Abstract
Introduction
Disruption of circadian rhythms is one of the proposed mechanisms linking late sleep timing to obesity risk but few studies have evaluated biological markers outside of the laboratory. The goal of this study was to determine the relationship between the timing and alignment of melatonin and sleep onset (phase angle) with BMI, body fat and obesity related behaviors. We hypothesized that circadian alignment (relationship of melatonin to sleep timing) rather than circadian (melatonin) timing would be associated with higher BMI, body fat, dietary intake and lower physical activity.
Subjects/Methods
Adults with sleep duration ≥6.5 hours completed 7 days of wrist actigraphy, food diaries and SenseWear arm band monitoring. Circadian timing, measured by dim light melatonin onset (DLMO) was measured in the clinical research unit. Circadian alignment was calculated as the duration between dim light melatonin onset and average sleep onset time in the prior week (phase angle). Body fat was evaluated using dual-energy absorptiometry (DXA). Data were analyzed using bivariate correlations and multivariable regression analyses controlling for age, sex, sleep duration and evening light exposure.
Results
Participants included 97 adults (61 F, age 26.8 ± 7.3 years) with average sleep duration 443.7 (SD= 50.4) minutes. Average phase angle was 2.2 hours (SD= 1.5). Circadian alignment was associated with circadian timing (p<0.001) and sleep duration (p=.005). In multivariable analyses, later circadian timing was associated with lower BMI (p=.04). Among males only, circadian alignment was associated with percent body fat (p=.02) and higher android/gynoid fat ratio (p=0.04). Circadian alignment was associated with caloric intake (p=0.049) carbohydrate intake (p=0.04) and meal frequency (p=0.03) among both males and females.
Conclusion
Circadian timing and alignment were not associated with increased BMI or body fat, among healthy adults with ≥6.5 hours of sleep, but circadian alignment was associated with dietary intake. There may be sex differences in the relationship between circadian alignment and body fat.
Introduction
The endogenous circadian system is involved in the regulation of sleep-wake behaviors as well as key metabolic pathways.1 Recent research has demonstrated both the timing and duration of sleep may influence risk for cardiometabolic disease.2, 3 In particular, several recent studies have reported higher BMI associated with later sleep timing, independent of sleep duration.4–6 However, a limitation of this literature is that few studies have included biological markers of circadian timing (e.g., melatonin). There are several pathways in which late sleep timing may affect weight regulation. Individuals may curtail their sleep in order to meet occupational or social demands. In addition, individuals with late sleep timing also may have greater barriers to engaging in healthful diet and exercise behaviors due to competing demands after work and school obligations.4, 7 Another factor may be that late timing may impact weight regulation through alignment of the circadian rhythm. The need to habitually sleep earlier in the circadian rhythm may lead to misalignment, which then contributes to changes in metabolism and behavior.8
Circadian misalignment has multiple definitions, but has primarily been defined as alteration of sleep-wake timing relative to the endogenous circadian rhythm. It is hypothesized that circadian rhythm misalignment among shift workers contributes to increased rates of obesity, diabetes and heart disease risk compared with non-shift workers.9–11 One of the primary effects of shifting the sleep period relative to the appropriate circadian time is sleep disruption, which has been well documented among shift workers.12 Therefore, it is difficult to tease apart the independent effects of circadian alignment versus sleep duration, particularly in real life settings. Several studies have used laboratory protocols, such as forced desynchrony or simulated shift work in attempts to isolate the effects of circadian misalignment, independent of sleep duration, and have demonstrated that circadian misalignment leads to impairments in glucose metabolism and alterations in appetite stimulating hormones even after controlling for sleep timing and quality.13–17 However, these studies typically evaluate major misalignment, such as that seen in shift work (e.g., 8–12 hours shifted from the individual’s typical overnight sleep wake schedule). Circadian misalignment can also be present in more minor forms, such as jet lag from only a few time zones, or due to changing sleep timing several hours to fit social or occupational obligations from weekdays to weekends. Less is known about the health effects of these minor degrees of circadian misalignment in non-shift workers.
One method of quantifying circadian alignment in daily life is by phase angle, which is calculated as the duration between dim light melatonin onset and sleep onset time. Dim light melatonin onset (DLMO), a marker of the nocturnal rise in melatonin, is a well-accepted measure of the timing of the endogenous circadian rhythm.19 The relationship between the timing of melatonin and other behavioral and biological markers (e.g. sleep onset) has been called “phase angle” and it is thought to be an excellent marker of alignment of the behavioral rhythm or other variables with the central circadian rhythm. On average, the duration between DLMO and sleep onset is 2 hours among individuals with controlled (i.e. 8 hours in bed, consistent bedtimes/wake times) and uncontrolled (i.e. self-selected) sleep schedules.20, 21 Individual differences in circadian alignment have been reported based on sex22, 23, age24, 25 and sleep timing preference.26 The determinants of circadian alignment in daily life have not been extensively studied but laboratory studies suggest light exposure and the length of their endogenous circadian period play a role.18 Individuals with later sleep/wake timing may be at greater risk for circadian misalignment, perhaps due to a mismatch between their preferred schedule and the typical work or school schedule.27 Prior research has demonstrated higher depressive symptoms among individuals with sleep onset closer to DLMO (i.e. short phase angles) but these studies did not measure associations with physical health variables.28–30 Two studies have reported that individuals with a greater difference between sleep times on work days and free days (social jet lag) had higher BMI but did not evaluate circadian timing or alignment.2, 31 Given the experimental literature linking phase alignment to metabolic outcomes, evaluating associations with circadian alignment in daily life would contribute to understanding potential mechanisms by which circadian alignment affects weight regulation in real world settings.
The goal of the current study was to determine the relationship between circadian timing and alignment of DLMO to sleep onset with BMI, body fat, diet and physical activity among healthy non-shift working adults. We tested these relationships among healthy individuals without psychiatric or sleep disorders with habitual sleep duration of at least 6.5 hours per night in order to assess the independent contribution of circadian timing and alignment. We predicted that later circadian timing would be associated with circadian alignment (i.e., sleep onset closer to melatonin onset), but effects of circadian alignment on BMI, body fat, diet and exercise behaviors would be independent of circadian timing and sleep duration.
Methods
Participants
Men and women were recruited from the community using flyers and web advertisements. Flyers were posted specifically to recruit healthy sleepers as well as healthy “night owls” in order to over recruit individuals who may have circadian misalignment. Inclusionary criteria included: age 18–50, habitual sleep duration ≥ 6.5 hours and ≤ 8.5 hours, and ability to read and write English. Exclusionary criteria included: High risk or presence of obstructive sleep apnea, insomnia, restless legs syndrome as assessed by the screening questionnaires and/or home sleep monitoring, history of cognitive or other neurological disorders, presence of any major psychiatric disorder, current alcohol or substance abuse as assessed by screening questionnaires, history of or concurrent unstable or serious medical illness (cancer, diabetes or cardiovascular disease), current use of psychoactive medications including antidepressants, anxiolytics, neuroleptics, anticonvulsants, hypnotics, stimulants, or beta blockers, shift work or travel over 2 time zones in the past 6 months, caffeine >300 mg per day, smoking, pregnancy or the desire to become pregnant during the study period.
Procedure
Screening procedures
Prospective participants first completed an initial web or telephone screening to screen for self-reported short sleep duration, comorbid conditions and health behaviors (smoking, alcohol and substance use). Next, potential participants attended an in-person screening visit. During this visit, they completed questionnaires and then one night of home sleep apnea screening and 7 days of actigraphy to determine if they met study criteria. After screening actigraphy and apnea screening data were reviewed, eligible participants were scheduled for one night in the Clinical Research Unit for melatonin assessment and DXA.
Pre-laboratory procedures
Participants completed 7 days of actigraphy, sleep and food diaries prior to the laboratory session. Participants were asked to keep their habitual bedtimes and wake times at screening stable, within ± 1 hour of their average at screening times.
Laboratory session
Participants arrived at approximately 1:00 PM. After admission, participants underwent a history and physical exam by a sleep medicine physician and female participants underwent a urine pregnancy screening. Then, participants completed a Dual-energy X-ray Absorptiometry (DXA) tissue measurement to assess body fat. Bedtime, wake time and blood sampling times were determined based on habitual bedtime. Circadian time 0 (CT 0) was determined by average sleep onset time plus 8 hours because participants were not required to spend 8 hours in bed in the week prior to the CRU visit. The lights were dimmed to <20 lux at CT 8. An IV was inserted by CT 10, and blood samples were taken every 30 min from CT 11-CT 18. Lights out (<5 lux) was CT 16. Wake time was scheduled for 8 hours after lights out to standardize the timing of the fasting blood draw. In the morning, participants were asked to void, and then their weight was taken without shoes in light clothing before eating or drinking anything. Participants were discharged after breakfast.
Measures
Demographics
A demographic questionnaire included age, sex, race, ethnicity, income, employment and marital status.
Depressive symptoms
Participants were screened for depressive symptoms using the Center for Epidemiologic Studies Questionnaire32. This 20-item measure assesses depressive symptoms over the past 2 weeks. Participants rate items from 0 (not at all or less than one day) to 3 (nearly every day for two weeks). Items measure a range of depressive symptoms, including low mood, appetite, anhedonia, and somatic symptoms. The CES-D is well established as a valid screening measure for depressive symptoms.32 Participants with scores ≥ 20 were excluded.
Obstructive sleep apnea risk
Risk for obstructive sleep apnea was first assessed via questionnaire at the phone/web screening. Then, those potential participants who did not score as high risk for obstructive sleep apnea on the phone screening completed an overnight screening at home using portable sleep apnea screening device (ApneaLink, Resmed Inc. Poway, CA). This 3 channel device includes a pulse oximeter, heart rate, respiratory effort and nasal airflow using a nasal pressure transducer. Apnea Hypopnea Index (AHI) is calculated based on events per hour of recording time. Previous studies have demonstrated this device has excellent sensitivity and specificity for AHI > 10, sensitivity 0.977 and specificity of 1.033. All recordings were both visually inspected and scored using the Apnea Link software. Participants who had an Apnea Hypopnea Index >5 on the Apnea Link recording were excluded from the study.
Subjective Sleep quality
Subjective sleep quality was measured by the the Pittsburgh Sleep Quality Questionnaire (PSQI).34 This measure assesses self-reported sleep quality and disturbances over a one-month time interval. A higher PSQI global score indicates greater sleep disturbance. Scores ≥5 are associated with clinically significant sleep disturbance.34 Subscores of the PSQI include sleep duration, sleep disturbance, sleep latency, daytime dysfunction, habitual sleep efficiency, sleep quality and medication use.
Sleep
Sleep/wake patterns were estimated using 7 days of wrist actigraphy at screening and before the laboratory session using the Actiwatch Spectrum (Philips/Respironics, Inc, Bend, OR). Actiwatches were worn on the non-dominant wrist and set with 30 second epoch length and medium sensitivity. Actigraphic sleep parameters were calculated using Actiware-Sleep 6.0 software with default settings and included the following variables: sleep onset time, sleep offset time and sleep duration. Sleep diary based measures of bedtime and wake time were manually entered in Actiware and used in scoring. Off wrist time was excluded based on the Spectrum’s off wrist detection. A day was not considered valid if there was any off-wrist time during the sleep period reported on the sleep diary. In order to be included in analyses, participants needed at least 5 days of valid actigraphy data.
Evening light exposure
White light exposure was measured using the Actiwatch Spectrum (Philips/Respironics, Inc, Bend, OR). We calculated evening light exposure as the sum of white light exposure (lux) in the 3 hours prior to bedtime on each day of recording and averaged over the valid recording days.
Circadian timing (DLMO)
Plasma melatonin levels were assayed using a commercially available radioimmunoassay for the in-vitro diagnostic quantitative determination of melatonin from IBL (IBL International GmbH, Hamburg, Germany). Lower limit of this assay is 3.5 kg/mL and upper limit is 281 pg/mL. After blood draws, samples were centrifuged, aliquoted and stored at −70 F until assayed. All melatonin profiles were first visually inspected. Dim light melatonin onset was determined using 2 SD above the baseline + 15% of the 3 highest values.35 Circadian alignment (also known as phase angle) was calculated as the duration of time between DLMO to average sleep onset time recorded on actigraphy during the prior 7 days.
Body Mass index
Body Mass Index (kg/m2) was calculated using measurements from the laboratory session: height measured at admission by nursing and weight taken in light clothing, without eating or drinking after the morning void.
Body fat and body fat distribution
Body Fat was measured using DXA on a whole body Hologic scanner (Version 13.1). Body fat values were calculated for total body fat mass, trunk fat mass, android and gynoid regions using automated calculations provided by Hologic. Values were calculated for total body fat % and the gynoid/android ratio, a measure of central adiposity.
Dietary Intake
Participants completed 7 days of written food diaries during the week before the laboratory assessment. On the food diaries, participants recorded time, location, type of food, amount consumed and a description of each component including brands and restaurant names. Participants were instructed to break down foods into component parts (e.g. turkey sandwich= 2 slices of white bread, 3 oz of deli turkey and 1 Tbs of reduced fat mayo) and to remember to record drinks and condiments. Participants emailed, faxed or called in diaries each day to ensure they were completed on time. At the laboratory session, study staff reviewed the food diaries with the participant and queried for missing foods and components (e.g. cream in coffee, drinks, condiments, cooking with oil or other missed foods). Food diaries were analyzed using the Food Processor software (ESHA, Inc, Salem, OR). For foods that were not in the database, they were looked up on company or restaurant websites. If caloric information was not available, the closest substitute was used. Total daily caloric intake, grams of protein, fat and carbohydrates each day was computed and averaged. Meal frequency was calculated as the number of eating occasions each day, with an eating occasion defined as >50 calories consumed >30 minutes after the previous meal or snack.
Physical activity
Physical Activity was assessed using the Sensewear Pro armband monitor (Bodymedia Inc., Pittsburgh, PA). The Sensewear monitor estimates physical activity and energy expenditure using several sensors including a 3 axis accelerometer, heat flux sensor, skin temperature sensor, near body ambient temperature sensor and galvanic skin response sensor). The Sensewear monitor has demonstrated accuracy and reliability for physical activity assessment.36–38 Physical activity values were calculated using Sensewear Professional software (Version 8.1). Sensewear recordings were considered valid if participants had at least 3 days of recording with ≥ 18 hours of wear time each day.
Statistical Analyses
Data were analyzed using SPSS (Version 22, IBM Inc., Chicago, IL). First, sample characteristics were calculated using means (SD) and frequencies. Next, we calculated Pearson correlations to examine associations between circadian timing and alignment with sleep characteristics, age and evening light exposure. We also used t-tests to evaluate sex differences in circadian timing and alignment. Next we conducted multivariable regression models predicting BMI, body fat, dietary intake and physical activity controlling for potentially confounding variables, age, sex and sleep duration. Models predicting diet variables were also adjusted for BMI. Both circadian timing and alignment were entered into the each model. Due to known sex differences in body fat39, circadian timing and alignment22, we conducted exploratory analyses testing for interactions with sex. There was one outlier for phase angle (phase angle −2.0 hours, which indicates DLMO occurred 2 hours after sleep onset). We examined the actigraphy data from this participant which indicated a sleep pattern consistent with study entry criteria and there was no clear behavioral reason why sleep onset was before DLMO (e.g., shift work). We then conducted our regression analyses with and without this participant and results were similar. Therefore data from this participant is included our analyses. Statistical significance was defined as p<0.05 on 2 tailed tests.
Results
Participant Characteristics
Of the 146 participants who were eligible for the study, 114 completed the protocol. Our sample includes 97 participants with valid DLMO and actigraphy data. Of the 17 participants excluded from the analyses, actigraph device failures occurred in 3 participants and we were unable to calculate DLMO among 14 participants due to lack of a baseline or other irregularities, such as no clear rise in melatonin or erratic profile. There were no differences in sleep timing, age, sex or BMI between participants with or without valid DLMO profiles. Sample statistics are listed in Table 1. Participants were on average in their middle 20’s. The majority (79%) reported work and or school during the week prior to the clinical research unit. Average BMI and body fat were in the normal weight range. BMI ranged from 16–38 kg/m2. Average sleep onset and sleep offset times were slightly later than average for the age of the sample, but reflect recruitment of strategy to over recruit self-described late sleep times. For example, 45% of the sample had midpoint of sleep after 5:00 am. The measure of circadian alignment (phase angle) had a normal distribution (ranging from −2.22 to 4.27 hours) with one participant with sleep onset before DLMO. The mean phase angle was 2.19 (SD= 0.95) hours.
Table 1.
Participant Characteristics
| Demographics | Total (n=97) M(SD) or N(%) |
Women (n=61) M(SD) or N(%) |
Men (n=36) M(SD) or N(%) |
|
|---|---|---|---|---|
| Age (years) | 26.8 (7.3) | 26.9 (8.4) | 26.5 (5.7) | |
| Ethnicity | Hispanic/Latino | 8 (8.2) | 4 (6.6) | 4 (11.1) |
| Non- Hispanic/Latino |
89 (91.8) | 57 (93.4) | 32 (88.9) | |
| Race | Asian | 22 (22.7) | 14 (23.0) | 8 (22.2) |
| Black/African American |
6 (6.2) | 5 (8.2) | 1 (2.8) | |
| White | 61 (62.9) | 38 (62.3) | 23 (36.8) | |
| More than one race | 7 (7.2) | 3 (4.9) | 4 (11.1) | |
| Do not wish to respond |
1 (1) | 1 (1.6) | 0 (0) | |
| Employment* | ||||
| Full time | 34 (35.1) | 21 (34.4) | 31 (36.1) | |
| Part time | 15 (15.5) | 11 (18.0) | 4 (11.1) | |
| Unemployed | 6 (6.2) | 4 (6.6) | 1 (2.8) | |
| Student | 42 (43.3) | 25 (41.0) | 17 (47.2) | |
|
Weight and Body Fat |
||||
| BMI (kg/m2) | 24.0 (4.6) | 23.9 (5.0) | 24.7 (3.8) | |
| Body Fat% | 30.4 (8.4) | 34.8 (6.6) | 22.9 (5.6) | |
| Sleep | ||||
| PSQI Global Score |
3.5 (2.0) | 3.6 (2.2) | 3.3 (1.6) | |
| Sleep Onset (hh:mm) |
00:47 (1:22) | 00:39 (1:22) | 01:02 (1:22) | |
| Sleep Offset (hh:mm) |
08:05 (1:14) | 08:06 (1:16) | 08:04 (1:12) | |
| Sleep midpoint (hh:mm) |
04:42 (1.24) | 04:37 (1:17) | 04:48 (1:10) | |
| Sleep Duration (min) |
443.7 (50.4) | 452.1 (48.1) | 429.5 (52.9) | |
| DLMO (hh:mm) | 22:36 (1:27) | 22:24 (1:18) | 22:57 (1:40) | |
| Circadian alignment (Phase angle of DLMO to sleep onset, hh:mm) |
2:11 (0:57) | 2:14 (0:59) | 2:05 (0:55) | |
| Dietary Intakea | ||||
| Caloric intake (kcal) |
2079 (656) | 1905 (594) | 2308 (673) | |
| Protein | 87.78 (44.87) | 76.72 (47.00) | 105.60 (34.97) | |
| Fat | 79.17 (33.09) | 71.45 (28.80) | 91.62 (36.09) | |
| Carbohydrates | 243.21 (81.04) | 229.98 (76.60) | 264.53 (84.48) | |
| Number of meals | 4.39 (1.22) | 4.46 (1.18) | 4.28 (1.28) | |
| Physical activityb | ||||
| Physical activity duration above 3.0 mets (hh:mm per day) |
1:49 (0:58) | 1:29 (0:49) | 2:26 (0:56) | |
Note. PSQI= Pittsburg Sleep Quality Index, DLMO= dim light melatonin onset,
79% of participants reported work or school obligations during the 7 days of actigraphy.
Number of participants varies due to missing data for some measures.
N=95 for dietary intake,
N= 72 for physical activity.
Relationships between demographic, circadian and sleep characteristics
We conducted bivariate correlations (Table 2) and t-tests to evaluate the relationships between demographics, sleep duration, sleep quality and evening light exposure with circadian timing and alignment. Later circadian timing was associated with younger age and circadian alignment (shorter duration between DLMO and sleep onset). Circadian alignment was associated with sleep duration but not associated with age, sleep quality (total score or subscores) or evening light exposure. Later circadian timing was associated with a higher score on the subjective sleep latency subscore but not the total score for subjective sleep quality, other sleep quality subscores or evening light exposure. Sex differences in circadian timing and alignment were not statistically significant (DLMO= 22:24 females, 22:57 males, p=0.07, alignment of melatonin and sleep onset (phase angle)=2.2 hours in females, 2.1 hours in men, p= ns).
Table 2.
Correlations between phase angle with demographics, sleep characteristics and evening light exposure
| Age | DLMO | Phase Angle |
Sleep Duration |
Sleep Quality |
Evening Light |
|
|---|---|---|---|---|---|---|
| Age | -- | |||||
| DLMO | 0.31** | -- | ||||
| Phase Angle | −0.17 | −0.42*** | -- | |||
| Sleep Duration | 0.16 | −0.15 | −0.28** | -- | ||
| Sleep Quality | −0.12 | 0.19 | −0.06 | −0.14 | -- | |
| Evening light | 0.30** | −0.001 | −0.09 | 0.02 | 0.03 | -- |
Note.
p< 0.01,
p< 0.001,
DLMO= Dim light melatonin onset time, Phase angle= DLMO to sleep onset,
BMI and Body Fat
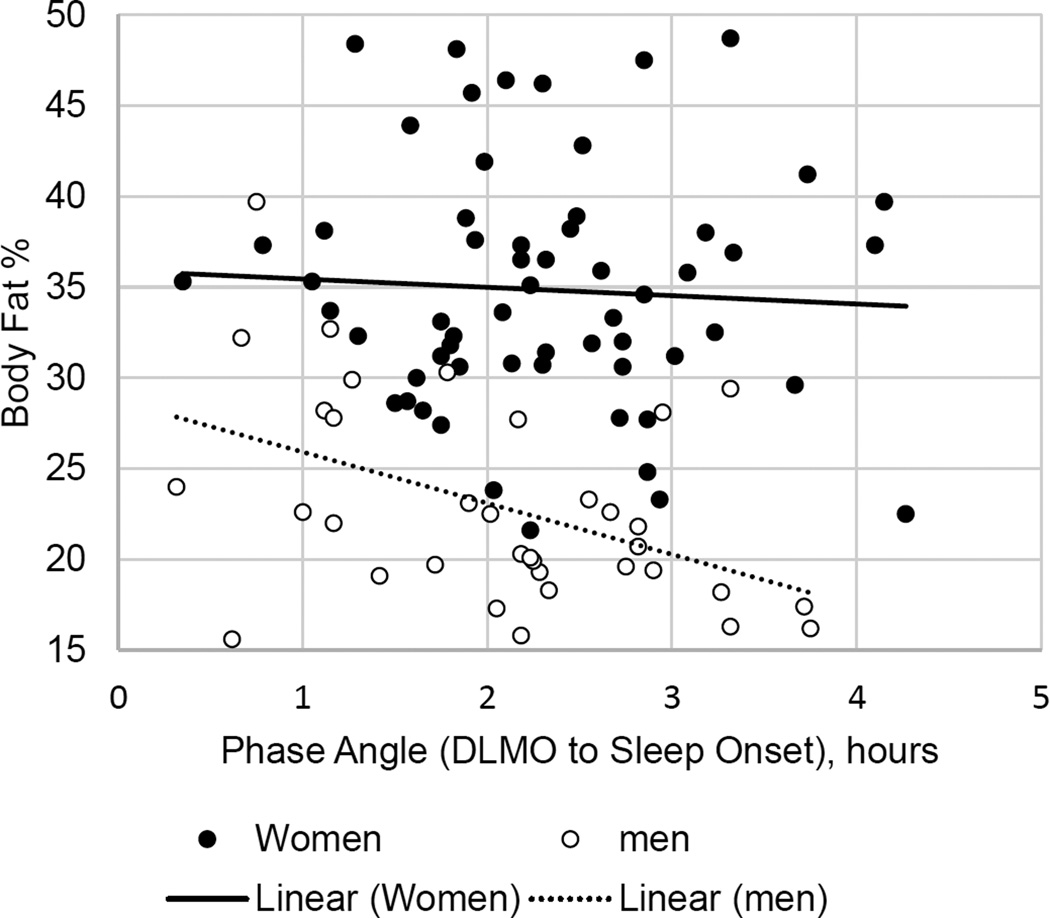
In the multivariable model including age, sex, sleep duration, phase angle, and evening light exposure, Circadian timing was inversely associated with BMI (B(SE)=−0.83 (0.40), p=0.04, r2Δ= 0.027). Circadian timing was not associated with body fat percentage or distribution. Circadian alignment was not associated with BMI or body fat but there were significant sex differences in the relationship with body fat. Circadian alignment was associated with total body fat % (B(SE)= −3.56 (1.48), p=0.02, r2Δ= 0.032; Figure 1) and android/gynoid fat ratio (B(SE)= −0.09 (.04), p=0.04, r2Δ= 0.044), indicating a stronger relationship between circadian alignment and body fat among men.
Figure 1.
Association between phase angle and body fat by sex
Dietary intake
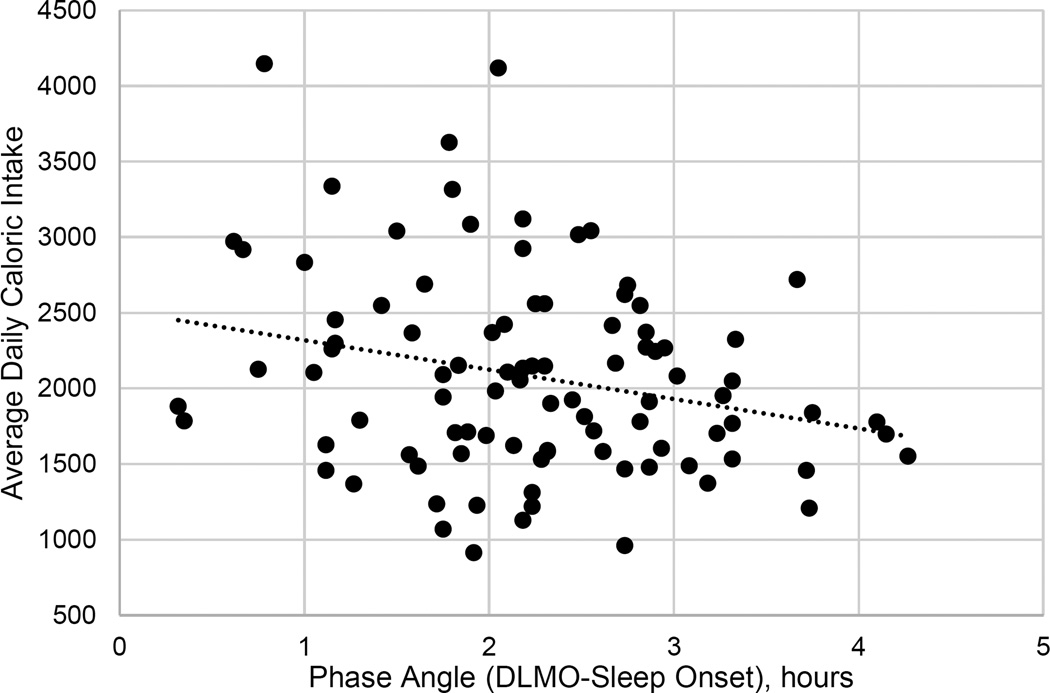
Circadian alignment was associated with higher caloric intake in the multivariable model (B(SE)= −168.85 (84.45), p=.049). There was a non-significant trend for a relationship between circadian alignment and carbohydrate intake (B(SE)= −21.23 (9.9), p=0.04) and no relationship between circadian alignment with protein or fat intake. Circadian timing was not associated with caloric or macronutrient intake. There were no sex differences for caloric intake. Circadian alignment but not timing was associated with meal frequency (B(SE)=−0.36 (0.16), p=0.03.
Associations with physical activity
Circadian timing and alignment were not associated with physical activity and there were no sex differences in these effects.
Discussion
In this study, we tested if later circadian timing or differences in circadian alignment were associated with BMI/body fat, diet and physical activity. Participants were carefully screened for confounds, most notably sleep duration and depression, which are often associated with later circadian timing. Results demonstrated that in this carefully screened population, later circadian timing was associated with lower BMI and circadian alignment (sleep onset closer to DLMO) was associated with higher caloric intake, carbohydrates and greater meal frequency. We did not observe associations between circadian alignment with BMI, body fat or physical activity however there were sex differences in the relationship with body fat, in that shorter duration between DLMO and sleep onset was associated with higher body fat and greater central body fat distribution among men.
The magnitude of effect for phase angle and caloric intake was moderate; interpretation of the regression coefficient of the multivariable model indicates that participants consumed an average of 168 more calories per day per 1 hour difference in phase angle, after accounting for demographics, sleep duration and circadian timing and that phase angle independently accounted for 4% of the variance in caloric intake. If not balanced by energy expenditure, it would amount to an increased weight 17 lbs per year. However, despite this difference in energy intake, phase angle was not associated with BMI or body fat. We also observed differences in other dietary behaviors associated with circadian alignment including greater carbohydrate intake and meal frequency associated with a shorter duration between DLMO and sleep onset. It is possible that differences in diet composition and eating pattern may contribute to the lack of an increased BMI despite higher caloric intake in our study. For example, higher meal frequency in the context of controlling overall calories has been associated with a lower postprandial insulin response and higher thermic effect of food.40, 41 Furthermore, previous studies, including those from our own laboratory, have observed eating occasions associated with higher caloric intake but not BMI in other samples of young and middle aged adults.42, 43
The association between circadian alignment and dietary intake is in line with several laboratory studies which use experimental protocols to test disruption of circadian rhythms on metabolism and weight regulation. One example is use of forced desynchrony, in which participants’ sleep schedules are moved several hours earlier or later each day. Using this protocol, researchers are able to observe effects of circadian misalignment controlling for sleep duration, and have observed the development of insulin resistance and decrease in leptin levels.13, 14, 16 Other studies have evaluated the effects of acute simulated shift work (3–7 days), and have also observed insulin resistance and changes in appetite regulating hormones.13, 15, 44 Results of our study are novel in that they demonstrate that even the relatively small differences in the internal phase relationship among healthy individuals in everyday life is associated with dietary behavior.
Contrary to prior studies which have demonstrated poorer sleep quality with circadian misalignment12, 45, 46 in our sample, a shorter phase angle was associated with longer sleep duration and was unrelated to subjective sleep quality. Our study criteria (recruitment of participants with ≥6.5 hours sleep duration and over recruitment of late sleepers) may have led to the selection of participants with more adaptable sleep schedules. It has been proposed that sleep is regulated by two main processes: homeostatic sleep pressure and the circadian rhythm.47 It is possible that due to our selection criteria, we selected individuals with later circadian timing but higher homeostatic pressure to sleep, which allowed them to fall asleep earlier, thus contributing to a shorter phase angle.
Given the sex differences in circadian timing, alignment22, 23,48 and body fat39, 49, we conducted exploratory analyses of sex differences and found that phase angle was associated with body fat and body fat distribution only in men. In our sample, consistent with previous research, women had higher body fat and slightly (non-significantly) longer phase angle than men.22, 39 We did not evaluate the role of sex hormones but previous studies have demonstrated changes in the circadian rhythm across the menstrual cycle. It should be noted that this study was not designed to study sex differences and we were therefore underpowered to observe interactions. Larger, more controlled samples should further explore the interaction of sex in circadian misalignment as well as the role of sex hormones that may moderate the effects of circadian alignment on health outcomes.
Limitations to our data include use of a convenience sample, which limits generalizability. Also, use of a healthy, young sample may have led to ceiling/floor effects in evaluation of BMI, physical activity and phase angle due to restricted range. In addition, our results in healthy sleepers without short sleep duration may under represent the effects of circadian misalignment among individuals who also have sleep duration < 6.5 hours. A previous study has demonstrated an additive effect of sleep loss and circadian misalignment in metabolic risk.15, We also assessed phase angle at a single point in time. Prior research suggests that circadian timing and alignment are stable over repeated assessment in weeks or months but little is known about changes in phase angle over months and years.50–52 Finally, further assessment of the type and intensity of light exposure is warranted. In this study, total white light exposure prior to bedtime was not associated with circadian timing and alignment. Given that light exposure is one of the determinants of phase angle in laboratory studies, known to suppress melatonin, and also associated with body fat, it may be useful to further characterize the role of the timing of light, wavelength and intensity in examining the metabolic correlates of phase angle.18, 53
In conclusion, these data suggest circadian alignment, rather than late timing alone, may play a role in the links between sleep timing and weight regulation. We observed lower BMI associated with later biological timing, rather than higher in this highly selected sample. Circadian alignment was associated with dietary intake even among healthy, non-shift workers with at least 6.5 hours habitual sleep duration. Our results did not support an association with circadian alignment and body fat in this sample as a whole. Future research should evaluate sex differences and seek to understand how differences in circadian alignment can affect weight regulation and risk for cardiometabolic disease.
Figure 2.
Association between phase angle and caloric intake
Acknowledgments
We thank Leland Bardsley, Leah Hecht, David Clough and Lori Koch for their assistance with data collection and analyses. Research reported in this publication was supported, in part, by the National Institutes of Health'sNational Center for Advancing Translational Sciences, Grant Number UL1TR000150 and by the National Institute of Health’s Heart Lung Blood and Sleep Institute Grant Number 1K23HL109110-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Dr. Zee reports she is a consultant for Merck, Philips, Research grants to Northwestern University from Jazz Pharmaceuticals and stock ownership in Teva Pharmaceuticals.
Footnotes
Conflicts of Interest
The other authors do not have conflicts of interest.
References
- 1.Arble DM, Ramsey KM, Bass J, Turek FW. Circadian disruption and metabolic disease: findings from animal models. Best Pract. Res. Clin. Endocrinol. Metab. 2010;24(5):785–800. doi: 10.1016/j.beem.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong PM, Hasler BP, Kamarck TW, Muldoon MF, Manuck SB. Social Jetlag, Chronotype, and Cardiometabolic Risk. J. Clin. Endocrinol. Metab. 2015;100(12):4612–4620. doi: 10.1210/jc.2015-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merikanto I, Lahti T, Puolijoki H, Vanhala M, Peltonen M, Laatikainen T, et al. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiol. Int. 2013;30(4):470–477. doi: 10.3109/07420528.2012.741171. [DOI] [PubMed] [Google Scholar]
- 4.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity. 2011;19(7):1374–1381. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- 5.Lucassen EA, Zhao X, Rother KI, Mattingly MS, Courville AB, de Jonge L, et al. Evening chronotype is associated with changes in eating behavior, more sleep apnea, and increased stress hormones in short sleeping obese individuals. PLoS ONE. 2013;8(3):e56519. doi: 10.1371/journal.pone.0056519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arora T, Taheri S. Associations among late chronotype, body mass index and dietary behaviors in young adolescents. Int. J. Obes. 2015;39(1):39–44. doi: 10.1038/ijo.2014.157. [DOI] [PubMed] [Google Scholar]
- 7.Sato-Mito N, Shibata S, Sasaki S, Sato K. Dietary intake is associated with human chronotype as assessed by both morningness-eveningness score and preferred midpoint of sleep in young Japanese women. Int. J. Food. Sci. Nutr. 2011;62(5):525–532. doi: 10.3109/09637486.2011.560563. [DOI] [PubMed] [Google Scholar]
- 8.Baron KG, Reid K. Circadian misalignment and heatlh. International Review of Psychiatry. 2014;26(2):139–154. doi: 10.3109/09540261.2014.911149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antunes LC, Levandovski R, Dantas G, Caumo W, Hidalgo MP. Obesity and shift work: chronobiological aspects. Nutr Res Rev. 2010;23(1):155–168. doi: 10.1017/S0954422410000016. [DOI] [PubMed] [Google Scholar]
- 10.Suwazono Y, Dochi M, Sakata K, Okubo Y, Oishi M, Tanaka K, et al. A longitudinal study on the effect of shift work on weight gain in male Japanese workers. Obesity (Silver Spring) 2008;16(8):1887–1893. doi: 10.1038/oby.2008.298. [DOI] [PubMed] [Google Scholar]
- 11.Ruger M, Scheer FA. Effects of circadian disruption on the cardiometabolic system. Rev. Endocr. Metab. Disord. 2009;10(4):245–260. doi: 10.1007/s11154-009-9122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP, Jr, Vitiello MV, et al. Circadian rhythm sleep disorders: part I, basic principles, shift work and jet lag disorders. An American Academy of Sleep Medicine review. Sleep. 2007;30(11):1460–1483. doi: 10.1093/sleep/30.11.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl. Acad. Sci. U. S. A. 2015 doi: 10.1073/pnas.1418955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. U. S. A. 2009;106(11):4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buxton OM, Cain SW, O'Connor SP, Porter JH, Duffy JF, Wang W, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4(129):129ra43. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gronfier C, Wright KP, Jr, Kronauer RE, Czeisler CA. Entrainment of the human circadian pacemaker to longer-than-24-h days. Proc. Natl. Acad. Sci. U. S. A. 2007;104(21):9081–9086. doi: 10.1073/pnas.0702835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckel RH, Depner CM, Perreault L, Markwald RR, Smith MR, McHill AW, et al. Morning Circadian Misalignment during Short Sleep Duration Impacts Insulin Sensitivity. Curr. Biol. 2015;25(22):3004–3010. doi: 10.1016/j.cub.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Wright KP, Jr, Gronfier C, Duffy JF, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J. Biol. Rhythms. 2005;20(2):168–177. doi: 10.1177/0748730404274265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, et al. Measuring melatonin in humans. J. Clin. Sleep. Med. 2008;4(1):66–69. [PMC free article] [PubMed] [Google Scholar]
- 20.Burgess HJ, Eastman CI. The dim light melatonin onset following fixed and free sleep schedules. J. Sleep. Res. 2005;14(3):229–237. doi: 10.1111/j.1365-2869.2005.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess HJ, Savic N, Sletten T, Roach G, Gilbert SS, Dawson D. The relationship between the dim light melatonin onset and sleep on a regular schedule in young healthy adults. Behav. Sleep. Med. 2003;1(2):102–114. doi: 10.1207/S15402010BSM0102_3. [DOI] [PubMed] [Google Scholar]
- 22.Cain SW, Dennison CF, Zeitzer JM, Guzik AM, Khalsa SB, Santhi N, et al. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J. Biol. Rhythms. 2010;25(4):288–296. doi: 10.1177/0748730410374943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Reen E, Sharkey KM, Roane BM, Barker D, Seifer R, Raffray T, et al. Sex of college students moderates associations among bedtime, time in bed, circadian phase angle. J. Biol. Rhythms. 2013;28(6):425–431. doi: 10.1177/0748730413511771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duffy JF, Dijk DJ, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am. J. Physiol. 1998;275(5 Pt 2):R1478–R1487. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- 25.Duffy JF, Zeitzer JM, Rimmer DW, Klerman EB, Dijk DJ, Czeisler CA. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. American Journal of Physiology Endocrinology and Metabolism. 2002;282(2):E297–E303. doi: 10.1152/ajpendo.00268.2001. [DOI] [PubMed] [Google Scholar]
- 26.Baehr EK, Revelle W, Eastman CI. Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. J. Sleep Res. 2000;9(2):117–127. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- 27.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol. Int. 2006;23(1–2):497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- 28.Lewy AJ, Emens JS, Songer JB, Sims N, Laurie AL, Fiala SC, et al. Winter Depression: Integrating mood, circadian rhythms, and the sleep/wake and light/dark cycles into a bio-psycho-social-environmental model. Sleep Med Clin. 2009;4(2):285–299. doi: 10.1016/j.jsmc.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emens J, Lewy A, Kinzie JM, Arntz D, Rough J. Circadian misalignment in major depressive disorder. Psychiatry Res. 2009;168(3):259–261. doi: 10.1016/j.psychres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Hasler BP, Buysse DJ, Kupfer DJ, Germain A. Phase relationships between core body temperature, melatonin, and sleep are associated with depression severity: further evidence for circadian misalignment in non-seasonal depression. Psychiatry Res. 2010;178(1):205–207. doi: 10.1016/j.psychres.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr. Biol. 2012;22(10):939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 32.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 33.Ng SS, Chan TO, To KW, Ngai J, Tung A, Ko FW, et al. Validation of a portable recording device (ApneaLink) for identifying patients with suspected obstructive sleep apnoea syndrome. Intern. Med. J. 2009;39(11):757–762. doi: 10.1111/j.1445-5994.2008.01827.x. [DOI] [PubMed] [Google Scholar]
- 34.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 35.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J. Biol. Rhythms. 1997;12(5):457–466. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- 36.Johannsen DL, Calabro MA, Stewart J, Franke W, Rood JC, Welk GJ. Accuracy of armband monitors for measuring daily energy expenditure in healthy adults. Med. Sci. Sports Exerc. 2010;42(11):2134–2140. doi: 10.1249/MSS.0b013e3181e0b3ff. [DOI] [PubMed] [Google Scholar]
- 37.Fruin ML, Rankin JW. Validity of a multi-sensor armband in estimating rest and exercise energy expenditure. Med. Sci. Sports Exerc. 2004;36(6):1063–1039. doi: 10.1249/01.mss.0000128144.91337.38. [DOI] [PubMed] [Google Scholar]
- 38.Drenowatz C, Eisenmann JC. Validation of the SenseWear Armband at high intensity exercise. Eur. J. Appl. Physiol. 2011;111(5):883–887. doi: 10.1007/s00421-010-1695-0. [DOI] [PubMed] [Google Scholar]
- 39.Li C, Ford ES, Zhao G, Balluz LS, Giles WH. Estimates of body composition with dual-energy X-ray absorptiometry in adults. Am. J. Clin. Nutr. 2009;90(6):1457–1465. doi: 10.3945/ajcn.2009.28141. [DOI] [PubMed] [Google Scholar]
- 40.Kanaley JA, Heden TD, Liu Y, Fairchild TJ. Alteration of postprandial glucose and insulin concentrations with meal frequency and composition. Br. J. Nutr. 2014;112(9):1484–1493. doi: 10.1017/S0007114514002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heden TD, Liu Y, Sims LJ, Whaley-Connell AT, Chockalingam A, Dellsperger KC, et al. Meal frequency differentially alters postprandial triacylglycerol and insulin concentrations in obese women. Obesity (Silver Spring) 2013;21(1):123–129. doi: 10.1002/oby.20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mills JP, Perry CD, Reicks M. Eating frequency is associated with energy intake but not obesity in midlife women. Obesity (Silver Spring) 2011;19(3):552–559. doi: 10.1038/oby.2010.265. [DOI] [PubMed] [Google Scholar]
- 43.Reid KJ, Baron KG, Zee PC. Meal timing influences daily caloric intake in healthy adults. Nutr Res. 2014;34(11):930–935. doi: 10.1016/j.nutres.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McHill AW, Melanson EL, Higgins J, Connick E, Moehlman TM, Stothard ER, et al. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc. Natl. Acad. Sci. U. S. A. 2014;111(48):17302–17307. doi: 10.1073/pnas.1412021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: implications for behavior. Ann. N. Y. Acad. Sci. 2004;1021:276–291. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- 46.Boivin DB, Czeisler CA, Dijk DJ, Duffy JF, Folkard S, Minors DS, et al. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Arch. Gen. Psychiatry. 1997;54(2):145–152. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- 47.Borbely AA. A two process model of sleep regulation. Hum. Neurobiol. 1982;1(3):195–204. [PubMed] [Google Scholar]
- 48.Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007;8(6):613–622. doi: 10.1016/j.sleep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 49.Heo M, Faith MS, Pietrobelli A, Heymsfield SB. Percentage of body fat cutoffs by sex, age, and race-ethnicity in the US adult population from NHANES 1999–2004. Am. J. Clin. Nutr. 2012;95(3):594–602. doi: 10.3945/ajcn.111.025171. [DOI] [PubMed] [Google Scholar]
- 50.Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L'Hermite-Baleriaux M, Zee PC. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J. Biol. Rhythms. 2005;20(2):178–188. doi: 10.1177/0748730404273983. [DOI] [PubMed] [Google Scholar]
- 51.Wyatt JK, Stepanski EJ, Kirkby J. Circadian phase in delayed sleep phase syndrome: predictors and temporal stability across multiple assessments. Sleep. 2006;29(8):1075–1080. doi: 10.1093/sleep/29.8.1075. [DOI] [PubMed] [Google Scholar]
- 52.Sletten TL, Vincenzi S, Redman JR, Lockley SW, Rajaratnam SM. Timing of sleep and its relationship with the endogenous melatonin rhythm. Front Neurol. 2010;1:137. doi: 10.3389/fneur.2010.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fonken LK, Nelson RJ. The Effects of Light at Night on Circadian Clocks and Metabolism. Endocr. Rev. 2014:er20131051. doi: 10.1210/er.2013-1051. [DOI] [PubMed] [Google Scholar]