SUMMARY
Exercise is a common component of weight loss strategies, yet exercise programs are associated with surprisingly small changes in body weight [1–4]. This may be due in part to compensatory adaptations, in which calories expended during exercise are counteracted by decreases in other aspects of energy expenditure [1, 5–10]. Here, we examined the relationship between a rodent model of voluntary exercise – wheel running – and total daily energy expenditure. Use of a running wheel for 3 to 7 days increased daily energy expenditure, resulting in a caloric deficit of approximately 1 kcal/day; however, total daily energy expenditure remained stable after the first week of wheel access, despite further increases in wheel use. We hypothesized that compensatory mechanisms accounted for the lack of increase in daily energy expenditure after the first week. Supporting this idea, we observed a decrease in off-wheel ambulation when mice were using the wheels, indicating behavioral compensation. Finally, we asked whether individual variation in wheel use within a group of mice would be associated with different levels of energy expenditure. Despite large variation in wheel running, we did not observe a significant relationship between the amount of daily wheel running and total daily energy expenditure or energy intake across mice. Together, our experiments support a model in which the transition from sedentary to light activity is associated with an increase in daily energy expenditure, but further increases in physical activity produce diminishingly small increments in daily energy expenditure.
Keywords: Behavioral compensation, energy expenditure, running wheel, voluntary exercise, wheel running
Graphical Abstract

RESULTS AND DISCUSSION
Exercise is commonly recommended in weight loss strategies for people with obesity [11]. Although important for overall health [12–15], exercise generally falls short on the goal of weight loss (effect size is typically less than 3% of body weight, even in studies lasting more than a year) [1–4]. This is surprising, as physical activity is associated with acute increases in energy expenditure [16, 17]. One explanation for the limited effect of exercise on weight is that exercise increases hunger, and the calories burned through exercise are thus recovered by increased food intake [18]. An alternative explanation suggests that compensatory adaptations counteract the calories expended during exercise [1, 5–10].
Additive models of energy expenditure predict that changes in physical activity result in proportional changes in daily energy expenditure [19]. However, these models fail to account for biomechanics or the kinetics of movement, whereby the transition from immobile to mobile involves a large conversion of potential energy to kinetic energy whereas maintenance of mobile states requires less energy [20]. Additionally, they discount the role of muscle training, in which repetition of a given movement increases muscle efficiency [21]. Perhaps most importantly, these models fail to account for alterations in other aspects of energy expenditure. Here, we examined this energy compensation of physical activity with a rodent model of voluntary exercise – wheel running.
Introduction of a running wheel increased total daily energy expenditure
Male C57BL/6 mice (n = 15) were housed for 9 days in indirect calorimetry chambers equipped with running wheels. The first three days were considered a habituation period and data were not included in further analyses. Days 3–6 were considered a “baseline” phase, in which the running wheels were mechanically locked to prevent use, and days 6–9 were considered the “wheel access” phase, in which the wheels were unlocked (Figure 1A). Five mice did not regularly use the wheels (average of 1.0 ± 0.7 wheel turns per day [mean ± SEM]), whereas the remaining mice used them consistently (5645 ± 1485 wheel turns per day, equating to approximately 1.5 km per day; Figure 1B). Total energy expenditure increased by approximately 4% between the baseline and wheel phases for the mice that used the wheel (termed “runners”; 10.6 ± 0.1 to 11.0 ± 0.3 kcal/day; t (9) = 2.6, p = 0.03; Figures 1C–1D). In contrast, total energy expenditure did not increase significantly for the mice that did not use the wheels regularly (termed “non-runners”; 10.7 ± 0.4 to 10.8 ± 0.2 kcal/day; t (4) = 0.6, p = 0.57). Subsequent analyses focused solely on “runners”, to focus on the effect of wheel running on energy intake and utilization. Importantly, body weight did not decrease during their time in the calorimetry chambers, but rather increased non-significantly by approximately 2% (one-way repeated-measures [RM] ANOVA, F (2, 18) = 2.6, p = 0.10; Figure 1E). Despite the lack of a reduction in body weight, average daily respiratory exchange ratio (RER, ratio of CO2 production to O2 consumption [VCO2: VO2]) slightly but significantly decreased during the wheel access phase (0.93 ± 0.00 to 0.91 ± 0.01; t (9) = 4.0, p = 0.003; Figure 1F), suggesting a shift towards the consumption of residual fat stores. This was consistent with a ~10% reduction in food intake throughout the wheel phase (11.5 ± 0.3 to 10.4 ± 0.2 kcal/day; t (9) = 3.7, p = 0.005; Figure 1G), and supports reported anorexic effects of short term wheel running [22]. Together, the decrease in food intake and increase in energy expenditure resulted in an average deficit of ~0.8kcal/day throughout the wheel access phase. This deficit would be predicted to reduce body fat by ~0.25g in 3 days. However, we did not detect any change in body weight, likely due to the short duration of the experiment (Figure 1E).
Figure 1. Short-term wheel running alters energy balance.
(A) Experimental design: mice (n = 15) were housed in indirect calorimetry chambers for a baseline phase (3 days; locked wheel) followed by a wheel access phase (3 days; unlocked wheel), and measures were collected throughout in 13 min bins. (B) Mice with regular use of running wheels (5645 ± 1485 wheel turns per day) were included in analyses (n = 10). (C) Energy expenditure (blue, left y-axis) and wheel running (orange, right y-axis) over the baseline and wheel access phases. (D) Total energy expenditure significantly increased by ~5% during the wheel access phase (p = 0.03). (E) Body weight was unchanged over the course of the baseline and wheel access phases (p = 0.10). (F) Respiratory exchange ratio (VCO2:VO2) was slightly but significantly decreased during the wheel access phase (p = 0.003). (G) Daily food intake decreased by ~10% during the wheel access phase (p = 0.005). Data shown as mean ± SEM or mean with individual mice; paired ttest or repeated-measures ANOVA followed by Fisher’s LSD post-hoc; *p < 0.05, **p < 0.01. Raw data for this figure presented in Supplemental tables online.
Further increments in wheel use was associated with minimal changes in daily energy expenditure
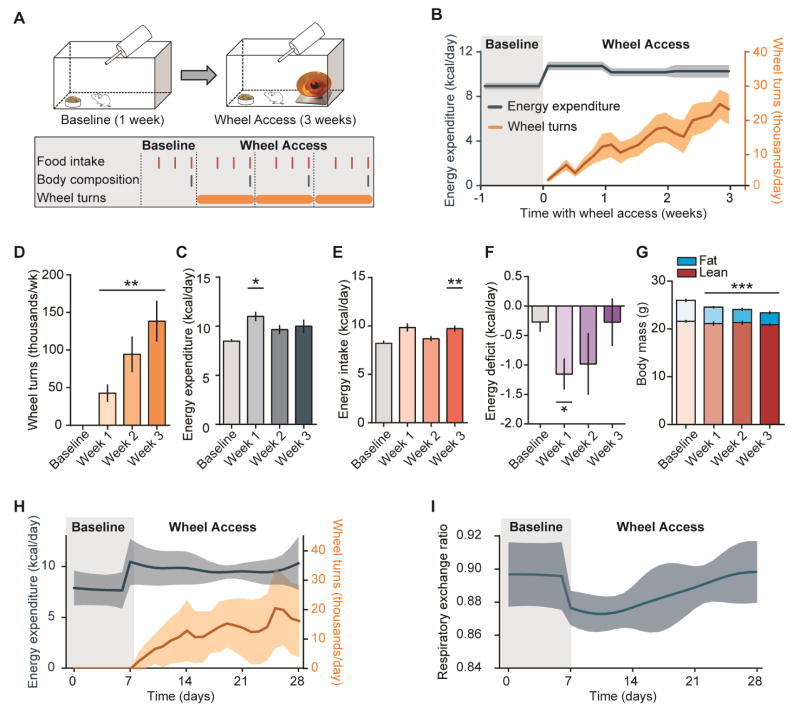
We examined whether the above effects would propagate into a larger energy deficit across a longer period of wheel access. Male C57BL/6 mice (n = 7) were habituated to home cages for 1 week, after which data was collected during a baseline phase (1 week); at the end of the baseline phase, running wheels were added to each cage and were freely available throughout the wheel access phase (3 weeks; Figure 2A). Throughout this and all following experiments energy expenditure was estimated using an energy balance technique (see Experimental Procedures), whereby the change in somatic energy stores were subtracted from total caloric intake to estimate energy expenditure [23, 24]. We observed a significant effect of week on energy expenditure (RM ANOVA, F (3, 18) = 4.1, p = 0.02), which we linked to an increase of approximately 30% in weekly energy expenditure from baseline to week 1 of the wheel access phase (8.5 ± 0.2 to 11.0 ± 0.5 kcal/day; p = 0.003); however, despite increments in wheel running of 52,000 between weeks 1 and 2 and an additional 44,000 between weeks 2 and 3 (Figure 2D), there was no further increase in energy expenditure (Figures 2B–C). This was surprising, and suggests that mice may compensate for the increased energetic demand of wheel running by reducing energy burned through other means. Consistent with our indirect calorimetry experiment, we observed a slight reduction in food intake across the first 3 days of wheel use (7.2± 0.7 to 6.1± 0.1 kcal/day), although this was not significant (p = 0.18). However, when analyzed across weeks, food intake increased significantly from 8.2 ± 0.3 in the baseline phase to 9.7 ± 0.3 kcal/day by week 3 (F (3, 18) = 6.6, p = 0.003; Figure 2E). Energetic balance (Energy intake – Energy expenditure) did not significantly change overall (F (3, 18) = 1.3, p = 0.29). However, independent t-tests revealed a significant deficit in caloric balance during the first (p = 0.04) but not second or third week of wheel access relative to baseline (both p > 0.24; Figure 2F). Finally, there was a reduction in body mass across the experiment (F (3, 18) = 28.14, p < 0.0001), which was attributable to a significant decrease in body fat (4.4 ± 0.2 to 2.5 ± 0.2g, F (3, 18) = 14.24, p < 0.0001), but not lean mass (21.6 ± 0.2 to 20.9 ± 0.2g, F (3, 18) = 1.75, p = 0.19; Figure 2G). These results support a model in which the transition from sedentary to mild activity is associated with increased total energy expenditure, but further increases in activity do not result in additional changes in total energy expenditure.
Figure 2. Changes in energy balance due to wheel running do not persist over time.
(A) Experimental design: mice (n = 7) were housed in home cages for a baseline phase (7 days) followed by a wheel access phase (21 days). (B) Energy expenditure (blue, left y-axis) and wheel running (orange, right y-axis) over the baseline and wheel access phases. (C) Wheel running significantly and continually increased throughout the wheel access phase (p = 0.002). (D) Energy expenditure increased upon initial wheel access (p = 0.0096) but did not significantly change over the course of the wheel access phase (p = 0.60). (E) By the end of the wheel access phase, daily food intake was significantly greater than during the baseline phase (p = 0.003). (F) Initial wheel access caused a significant energetic deficit (p = 0.02) that waned over the following weeks of wheel access despite sustained wheel use. (G) Body mass decreased across the experiment (p < 0.0001), which was attributed to a change in fat mass. (H–I) Modeled (H) energy expenditure and (I) respiratory exchange ratio as functions of time. See also Figure S1. Data shown as mean ± SEM (shaded region) or mean with individual mice; paired t-test or repeated-measures ANOVA followed by Bonferroni’s or Fisher’s LSD post-hoc; *p < 0.05; **p < 0.01, ***p < 0.001. Raw data for this figure presented in Supplemental tables online.
To gain a better understanding of the time-course of changes in energy utilization with wheel access, we employed a mathematical model encompassing food intake (measured continuously, with sampling every other day) and weekly body composition measurements to make daily predictions about energy expenditure, fat oxidation rates, oxygen consumption, carbon dioxide production, and RER [25]. In contrast to the linear increase in wheel running across days, changes in modeled energy expenditure peaked in the first days of wheel access and plateaued across the next two weeks (Figure 2H), resulting in an energy deficit for the first week that recovered over the next two weeks despite increased wheel running (Figure 2I). RER and fat oxidation also changed most strongly in the first week and then regressed towards basal values, despite further increases in wheel running (Figure S1).
Wheel running was associated with an increase in off-wheel physical inactivity
Next, we repeated the prior experiment in behavioral chambers (Noldus PhenoTypers, see Experimental Procedures) that were equipped with over-head cameras for continuous video monitoring and quantification of off-wheel locomotion and physical inactivity (Figure 3A). In these mice (male C57BL/6 mice; n = 8), wheel running did not progressively increase as in our prior experiment, but instead stabilized after week 2 (F (3, 21) = 11.60, p = 0.0001; Figure 3B). Again, changes in wheel running were not accompanied by increases in total energy expenditure (F (3, 21) = 1.41, p = 0.27; Figure 3C) or food intake (F (3, 21) = 0.81, p = 0.50; Figure 3D). Body fat decreased by 16% during the wheel access phase (2.4g to 2.0g; F (3, 21) = 15.60, p < 0.0001), while lean mass (21.9 to 22.0; F (3, 21) = 0.43, p = 0.73) did not change (Figure 3E). The decrease in body fat is consistent with the shift in RER we observed in the indirect calorimetry experiment, as well as in our modeling analysis. Energy balance also followed a similar pattern as in our prior experiment, with a deficit following initial addition of running wheels, that rebounded by the end of week 3 (F (3, 21) = 11.07, p = 0.002; Figure 3F). Off-wheel ambulation decreased significantly after addition of running wheels, an effect that persisted throughout the wheel access phase (F (25, 175) = 9.67, p = 0.0002; Figure 3G), possibly reflecting a compensatory adaptation to the energetic demand of increased wheel use.
Figure 3. Wheel running was associated with an increase in off-wheel physical inactivity.
(A) Experimental design: mice (n = 8) were housed in behavioral chambers with ceiling-mounted cameras for a baseline phase (7 days) followed by a wheel phase (21 days). (B) Wheel running increased during the wheel access phase (p = 0.006). (C–D) Neither energy expenditure (p = 0.28) nor daily food intake (p = 0.46) significantly changed as a result of wheel access or use. (E) Body mass did not change significantly across this experiment. (F) Initial wheel access resulted in negative energy balance that reversed over three additional weeks of wheel access (p = 0.002). (G) Representative activity paths from the baseline (top) and wheel access (bottom) phases. During the wheel phase, off-wheel ambulation (green, left y-axis) significantly decreased (p < 0.0001) while wheel running (orange, right y-axis) significantly increased (p = 0.006) over time. Data shown as mean ± SEM; repeated-measures ANOVA followed by Bonferroni’s post-hoc; **p < 0.01, ***p < 0.001. Raw data for this figure presented in Supplemental tables online.
Natural variation in wheel running did not correlate with total daily energy use
Finally, we investigated whether natural variation in wheel running would be associated with different levels of total daily energy expenditure. Male C57BL/6 mice (n = 41) were habituated to home cages for 1 week, after which data was collected during a baseline phase (1 week); at the end of the baseline phase, running wheels were added to each cage and were freely available throughout the wheel access phase (2–3 weeks). There was a large variance in dialy wheel use among mice (Figure 4A). However, the amount of daily wheel use did not correlate with total energy expenditure (R2 = 0.08, p = 0.08; Figure 4B) or food intake (R2 = 0.00, p = 0.93; Figure 4C). We identified a slight but significant inverse correlation between wheel running and body mass (R2 = 0.12, p = 0.02; Figure 4D), which appeared to be attributed to mice with very low wheel counts having higher body fat (R2 = 0.49; Figure 4E) and not differences in lean mass (Figure 4F). We conclude that natural variation in wheel running does not translate into detectable differences in energy intake or expenditure, but may underlie differences in body composition.
Figure 4. Natural variation in wheel running does not correlate with energy intake or expenditure.
(A) Mice (n = 41) had substantial variance in daily wheel running activity. (B–C) Neither energy expenditure nor daily food intake significantly correlated with wheel use. (D–E) Both total body mass and fat mass formed significant inverse relationships with wheel use. (F). Lean mass was not significantly correlated with wheel use. Data shown as mean ± SEM or as individual mice; linear or non-linear regressions. Raw data for this figure presented in Supplemental tables online.
Compensatory adaptations restricted increases in energy expenditure following wheel running
In these experiments, the transition from sedentary to mild activity caused an increase in daily energy expenditure, while further increments in activity translated into diminishingly small increases in daily energy expenditure. The incremental cost of wheel use in mice has been estimated at ~100 to 200 calories per 1000 wheel counts, accounting for ~5–10% of daily energy demands [26–28]. Without compensatory mechanisms, these increases in wheel counts would have been predicted to increase daily energy expenditure by ~10–20%, an effect size that we observed in the early phases of wheel access. However, this effect failed to propagate across subsequent weeks, despite further increases in wheel use.
The contribution of physical activity to daily energy expenditure in humans and mice cover a large range, estimated at ~10–30% of daily energy expenditure [29, 30]. Unlike humans, thermogenesis represents the greatest contributor to energy expenditure for mice housed below thermoneutrality [29, 31]. Active skeletal muscle is a highly thermogenic organ, converting the bulk of utilized energy into heat and a minority into work [32, 33]. Therefore, muscle thermogenesis during wheel running may have caused mice to downregulate thermogenesis by brown adipose and other tissues. In support of this idea, while voluntary physical activity at thermoneutrality increased energy expenditure in mice, physical activity below thermoneutrality had no effect on daily energy expenditure [34]. Mice on wheels at lower ambient temperatures also exhibit a shallower slope between acute running speed and rate of energy expenditure, likely reflecting the larger contribution of muscle thermogenesis at lower ambient temperatures [27]. Finally, a study employing high-resolution (1 sample every 2 seconds) indirect calorimetry noted that resting metabolic rate of mice at 22°C dropped below basal levels for 15–30 minutes following bouts of motor activity. The authors hypothesized that this was due to muscle thermogenesis during activity reducing the need for other forms of thermogenesis [35]. Since humans operate in an approximate state of thermoneutrality, this aspect of energy expenditure may be less relevant to humans than mice.
Although exercise acutely increases energy expenditure, this may be compensated for by a comparable reduction in non-exercise physical activity. Accordingly, we observed a decrease in off-wheel locomotion in mice that were provided with running wheel access, consistent with findings of other researchers [36, 37]. Muscle efficiency can also increase with exercise repetition and training such that performing the same exercise over time utilizes less energy [21]. While we did not measure the biomechanics or dynamics of how the mice ran on the wheels in this study, it is possible that such adaptations further affected energy expenditure in this study.
Our results may relate to why exercise results in modest changes in weight [1–4]. However, exercise confers many health benefits even in the absence of weight loss, including improvements in glucose regulation, cardiac function, neurological health, and muscle and joint function [12–15, 38]. We therefore believe that physical activity should be encouraged for its overall health benefits, while expectations concerning its role in weight loss should be kept realistic. Understanding the mechanisms governing energy expenditure may lead to better understanding of levels of physical activity required to achieve health goals, regardless of changes in body weight.
EXPERIMENTAL PROCEDURES
Energy expenditure measurements
Indirect calorimetry chambers (Oxymax/CLAMS; Columbus Instruments) were used to measure energy expenditure through detection of O2 consumption and CO2 production within each chamber (22 °C, 2.5 L volume, flow rate of 0.5 L/min, sampling flow of 0.4 L/min) in 13 min bins. Alternatively, using the assumption that the energy density of fat and lean mass represent 9.4 kcal/g and 1.0 kcal/g, respectively, energy expenditure was estimated using the mass balance method [23, 24] as follows:
Raw data for all analyses is provided in supplemental data tables online.
Statistical analyses
Measures of physical activity, energy homeostasis, and body composition were independently analyzed in GraphPad Prism or Microsoft Excel using paired t-tests or repeated-measures analyses of variance (RM-ANOVAs; one-way or 2 x 5), according to experimental design.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). We would like to thank the Mouse Metabolism Core at the National Institute of Diabetes and Digestive and Kidney Diseases for assistance with indirect calorimetry experiments. We thank members of the Kravitz lab, Marc Reitman, and Oksana Gavrilova for helpful discussions and insight on the manuscript. The authors declare no conflict of interests.
Footnotes
AUTHOR CONTRIBUTIONS
Conceptualization, T.J.O., D.M.F., and A.V.K.; Methodology, T.J.O., D.M.F., A.V.K.; Investigation, T.J.O. and D.M.F.; Data Analysis, T.J.O., D.M.F., J.G., K.D.H., and A.V.K.; Writing, T.J.O., D.M.F., K.D.H., and A.V.K.; Supervision, D.M.F. and A.V.K.; Funding, K.D.H. and A.V.K.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Byrne NM, Wood RE, Schutz Y, Hills AP. Does metabolic compensation explain the majority of less-than-expected weight loss in obese adults during a short-term severe diet and exercise intervention? International journal of obesity. 2012;36:1472–1478. doi: 10.1038/ijo.2012.109. [DOI] [PubMed] [Google Scholar]
- 2.Church TS, Martin CK, Thompson AM, Earnest CP, Mikus CR, Blair SN. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PloS one. 2009;4:e4515. doi: 10.1371/journal.pone.0004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross R, Janssen I. Physical activity, total and regional obesity: dose-response considerations. Medicine and science in sports and exercise. 2001;33:S521–S527. doi: 10.1097/00005768-200106001-00023. [DOI] [PubMed] [Google Scholar]
- 4.Shaw K, Gennat H, O’Rourke P, Del Mar C. Exercise for overweight or obesity. The Cochrane database of systematic reviews. 2006:CD003817. doi: 10.1002/14651858.CD003817.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johannsen DL, Knuth ND, Huizenga R, Rood JC, Ravussin E, Hall KD. Metabolic slowing with massive weight loss despite preservation of fat-free mass. The Journal of clinical endocrinology and metabolism. 2012;97:2489–2496. doi: 10.1210/jc.2012-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westerterp KR, Meijer GA, Janssen EM, Saris WH, Ten Hoor F. Long-term effect of physical activity on energy balance and body composition. Br J Nutr. 1992;68:21–30. doi: 10.1079/bjn19920063. [DOI] [PubMed] [Google Scholar]
- 7.Ridgers ND, Timperio A, Cerin E, Salmon J. Compensation of physical activity and sedentary time in primary school children. Medicine and science in sports and exercise. 2014;46:1564–1569. doi: 10.1249/MSS.0000000000000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drenowatz C, Grieve GL, DeMello MM. Change in energy expenditure and physical activity in response to aerobic and resistance exercise programs. SpringerPlus. 2015;4:798. doi: 10.1186/s40064-015-1594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mansoubi M, Pearson N, Biddle SJ, Clemes SA. Using Sit-to-Stand Workstations in Offices: Is There a Compensation Effect? Medicine and science in sports and exercise. 2016;48:720–725. doi: 10.1249/MSS.0000000000000802. [DOI] [PubMed] [Google Scholar]
- 10.King NA, Caudwell P, Hopkins M, Byrne NM, Colley R, Hills AP, Stubbs JR, Blundell JE. Metabolic and behavioral compensatory responses to exercise interventions: barriers to weight loss. Obesity. 2007;15:1373–1383. doi: 10.1038/oby.2007.164. [DOI] [PubMed] [Google Scholar]
- 11.P.A. Division of Nutrition, and Obesity, editor. Physical Activity for a Healthy Weight. 2015 ( https://www.cdc.gov/healthyweight/physical_activity/)
- 12.Michigan A, Johnson TV, Master VA. Review of the relationship between C-reactive protein and exercise. Molecular diagnosis & therapy. 2011;15:265–275. doi: 10.1007/BF03256418. [DOI] [PubMed] [Google Scholar]
- 13.Benatti FB, Pedersen BK. Exercise as an anti-inflammatory therapy for rheumatic diseases-myokine regulation. Nature reviews Rheumatology. 2015;11:86–97. doi: 10.1038/nrrheum.2014.193. [DOI] [PubMed] [Google Scholar]
- 14.Roemmich JN, Lambiase MJ, Balantekin KN, Feda DM, Dorn J. Stress, behavior, and biology: risk factors for cardiovascular diseases in youth. Exercise and sport sciences reviews. 2014;42:145–152. doi: 10.1249/JES.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverman MN, Deuster PA. Biological mechanisms underlying the role of physical fitness in health and resilience. Interface focus. 2014;4:20140040. doi: 10.1098/rsfs.2014.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Booyens J, Keatinge WR. The expenditure of energy by men and women walking. The Journal of physiology. 1957;138:165–171. doi: 10.1113/jphysiol.1957.sp005844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter SE, Jones M, Gladwell VF. Energy expenditure and heart rate response to breaking up sedentary time with three different physical activity interventions. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2015;25:503–509. doi: 10.1016/j.numecd.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Stensel D. Exercise, appetite and appetite-regulating hormones: implications for food intake and weight control. Annals of nutrition & metabolism. 2010;57(Suppl 2):36–42. doi: 10.1159/000322702. [DOI] [PubMed] [Google Scholar]
- 19.Human energy requirements: report of a joint FAO/WHO/UNU expert consultation. Food and nutrition bulletin. 2005:166. [PubMed] [Google Scholar]
- 20.Cavagna GA, Heglund NC, Taylor CR. Mechanical work in terrestrial locomotion: two basic mechanisms for minimizing energy expenditure. The American journal of physiology. 1977;233:R243–261. doi: 10.1152/ajpregu.1977.233.5.R243. [DOI] [PubMed] [Google Scholar]
- 21.Gibala MJ, Little JP, van Essen M, Wilkin GP, Burgomaster KA, Safdar A, Raha S, Tarnopolsky MA. Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol. 2006;575:901–911. doi: 10.1113/jphysiol.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis DY, Brett RR. Activity-based anorexia in C57/BL6 mice: effects of the phytocannabinoid, Delta9-tetrahydrocannabinol (THC) and the anandamide analogue, OMDM-2. Eur Neuropsychopharmacol. 2010;20:622–631. doi: 10.1016/j.euroneuro.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Ravussin Y, Gutman R, LeDuc CA, Leibel RL. Estimating energy expenditure in mice using an energy balance technique. International journal of obesity. 2013;37:399–403. doi: 10.1038/ijo.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo J, Jou W, Gavrilova O, Hall KD. Persistent diet-induced obesity in male C57BL/6 mice resulting from temporary obesigenic diets. PloS one. 2009;4:e5370. doi: 10.1371/journal.pone.0005370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo J, Hall KD. Estimating the continuous-time dynamics of energy and fat metabolism in mice. PLoS computational biology. 2009;5:e1000511. doi: 10.1371/journal.pcbi.1000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koteja P, Swallow JG, Carter PA, Garland T., Jr Energy cost of wheel running in house mice: implications for coadaptation of locomotion and energy budgets. Physiological and biochemical zoology: PBZ. 1999;72:238–249. doi: 10.1086/316653. [DOI] [PubMed] [Google Scholar]
- 27.Chappell MA, Garland T, Jr, Rezende EL, Gomes FR. Voluntary running in deer mice: speed, distance, energy costs and temperature effects. The Journal of experimental biology. 2004;207:3839–3854. doi: 10.1242/jeb.01213. [DOI] [PubMed] [Google Scholar]
- 28.Rezende EL, Gomes FR, Chappell MA, Garland T., Jr Running behavior and its energy cost in mice selectively bred for high voluntary locomotor activity. Physiological and biochemical zoology: PBZ. 2009;82:662–679. doi: 10.1086/605917. [DOI] [PubMed] [Google Scholar]
- 29.Abreu-Vieira G, Xiao C, Gavrilova O, Reitman ML. Integration of body temperature into the analysis of energy expenditure in the mouse. Molecular metabolism. 2015;4:461–470. doi: 10.1016/j.molmet.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maclean PS, Bergouignan A, Cornier MA, Jackman MR. Biology’s response to dieting: the impetus for weight regain. Am J Physiol Regul Integr Comp Physiol. 2011;301:R581–600. doi: 10.1152/ajpregu.00755.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garland T, Jr, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, Acosta W, Drenowatz C, Maciel RC, van Dijk G, et al. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. The Journal of experimental biology. 2011;214:206–229. doi: 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaesser GA, Brooks GA. Muscular efficiency during steady-rate exercise: effects of speed and work rate. Journal of applied physiology. 1975;38:1132–1139. doi: 10.1152/jappl.1975.38.6.1132. [DOI] [PubMed] [Google Scholar]
- 33.Block BA. Thermogenesis in muscle. Annual review of physiology. 1994;56:535–577. doi: 10.1146/annurev.ph.56.030194.002535. [DOI] [PubMed] [Google Scholar]
- 34.Virtue S, Even P, Vidal-Puig A. Below thermoneutrality, changes in activity do not drive changes in total daily energy expenditure between groups of mice. Cell metabolism. 2012;16:665–671. doi: 10.1016/j.cmet.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Even PB, Anne Increased Cost of Motor Activity and Heat Transfer between Non-Shivering Thermogenesis, Motor Activity, and Thermic Effect of Feeding in Mice Housed at Room Temperature – Implications in Pre-Clinical Studies. Front Nutr. 2016 doi: 10.3389/fnut.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Copes LE, Schutz H, Dlugosz EM, Acosta W, Chappell MA, Garland T., Jr Effects of voluntary exercise on spontaneous physical activity and food consumption in mice: Results from an artificial selection experiment. Physiol Behav. 2015;149:86–94. doi: 10.1016/j.physbeh.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 37.de Carvalho FP, Benfato ID, Moretto TL, Barthichoto M, de Oliveira CA. Voluntary running decreases nonexercise activity in lean and diet-induced obese mice. Physiol Behav. 2016;165:249–256. doi: 10.1016/j.physbeh.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Camps SG, Verhoef SP, Westerterp KR. Physical activity and weight loss are independent predictors of improved insulin sensitivity following energy restriction. Obesity. 2016;24:291–296. doi: 10.1002/oby.21325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.