of EphB1 receptor. Mol Pain. 2009;5:29. doi: 10.1186/1744-8069-5-29. (Available from: http://dx.doi.org/10.1186/1744-8069-5-29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lustig M, Sakurai T, Grumet M. Nr-CAM promotes neurite outgrowth from peripheral ganglia by a mechanism involving axonin-1 as a neuronal receptor. Dev Biol. 1999;209(2):340–351. doi: 10.1006/dbio.1999.9250. (Available from: http://dx.doi.org/10.1006/dbio.1999.9250) [DOI] [PubMed] [Google Scholar]
- 18.McGowan LD, Alaama RA, Striedter GF. FGF2 delays tectal neurogenesis, increases tectal cell numbers, and alters tectal lamination in embryonic chicks. PLoS ONE. 2013;8(11):e79949. doi: 10.1371/journal.pone.0079949. (Available from: http://dx.doi.org/10.1371/journal.pone.0079949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishra S, Maurya SK, Srivastava K, et al. Pax6 influences expression patterns of genes involved in neuro-degeneration. Ann Neurosci. 2015;22(4):226–231. doi: 10.5214/ans.0972.7531.220407. (Available from: http://dx.doi.org/10.5214/ans.0972.7531.220407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mlechkovich G, Peng SS, Shacham V, et al. Distinct cytoplasmic domains in Plexin-A4 mediate diverse responses to semaphorin 3A in developing mammalian neurons. Sci Signal. 2014;7(316):ra24. doi: 10.1126/scisignal.2004734. (Available from: http://dx.doi.org/10.1126/scisignal.2004734) [DOI] [PubMed] [Google Scholar]
- 21.Nair J, Ghatge M, Kakkar VV, et al. Network analysis of inflammatory genes and their transcriptional regulators in coronary artery disease. PLoS ONE. 2014;9(4):e94328. doi: 10.1371/journal.pone.0094328. (Available from: http://dx.doi.org/10.1371/journal.pone.0094328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nandy SB, Mohanty S, Singh M, et al. Fibroblast Growth Factor-2 alone as an efficient inducer for differentiation of human bone marrow mesenchymal stem cells into dopaminergic neurons. J Biomed Sci. 2014;21(1):83. doi: 10.1186/s12929-014-0083-1. (Available from: http://dx.doi.org/10.1186/s12929-014-0083-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson AD, Svendsen CN. Low concentrations of extracellular FGF-2 are sufficient but not essential for neurogenesis from human neural progenitor cells. Mol Cell Neurosci. 2006;33(1):29–35. doi: 10.1016/j.mcn.2006.06.003. (Available from: http://dx.doi.org/10.1016/j.mcn.2006.06.003) [DOI] [PubMed] [Google Scholar]
- 24.Parivar K, Baharara J, Sheikholeslami A. Neural differentiation of mouse bone marrow-derived mesenchymal stem cells treated with sex steroid hormones and basic fibroblast growth factor. Cell J. 2015;17(1):27–36. doi: 10.22074/cellj.2015.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira L, Medina R, Baena M, et al. IFN gamma regulates proliferation and neuronal differentiation by STAT1 in adult SVZ niche. Front Cell Neurosci. 2015;9:270. doi: 10.3389/fncel.2015.00270. (Available from: http://dx.doi.org/10.3389/fncel.2015.00270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratie L, Ware M, Jagline H, et al. Dynamic expression of Notch-dependent neurogenic markers in the chick embryonic nervous system. Front Neuroanat. 2014;8:158. doi: 10.3389/fnana.2014.00158. (Available from: http://dx.doi.org/10.3389/fnana.2014.00158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richards AB, Scheel TA, Wang K, et al. EphB1 null mice exhibit neuronal loss in substantia nigra pars reticulata and spontaneous locomotor hyperactivity. Eur J Neurosci. 2007;25(9):2619–2628. doi: 10.1111/j.1460-9568.2007.05523.x. (Available from: http://dx.doi.org/10.1111/j.1460-9568.2007.05523.x) [DOI] [PubMed] [Google Scholar]
- 28.Round JE, Sun H. The adaptor protein Nck2 mediates Slit1-induced changes in cortical neuron morphology. Mol Cell Neurosci. 2011;47(4):265–273. doi: 10.1016/j.mcn.2011.04.009. (Available from: http://dx.doi.org/10.1016/j.mcn.2011.04.009) [DOI] [PubMed] [Google Scholar]
- 29.Sakurai T. The role of NrCAM in neural development and disorders–beyond a simple glue in the brain. Mol Cell Neurosci. 2012;49(3):351–363. doi: 10.1016/j.mcn.2011.12.002. (Available from: http://dx.doi.org/10.1016/j.mcn.2011.12.002) [DOI] [PubMed] [Google Scholar]
- 30.Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. (Available from: http://dx.doi.org/10.1101/gr.1239303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherman BT, Huangda W, Tan Q, et al. DAVID Knowledgebase: a gene-centered database integrating heterogeneous gene annotation resources to facilitate high-throughput gene functional analysis. BMC Bioinformatics. 2007;8(1):8426. doi: 10.1186/1471-2105-8-426. (Available from: http://dx.doi.org/10.1186/1471-2105-8-426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snel B, Lehmann G, Bork P, et al. STRING: a web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res. 2000;28(18):3442–3444. doi: 10.1093/nar/28.18.3442. (Available from: http://dx.doi.org/10.1093/nar/28.18.3442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun T, Li W, Ling S. miR-30c and semaphorin 3A determine adult neurogenesis by regulating proliferation and differentiation of stem cells in the subventricular zones of mouse. Cell Prolif. 2016;49(3):270–280. doi: 10.1111/cpr.12261. (Available from: http://dx.doi.org/10.1111/cpr.12261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torres L, Juarez U, Garcia L, et al. External ear microRNA expression profiles during mouse development. Int J Dev Biol. 2015;59(10-12):497–503. doi: 10.1387/ijdb.150124sf. (Available from: http://dx.doi.org/10.1387/ijdb.150124sf) [DOI] [PubMed] [Google Scholar]
- 35.Wu CY, Persaud SD, Wei LN. Retinoic acid induces ubiquitination-resistant RIP140/LSD1 complex to fine-tune Pax6 gene in neuronal differentiation. Stem Cells. 2016;34(1):114–123. doi: 10.1002/stem.2190. (Available from: http://dx.doi.org/10.1002/stem.2190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu KY, He M, Hou QQ, et al. Semaphorin 3A activates the guanosine triphosphatase Rab5 to promote growth cone collapse and organize callosal axon projections. Sci Signal. 2014;7(340):ra81. doi: 10.1126/scisignal.2005334. (Available from: http://dx.doi.org/10.1126/scisignal.2005334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Yang C, Liu C, et al. Paradoxical effects of VEGF on synaptic activity partially involved in notch1 signaling in the mouse hippocampus. Hippocampus. 2016;26(5):589–600. doi: 10.1002/hipo.22544. (Available from: http://dx.doi.org/10.1002/hipo.22544) [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Zheng H, Zhu HY, et al. Acute effects of transforming growth factor-β1 on neuronal excitability and involvement in the pain of rats with chronic pancreatitis. J Neurogastroenterol Motil. 2016;22(2):333–343. doi: 10.5056/jnm15127. (Available from: http://dx.doi.org/10.5056/jnm15127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou X, Zoller T, Krieglstein K, et al. TGFβ1 inhibits IFNγ-mediated microglia activation and protects mDA neurons from IFNγ-driven neurotoxicity. J Neurochem. 2015;134(1):125–134. doi: 10.1111/jnc.13111. (Available from: http://dx.doi.org/10.1111/jnc.13111) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Information of differentially expressed genes between neurons and BMSCs
Gene ontology enrichment analysis of biological process