Abstract
We have previously shown that activated γδ T cells have a much stronger proinflammatory effect in the development of experimental autoimmune uveitis (EAU) than their non-activated counterparts. Our present study explored γδ T cell subsets are functionally distinct in autoimmune pathogenesis and determined the pathogenic contribution of biased Vγ4+ γδ T cell activation in this disease. By systematically comparing two major peripheral γδ T cell subsets, the Vγ1+ and the Vγ4+ cells, we found that the Vγ4+ cells were readily activated in B6 mice during EAU development, whereas Vγ1+ cells remained non-activated. Cytokines that were abundantly found in the serum of immunized mice activated Vγ4+, but did not activate Vγ1+, cells. The Vγ4+ cells had a strong pro-inflammatory activity, whereas the Vγ1+ cells remained non-activated when tested immediately after isolation from immunized mice. However, when the Vγ1+ cells were activated in vitro, they promoted inflammation. Our results demonstrated that activation is a major factor in switching the enhancing and inhibiting effects of both Vγ1+ and Vγ4+ γδ T cell subsets and that γδ T cell subsets differ greatly in their activation requirements. Whether the enhancing or inhibiting function of γδ T cells is dominant is mainly determined by the proportion of the γδ T cells that are activated versus the proportion not activated.
Keywords: autoimmunity, experimental autoimmune uveitis, γδ T cells, Interleukin-17, Th17, uveitis
Introduction
γδ T cells play a major role in both innate and adaptive immunity (1, 2) and are the early infiltrating cells in inflammatory disorders such as autoimmune diseases (3–10). Studies have shown that γδ T cells can either enhance (9, 11–15) or inhibit (8, 15–20) an adaptive immune response. This functional diversity has been previously credited to γδ T cell subsets that express distinct TCRs (21–25). Later studies have also demonstrated that the enhancing and inhibiting activities of γδ T cells could be reversed if γδ T cells were pre-exposed to bacterial products (23, 25). Clinical approaches have been developed to use γδ T cells as a therapeutic modality (26–28). A better knowledge of how these cells exert their enhancing and inhibitory functions should improve their therapeutic use.
More than 90% of the γδ T cells in the peripheral lymphoid tissues of naïve mouse are either Vγ1+ or Vγ4+ γδ T cells; among these, Vγ1+ γδ T cells are the major components (29). This dominance shifts during disease. Thus, at the peak of the peripheral immune response in induced autoimmune diseases such as experimental autoimmune encephalomyelitis and autoimmune uveitis (EAU), the dominant Vγ1+ γδ T cells are rapidly replaced by Vγ4+ γδ T cells; meanwhile, the total γδ T cell number is increased by 5- to 10-fold (11, 30–33). We have previously shown that activated γδ T cells have a much stronger proinflammatory effect than their non-activated counterparts (34–36). Studies on the mechanism of how γδ T cells become activated and whether manipulation of γδ activation could allow us to control disease progression should have implications for their therapeutic use.
Using a mouse EAU model that consistently demonstrated a dominant activation of Vγ4+ γδ T cells in a pre-clinical stage (30), we investigated the mechanism leading to this biased γδ activation and the correlation of disease pathogenesis and the dominance shift. Our results showed that activation of macrophages/dendritic cells (DCs) in the immunized mice produces increased amounts of cytokines; these cytokines have a biased stimulatory effect on Vγ4+ cells, but not on Vγ1+ cells, leading to a preferential activation and expansion of the Vγ4+ γδ subset.
Structural and functional comparison between Vγ1+ and Vγ4+ γδ T cell subsets isolated from EAU-prone B6 mice before or after immunization showed that the enhancing and inhibitory functions of both Vγ1+ and Vγ4+ T cells are determined by their activation status. The Vγ4+ cells are dominant among the activated γδ T cells in immunized mice, and these cells possessed greatly increased pro-inflammatory activity. By contrast, the Vγ1+ cells remained non-activated in immunized mice and are functionally suppressive. However, the suppressive effect of Vγ1+ cells could also be converted to an enhancing effect if these cells were rendered activated. The balance of enhancing or inhibiting function of γδ T cells is mainly determined by the proportion of the γδ T cells that are activated versus those that are not activated.
Our results demonstrated that activation is a major factor in switching the enhancing and inhibitory functions of both the Vγ1+ and the Vγ4+ γδ T subsets. The difference in activation requirements accounted for the selective dominance of a specific γδ T subset. The net functional balance between the enhancing and inhibiting effects of γδ T cells is mainly determined by the number of activated γδ T cells and the proportion of activated versus non-activated γδ T cells.
Materials and Methods
Animals and reagents
Female C57BL/6 (B6) and TCR-δ−/− mice on the B6 background, purchased from Jackson Laboratory (Bar Harbor, ME), were housed and maintained in the animal facilities of the University of California, Los Angeles, and were used at 12–16 weeks of age. The experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of California, Los Angeles (Protocol number: ARC#2014-029-03A). Recombinant murine IL-12, IL-23, and GM-CSF were purchased from R & D Systems (Minneapolis, MN). FITC-, PE-, or APC-conjugated mouse monoclonal antibodies against mouse αβ T cell receptor (TCR, clone H57–597), mouse γδ TCR-Vγ4 (UC3), mouse γδ TCR-Vγ1 (211), mouse γδ TCR (clone GL3), mouse IL-17, mouse IFN-γ, mouse CD73, or mouse CD44 and isotype control antibodies were all purchased from BioLegend (San Diego, CA).
Cell preparation
At day 13 post-immunization, CD3+ T cells were purified from the spleen or draining lymph nodes of B6 or TCR-δ−/− mice immunized with peptide interphotoreceptor retinoid-binding protein (IRBP)1–20 (amino acids 1–20 of human IRBP, LifeTein, LLC, Hillsborough, NJ) by positive selection using a combination of FITC-conjugated anti-mouse CD3 antibodies and anti-FITC antibody-coated Microbeads. Cells were then separated on an auto-MACS separator according to the manufacturer’s suggested protocol (Miltenyi Biotec, Auburn, CA).
γδ T cells were isolated from IRBP1–20-immunized mice at 13 days post-immunization, using a combination of FITC-conjugated anti-TCR-δ antibodies and anti-FITC antibody-coated Microbeads, followed by separation using an auto-MACS. To test the effect of activation by either cytokines or DCs, freshly prepared γδ T cells from IRBP1–20-immunized B6 mice were cultured in cytokine-free medium for 5 days to generate the resting state, since γδ T cells freshly isolated from IRBP1–20-immunized mice are activated. The cells were then incubated for 48 h with a combination of IL-1, IL-7, and IL-23 (10 ng/ml of each) as described previously (35) or for 48 h with bone marrow dendritic cells (BMDCs) and anti-CD3 antibodies.
EAU induction and evaluation
To induce EAU, B6 mice were injected subcutaneously at 6 spots at the tail base and on the flank with a total of 200 μl of emulsion consisting of equal volumes of 150 μg of peptide IRBP1–20 in PBS and CFA (BD Biosciences, San Diego, CA), and intraperitoneally (i.p.) with 200 ng of pertussis toxin (Sigma-Aldrich, St. Louis, MO).
Assessment of Th17 responses
Responder TCRαβ+ T cells (3 × 106) prepared from IRBP1–20-immunized TCR-δ−/− mice were co-cultured for 48 h with IRBP1–20 (10 μg/ml) and irradiated spleen cells (2 × 106/well) as APCs in a 12-well plate under Th17 polarized conditions (culture medium supplemented with 10 ng/ml of IL-23), with or without the addition of γδ T cells. IL-17 levels in the culture medium were then measured using ELISA kits (R & D Systems), and the number of antigen-specific T cells expressing IL-17 was determined by intracellular staining, followed by FACS analysis, as described below (35, 37).
ELISA measurement of cytokine levels in serum and culture supernatants
ELISA was used to measure cytokine (IL-1, IL-7, IL-12 IL-23 and IL-17) levels in the serum on day 13 post-immunization and in the 48 h culture supernatants of responder T cells isolated from immunized TCR-γ−/− mice in the absence or presence of Vγ1 or Vγ4 cells.
Immunofluorescence flow cytometry for surface and cytoplasmic antigens
In vivo primed T cells were stimulated with the immunizing antigen and APCs for 5 days; then the T cells were separated using Ficoll gradient centrifugation and stimulated in vitro for 4 h with 50 ng/ml of PMA, 1 μg/ml of ionomycin, and 1 μg/ml of brefeldin A (all from Sigma). Aliquots of cells (2 × 105 cells) were then fixed, permeabilized overnight with Cytofix/Cytoperm buffer (eBioscience, San Diego, CA), and intracellularly stained with PE-conjugated anti-mouse IFN-γ antibodies or FITC-labeled anti-mouse IL-17 antibodies. Data collection and analysis were performed on a FACScalibur flow cytometer using CellQuest software.
Generation of bone marrow dendritic cells
Bone marrow dendritic cells were generated by incubating bone marrow cells from B6 mice for 5 days in the presence of 10 ng/ml of recombinant murine granulocyte macrophage colony-stimulating factor (R&D Systems), as described previously (38). To test the stimulating effect of these cells on γδ T cells, the BMDCs were pre-treated for 48 h with 100 ng/ml of LPS (39).
CFSE assays
Purified αβTCR+ T cells from IRBP1–20-immunized TCR-δ−/− mice were stained with CFSE (Sigma-Aldrich) as described previously (40). Briefly, the cells were washed and suspended at 50 × 106 cells/ml in serum-free RPMI 1640 medium; cells were then incubated at 37°C for 10 min with gentle shaking with a final concentration of 5 μM CFSE before being washed twice with, and suspended in, complete medium, stimulated with immunizing peptide in the presence of APCs, and analyzed by flow cytometry.
Measurement of adenosine receptor mRNA levels
Cytokine (IL-1, IL-7 and IL-23) receptor mRNA levels were determined by real-time PCR. Vγ1+ and Vγ4+ γδ T cells were purified from IRBP1–20-immunized B6 mice by autoMACS separation. Total RNA was extracted from 2 × 105 cells using an RNA isolation kit (Invitrogen, Carlsbad, CA) and treated with DNase I (GE Healthcare, Piscataway, NJ); then 0.1 μg was reverse transcribed into cDNA using a Moloney murine leukemia virus RT kit (Invitrogen) and tested in a Cyber green real-time PCR assay. Levels of each cDNA were measured in triplicate, using Gapdh cDNA as reference. Each cDNA sample was amplified for the gene of interest. The concentration of the mRNA for the gene of interest was determined using the comparative threshold cycle number and normalized to that of the internal Gapdh control. Results were shown as 2-ΔCt.
Statistical analysis
All experiments were repeated 3–5 times. Experimental groups typically consisted of six mice, and the figures show the data from a representative experiment. The statistical significance of differences between the values for different groups was examined using the Mann Whitney U-test.
Results
Vγ4+ cells gradually become the dominant γδ cells in the spleen of immunized B6 mice
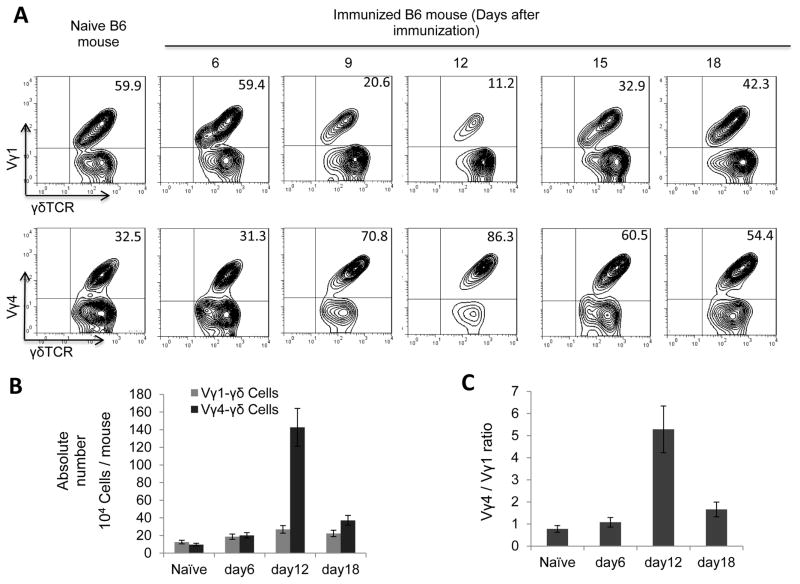
Kinetic examination of the intensity of induced autoimmune responses and the appearance of Vγ1+ and Vγ4+ T cells in the spleen of immunized B6 mice demonstrated that the maximal responses to the immunizing antigen were constantly detected around 13 days after immunization, about a week before clinical symptoms were detected. The Vγ1+ γδ T cells constitute approximately two-thirds of the total splenic γδ T cells in naïve mice, and in immunized mice until 6 days post immunization, whereas the Vγ4+ γδ T cells constitute only one-third (Fig. 1A). The non-Vγ4 non-Vγ1 cells are very few. These proportional numbers shifted dramatically, starting 6–7 days post-immunization. By 12 days post immunization, the Vγ1+ γδ T cells had declined quickly to 11.2% of the total splenic γδ T cells, whereas the Vγ4+ γδ T cells had increased to 86.5%, leading to a change in the ratio of Vγ1: Vγ4 from 7:3 to 1:8. Estimation of the absolute numbers of Vγ1 and Vγ4 cells showed, additionally, the absolute number of Vγ1+ γδ T cells remained largely unchanged whereas that of the Vγ4+ γδ T cells increased 5- to 10-fold (Fig. 1B&C).
FIGURE 1. Vγ4+ γδ T cells gradually become the dominant γδ subset in the periphery of immunized B6 mice.
(A) Relative number of Vγ1+ and Vγ4+ γδ T cells. Splenic T cells from IRBP1–20-immunized B6 mice (n=6) were double-stained with anti-mouse Vγ1 (upper panels) or anti-mouse Vγ4 (lower panels) and anti-mouse γδTCR antibodies, on the indicated days after immunization. Pan γδ T cells were gated for FACs analysis.
(B) (B&C) Absolute number of Vγ1+ and Vγ4+ γδ T cells. Total numbers of splenic Vγ1+ and Vγ4+ γδ T cells were evaluated from immunized B6 mice (B) and the ratio change between Vγ1+ and Vγ4+ γδ T cells is shown in (C). The results are from a single experiment (n=6) and are representative of three independent studies.
Vγ4+ cells in immunized B6 mice are activated, whereas the Vγ1+ γδ T cells remain non-activated
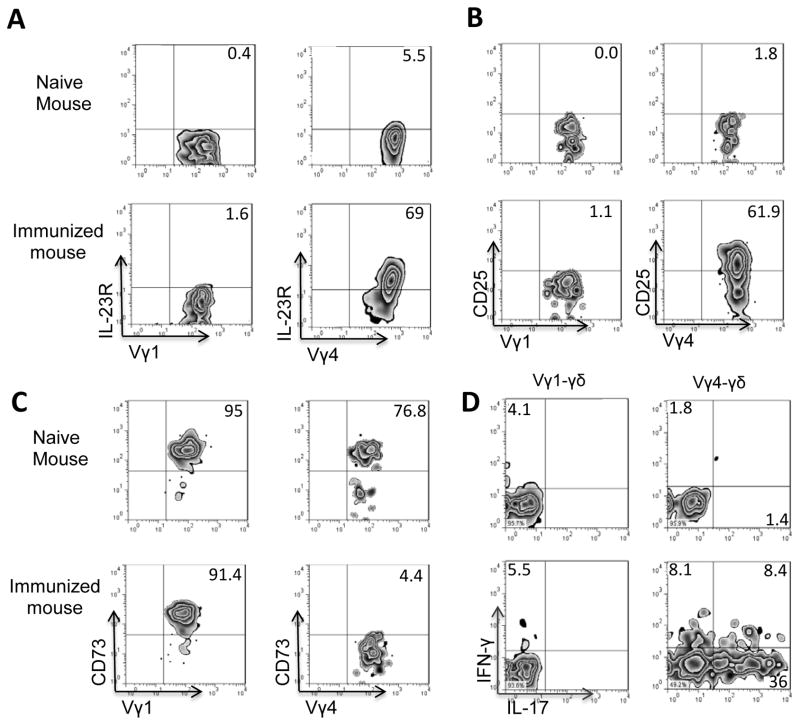
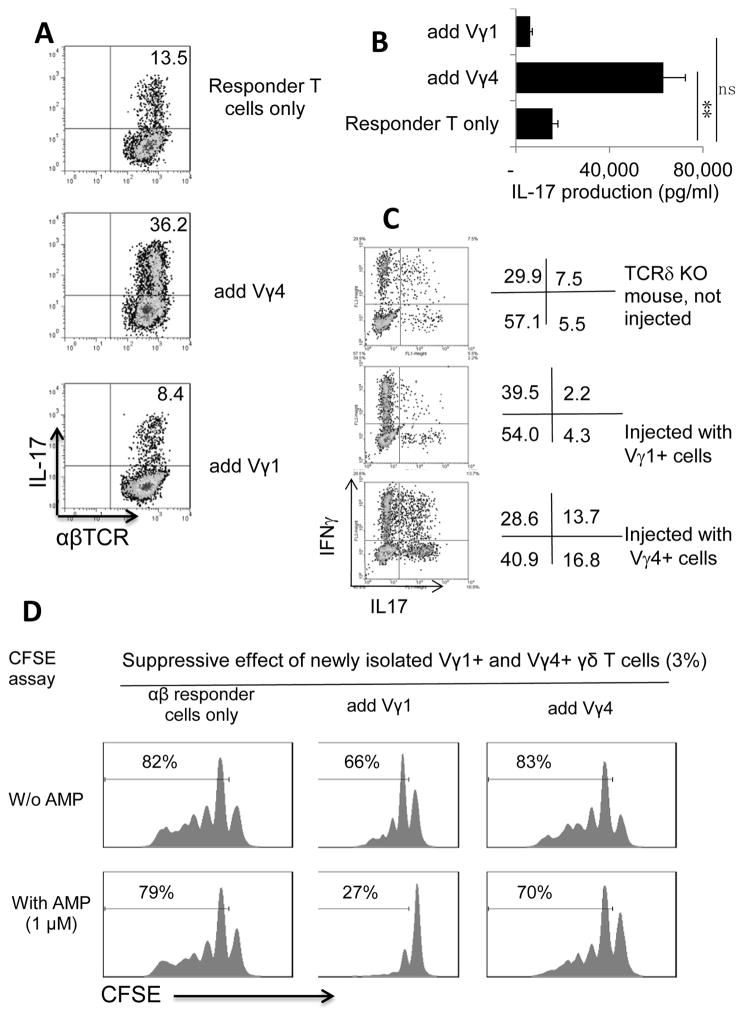
We have assessed the activation status of Vγ1 and Vγ4 cells immediately after isolation from splenic T cells of naïve or immunized mice. Figure 2A shows that both Vγ1 and Vγ4 cells from naïve mice were CD25lowCD44low. In immunized mice, the Vγ4+ cells became CD25highCD44high but the Vγ1+ T cells of immunized mice remained CD25lowCD44low. As our previous study found activated γδ T cells expressed downregulated CD73 (41, 42), we examined whether Vγ1+ and Vγ4+ γδ T cells express different levels of CD73. The results showed that a majority of the Vγ4+ cells from immunized mice were CD73low or CD73−; by contrast, the Vγ1+ cells from immunized mice remained CD73high (Fig. 2C). In addition, a significant portion of the Vγ4+ T cells, but not of the Vγ1+ T cells, expressed IL-17, in the absence of additional in vitro stimulation (Fig. 2D). We have previously reported that activated γδ T cells have a strong enhancing effect on Th17 responses (30, 34, 35, 42). We then determined whether Vγ1+ γδ T cells isolated from immunized mice differed in their enhancing autoimmune response from the Vγ4+ cells isolated from immunized mice. Responder αβ T cells isolated from immunized TCR-δ−/− mice were stimulated with the immunizing antigen and APCs, with an addition of Vγ1+ or Vγ4+ cells isolated from immunized mice. Five days after in vitro stimulation, the IL-17+ cells among the responder T cells were assessed by intracellular staining with IL-17 (Fig. 3A). In addition, T cell supernatants were tested for IL-17 production by ELISA 48 h after stimulation (Fig. 3B). Our results showed that the addition of a small number (2%) of Vγ4+ cells strongly enhanced the Th17 response, whereas the addition of Vγ1+ cells was ineffective or slightly suppressive (Fig. 3A&B). In vivo test agreed with this prediction: TCR-δ−/− recipient mice injected with Vγ4+, but not with Vγ1+, γδ T cells showed significantly enhanced Th17 responses (Fig. 3C), as examined by intracellular staining of the responder T cells, or by assessing cytokine production in 48 cultured supernatants by ELISA (data not shown). In addition, when the suppressive effect was tested, the Vγ1+ cells, but not Vγ4+ cells, that were freshly isolated from immunized mice showed greater suppressive effect, particularly when in the presence of exogenously added AMP (Fig. 3D).
FIGURE 2. Vγ4+ cells became activated whereas the Vγ1+ γδ T cells remained non-activated in EAU-induced B6 mice.
Splenic CD3+ cells were enriched from a group (n=6) of naïve mice or from immunized mice 13 days post-immunization using MACS column. They were double-stained with anti-mouse Vγ1/Vγ4 antibodies and anti-IL23R (A); anti-mouse CD25 antibody (B); or anti-CD73 antibody (C). Alternatively, they were intracellular staining of IFN-γ and IL-17 (D).
Vγ1 and Vγ4 cells were selectively gated for FACs analysis.
FIGURE 3. The Vγ4+ cells, but not the Vγ1+ cells isolated from immunized mice, had an enhancing effect on activation of autoreactive T cells.
A) Splenic αβ T cells were enriched from a group of immunized TCR-δ−/− mice 13 days post-immunization using MACS column. They were stimulated with the immunizing antigen and APCs for 5 days, in the presence of Vγ1 or Vγ4 T cells, before they were stained with anti-IL17 and anti-mouse αβ antibodies; and αβ T cells were gated for FACs analysis. Results are from one experiment representing 5 separate studies.
B) ELISA test of IL-17 production of the responder T cells activated in the presence of Vγ1 or Vγ4 T cells; Groups (n=6) of TCR-δ−/− mice with or without injection of Vγ1+ and Vγ4+ γδ T cells (1 × 106/mouse) were immunized one day later with a pathogenic dose of IRBP1–20/CFA, and the IL17 production of the responder T cells was assessed 2 days after in vitro stimulation under Th17 polarized conditions as described earlier. The numbers indicated are calculated from triplicated samples. Data are from a single experiment, representative of three independent experiments. **P < 0.01; ns, not significant.
C) Injection of TCR-δ−/− mice with Vγ4+, but not Vγ1+, γδ T cells, before IRBP1–20 immunization increases the generation of IL-17+ IRBP-specific T cells. Groups (n=6) of TCR-δ−/− mice with or without injection of Vγ4+, but not Vγ1+, γδ T cells (1 × 106/mouse) were immunized one day later with a pathogenic dose of IRBP1–20. The IFN-γ+ and IL-17+ T cells were assessed 5 days after in vitro stimulation. αβ T cells were gated for FACs analysis.
D) Vγ1+, but not Vγ4+, cells newly isolated from immunized mice, showed greatly augmented suppressive effect in the presence of exogenous AMP. Responder T cells isolated from TCR-δ−/− mice were labeled with CFSE and stimulated with the immunizing IRBP1–20 and APCs, in the absence or presence of added (2%) Vγ1+ or Vγ4+ cells, freshly prepared from immunized mice. The test was conducted in the absence of additions (left panels), in the presence of AMP (middle panels), and in the presence of AMP and APCP, the CD73 inhibitor (right panels).
Cytokine exposure activates the Vγ4+, but not the Vγ1+, γδ T cells in vitro
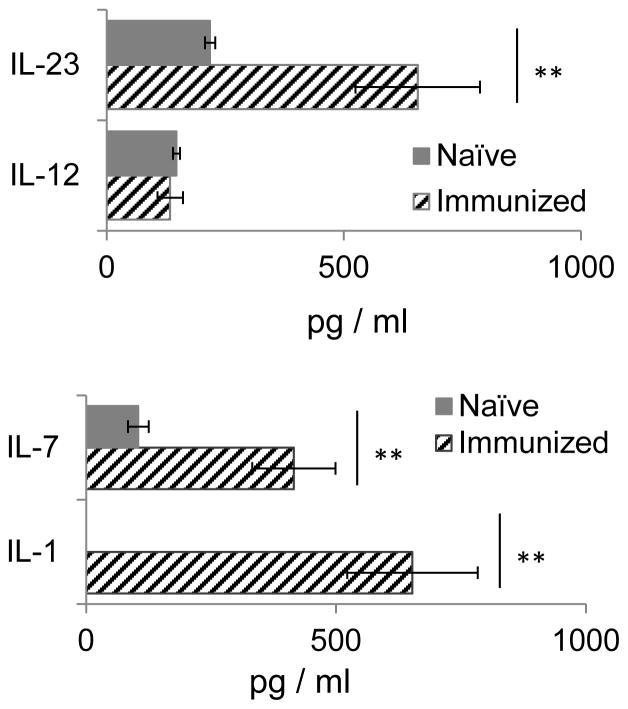
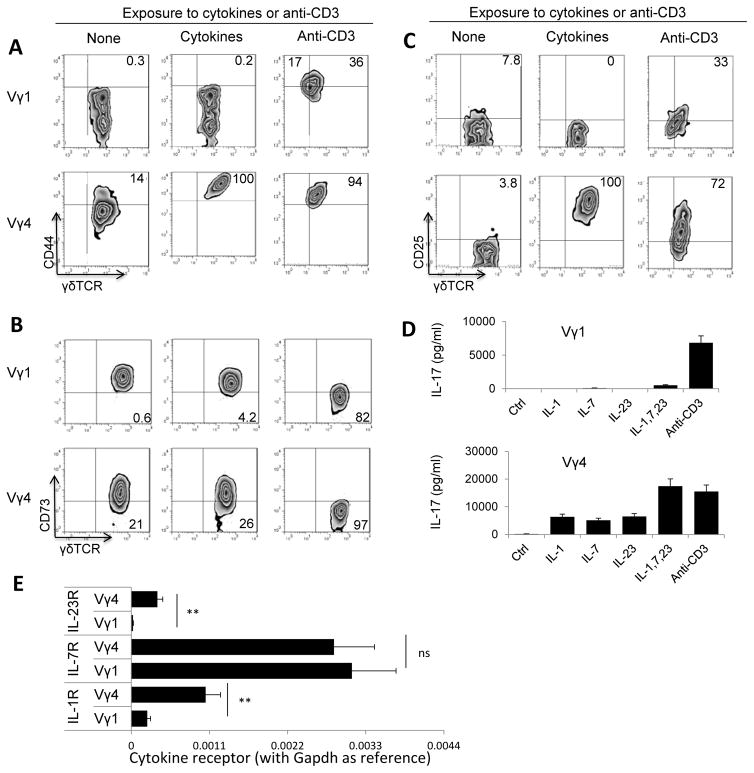
To determine whether different activation requirements were due to the biased activation of Vγ4+ γδ subset during autoimmune induction, we examined the serum cytokines that were increased in immunized mice and found IL-1, IL-23 and IL-7 increased significantly in the serum of immunized mice, as compared to that of naïve mice (Fig. 4). When separated Vγ1+ and Vγ4+ γδ T cells were exposed to a combination of IL-1, IL-23 and IL-7, only the Vγ4+, but not the Vγ1+, γδ T cells were activated (Fig. 5A–C). The stimulated Vγ4+ T cells produced significantly increased amounts of IL-17 after exposure to either cytokines or anti-CD3 antibody (Fig. 5D). The Vγ1+ cells, on the other hand, expressed increased levels of CD44 and CD25 only and downregulated CD73 after exposure to anti-CD3 antibodies, but not to cytokines (Fig. 5A–C). To determine the mechanism by which cytokines biasedly activated Vγ4+ γδ T cells, we have compared the cytokine receptor expression between Vγ1+ and Vγ4+ γδ T cells. The new RT-PCR results (Fig. 5E) showed that Vγ4+, but not Vγ1+ cells in immunized mice expressed increased amounts of IL-1 and IL-23 receptors, even though expression of IL-7 receptor was indistinguishable between the two cells.
FIGURE 4. Serum cytokine detection of naïve and immunized B6 mice.
Blood samples collected from groups (n=6) of naïve and immunized mice (on day 13 post immunization) were pooled and tested in triplicate by ELISA. The standard errors were calculated from triplicated samples. Data are from a single experiment, representative of three independent experiments. The standard errors were calculated from triplicated samples. Data are from a single experiment, representative of three independent experiments. **p < 0.01.
FIGURE 5.
The cytokine combination (IL-1+7+23) activated Vγ4+, but not Vγ1+, γδ T cells. (A–C) Expression of CD44 (A), CD73 (B), and CD25 (C) by Vγ1+ and Vγ4+ cells was assessed before and after an exposure to cytokine or anti-CD3 antibody. Vγ1+ and Vγ4+ γδ subsets were separated from immunized B6 mice 13 days post immunization. They were exposed for 48h in culture to a combination of IL-1, IL-23 and IL-7 (10 ng/ml) or anti-CD3 antibody (1 μg/ml). Expression of CD44 (A), CD73 (B) and CD25 (C) of Vγ1+ and Vγ4+ cells before and after cytokine or antibody stimulation was compared, after staining with related antibodies followed by FACS analysis. A representative experiment of five separate repeats.
(D) Cytokine production assay. Supernatants of cultured Vγ1+ and Vγ4+ cells were assessed in triplicate for IL-17 before and after an exposure to single or pooled cytokines of IL-1+7+23 (10 ng/ml) as indicated.
(E) Real-time RT-PCR analysis of IL-1R, IL-7R and IL-23R transcripts among total RNA isolated from Vγ1+ and Vγ4+ γδ T cells isolated from IRBP1–20-immunized B6 mice. Vγ1+ and Vγ4+ cells were purified from splenocytes and drainage lymphocytes of immunized B6 mice by auto-MACs purification, using (PE)-conjugated anti-Vγ1 or anti-Vγ4 antibodies and anti-PE antibodies conjugated magnetic beads. qPCR was performed with Gapdh as the internal reference. Results were represented as 2−ΔCt. **, p < 0.01; ns, not significant.
Role of DC in biased Vγ4+ T cell activation
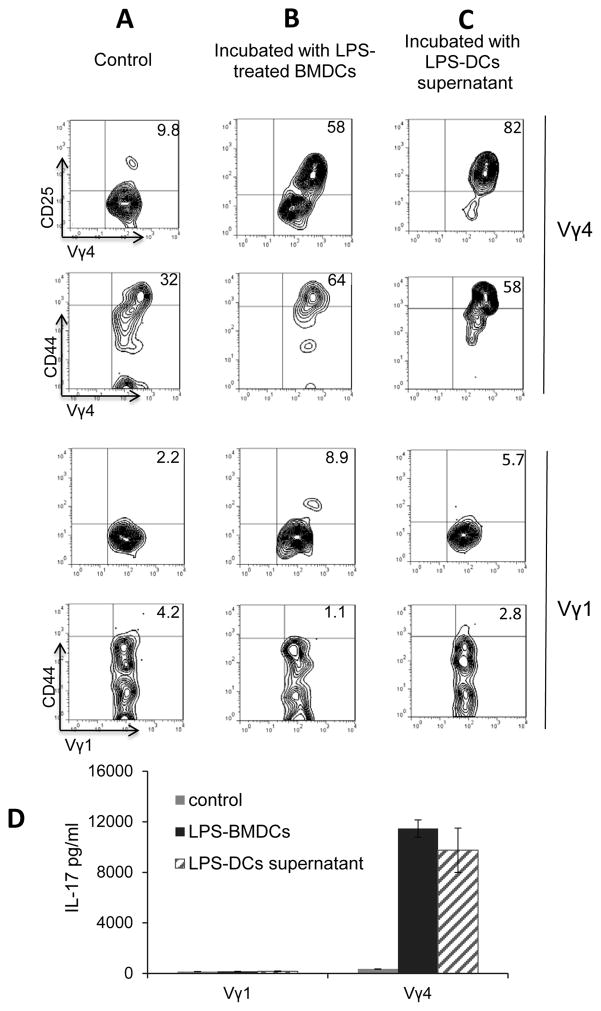
Our previous studies showed that DCs have a stimulating effect on γδ T cells (36, 43, 44), if pre-exposed to TLR ligands such as LPS (39, 43). Bone marrow dendritic cells were cultured from bone marrow cells of immunized mice in medium containing GM-CSF. To determine whether LPS-treated BMDCs stimulated Vγ1+ and Vγ4+ γδ subsets, CD3+ responder T cells were isolated from the spleens of naïve B6 mice, and the gated Vγ1+ and Vγ4+ γδ cells were assessed for expression of CD25 and CD44 by FACS analysis, 2 days after co-culture with BMDCs. Our results showed that the Vγ4+ cells expressed greatly increased amounts of CD25 and CD44, whereas the Vγ1+ did not (Fig. 6B compares to Fig. 6A) after exposure to LPS-treated BMDCs. We have also examined whether direct cell-cell contact is mandatory for DCs to render a Vγ4+ T cell activated. Vγ1+ and Vγ4+ cells were cultured in medium with supernatants of LPS-stimulated BMDCs (1:10 dilution) for 48h, before assessment of γδ T cell activation molecules, CD44 and CD25 (42). The results showed that LPS-treated BMDC supernatant has a strong stimulating effect on Vγ4+ but not on Vγ1+ cells (Fig. 6C). Cytokine test (Fig. 6D) detected increased IL-17 production from Vγ4+ but not from Vγ1+ cells after incubation with BMDCs.
FIGURE 6. LPS-treated BMDCs activated Vγ4+ but not Vγ1+ γδ T cells.
Resting Vγ1+ and Vγ4+ cells were co-incubated with medium alone (A) or with LPS-treated (100 ng/ml) BMDCs (B) (ratio γδ:DC=10:1) in 6-well-plates for 3 days. The treated Vγ1+ and Vγ4+ T cells were separated by Ficoll gradient centrifugation and stained with anti-CD44 and anti-CD25 antibodies, followed by FACS analysis. (C) Separated Vγ1+ and Vγ4+ cells (n=6) were incubated with LPS-treated (100ng/ml) BMDCs supernatants (1:10 diluted) for 3 days, the T cells were separated by Ficoll gradient centrifugation, stained with anti-CD44 and anti-CD25 antibodies, respectively, followed by FACS analysis. D). IL-17 in the supernatant of treated Vγ1+ and Vγ4+ cells was determined by ELISA, (n=6)
Vγ1+ γδ T cells’ enhancing activity is increased but suppressive activity is decreased after exposure to anti-CD3 MAb
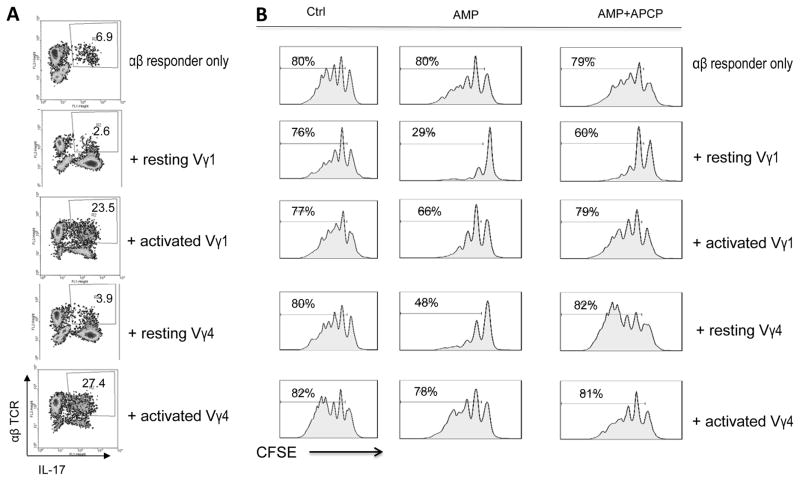
To determine whether activation can convert the enhancing and suppressive effect of both Vγ1+ and Vγ4+ subsets, Vγ1+ and Vγ4+ T cells were assessed for their enhancing and inhibiting effects before or after exposure to anti-mouse CD3 mAb (34, 42). The enhancing effect on Th17 response was assessed by testing the response of in vivo primed αβ T cells stimulated with the immunizing antigen and APCs under Th17 polarized conditions (culture medium containing IL-23) and in the absence or presence of added γδ T cells (Fig. 7A). The enhancing effect was significantly augmented when Vγ1+ or Vγ4+ cells were added after anti-CD3 stimulation. As expected, the enhancing effect was minimal when non-activated cells were added to responder T cells.
FIGURE 7. Both Vγ1+ and Vγ4+ γδ T cells possessed an enhancing effect on autoimmune response in their activated state and suppressive effect in their non-activated state.
(A) Enhancing activity of activated Vγ1+ and Vγ4+ γδ T cells. Responder T cells were isolated from TCR-δ−/− mice 13 days post immunization. They were stimulated with the immunizing IRBP1–20 and APCs, in the absence or presence of added (2%) Vγ1+ and Vγ4+cells, with or without a prior exposure to anti-CD3 MAb. The activated T cells were then separated by Ficoll centrifugation and stained with ant-αβTCR and anti-IL-17 antibodies followed by FACS analysis. Results of gated αβ T cells were shown. A representative study of five separate experiments.
(B) Non-activated Vγ1+ and Vγ4+ γδ T cells are both suppressive. Responder T cells were isolated from TCR-δ−/− mice 13 days post immunization. They were labeled with CFSE and stimulated with the immunizing IRBP1–20 and APCs, in the absence or presence of added (2%) Vγ1+ or Vγ4+ cells. Activated Vγ1+ or Vγ4+ cells were prepared by exposure to anti-CD3 antibody. The test was conducted in the absence of additions (left panels), in the presence of AMP (middle panels), and in the presence of AMP and APCP, the CD73 inhibitor (right panels). FACS analysis was conducted 7 days after in vitro stimulation. The demonstrated results were from a single experiment representative of five independent studies.
We previously reported that the inhibitory effect of γδ T cells was better demonstrated if exogenous AMP was provided because non-activated γδ T cell expresses higher amounts of CD73, which more effectively degrades AMP to adenosine (42, 45, 46). Activated γδ T cells, on the other hand, expressed decreased levels of CD73, which weakened their ability to convert AMP to adenosine (42). Inhibition test used a CFSE assay, in which the responder αβ T cells were pre-labeled with CFSE before stimulation with the immunizing peptide and APCs (Fig. 7B). Unlabeled Vγ1+ and Vγ4+ γδ T cells, with or without prior anti-CD3 mAb stimulation, were added to responder cells at a ratio of γδ : αβ=1:20. The results, assessed 7 days after in vitro stimulation, showed that in the presence of AMP, both Vγ1+ and Vγ4+ resting cells showed a suppressive effect, whereas both Vγ1+ and Vγ4+ activated cells showed a significantly diminished suppressive activity. Importantly, the suppressive effect was partially neutralized by the CD73 inhibitor, the adenosine 5′-(alpha,beta-methylene)diphosphate (APCP) (Fig. 7B) (47, 48).
Discussion
Although a regulatory effect of γδ T cells on adaptive immunity has been repeatedly observed (22, 26–28), knowledge of how these cells regulate remains very limited, and the mechanisms by which they enhance an immune response in some cases (49–51) but inhibit it in others (16–18, 52) remain largely obscure. A better understanding of the mechanisms by which γδ T cells regulate immune response should facilitate the development of γδ T cell-related therapeutic approaches. In a number of induced autoimmune diseases, including EAU (9, 11, 30, 53), a prevailing activation of Vγ4+ cells was observed; however, the mechanism of such a biased γδ activation to disease pathogenesis remains unclear. The current study is aimed at determining the mechanism that causes the biased γδ T cell activation and determining the factors that convert the enhancing and inhibiting effect of γδ T cells. Previous studies have shown that the functional diversity of γδ T cell subsets is correlated to distinct TCRs they express (22, 54–56). Later studies also demonstrated that the enhancing and inhibiting activity of γδ T cells could be converted by a pre-exposure of these cells to bacterial products (23, 25). Furthermore, studies by our laboratory have demonstrated that activated γδ T cells possessed a greatly increased ability to enhance autoimmune response (34, 35, 43). Mice deficient of γδ T cells (TCR-δ−/−) demonstrated milder Th17 response when induced for EAU as compared to wt-B6 mice (30, 34), and administration of γδ T cells to TCR-δ−/− mice before immunization greatly enhanced the disease susceptibility associated with an augmented Th17 response (34, 35, 43). In vitro study also showed that the addition of a small number (2%) of γδ T cells to responder αβ T cells greatly enhanced their Th17 responses in vitro (30, 35, 41, 57).
Development of induced EAU in the EAU-prone B6 mouse is associated with an increased activation and dominance of Vγ4+ γδ T cells (30). The contribution of these biased γδ T cells to disease development remained unclear. In this study, we show that in the pre-clinical phases of EAU, a vigorous activation and expansion of Vγ4+ cells is caused by cytokines produced by activated myeloid cells. The Vγ1+ γδ T cells are the poor responder cells of cytokines in this inflammatory environment. As a result, Vγ4+ cells are activated and become the dominant γδ subset. Our results demonstrated that this dominance shift of γδ subsets is due to the preferred activation requirements of the different γδ subsets. We conclude that the appearance of a larger number of activated γδ T cells, rather that the γδ subset expressing a specific TCR segment, or the Vγ4+ subset, is the major factor leading to disease progression. This assumption is supported by the evidence that Vγ1+ cells also gained enhancing activity after being activated.
We previously observed that administration of γδ-specific antibody removed γδ T cells more effectively in naïve mice, whereas the γδ T cells of immunized mice were more resistant to the depletion, because the activated γδ T cells express decreased numbers of surface expressed TCR which allowed them to escape removal by the injected antibodies (31, 57). Injection of γδ-specific antibody may remove the non- or less activated γδ T cell population more easily than activated cells. The outcome of this treatment will weaken the suppressive effect, shifting the balance from suppression toward enhancement. Our results showing that the γδ T subsets differed in activation requirements suggested that both Vγ1+ and Vγ4+ γδ T cell subsets can either enhance or inhibit, depending on the disease-associated microenvironment that determines γδ T cell activation. Such a prediction is supported by observations that Vγ1+ γδ T cells are dominantly activated in many different infectious disease models (58–63).
We found that cytokines (IL-1, IL-23, IL-7) that are stimulatory to Vγ4+ γδ T cells were mainly produced by myeloid rather than by T cells (data not shown). Indeed, cytokines in LPS-stimulated BMDC supernatants showed a strong stimulatory effect, indicating that DC activation and release of cytokines play a major role in γδ activation in the early stage of induced autoimmune disease.
In studies clarifying the mechanism by which activated γδ T cells gain increased enhancing activity, we have made efforts to identify molecules that contribute to such a functional switch. We were able to show that activated γδ T cells express altered levels of IL-23R (35), giving γδ T cells a competitive ability to bind IL-23, which would abate subsequently initiated Th17 αβ T cell responses that require IL-23 (35). In recent studies (41, 42), we also demonstrated that activated γδ T cells express low levels of CD73 molecules, an ecto-enzyme that converts proinflammatory extracellular ATP to adenosine, which is suppressive for adaptive responses (64, 65). Expression of low levels of CD73 causes activated γδ T cells to convert less adenosine from extracellular ATP, which would predispose to stronger T cell responses. Such a hypothesis has been supported by the evidence that the suppressive effect of γδ T cells is readily amplified if exogenous AMP, a precursor molecule of adenosine, is provided; and the suppressive effect is abolished in the presence of a CD73 inhibitor (41, 42), indicating expression of different amounts of CD73 allows γδ T cells to modulate their regulatory effect. Results in the current study further confirmed this hypothesis by showing that the Vγ1+ cells in EAU-induced mice retained the ability to express higher amounts of CD73; thus, the inhibiting effect prevailed in the Vγ1+ cells. Nevertheless, the enhancing activity prevailed when the function of the entire γδ T cells is assessed, because the Vγ1+ cells in immunized mice were greatly outnumbered by activated Vγ4+ T cells. The fact that anti-CD3 mAB-activated Vγ1+ γδ T cells showed an increased enhancing effect but a decreased suppressive activity appeared to support the notion that activation status is an important factor balancing the enhancing and suppressive effects in the two γδ T cell subsets.
In summary, our previous results demonstrated that activation switches the inhibitory and enhancing effect of γδ T cell (34, 35, 42, 43). In the current study, we further demonstrate that the activation-induced functional change is not restricted to Vγ4+ cells, but also applies to Vγ1+ cells. An abundance of activated Vγ4+ γδ T cells shifts the enhancing and suppressing activities towards the former, leading to enhanced disease susceptibility.
Acknowledgments
We thank Susan Clarke for the editorial assistance of the manuscript.
This work was supported by NIH grants EY 0022403 and EY018827 and by Research to Prevent Blindness, Inc., New York, New York.
Abbreviations
- EAU
experimental autoimmune uveitis
- DC
dendritic cell
- IRBP
interphotoreceptor retinoid-binding protein
- TCR
T cell receptor
- BMDC
bone marrow dendritic cells
References
- 1.Mak TW, Ferrick DA. The γδ T-cell bridge - Linking innate and acquired immunity. Nat Med. 1998;4:764–765. doi: 10.1038/nm0798-764. [DOI] [PubMed] [Google Scholar]
- 2.Chien Y-h, Meyer C, Bonneville M. γδ T Cells: First line of defense and beyond. Ann Rev Immunol. 2014;32:121–155. doi: 10.1146/annurev-immunol-032713-120216. [DOI] [PubMed] [Google Scholar]
- 3.Selmaj K, Raine CS, Farooq M, Norton WT, Brosnan CF. Cytokine cytotoxicity against oligodendrocytes: Apoptosis induced by lymphotoxin. J Immunol. 1991;147:1522–1529. [PubMed] [Google Scholar]
- 4.Wucherpfennig KW, Newcombe J, Li H, Keddy C, Cuzner ML, Hafler DA. γδ T-cell receptor repertoire in acute multiple sclerosis lesions. Proc Natl Acad Sci USA. 1992;89:4588–4592. doi: 10.1073/pnas.89.10.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimonkevitz R, Colburn C, Burnham JA, Murray RS, Kotzin BL. Clonal expansions of activated γ/δ T cells in recent- onset multiple sclerosis. Proc Natl Acad Sci U S A. 1993;90:923–927. doi: 10.1073/pnas.90.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng SL, Madaio MP, Hughes DPM, Crispe IN, Owen MJ, Wen L, Hayday AC, Craft J. Murine lupus in the absence of αβ T cells. J Immunol. 1996;156:4041–4049. [PubMed] [Google Scholar]
- 7.Bucht A, Söderström K, Hultman T, Uhlén M, Nilsson E, Kiessling R, Grönberg A. T cell receptor diversity and activation markers in the Vδ1 subset of rheumatoid synovial fluid and peripheral blood T lymphocytes. Eur J Immunol. 1992;22:567–574. doi: 10.1002/eji.1830220240. [DOI] [PubMed] [Google Scholar]
- 8.Peterman GM, Spencer C, Sperling AI, Bluestone JA. Role of γδ T cells in murine collagen-induced arthritis. J Immunol. 1993;151:6546–6558. [PubMed] [Google Scholar]
- 9.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KHG. Interleukin-1 and IL-23 induce innate IL-17 production from gd T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Mukasa A, Lahn M, Pflum EK, Born W, O’Brien RL. Evidence that the same γδ T cells respond during infection-induced and autoimmune inflammation. J Immunol. 1997;159:5787–5794. [PubMed] [Google Scholar]
- 11.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O’Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing γδ T cells. J Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moser B, Eberl M. γδ T cells: novel initiators of adaptive immunity. Immunol Rev. 2007;215:89–102. doi: 10.1111/j.1600-065X.2006.00472.x. [DOI] [PubMed] [Google Scholar]
- 13.Carding SR, Egan PJ. The importance of γδ T cells in the resolution of pathogen-induced inflammatory immune responses. Immunol Rev. 2000;173:98–108. doi: 10.1034/j.1600-065x.2000.917302.x. [DOI] [PubMed] [Google Scholar]
- 14.Nanno M, Kanari Y, Naito T, Inoue N, Hisamatsu T, Chinen H, Sugimoto K, Shimomura Y, Yamagishi H, Shiohara T, Ueha S, Matsushima K, Suematsu M, Mizoguchi A, Hibi T, Bhan AK, Ishikawa H. Exacerbating role of γδ T cells in chronic colitis of T-cell receptor α mutant mice. Gastroenterology. 2008;134:481–490. doi: 10.1053/j.gastro.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 15.Ponomarev ED, Dittel BN. γδ T cells regulate the extent and duration of inflammation in the central nervous system by a fas ligand-dependent mechanism. J Immunol. 2005;174:4678–4687. doi: 10.4049/jimmunol.174.8.4678. [DOI] [PubMed] [Google Scholar]
- 16.Tsuchiya T, Fukuda S, Hamada H, Nakamura A, Kohama Y, Ishikawa H, Tsujikawa K, Yamamoto H. Role of γδ T cells in the inflammatory response of experimental colitis mice. J Immunol. 2003;171:5507–5513. doi: 10.4049/jimmunol.171.10.5507. [DOI] [PubMed] [Google Scholar]
- 17.Uezu K, Kawakami K, Miyagi K, Kinjo Y, Kinjo T, Ishikawa H, Saito A. Accumulation of γδ T cells in the lungs and their regulatory roles in Th1 response and host defense against pulmonary infection with Cryptococcus neoformans. J Immunol. 2004;172:7629–7634. doi: 10.4049/jimmunol.172.12.7629. [DOI] [PubMed] [Google Scholar]
- 18.D’Souza CD, Cooper AM, Frank AA, Mazzaccaro RJ, Bloom BR, Orme IM. An anti-inflammatory role for γδ T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217–1221. [PubMed] [Google Scholar]
- 19.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial γδ T cells. Proc Natl Acad Sci U S A. 2002;99:14338–14343. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inagaki-Ohara K, Chinen T, Matsuzaki G, Sasaki A, Sakamoto Y, Hiromatsu K, Nakamura-Uchiyama F, Nawa Y, Yoshimura A. Mucosal T Cells bearing TCRγδ play a protective role in intestinal inflammation. J Immunol. 2004;173:1390–1398. doi: 10.4049/jimmunol.173.2.1390. [DOI] [PubMed] [Google Scholar]
- 21.Dalton JE, Pearson J, Scott P, Carding SR. The interaction of γδT cells with activated macrophages is a property of the Vγ1 subset. J Immunol. 2003;171:6488–6494. doi: 10.4049/jimmunol.171.12.6488. [DOI] [PubMed] [Google Scholar]
- 22.Huber SA, Graveline D, Newell MK, Born WK, O’Brien RL. Vγ1+ T cells suppress and Vγ4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J Immunol. 2000;165:4174–4181. doi: 10.4049/jimmunol.165.8.4174. [DOI] [PubMed] [Google Scholar]
- 23.Spencer CT, Abate G, Blazevic A, Hoft DF. Only a subset of phosphoantigen-responsive γ9δ2 T cells mediate protective tuberculosis immunity. J Immunol. 2008;181:4471–4484. doi: 10.4049/jimmunol.181.7.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Heyde HC, Elloso MM, Chang WL, Pepper BJ, Batchelder J, Weidanz WP. Expansion of the γδ T cell subset in vivo during bloodstage malaria in B cell-deficient mice. J Leukoc Biol. 1996;60:221–229. doi: 10.1002/jlb.60.2.221. [DOI] [PubMed] [Google Scholar]
- 25.Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-infiltrating γδ T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27:334–348. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Kabelitz D, Wesch D, He W. Perspectives of γδ T cells in tumor immunology. Cancer Res. 2007;67:5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- 27.Girardi M. Immunosurveillance and immunoregulation by γδ T cells. J Invest Dermatol. 2006;126:25–31. doi: 10.1038/sj.jid.5700003. [DOI] [PubMed] [Google Scholar]
- 28.Poccia F, Agrati C, Martini F, Mejia G, Wallace M, Malkovsky M. Vγ9Vδ2 T cell-mediated non-cytolytic antiviral mechanisms and their potential for cell-based therapy. Immunol Lett. 2005;100:14–20. doi: 10.1016/j.imlet.2005.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayday AC. γδ Cells: a right time and a right place for a conserved third way of protection. Ann Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 30.Cui Y, Shao H, Lan C, Nian H, O’Brien RL, Born WK, Kaplan HJ, Sun D. Major role of γδ T cells in the generation of IL-17+ uveitogenic T cells. J Immunol. 2009;183:560–567. doi: 10.4049/jimmunol.0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng L, Cui Y, Shao H, Han G, Zhu L, Huang Y, O’Brien RL, Born WK, Kaplan HJ, Sun D. Mouse γδ T cells are capable of expressing MHC class II molecules, and of functioning as antigen-presenting cells. J Neuroimmunol. 2008;203:3–11. doi: 10.1016/j.jneuroim.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hahn YS, Taube C, Jin N, Takeda K, Park JW, Wands JM, Aydintug MK, Roark CL, Lahn M, O’Brien RL, Gelfand EW, Born WK. Vγ4+ γδ T cells regulate airway hyperreactivity to methacholine in ovalbumin-sensitized and challenged mice. J Immunol. 2003;171:3170–3178. doi: 10.4049/jimmunol.171.6.3170. [DOI] [PubMed] [Google Scholar]
- 33.Lahn M, Kanehiro A, Takeda K, Terry J, Hahn YS, Aydintug MK, Konowal A, Ikuta K, O’Brien RL, Gelfand EW, Born WK. MHC class I-dependent Vγ4+ pulmonary T cells regulate αβ T cell-independent airway responsiveness. Proc Natl Acad Sci U S A. 2002;99:8850–8855. doi: 10.1073/pnas.132519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nian H, Shao H, O’Brien BA, Born WK, KHJ, Sun D. Activated γδ cells promote the activation of uveitogenic T cells and exacerbate EAU development. Invest Ophthalmol Vis Sci. 2011;52:5920–5927. doi: 10.1167/iovs.10-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang D, Zuo A, Shao H, Born WK, O’Brien RL, Kaplan HJ, Sun D. IL-23 Receptor expression on γδ T cells correlates with their enhancing or suppressive effects on autoreactive T cells in experimental autoimmune uveitis. J Immunol. 2013;191:1118–1125. doi: 10.4049/jimmunol.1300626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang D, Zuo A, Shao H, Born WK, O’Brien RL, Kaplan HJ, Sun D. Role of CD25+ dendritic cells in the generation of Th17 autoreactive T cells in autoimmune experimental uveitis. J Immunol. 2012;188:5785–5791. doi: 10.4049/jimmunol.1200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng Y, Han G, Shao H, Wang Y, Kaplan HJ, Sun D. Characterization of IL-17+ interphotoreceptor retinoid-binding protein-specific T cells in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2007;48:4153–4161. doi: 10.1167/iovs.07-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen M, Liang D, Zuo A, Shao H, Kaplan HJ, Sun D. An A2B adenosine receptor agonist promotes Th17 autoimmune responses in experimental autoimmune uveitis (EAU) via dendritic cell activation. PLoS One. 2015;10:e0132348. doi: 10.1371/journal.pone.0132348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 41.Liang D, Zuo A, Shao H, Chen M, Kaplan HJ, Sun D. Roles of the adenosine receptor and CD73 in the regulatory effect of γδ T cells. PLoS One. 2014;9:e108932. doi: 10.1371/journal.pone.0108932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang D, Zuo A, Zhao R, Shao H, Born WK, O’Brien RL, Kaplan HJ, Sun D. CD73 expressed on γδ T cells shapes their regulatory effect in experimental autoimmune uveitis. PLoS One. 2016;11:e0150078. doi: 10.1371/journal.pone.0150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang D, Zuo A, Shao H, Born WK, O’Brien RL, Kaplan HJ, Sun D. Retinoic Acid inhibits CD25+ dendritic cell expansion and γδ T-cell activation in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2013;54:3493–3503. doi: 10.1167/iovs.12-11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang D, Zuo A, Shao H, Chen M, Kaplan HJ, Sun D. A2B adenosine receptor activation switches differentiation of bone marrow cells to a CD11c+Gr-1+ dendritic cell subset that promotes the Th17 response. Immun, Inflamm Dis. 2015;3:360–373. doi: 10.1002/iid3.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antonioli L, Pacher P, Vizi ES, Haskó G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19:355–367. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beavis PA, Stagg J, Darcy PK, Smyth MJ. CD73: a potent suppressor of antitumor immune responses. Trends Immunol. 2012;33:231–237. doi: 10.1016/j.it.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Forte G, Sorrentino R, Montinaro A, Luciano A, Adcock IM, Maiolino P, Arra C, Cicala C, Pinto A, Morello S. Inhibition of CD73 improves B cell-mediated anti-tumor immunity in a mouse model of melanoma. J Immunol. 2012;189:2226–2233. doi: 10.4049/jimmunol.1200744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romio M, Reinbeck B, Bongardt S, Hüls S, Burghoff S, Schrader J. Extracellular purine metabolism and signaling of CD73-derived adenosine in murine Treg and Teff cells. Am J Physiol. 2011;301:C530–C539. doi: 10.1152/ajpcell.00385.2010. [DOI] [PubMed] [Google Scholar]
- 49.Rajan AJ, V, Asensio C, Campbell IL, Brosnan CF. Experimental autoimmune encephalomyelitis on the SJL mouse: effect of γδ T cell depletion on chemokine and chemokine receptor expression in the central nervous system. J Immunol. 2000;164:2120–2130. doi: 10.4049/jimmunol.164.4.2120. [DOI] [PubMed] [Google Scholar]
- 50.Spahn TW, Issazadah S, Salvin AJ, Weiner HL. Decreased severity of myelin oligodendrocyte glycoprotein peptide 33–35-induced experimental autoimmune encephalomyelitis in mice with a disrupted TCR δ chain gene. Eur J Immunol. 1999;29:4060–4071. doi: 10.1002/(SICI)1521-4141(199912)29:12<4060::AID-IMMU4060>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 51.Tagawa T, Nishimura H, Yajima T, Hara H, Kishihara K, Matsuzaki G, Yoshino I, Maehara Y, Yoshikai Y. Vδ1+ γδ T cells producing CC chemokines may bridge a gap between neutrophils and macrophages in innate immunity during Escherichia coli infection in mice. J Immunol. 2004;173:5156–5164. doi: 10.4049/jimmunol.173.8.5156. [DOI] [PubMed] [Google Scholar]
- 52.Born W, Cady C, Jones-Carson J, Mukasa A, Lahn M, O’Brien R. Immunoregulatory functions of γδ T cells. Adv Immunol. 1999;71:77–144. [PubMed] [Google Scholar]
- 53.Blink SE, Caldis MW, Goings GE, Harp CT, Malissen B, Prinz I, Xu D, Miller SD. γδ T cell subsets play opposing roles in regulating experimental autoimmune encephalomyelitis. Cell Immunol. 2014;290:39–51. doi: 10.1016/j.cellimm.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dalton JE, Pearson J, Scott P, Carding SR. The interaction of γδ T cells with activated macrophages is a property of the Vγ1 subset. J Immunol. 2003;171:6488–6494. doi: 10.4049/jimmunol.171.12.6488. [DOI] [PubMed] [Google Scholar]
- 55.Hahn YS, Taube C, Jin N, Sharp L, Wands JM, Aydintug MK, Lahn M, Huber SA, O’Brien RL, Gelfand EW, Born WK. Different potentials of γδ T cell subsets in regulating airway responsiveness: Vγ1+ cells, but not Vγ4+ cells, promote airway hyperreactivity, Th2 cytokines, and airway inflammation. J Immunol. 2004;172:2894–2902. doi: 10.4049/jimmunol.172.5.2894. [DOI] [PubMed] [Google Scholar]
- 56.O’Brien RL, Yin X, Huber SA, Ikuta K, Born WK. Depletion of a γδ T cell subset can increase host resistance to a bacterial infection. J Immunol. 2000;165:6472–6479. doi: 10.4049/jimmunol.165.11.6472. [DOI] [PubMed] [Google Scholar]
- 57.Nian H, Shao H, Zhang G, Born WK, O’Brien R, Kaplan HJ, Sun D. Regulatory effect of γδ T cells on IL-17+ uveitogenic T cells. Invest Ophthalmol Vis Sci. 2010;51:4661–4667. doi: 10.1167/iovs.09-5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dieli F, Ivanyi J, Marsh P, Williams A, Naylor I, Sireci G, Caccamo N, Di Sano C, Salerno A. Characterization of lung γδ T cells following intranasal infection with Mycobacterium bovis bacillus Calmette-Guerin. J Immunol. 2003;170:463–469. doi: 10.4049/jimmunol.170.1.463. [DOI] [PubMed] [Google Scholar]
- 59.Belles C, Kuhl AK, Donoghue AJ, Sano Y, O’Brien RL, Born W, Bottomly K, Carding SR. Bias in the γδ T cell response to Listeria monocytogenes. V delta 6.3+ cells are a major component of the gamma delta T cell response to Listeria monocytogenes. J Immunol. 1996;156:4280–4289. [PubMed] [Google Scholar]
- 60.Naiki Y, Nishimura H, Itohara S, Yoshikai Y. γδ T cells may dichotomously modulate infection with avirulent Salmonella choleraesuis via IFN-γ and IL-13 in mice. Cell Immunol. 2000;202:61–69. doi: 10.1006/cimm.2000.1659. [DOI] [PubMed] [Google Scholar]
- 61.Ninomiya T, Takimoto H, Matsuzaki G, Hamano S, Yoshida H, Yoshikai Y, Kimura G, Nomoto K. Vγ1+γδ T cells play protective roles at an early phase of murine cytomegalovirus infection through production of interferon-γ. Immunology. 2000;99:187–194. doi: 10.1046/j.1365-2567.2000.00938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huber SA, Moraska A, Choate M. T cells expressing the γδ T-cell receptor potentiate coxsackievirus B3-induced myocarditis. J Virol. 1992;66:6541–6546. doi: 10.1128/jvi.66.11.6541-6546.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hou S, Katz JM, Doherty PC, Carding SR. Extent of γδ T cell involvement in the pneumonia caused by Sendai virus. Cell Immunol. 1992;143:183–193. doi: 10.1016/0008-8749(92)90015-h. [DOI] [PubMed] [Google Scholar]
- 64.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 65.Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]