Abstract
Foxp3+ T regulatory cells (Tregs), conventional CD4+Foxp3− T cells, and CD8+ T cells represent heterogeneous populations composed of naïve-phenotype (NP, CD44low) and memory-phenotype (MP, CD44high) subpopulations. NP and MP subsets differ in their activation state, contribution to immune function, and capacity to proliferate in vivo. To further understand the factors that contribute to the differential homeostasis of NP/MP subsets, we examined the differential effects of CD28 and CTLA-4 interaction with CD80/CD86, as well as MHC class II-TCR interaction within mouse Treg, CD4+, and CD8+ T cell pools. Blockade of CD80/CD86 with CTLA-4-Ig markedly reduced the cycling and absolute numbers of MP Tregs and MP CD4+ T cells, with minimal effect on the NP T cell subpopulations. Blockade of MHC class II-TCR interaction led to selective expansion of MP Tregs and MP CD4+ and CD8+ T cells that was reversed upon co-treatment with CTLA-4-Ig. Treatment with anti-CTLA-4 mAb altered MP Treg and MP CD4+ and CD8+ T cell homeostasis in a manner similar to that observed with anti-MHC class II. We postulate a complex pathway in which CD28 is the primary driver of Treg proliferation and CTLA-4 functions as the main brake but is likely dependent upon TCR signals and CD80/CD86. These findings have important implications for the use of biologic agents targeting such pathways to modulate autoimmune and neoplastic disease.
Introduction
Foxp3+ T regulatory cells (Tregs) are critical to the maintenance of immune homeostasis and tolerance (1, 2). Tregs, conventional CD4+Foxp3− T cells (Tconvs, herein referred to as CD4+), and CD8+ T cells represent heterogeneous populations composed of memory/effector/activated-phenotype (MP, CD44high) and naive/quiescent/resting-phenotype (NP, CD44low) subpopulations (3). While the expression of CD44 on CD4+ and CD8+ T cells is bimodal, its expression on Tregs is more broadly distributed making it somewhat difficult to clearly delineate distinct populations (4, 5). Recently, expression of the GPI-linked surface protein, Ly-6C, has been used to discriminate between high and low affinity self-reactive T conventional (Tconvs) cells (6) and has further proven useful as a more specific marker to divide peripheral Tregs into NP (~30%, Ly-6C+) and MP (~70%, Ly-6C−) subpopulations (7). The Ly-6C− Treg population was proposed to undergo increased TCR signaling events compared to Ly-6C+ Tregs based upon increased level of CD5 expression as well as CD3ζ phosphorylation and an augmented proportion of nuclear NFAT. This correlated with increased an activation status for the Ly-6C− subset, including higher levels of CTLA-4, increased cycling in the steady state and heighted suppressor function in vivo and in vitro. One of the more prominent characteristics of MP Tregs and MP CD4+ T cells is their high degree of cell cycling in vivo (~10% proliferate/day) (5, 8). While the cytokines IL-7 and IL-15 have been shown to drive MP CD8+ T cell proliferation (9), the cellular or soluble factors that regulate MP Tregs and MP CD4+ T cell proliferation in vivo are poorly characterized.
Optimal T cell activation and expansion involves TCR engagement of cognate peptide presented in the context of MHC as well as a second, co-stimulatory signal. CD28 represents a major co-stimulatory molecule constitutively expressed by T cells that binds the B7 family members B7-1 (CD80) and B7-2 (CD86) present on APC, resulting in enhancement of T cell proliferation, cytokine production, and survival (10, 11). Such action is opposed by the key inhibitory molecule CTLA-4, which competes with CD28 for engagement with CD80 and CD86 and leads to downregulation of T cell responsiveness (12). Unopposed CD28 stimulation, as in the case of genetic disruption of CTLA-4, results in profound immune dysregulation and autoimmune disease, akin to scurfy mice, which is fatal within 3-4 weeks of birth (13, 14). The critical role of CTLA-4 in regulation of CD28-dependent T cell costimulation is demonstrated by the lack of autoimmunity in CTLA-4-deficient mice when crossed with mice lacking CD80 and CD86 (15).
The co-receptors CD28 and CTLA-4 and their ligands CD80/CD86 have been demonstrated to play critical roles in the generation, maintenance, and function of Tregs. While CTLA-4 is upregulated on conventional T cells following activation, it is constitutively expressed by Tregs in the steady state. Blockade of CD80/CD86 with CTLA-4-Ig leads to a rapid decrease in both thymic and peripheral Tregs (16, 17). Mice with conditional deletion of CD28 in Tregs have a 25% decrease in the number of thymic Tregs, while peripheral Treg numbers are maintained (18). However, between 8-12 weeks of age, such mice develop signs of autoimmunity that in many respects phenotypically resembles the much more rapid development of autoimmunity in scurfy mice. Surviving CD28 deficient Tregs do exhibit a more naive phenotype characterized by lower levels of CTLA-4, PD-1, and CD103. Treatment of mice with anti-CTLA-4 antibody results in enhanced Treg proliferation and frequency (17, 19). The role for CTLA-4 in modulating Treg function has been shown to include a cell intrinsic mechanism controlling TCR signaling (20), along with several distinct cell extrinsic mechanisms (21, 22) including competition with CD28 on Tconvs for CD80/CD86 signaling (23, 24), negatively signaling antigen presenting cells (APC) via CD80/CD86 (25), and actively removing CD80/CD86 from the APC surface via transendocytosis (26). Furthermore, targeted deletion of CTLA-4 in Tregs of adult mice resulted in enhanced Treg proliferation suggesting that the major function of CTLA-4 in Tregs was to act as an intrinsic brake on their proliferation (27).
While the roles of CD28 and CTLA-4 in Treg homeostasis have received considerable attention, much less is known about the contribution of TCR signals alone or the interrelationships between TCR and co-stimulatory/co-inhibitory signals on Treg homeostasis. Two studies in which TCR expression was deleted among peripheral Tregs (28, 29) concluded that TCR deficient Tregs maintain Foxp3 expression and remain hypo-responsive but lose Treg suppressor function. Here, we examine the differential effects of CD28 and CTLA-4 interaction with CD80/CD86, as well as MHC Class II-TCR interaction, on Treg subset homeostasis and compare these results to the effects of manipulation of such pathways on the homeostasis of NP and MP CD4+ and CD8+ T cell subpopulations. Our results are consistent with a complex pathway in which CD28 is the primary driver of Treg proliferation and CTLA-4 functions as the main brake but is also dependent upon TCR signals and interactions with CD80/CD86. These results have important implications for the use of biologic agents to these targets to modulate T effector cell function.
Materials and Methods
Mice
Female C57BL/6 mice were obtained from The Jackson Laboratory, Taconic Farms and Charles River Laboratories. Fcεr1γ−/− (FcRγ−/−; B6.129P2-Fcer1gtm1RavN12) mice were obtained from Taconic Farms. Spleens of C57BL/6 germ-free (GF) and the related specific-pathogen-free (SPF) mice were a generous gift from Dr. Yasmine Belkaid (Laboratory of Parasitic Diseases, NIAID, NIH). All mice were sex- and age-matched for experimentation and used between 7-12 weeks of age. All animal protocols used in this study were approved by the NIAID Animal Care and Use Committee.
Flow cytometry and antibodies
Single cell suspensions were obtained from the spleens of mice and stained with the following anti-mouse mAbs: B220 (clone RA3-6B2), CD4 (clone RM4-5), CD8 (clone 53-6.7), CD44 (clone IM7), CTLA-4 (clone UC10-4B9), Foxp3 (clone FJK-16s), and TCRβ (clone H57-597) from eBioscience and anti-human Ki-67 (clone B56) from BD Pharmingen. For intracellular staining of Foxp3 and Ki-67, cells were first stained for surface markers and then fixed and permeabilized using the Foxp3/Transcription Factor Staining Buffer Set (eBioscience) according to the manufacturer’s instructions. Flow cytometry data were acquired using a LSR II Flow Cytometer (BD Biosciences) and analyzed using FlowJo software (Treestar).
In vivo treatment
Mice were treated with the following: 450 μg/dose (i.p.) of anti-mouse IL-2 mAb (clone S4B6-1; BioXCell) or PBS on d 0, 2, 4, and 6 and sacrificed on d 9; 250 μg/dose (i.p.) of human CTLA-4-Ig (Orencia; Bristol-Myers Squibb) or PBS on d 0, 2, 4, and 6 and sacrificed on d 8; 700 μg/dose (i.p.) of anti-mouse I-Ab mAb (clone Y-3P; BioXCell) or mouse IgG2a (clone C1.18.4) or PBS on d 0, 2, and 3 and sacrificed on d 6; 250 μg/dose (i.p.) of anti-mouse CTLA-4 mAb (clone UC10-4F10-11; BioXCell) or Armenian hamster IgG on d 0, 2, 4, and 6 and sacrificed on d 8. For co-treatment studies, mice were treated with CTLA-4-Ig on d 0, 2, and 4 along with anti-I-Ab mAb on d 0, 2, and 3 and sacrificed on d 6.
Statistical analysis
Comparisons between groups were tested by two-tailed unpaired Student's t test or one-way ANOVA using Prism 6 (GraphPad Software, Inc). Statistical significance was determined based on the following P values: *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001
Results
Acute blockade of IL-2 does not severely impact Treg homeostatic proliferation
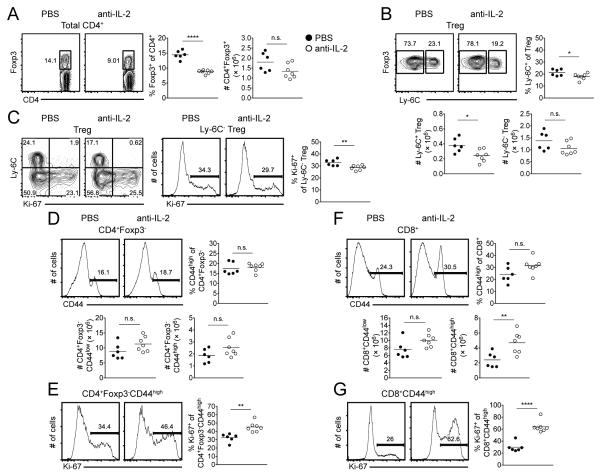
The partitioning of Tregs into distinct subsets based on the expression of CD44 and CD62L with unique expression profiles revealed that IL-2 is important in selectively maintaining a quiescent Treg population (5). Furthermore, blockade of IL-2 signaling did not interfere with Treg homeostatic proliferation. While CD44 is a useful marker to delineate naive versus memory cells in the Tconv cell pool, its use to divide Tregs remains challenging (Supplemental Fig. 1A, B). We therefore incorporated the cell surface marker Ly-6C that has recently been identified to separate two distinct Treg populations (7) in order to further examine the role of IL-2 in Treg homeostasis. Wild-type (WT) C57BL/6 mice were treated with anti-IL-2 mAb every other day for 6 days prior to isolation of splenocytes on d 9. The percentage of Tregs decreased following anti-IL-2 mAb treatment, although their absolute numbers remained unchanged (Fig. 1A). In the steady state, approximately 20-30% of Tregs express Ly-6C within the spleen. While no change in the distribution of Ly-6C+ and Ly-6C− Tregs was observed following blockade of IL-2 (Fig. 1B), the number of Ly-6C+ Tregs decreased slightly (Fig. 1B). In comparison to the mainly quiescent Ly-6C+ Tregs, the Ly-6C− subset undergoes pronounced proliferation in the steady state (7; Supplemental Fig. 1C). Treatment of mice with anti-IL-2 mAb slightly diminished the homeostatic cycling of Ly-6C− Tregs (Fig. 1C), evident from Ki-67 expression, a marker of cell cycle progression. Although analysis of the CD4+ and CD8+ T cell compartments revealed no significant change in the percentage of MP T cells (Fig. 1D, 1F), a modest increase in the proliferation of MP CD4+ T cells and a marked in increase in the proliferation of CD8+ T cells following blockade of IL-2 (Fig. 1E, 1G). A significant increase in the absolute number of MP CD8+ T cells was also observed (Fig. 1F).
FIGURE 1.
Acute blockade of IL-2 does not severely impact Treg homeostatic proliferation. WT C57BL/6 mice were administered anti-IL-2 mAb (or PBS) on d 0, 2, 4, and 6 and spleens harvested on d 9. (A) Representative contour plots of Foxp3 expression among total CD4+ T cells (left). Pooled frequency and number of Foxp3+ cells among total CD4+ T cells (right). (B) Representative contour plots of Ly-6C expression among Tregs. Pooled frequency and number of Ly-6C+ cells and number of Ly-6C− cells among Tregs. (C) Representative contour plots of Ly-6C versus Ki-67 expression among Tregs (left). Representative histograms of Ki-67 expression among Ly-6C− Tregs; pooled frequency of Ki-67 expression among Ly-6C− Tregs (right). Representative histograms of CD44 expression among CD4+ T cells (D) or CD8+ T cells (F). Pooled frequency (top) and number (bottom) of MP CD4+ T cells (D) or MP CD8+ T cells (F). Representative histograms (left) and pooled frequency (right) of Ki-67 expression among MP CD4+ T cells (E) or MP CD8+ T cells (G). Filled circles represent PBS treatment; open circles represent anti-IL-2 mAb treatment. Each symbol represents an individual mouse from two independent experiments with three or four mice per group per experiment (PBS, six total mice; anti-IL-2 mAb, seven total mice). Small horizontal lines indicate the mean. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (unpaired Student's t test).
We further examined the impact of commensal bacteria on the homeostasis of Ly-6C+ and Ly-6C− Tregs as well as NP and MP CD4+ and CD8+ T cells. Analysis of the spleen between SPF and germ-free GF mice in the steady state revealed no difference in the frequency of the various T cell subsets (Supplemental Fig. 2). Furthermore, we did not observe a change in the proliferation of MP CD4+ and CD8+ T cell populations, although a small, yet significant, reduction in Ki-67 expression was evident among the Ly-6C− Treg subset (Supplemental Fig. 2C). Such results implicate non-bacteria driven signals are key mediators of T cell subset homeostasis.
Steady state cycling of T cells requires intact CD80/CD86 signaling
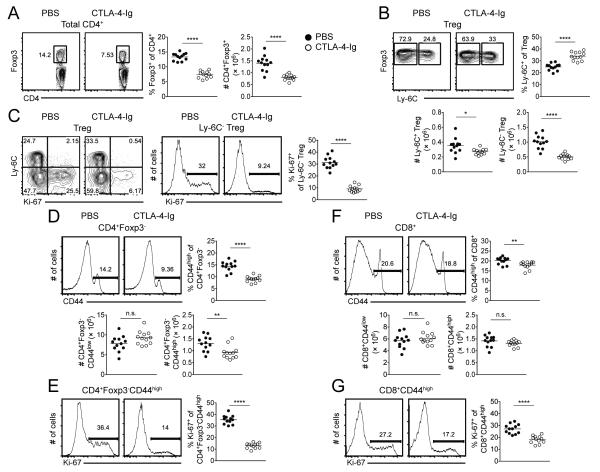
To assess the role of co-stimulation in the peripheral homeostasis of NP and MP T cell populations, human CTLA-4-Ig was administered to WT C57BL/6 mice on d 0, 2, 4, and 6. CTLA-4-Ig can block CD28 signaling by binding to and decreasing the availability of CD80 and CD86 and has been shown to prevent the fatal autoimmune disease in CTLA-4-deficient mice (30). Examination of the spleen on d 8 revealed a significant reduction in both the frequency and absolute number of Tregs (Fig. 2A) accompanied by a reciprocal increase/decrease in the frequency of the Ly-6C+/Ly-6C− subsets, respectively (Fig. 2B). The reduction in Treg number was more prominent among the Ly-6C− population (Fig. 2B), which demonstrated a 3.3-fold reduction in their expression of Ki-67 (Fig. 2C). In contrast to the NP T cell subset, MP CD4+ and CD8+ T cells undergo prominent proliferation in the steady state. The loss of co-stimulation further resulted in a significant reduction in the frequency, absolute number, and cycling of MP CD4+ T cells (Fig. 2D, 2E), while the number of NP CD4+ T cells remained relatively unchanged (Fig. 2D). A portion of the proliferative response of MP CD4+ T cells depends on IL-7 (8) and that may account for the fraction of Ki-67 expression not blocked by CTLA-4-Ig. Although CTLA-4-Ig treatment did not alter the frequency or number of either NP or MP CD8+ T cells (Fig. 2F), we did observe a significant decrease in the expression of Ki-67 among MP CD8+ T cells (Fig. 2G). Overall, these results suggest that CD80/CD86 signaling is required for the homeostatic proliferation of both the Ly-6C− Treg and MP CD4+ T cell pools and to a lesser extent MP CD8+ T cells.
FIGURE 2.
Steady state cycling of T cells requires intact CD80/CD86 signaling. WT C57BL/6 mice were administered human CTLA-4-Ig (Orencia) or PBS on d 0, 2, 4, and 6 and spleens harvested on d 8. (A) Representative contour plots of Foxp3 expression among total CD4+ T cells (left). Pooled frequency and number of Foxp3+ cells among total CD4+ T cells (right). (B) Representative contour plots of Ly-6C expression among Tregs. Pooled frequency and number of Ly-6C+ cells and number of Ly-6C− cells among Tregs. (C) Representative contour plots of Ly-6C versus Ki-67 expression among Tregs (left). Representative histograms of Ki-67 expression among Ly-6C− Tregs; pooled frequency of Ki-67 expression among Ly-6C− Tregs (right). Representative histograms of CD44 expression among CD4+ T cells (D) or CD8+ T cells (F). Pooled frequency (top) and number (bottom) of MP CD4+ T cells (D) or MP CD8+ T cells (F). Representative histograms (left) and pooled frequency (right) of Ki-67 expression among MP CD4+ T cells (E) or MP CD8+ T cells (G). Filled circles represent PBS treatment; open circles represent CTL4-Ig treatment. Each symbol represents an individual mouse from three independent experiments with three - five mice per group per experiment (twelve mice total). Small horizontal lines indicate the mean. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (unpaired Student's t test).
Blockade of MHC Class II results in prominent expansion of Ly-6C− Tregs as well as MP CD4+ and MP CD8+ T cells
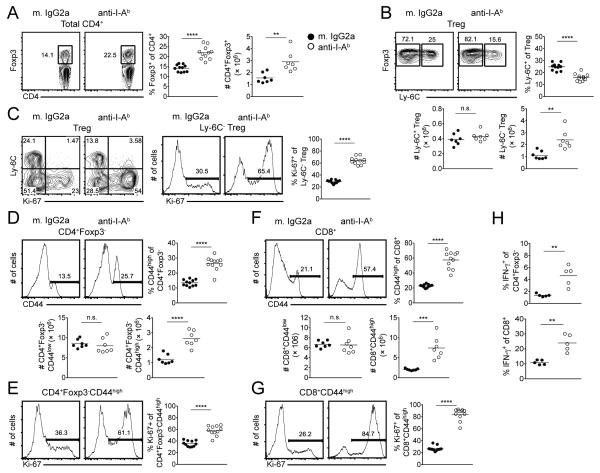
One possible explanation for the effects of co-stimulatory blockade on the homeostasis of Ly-6C− Tregs and MP CD4+ T cells is that these populations depend on TCR signaling together with co-stimulation via CD80/CD86 in a manner similar to the requirements for naive T cell activation. Although the role for MHC Class II-TCR interaction in the survival of NP CD4+ T cells remains controversial, the long-term survival of MP CD4+ T cells is agreed to be MHC-independent (3). To initially test this hypothesis, we administered an anti-MHC Class II mAb to WT C57BL/6 mice on d 0, 2, and 3 with analysis of the spleen on d 6. The in vivo use of such an antibody has been shown to disrupt TCR signaling, evident by a reduction in the phosphorylation of CD3ζ and ZAP-70 as well as NFAT localization among T cells (7, 31, 32). Consistent with previous reports (33), treatment of WT mice with anti-I-Ab mAb led to a substantial depletion of antigen presenting cells, such as B cells, occurring as early as 24 h following antibody administration (data not shown). To avoid this confounding factor of antibody-mediated depletion, FcRγ-deficient (FcRγ−/−) mice that lack the γ-chain subunit of the Fc receptor were used. In marked contrast to the results seen with co-stimulatory blockade, treatment of FcRγ−/− mice with anti-I-Ab mAb resulted in a significant expansion of Treg frequency and absolute number (Fig. 3A) that corresponded with selective expansion and proliferation of the Ly-6C− Treg subset (Fig. 3B, 3C). Blockade of MHC Class II also resulted in a significant expansion and enhanced proliferation of MP CD4+ T cells (Fig. 3D, 3E) with no effect on NP CD4+ T cell number (Fig. 3D). While the number of NP CD8+ T cells was unchanged, the MP CD8+ T cell pool expanded to comprise ~55% of the CD8+ T population (Fig. 3F), with ~80% of MP CD8+ T cells expressing Ki-67 versus <30% in the steady state (Fig. 3G).
FIGURE 3.
Blockade of MHC Class II results in prominent expansion of Ly-6C− Tregs as well as MP CD4+ and CD8+ T cells. FcRγ−/− mice were administered anti-I-Ab mAb (or mouse IgG2a) on d 0, 2, and 3 and spleens harvested on d 6. (A) Representative contour plots of Foxp3 expression among total CD4+ T cells (left). Pooled frequency and number of Foxp3+ cells among total CD4+ T cells (right). (B) Representative contour plots of Ly-6C expression among Tregs. Pooled frequency and number of Ly-6C+ cells and number of Ly-6C− cells among Tregs. (C) Representative contour plots of Ly-6C versus Ki-67 expression among Tregs (left). Representative histograms of Ki-67 expression among Ly-6C− Tregs; pooled frequency of Ki-67 expression among Ly-6C− Tregs (right). Representative histograms of CD44 expression among CD4+ T cells (D) or CD8+ T cells (F). Pooled frequency (top) and number (bottom) of MP CD4+ T cells (D) or MP CD8+ T cells (F). Representative histograms (left) and pooled frequency (right) of Ki-67 expression among MP CD4+ T cells (E) or MP CD8+ T cells (G). (H) Total splenocytes were stimulated in vitro with PMA/ionomycin in the presence of Golgistop for 3.5 hours. Pooled frequency of IFN-γ expression among CD4+ cells (left) and CD8+ T cells (right). Filled circles represent mouse IgG2a treatment; open circles represent anti-I-Ab mAb treatment. For pooled frequency data (A-G), each symbol represents an individual mouse from three independent experiments with three or four mice per group per experiment (eleven mice total). For pooled number data (A, B, D, F), each symbol represents an individual mouse from two independent experiments with three or four mice per group per experiment (seven mice total). For pooled frequency data (H), each symbol represents an individual mouse from two independent experiments with two or three mice per group per experiment (five mice total). Small horizontal lines indicate the mean. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (unpaired Student's t test).
The conditional deletion of TCR on Tregs (in a homozygous setting) has been shown to result in a severe inflammatory condition (28). Consistent with such findings, the disruption of MP T cell homeostasis following MHC Class II blockade in FcRγ−/− mice also correlated with higher levels of IFN-γ expression among CD4+ and CD8+ T cells following in vitro stimulation (Fig. 3H). This enhanced cytokine production reflected an increase in the frequency of MP cells, as the amount of IFN-γ among MP CD4+ and CD8+ T cells, on a per cell basis, was comparable between mouse IgG2a and anti-I-Ab mAb treated groups (data not shown).
While the co-inhibitory molecule CTLA-4 is upregulated on non-Tregs following activation, it is constitutively expressed by Tregs and plays a major role in their suppressive function and maintenance of immune homeostasis (21, 34). Furthermore, it has been shown that CTLA-4 undergoes intracellular trafficking and focal localization towards sites of TCR engagement (35). We questioned whether the transient and global blockade of MHC Class II resulted in a potential disruption of CTLA-4 expression among Tregs. In spite of the constitutive expression of CTLA-4 on Tregs compared to Tconv cells, quantitation of cell-surface CTLA-4 is difficult secondary to the high rate of cycling within intracellular compartments. Previous reports have shown that Ly-6C+ Tregs possess lower levels of intracellular CTLA-4 in the steady state compared to the Ly-6C− population (7). While no change in CTLA-4 expression was evident among the Ly-6C+ subset after treatment of FcRγ−/− mice with anti-I-Ab mAb, we did observe a significant increase in CTLA-4 MFI among the Ly-6C− Tregs (Supplemental Fig. 3). Whether this increase in intracellular CTLA-4 level reflects a reduced ability to cycle to the cell surface resulting in accumulation within the cell or simply a reflection of increased cellular activation resulting from increased proliferation remains to be determined.
Expansion of Ly-6C− Tregs and MP CD4+ T cells following MHC Class II blockade is dependent on CD80/CD86 co-stimulation
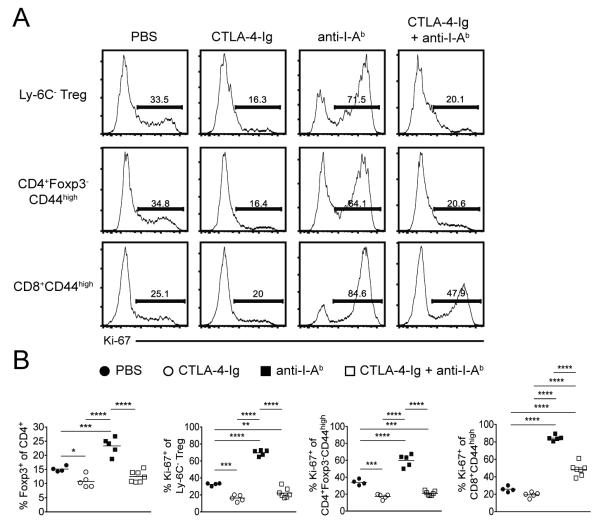
To determine whether the enhanced proliferation of Ly-6C− Tregs and MP CD4+ T cells following global MHC Class II blockade was mediated by co-stimulation via CD28, we administered anti-I-Ab mAb (on d 0, 2, and 3) to FcRγ−/− mice in conjunction with CTLA-4-Ig (on d 0, 2, and 4). The expansion of Ly-6C− Tregs and MP CD4+ T cells observed on d 6 following blockade of MHC Class II was markedly abrogated in the presence of CTLA-4-Ig (Fig. 4), strongly suggesting a requirement for CD28 stimulation via CD80/CD86 in this process. While a pronounced Ki-67 expression peak was still observed among MP CD8+ T cells during this co-treatment, it was significantly diminished compared to anti-I-Ab mAb alone (Fig. 4). Differences in the duration of treatment as well as mouse strain may account for variation in the degree to which Ki-67 expression was reduced following CTLA-4-Ig administration alone between Fig. 2 and Fig. 4.
FIGURE 4.
Expansion of Ly-6C− Tregs and MP CD4+ T cells following MHC Class II blockade is dependent on CD80/CD86 availability. FcRγ−/− mice were administered PBS, CTLA-4-Ig alone (on d 0, 2, and 4), anti-I-Ab mAb alone (on d 0, 2, and 3) or co-treatment of CTLA-4-Ig and anti-I-Ab mAb; spleens were harvested on d 6. (A) Representative histograms and (B) pooled frequency of Ki-67 expression among Ly-6C− Tregs, MP CD4+ T cells, or MP CD8+ T cells. Filled circles represent PBS treatment; open circles represent CTLA-4-Ig treatment; filled squares represent anti-I-Ab mAb treatment; open squares represent co-treatment of CTLA-4-Ig and anti-I-Ab mAb. For pooled frequency data, each symbol represents an individual mouse from two independent experiments (PBS, four total mice; CTLA-4-Ig, five total mice; anti-I-Ab mAb, five total mice; CTLA-4-Ig + anti-I-Ab mAb, seven total mice). Small horizontal lines indicate the mean. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (one-way ANOVA).
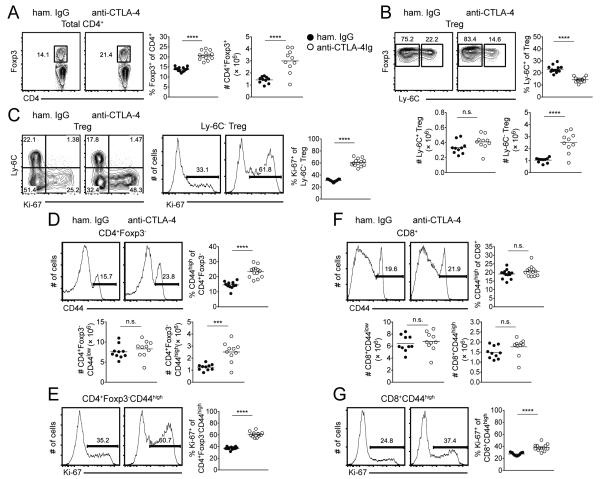
CTLA-4-CD80/CD86 interactions are necessary to control homeostatic proliferation of Ly-6C− Tregs and MP CD4+ and CD8+ T cells
The above results raise the possibility that the proliferation of Ly-6C− Tregs and MP CD4+ T cells is dependent on CD80/CD86 derived co-stimulatory signals and furthermore that the role of TCR signaling is to directly (in terms of CD4+ T cells) or indirectly (in terms of CD8+ T cells) restrain Treg and MP T cell expansion under steady-state conditions. One potential mechanism by which MHC Class II signals might regulate such T cell homeostasis is by activation of the co-inhibitory receptor CTLA-4. To address this possibility, we administered a blocking anti-CTLA-4 mAb (or Armenian hamster IgG) to WT C57BL/6 mice on d 0, 2, 4, and 6 with analysis of the spleen on d 8. Indeed, the effects of anti-CTLA-4 mAb resembled those seen after anti-MHC Class II mAb treatment, as a significant expansion in the frequency and absolute number of Tregs (Fig. 5A) and an increase in the number and proliferation of the Ly-6C− Treg subset (Fig. 5B, 5C) were observed. Analysis of the spleen on d 8 resulted in a significant increase in the percentage, absolute number, and proliferation of MP CD4+ T cells (Fig. 5D, 5E) with minimal change in NP CD4+ T cells (Fig. 4D). While the size of the NP and MP CD8+ T cell pools were not impacted by such treatment (Fig. 5F), the expression of Ki-67 among MP CD8+ T cells was significantly enhanced (Fig. 5G). The extent of Ki-67 expression following anti-CTLA-4 mAb treatment was less than that observed upon blockade of MHC Class II, suggesting additional mechanisms may be involved during anti-I-Ab mAb treatment. Regardless, the effects of CTLA-4 blockade were not secondary to antibody-mediated deletion of T cells as similar results were observed using FcRγ−/− mice (Supplemental Fig. 4).
FIGURE 5.
CTLA-4-CD80/CD86 interactions are necessary to control homeostatic proliferation of Ly-6C− Tregs and MP CD4+ and CD8+ T cells. WT C57BL/6 mice were administered anti-CTLA-4 mAb (or hamster IgG) on d 0, 2, 4, and 6 and spleens harvested on d 8. (A) Representative contour plots of Foxp3 expression among total CD4+ T cells (left). Pooled frequency and number of Foxp3+ cells among total CD4+ T cells (right). (B) Representative contour plots of Ly-6C expression among Tregs. Pooled frequency and number of Ly-6C+ cells and number of Ly-6C− cells among Tregs. (C) Representative contour plots of Ly-6C versus Ki-67 expression among Tregs (left). Representative histograms of Ki-67 expression among Ly-6C− Tregs; pooled frequency of Ki-67 expression among Ly-6C− Tregs (right). Representative histograms of CD44 expression among CD4+ T cells (D) or CD8+ T cells (F). Pooled frequency (top) and number (bottom) of MP CD4+ T cells (D) or MP CD8+ T cells (F). Representative histograms (left) and pooled frequency (right) of Ki-67 expression among MP CD4+ T cells (E) or MP CD8+ T cells (G). Filled circles represent hamster IgG treatment; open circles represent anti-CTLA-4 mAb treatment. For pooled frequency data (A-G), each symbol represents an individual mouse from three independent experiments with three or five mice per group per experiment (thirteen total mice). For pooled number data (A, B, D, F), each symbol represents an individual mouse from two independent experiments with five mice per group per experiment (ten total mice). Small horizontal lines indicate the mean. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (unpaired Student's t test).
Discussion
We have examined in depth the differential roles of CD28, CTLA-4, and TCR signaling on the homeostasis of NP and MP T cell populations. Taken together, our results demonstrate that the homeostasis of MP Tregs and MP CD4+ and CD8+ T cells is differentially regulated in comparison to their NP counterparts, as the changes we have observed by blocking C80/CD86, MHC Class II, and CTLA-4 signaling were for the most part only observed in the MP subsets. Our results support the concept that a major portion of the homeostatic proliferation of Treg is CD28-dependent and cytokine independent. However, CTLA-4-Ig blockade never completely reduced Treg proliferation and it remains possible that a fraction of the response in vivo is cytokine mediated, most likely, IL-7 driven (8). While CTLA-4-Ig treatment might compromise the function of Tregs, the negative effects of CTLA-4-Ig on Tregs would be neutralized by the inhibitory effects of CTLA-4-Ig on the activation of T effector cell functions. Although the profound effects of CD80/CD86 blockade on MP Treg proliferation raised the possibility that both TCR and co-stimulatory signals were driving their proliferation, the marked expansion of this subset following MHC class II blockade and CTLA-4 blockade are consistent with a more complex regulatory control of MP Treg homeostasis.
One explanation for the increased expansion of MP CD4+ T cells and particularly the MP CD8+ T cell population following blockade of MHC Class II is impairment in the cell intrinsic effects of CTLA-4 function leading to decreased Treg-mediated suppression of MP4 CD4+ and CD8+ T cells in the steady state. While it is widely accepted that CTLA-4 plays an important role in downregulating TCR signaling via recruitment of the phosphatases, SHP-2 and PP2A, to its cytoplasmic tail (36-38), it has recently been reported that downstream signals from the TCR, including phosphorylation of SLP-76, are critical for optimal Treg suppression as well as expression of CTLA-4 and other Treg activation antigens (39). Deletion of the TCR from Tregs also has been shown to reduce the expression of CTLA-4 under some conditions (28). As the effects of MHC Class II blockade were mimicked by treatment with anti-CTLA-4 mAb, it is possible that both TCR signals and signals delivered by CD80/CD86 are required for optimal CTLA-4 function. We cannot exclude the possibility that the effects of anti-MHC class II treatment and anti-CTLA-4 treatment are completely unrelated. It is also possible that the effects of both of these treatments on Treg homeostasis are indirect and mediated by the action of these agents on other cells such as tolerogenic dendritic cells, regulatory B cells or via co-inhibitory receptors such as PD-1.
In vivo use of anti-MHC Class II mAb has been shown to disrupt TCR signaling, resulting in reduction in the phosphorylation of the CD3ζ chain and ZAP-70, as well as disruption in NFAT localization in T cells (7, 31, 32). We cannot rule out the possibility that in addition to the disruption of MHC Class II-TCR interactions, anti-I-Ab mAb treatment may alter the interaction of MHC class II with other potential ligands. CD4 has been shown to directly interact with MHC Class II, functioning as a cell surface adhesion molecule (40). MHC Class II is a ligand for lymphocyte activation gene 3 (LAG-3) (41), which is expressed on activated T cells and has been shown to negatively regulate cellular proliferation and homeostasis in a manner somewhat similar to CTLA-4 (42, 43). In particular, LAG-3 has been shown to regulate the accumulation and effector function of antigen-specific CD8+ T cells (44). Although we cannot exclude the possibility that the anti-I-Ab mAb may alter the interaction of other molecules with MHC Class II, we favor the hypothesis that the primary role of TCR signaling in Tregs is the regulation of CTLA-4 function.
A small, yet significant decrease in Ki-67 expression among the Ly-6C− Tregs was apparent, which may be secondary to variability among the control group and not reflective of a true influence by the commensal bacteria. Anti-IL-2 treatment resulted in a decrease in the number of Ly-6C+ Treg, which is consistent with the dependence of this subset on IL-2 for survival (5). Anti-IL-2 treatment also resulted in a small, but significant, decrease in the proliferation of the Ly-6C− subset. These results differ slightly from previous studies and the differences likely reflect the different anti-IL-2 treatment regimens. Most notably, anti-IL-2 mAb enhanced the proliferation of the MP CD4+ and CD8+ subsets and resulted in an increase in the absolute number of MP CD8+ cells, but not MP CD4+ T cells. We believe the increase in the numbers of MP CD8+ T cells is secondary to the production of anti-IL-2/IL-2 immune complexes in vivo with the anti-IL-2 mAb (S4B6) used which specifically targets the immune complexes to the IL-2R β-chain expressed on MP CD8+ T cells (45-48). Alternatively, anti-IL-2 may impair the suppressive function of Treg resulting in MP CD4+ and CD8+ T cell activation (49). We also considered the potential effects of the gut microbiota Treg homeostasis. Consistent with previous reports (7), we did not observe any effect of the gut microbiota on the percentages of Ly-6C+ or Ly-6C− Tregs.
Our results are consistent with a model in which CD28 signaling provides the throttle and CTLA-4 signaling provides the brake to coordinate MP Treg proliferation. The results extend previous studies (27) in which deletion of CTLA-4 expression from the entire Treg population in the adult mouse resulted in enhanced Treg proliferation, activation of CD4+ T cells, and mediated increased Treg suppressor function in vivo. In contrast, the enhanced proliferation of MP CD4+ T cells and particularly MP CD8+ T cells following either short-term anti-MHC class II or anti-CTLA-4 mAb treatment is more consistent with a loss of Treg-mediated suppression. Indeed, enhanced MP T cell proliferation is seen following depletion of Tregs (50; Punkosdy and Shevach, unpublished observations) and loss of suppressor function following deletion of the TCR on the MP Treg subpopulation (28, 29). Further studies on the relationship between Treg suppressive function and proliferation will be needed to clarify this issue.
The profound effects of anti-CTLA-4 mAb treatment on MP Treg and MP CD4+ and CD8+ T cell proliferation have important implication for the use of this checkpoint inhibitor for tumor immunotherapy. While the effects of anti-CTLA-4 mAb on Treg-mediated suppression in tumor models versus the effects of this antibody on effector T cell function remain unclear (51-53), Treg-specific deficiency results in enhanced tumor immunity (21). In our studies, anti-CTLA-4 mAb treatment resulted in marked enhancement in the number of Ly-6C− Tregs but simultaneously resulted in increased proliferation and absolute numbers of MP CD4+ T cells and increased the proliferation of MP CD8+ T cells. These results suggest that the anti-CTLA-4 mAb may transiently disrupt Treg-mediated suppression, even under non-depleting conditions. Whether these changes in the homeostasis of MP CD4+ and CD8+ T cells can translate into a more effective anti-tumor response remains to be investigated. Lastly, while global blockade of MHC Class II is usually regarded as an immunosuppressive, its use in our studies revealed marked expansion of MP CD4+ and CD8+ T cells suggesting that in the primed host, inhibition of MHC Class II-TCR interaction might blunt Treg suppression and promote CD4+ and CD8+ T cell effector function in the tumor bearing host.
Supplementary Material
Acknowledgements
We thank members of the Shevach lab as well as Drs. William E. Paul and Jeff Zhu for their insight and suggestions to this project.
This work was supported by funds from the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Abbreviations
- GF
germ free
- LAG-3
lymphocyte activation gene-3
- MP
memory phenotype
- NP
naïve phenotype
- SPF
specific pathogen free
- Tregs
T regulatory cells
- Tconv
T conventional cells
- WT
wild type
References
- 1.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 2.Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, Hamann A, Wagner H, Huehn J, Sparwasser T. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Fisson S, Darrasse-Jeze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon BL. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J. Exp. Med. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smigiel KS, Richards E, Srivastava S, Thomas KR, Dudda JC, Klonowski KD, Campbell DJ. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J. Exp. Med. 2014;211:121–136. doi: 10.1084/jem.20131142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin B, Auffray C, Delpoux A, Pommier A, Durand A, Charvet C, Yakonowsky P, de Boysson H, Bonilla N, Audemard A, Sparwasser T, Salomon BL, Malissen B, Lucas B. Highly self-reactive naive CD4 T cells are prone to differentiate into regulatory T cells. Nat. Commun. 2013;4:2209. doi: 10.1038/ncomms3209. [DOI] [PubMed] [Google Scholar]
- 7.Delpoux A, Yakonowsky P, Durand A, Charvet C, Valente M, Pommier A, Bonilla N, Martin B, Auffray C, Lucas B. TCR signaling events are required for maintaining CD4 regulatory T cell numbers and suppressive capacities in the periphery. J. Immunol. 2014;193:5914–5923. doi: 10.4049/jimmunol.1400477. [DOI] [PubMed] [Google Scholar]
- 8.Younes SA, Punkosdy G, Cauchtexeux S, Chen T, Grossman Z, Paul WE. Memory phenotype CD4 T cells undergoing rapid, nonburst-like, cytokine-driven proliferation can be distinguished from antigen-experienced memory cells. PLoS Biol. 2011;9(10):e1001171. doi: 10.1371/journal.pbio.1001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 11.Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK. Binding of the B cell antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J. Exp. Med. 1991;173:721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulatory of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 13.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 14.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 15.Mandelbrot DA, McAdam AJ, Sharpe AH. B7-1 or B7-2 is required to produce the lymphoproliferative phenotype in mice lacking cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) J. Exp. Med. 1999;189:435–440. doi: 10.1084/jem.189.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, Zheng XX, Strom TB, Bluestone JA. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J. Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 17.Tang AL, Teijaro JR, Njau MN, Chandran SS, Azimzadeh A, Nadler SG, Rothstein DM, Farber DL. CTLA4 expression is an indicator and regulator of steady-state CD4+ FoxP3+ T cell homeostasis. J. Immunol. 2008;181:1806–1813. doi: 10.4049/jimmunol.181.3.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang R, Huynh A, Whitcher G, Chang J, Maltzman JS, Turka LA. An obligage cell-intrinsic function for CD28 in Tregs. J.Clin. Invest. 2013;123:580–593. doi: 10.1172/JCI65013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kavanagh B, O'Brien S, Lee D, Hou Y, Weinberg V, Rini B, Allison JP, Small EJ, Fong L. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 2008;112:1175–1183. doi: 10.1182/blood-2007-11-125435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tai X, Van Laethem F, Pobezinsky L, Guinter T, Sharrow SO, Adams A, Granger L, Kruhlak M, Lindsten T, Thompson CB, Feigenbaum L, Singer A. Basis of CTLA-4 function in regulatory and conventional CD4+ T cells. Blood. 2012;119:5155–5163. doi: 10.1182/blood-2011-11-388918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 22.Walker LS, Sansom DM. Confusing signals: Recent progress in CTLA-4 biology. Trends in Immunol. 2015;36:63–70. doi: 10.1016/j.it.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J. Exp. Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunner MC, Chambers CA, Chan FK, Hanke J, Winoto A, Allison JP. CTLA-4-Mediated inhibition of early events of T cell proliferation. J. Immunol. 1999;162:5813–5820. [PubMed] [Google Scholar]
- 25.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 26.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, Sansom DM. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paterson AM, Lovitsh SB, Sage PT, Juneja VR, Lee Y, Trombley JD, Arancibia-Carcamo CV, Sobel RA, Rudensky AY, Kuchroo VK, Freeman GJ, Sharpe AH. Deletion of CTLA-4 on regulatory T cells during adulthood leads to resistance to autoimmunity. J. Exp. Med. 2015;212:1603–1621. doi: 10.1084/jem.20141030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat. Immunol. 2014;15:1070–1078. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vahl JC, Drees C, Heger K, Heink S, Fischer JC, Nedjic J, Ohkura N, Morikawa H, Poeck H, Schallenberg S, Riess D, Hein MY, Buch T, Polic B, Schonle A, Zeiser R, Schmitt-Graff A, Kretschmer K, Klein L, Korn T, Sakaguchi S, Schmidt-Supprian M. Continuous T cell receptor signals maintain a functional regulatory T cell pool. Immunity. 2014;41:722–736. doi: 10.1016/j.immuni.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Tivol EA, Boyd SD, McKeon S, Borriello F, Nickerson P, Strom TB, Sharpe AH. CTLA4Ig prevents lymphoproliferation and fatal multiorgan tissue destruction in CTLA-4-deficient mice. J. Immunol. 1997;158:5091–5094. [PubMed] [Google Scholar]
- 31.Dorfman JR, Stefanova I, Yasutomo K, Germain RN. CD4+ T cell survival is not directly linked to self-MHC-induced TCR signaling. Nat. Immunol. 2000;1:329–335. doi: 10.1038/79783. [DOI] [PubMed] [Google Scholar]
- 32.Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 33.Andersson J, Stefanova I, Stephens GL, Shevach EM. CD4+CD25+ regulatory T cells are activated in vivo by recognition of self. Int. Immunol. 2007;19:557–566. doi: 10.1093/intimm/dxm021. [DOI] [PubMed] [Google Scholar]
- 34.Friedline RH, Brown DS, Nguyen H, Kornfeld H, Lee J, Zhang Y, Appleby M, Der SD, Khang J, Chambers CA. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J. Exp. Med. 2009;206:421–434. doi: 10.1084/jem.20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linsely PS, Bradshaw J, Greene J, Peach R, Bennett KL, Mittler RS. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4:535–543. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- 36.Chuang E, Lee KM, Robbins MD, Duerr JM, Alegre ML, Hambor JE, Neveu MJ, Bluestone JA, Thompson CB. Regulation of cytotoxic T lymphocyte-associated molecule-4 by Src kinases. J. Immunol. 1999;162:1270–1277. [PubMed] [Google Scholar]
- 37.Chuang E, Fisher TS, Morgan RW, Robbins MD, Duerr JM, Vander Heiden MG, Gardner JP, Hambor JE, Neveu MJ, Thompson CB. The CD28 and CTLA-4 receptors associate with the serine/threonine phosphatase PP2A. Immunity. 2000;13:313–322. doi: 10.1016/s1074-7613(00)00031-5. [DOI] [PubMed] [Google Scholar]
- 38.Marengere LE, Waterhouse P, Duncan GS, Mittrucker HW, Feng GS, Mak TW. Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science. 1996;272:1170–1173. doi: 10.1126/science.272.5265.1170. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt AM, Lu W, Sindhava VJ, Huang Y, Burkhardt JK, Yang E, Riese MJ, Maltzman JS, Jordan MS, Kambayashi T. Regulatory T cells required TCR signaling for their suppressive function. J. Immunol. 2015;194:4362–4370. doi: 10.4049/jimmunol.1402384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doyle C, Strominger JL. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987;330:256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- 41.Huard B, Prigent P, Tournier M, Bruniquel D, Triebel F. CD4/major histocompatibility complex class II interaction analyzed with CD4− and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. Eur. J. Immunol. 1995;25:2718–2721. doi: 10.1002/eji.1830250949. [DOI] [PubMed] [Google Scholar]
- 42.Workman CJ, Vignali DA. The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. Eur. J. Immunol. 2003;33:970–979. doi: 10.1002/eji.200323382. [DOI] [PubMed] [Google Scholar]
- 43.Workman CJ, Cauley LS, Kim IJ, Blackman MA, Woodland DL, Vignali DA. Lymphocyte activation gene-3 (CD233) regulates the size of the expanding T cell population following antigen activation in vivo. J. Immunol. 2004;172:5450–5455. doi: 10.4049/jimmunol.172.9.5450. [DOI] [PubMed] [Google Scholar]
- 44.Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, Anders R, Netto G, Getnet D, Bruno TC, Goldberg MV, Pardoll DM, Drake CG. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J. Clin. Invest. 2007;117:3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 46.Murakami M, Sakamoto A, Bender J, Kapler J, Marrack P. CD25+CD4+ T cells contribute to the control of memory CD8+ T cells. Proc. Natl. Acad. Sci. USA. 2002;99:8832–8837. doi: 10.1073/pnas.132254399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 48.Kamimura D, Sawa Y, Sato M, Agung E, Hirano T, Murakami M. IL-2 in vivo activities and antitumor efficacy enhanced by an anti-IL-2 mAb. J. Immunol. 2006;177:306–314. doi: 10.4049/jimmunol.177.1.306. [DOI] [PubMed] [Google Scholar]
- 49.Chinen T, Kannan AK, Levine AG, Fan X, Klein U, Zheng Y, Gasteiger G, Feng Y, Fontenot JD, Rudensky AY. An essential role for the IL-2 receptor in Treg cell function. Nat. Immunol. 2016;17:1322–1333. doi: 10.1038/ni.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arvey A, van der Veeken J, Samstein RM, Feng Y, Stamatoyannopoulos JA, Rudensky AY. Inflammation-induced repression of chromatin bound by the transcription factor Foxp3 in regulatory T cells. Nat. Immunol. 2014;15:580–587. doi: 10.1038/ni.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, Peggs KS, Ravetch JV, Allison JP, Quezada SA. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim JM, Ashkenazi A. Fcγ receptors enable anticancer action of proapoptotic and immune-modulatory antibodies. J. Exp. Med. 2013;210:1647–1651. doi: 10.1084/jem.20131625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.