Abstract
Background
Posttranslational histone modifications (PTHMs) are altered by arsenic, an environmental carcinogen. PTHMs are also influenced by nutritional methyl donors involved in one-carbon metabolism (OCM), which may protect against epigenetic dysregulation.
Methods
We measured global levels of three PTHMs, which are dysregulated in cancers (H3K36me2, H3K36me3, H3K79me2), in peripheral blood mononuclear cells (PBMCs) from 324 participants enrolled in the Folic Acid and Creatine Trial, a randomized trial in arsenic-exposed Bangladeshi adults. Sex-specific associations between several blood OCM indices (folate, vitamin B12, choline, betaine, homocysteine) and PTHMs were examined at baseline using regression models, adjusted for multiple tests by controlling for the false discovery rate (PFDR). We also evaluated the effects of FA supplementation (400 µg/day for 12 weeks), compared with placebo, on PTHMs.
Results
Associations between choline and H3K36me2 and between vitamin B12 and H3K79me2, differed significantly by sex (Pdiff < 0.01 and < 0.05, respectively). Among men, plasma choline was positively associated with H3K36me2 (PFDR < 0.05), and among women, plasma vitamin B12 was positively associated with H3K79me2 (PFDR < 0.01). FA supplementation did not alter any of the PTHMs examined (PFDR = 0.80).
Conclusion
OCM indices may influence PTHMs in a sex-dependent manner, and FA supplementation, at this dose and duration, does not alter PTHMs in PBMCs.
Impact
This is the first study to examine the influences of OCM indices on PTHMs in a population that may have increased susceptibility to cancer development due to widespread exposure to arsenic-contaminated drinking water and a high prevalence of hyperhomocysteinemia.
Keywords: one-carbon metabolism, histone modifications, arsenic
Introduction
DNA methyltransferases and lysine histone methyltransferases depend on methyl donations from S-adenosylmethionine (SAM) [1]. Synthesis of SAM via one-carbon metabolism (OCM) involves the remethylation of homocysteine (Hcys) to methionine, which requires nutritional methyl donors and cofactors, such as folate, vitamin B12, choline, and betaine. There are important sex differences in the OCM pathway. For example, plasma Hcys concentrations are higher in men, and there is evidence that this may be due to both higher methyl demand for creatine synthesis as a result of greater muscle mass [2] and also lower remethylation and transmethylation rates among men [3]. Circulating concentrations of folate, vitamin B12, and choline also differ by sex [4, 5].
Hyperhomocysteinemia (HHcys) and insufficient intake of nutritional methyl donors have been implicated in the development of human cancers [6]. This may be mediated by alterations in epigenetic modifications [7, 8], including PTHMs [9, 10]. Nutritional methyl donors have also been shown to modify, or buffer against, epigenetic dysregulation caused by environmental toxicants. For example, mice exposed in utero to the endocrine disruptor bisphenol A have reduced levels of DNA methylation in several tissues, but this can be prevented with maternal supplementation with folic acid (FA), vitamin B12, choline, and betaine [11]. Similarly, Bangladeshi adults exposed chronically to arsenic, a human carcinogen, have higher global levels of DNA methylation in leukocytes, but only in those that are folate sufficient (plasma folate > 9 nmol/L), which may be a protective compensatory mechanism [12]. Concurrent exposure to arsenic and a methyl deficient diet also alters global DNA methylation in the mouse liver in a sex-dependent manner [13]. We, and others, have previously observed sex-specific effects of arsenic exposure on global levels of DNA methylation and PTHMs in human populations [14–17]. However, the relationships between OCM indices and PTHMs, and potential differences by sex, have not been investigated. We therefore examined this in a population in Bangladesh that may have increased susceptibility to cancer development due to a high prevalence of HHcys and widespread exposure to arsenic-contaminated drinking water. We also examined the effect of folic acid (FA) supplementation (400 µg/day for 12 weeks) on PTHMs. We selected three PTHMs (histone H3 lysine 36 di- and tri-methylation (H3K36me2 and H3K36me3, respectively), and histone H3 lysine 79 di-methylation (H3K79me2)), which are dysregulated in several types of cancer [18–23] and are altered by arsenic and/or nutritional methyl donors in experimental models [24–27]. PTHMs were measured in peripheral blood mononuclear cells (PBMCs) collected from participants in the Folic Acid and Creatine Trial (FACT). FACT is a randomized clinical trial that was originally designed to examine whether FA and/or creatine supplementation can be used as therapeutic approaches to reduce blood arsenic concentrations; the primary findings of this trial have been published [28].
Materials and methods
Region and Participants
Participants for the FACT study were recruited from the Health Effects of Arsenic Longitudinal Study, a prospective cohort that initially recruited 11,746 adults living in a 25 km2 region in Araihazar, Bangladesh [29]. FACT is a double-blind randomized, placebo-controlled trial [28]. FACT participants were between the ages of 20 and 65 and had been drinking from household wells with water arsenic ≥ 50 µg/L, the Bangladesh standard for safe drinking water. Exclusion criteria included: pregnancy, nutritional supplement use, and known health problems, including cancers. Informed consent was obtained by Bangladeshi field staff physicians, and this study was approved by the Institutional Review Board of Columbia University Medical Center and the Bangladesh Medical Research Council.
Study Design
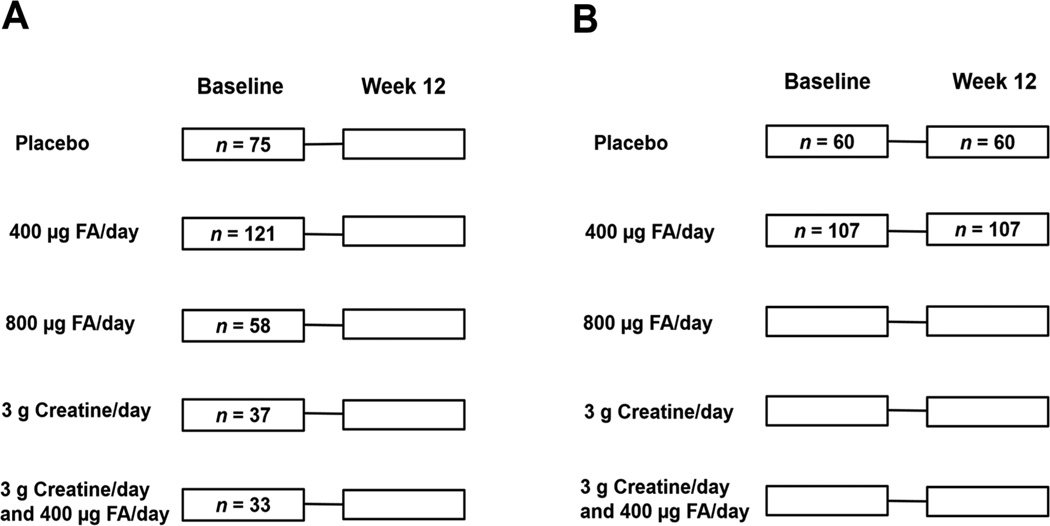
As described previously [28], FACT participants (n = 622) were randomized to one of five treatment arms: placebo (n = 104), 400 µg FA/day (n = 156), 800 µg FA/day (n = 154), 3 g creatine/day (n = 104), and 3 g creatine + 400 µg FA/day (n = 104) (Figure 1). Due to ethical considerations, all participants received arsenic-removal water filters (READ-F filter, Brota Services International, Bangladesh) at baseline.
Figure 1. FACT Design and Sampling for Current Study.
A) Participants selected for cross-sectional analyses. Baseline-collected samples from 324 FACT participants were included in cross-sectional analyses for the current study. All participants from the placebo and 400 µg FA/day treatment arms who had complete information for predictors (one-carbon metabolism indices), PTHMs, and potential confounders were included in these analyses (n = 75 for placebo group, n = 121 for 400 µg FA/day group); an additional 128 participants with complete information for all relevant variables were randomly selected from the remaining three treatment arms. The distribution of these participants are shown by treatment arm. B) Participants selected for examination of FA treatment effects. Participants with at least one PTHM measure at both time points of interest (i.e., baseline and week 12) were included in analyses examining the effect of 400 µg FA/day (n = 107) vs placebo (n = 60) on PTHMs.
Whole blood samples were collected from participants at baseline, week 12, and week 24; sample collection and handling have been described previously [14, 28]. For the current study, we used histones isolated from baseline-collected PBMCs. We selected a subset of participants who had necessary biological samples and complete data for relevant covariates; specifically, we included all participants in the placebo (n = 75) and 400 µg FA group (n = 121) and an additional 128 participants, who were randomly selected from the other three treatment arms (total n = 324). We also used available PBMCs collected at week 12 from participants in the placebo (n = 60) and 400 µg FA (n = 107) treatment arms (Figure 1). We evaluated FA treatment effects from baseline to week 12, rather than from baseline to week 24, because 1) we had a larger number of PBMC samples available at week 12 and 2) half of the participants in each FA treatment arm were switched to placebo at week 12, so our sample size and statistical power for week 24 analyses would have been further reduced. The 400 µg FA dose was selected based on the U.S. recommended dietary allowance for adults [30].
Folate and Vitamin B12
Plasma folate and vitamin B12 were measured by radio-protein-binding assay (SimulTRAC-SNB, MP Biomedicals). The intra- and inter-assay CVs were 5% and 13%, respectively, for folate and 6% and 17%, respectively, for vitamin B12. Folate in whole blood hemolysate was also measured by radio-protein-binding assay (SimulTRAC-S, MP Biomedicals) in participants from the placebo, 400 µg FA/day, and 800 µg FA/day treatment arms, as described previously [28]; red blood cell (RBC) folate was calculated by dividing these measures by [%hematocrit/100]. The intra- and inter-assay CVs for RBC folate were 4% and 9%, respectively.
Plasma Choline and Betaine
Plasma choline and betaine concentrations were measured by LC-MS/MS, using the method of Holm et al. [31], with some modifications, as described previously [32]. The intra- and inter-assay CVs for plasma choline were 2.2% and 5.8%, respectively, and were 2.5% and 5.6%, respectively, for plasma betaine.
Plasma Homocysteine
Plasma total homocysteine (Hcys) was measured by HPLC with fluorescence detection, based on a method described by Pfeiffer et al. [33]. The intra- and inter-assay CVs were 5% and 7%, respectively.
Blood Arsenic and Selenium
Total blood arsenic and selenium (bSe) concentrations are routinely measured simultaneously, using a Perkin-Elmer Elan DRC II ICP-MS equipped with an AS10+ autosampler based on a previously described method [34]. The intra- and inter-assay CVs for arsenic were 2.7% and 5.7%, respectively, and were 1.5% and 4.6%, respectively, for bSe.
Urinary Creatinine
Urinary creatinine (uCr) was measured by a method based on the Jaffe reaction [35], and the intra- and inter-assay CVs were 1.3% and 2.9%, respectively
Histone Isolation
Histones were isolated from PBMCs by acid extraction, as described previously [14, 15]. Isolated histones were diluted in 4 M urea, and aliquots were stored at −80°C.
H3K36me2, H3K36me3, H3K79me2
Although we previously identified a specific cleavage product of histone H3, which interferes with the measurement of downstream PTHMs, H3K36me2, H3K36me3, and H3K79me2 are not impacted by H3 cleavage [36]. These PTHMs were measured by sandwich ELISA, as described previously [15]. Samples were run in duplicate. The intra- and inter-assay CVs, respectively, for each ELISA method were as follows: H3K36me2: 3.4% and 9.6%, H3K36me3: 4.9% and 11.9%, and H3K79me2: 7.1% and 7.0%. Since there were limited histone aliquots for the final assays, and since samples with poor reproducibility (intra-assay CV > 15%) were excluded, final sample sizes for H3K36me2 (n = 318) and H3K36me3 (n = 306) were smaller than the final sample size for H3K79me2 (n = 321).
Statistical methods
Differences in continuous and categorical variables between men and women and between participants with and without PTHM measures, or with and without RBC folate measures, were assessed using Wilcoxon rank-sum and Chi-square tests, respectively. Transformations were applied to variables with skewed distributions to stabilize variances for parametric model assumptions and to reduce the influence of extreme values. Natural log-transformations were applied to H3K36me3, H3K79me2, bSe, RBC folate, and plasma folate, choline, betaine, vitamin B12, and Hcys. An inverse transformation (1/×) was applied to H3K36me2.
Due to the distribution of H3K36me2, a generalized linear model with an inverse-link function (which effectively back-transforms the regression coefficient) was used to examine associations between the ln-transformed OCM indices and the harmonic mean of H3K36me2. Linear models were used to examine associations between ln-transformed OCM indices and ln-H3K36me3 and ln-H3K79me2. Plasma folate, choline, betaine, vitamin B12, and Hcys were included simultaneously in models. Alternative models replacing plasma folate with RBC folate were applied to the subset of participants with RBC folate measures (n = 250). Models were run separately by sex. The Wald test was used to detect differences by sex [37]. Models were additionally adjusted for hypothesized confounders of the relationships between OCM indices and PTHMs, and any variables that were associated with PTHMs in bivariate analyses: age, education, TV ownership (an indicator of socioeconomic status in this population), ln-bSe, and cigarette smoking (for analyses of H3K36me3 and H3K79me2 in men) were included as covariates in final models; bSe was considered as a potential confounder, because selenium is known to influence the OCM pathway [38] and has also been shown to alter epigenetic marks, including PTHMs [39]. Models did not adjust for alcohol consumption, because alcohol use is very rare in Bangladesh [40]. All covariates were included as continuous variables, except for TV ownership, cigarette smoking status, and education; the latter was included as a binary variable (education > 5 years vs. ≤ 5 years) because many participants had few years of education. In sensitivity analyses, we also examined models that 1) were additionally adjusted for ln-BMI and ln-transformed arsenic measures, 2) evaluated OCM indices individually, and 3) were stratified by median age or blood arsenic concentration. Since we present multiple tests examining associations between OCM indices and PTHMs by sex, p-values were adjusted for multiple tests by controlling for the false discovery rate (PFDR).
The difference in the within-person change for each PTHM between the two treatment arms (400 µg FA vs. placebo) was examined using the Wilcoxon rank-sum test. In exploratory analyses, we also investigated potential differences in the effects of FA on PTHMs separately by sex. In secondary analyses, we additionally evaluated whether the change in each OCM index from baseline to week 12 was associated with a significant change in each PTHM during the same time period. To meet assumptions of linear regression models, the change in the ln-OCM index was examined in relation to the change in the ln-PTHM.
The significance level was set at 0.05 for all statistical tests. Analyses were conducted using SAS (version 9.3, Cary, NC) and R (version 3.1.3).
Results
General Characteristics, Nutritional Indices, and PTHMs
General characteristics of the study participants have been described previously [29], and are presented separately by sex in Table 1 and by sex and treatment arm in Table 2. Participants were between 24 and 54 years old with a median BMI of 19.2 kg/m2. Median plasma choline and betaine concentrations were 11.0 and 42.8 µmol/L, respectively. Approximately, 23% of participants were folate deficient (plasma folate < 9 nmol/L [38]) and 24% were vitamin B12 deficient (plasma vitamin B12 < 151 pmol/L [38]). The prevalence of HHcys (plasma Hcys ≥ 13 µmol/L) was 40.7%. Compared with women, men were generally older; were less likely to own a TV; had lower BMIs; had higher bAs, choline, betaine, and Hcys concentrations; and were more likely to be folate deficient and to have HHcys. Men were also much more likely to have ever smoked cigarettes. Baseline measures of PTHMs did not differ significantly between men and women.
Table 1.
General baseline characteristics by sex for FACT participants with PTHM measures
| All Participants (n = 324) | Males (n = 162) | Females (n = 162) | ||
|---|---|---|---|---|
| Characteristic | Median (IQR) | Median (IQR) | Median (IQR) | Pa |
| Age (years) | 39 (34–44) | 42 (36–46) | 37 (31–42) | <0.01 |
| BMI (kg/m2)b | 19.2 (17.7–21.3) | 18.7 (17.7–20.3) | 20.0 (17.9–22.5) | <0.01 |
| Blood Arsenic (µg/L) | 8.7 (6.0–12.4) | 9.5 (6.5–12.4) | 7.9 (5.3–12.4) | 0.04 |
| bSe (µg/L) | 134 (122–149) | 135 (123–151) | 132 (120–146) | 0.16 |
| RBC Folate (nmol/L)c | 451 (363–603) | 434 (362–572) | 461 (364–607) | 0.34 |
| Plasma Folate (nmol/L) | 12 (9–17) | 12 (8–16) | 13 (10–18) | 0.10 |
| Plasma Vitamin B12 (pmol/L) | 215 (153–319) | 217 (154–296) | 213 (153–338) | 0.49 |
| Plasma Choline (µmol/L) | 11.0 (9.8–13.1) | 11.9 (10.4–13.3) | 10.7 (9.0–12.4) | <0.01 |
| Plasma Betaine (µmol/L) | 42.8 (33.9–52.0) | 47.0 (40.2–57.5) | 37.3 (28.9–44.9) | <0.01 |
| Plasma Hcys (µmol/L) | 11.4 (8.8–15.5) | 14.2 (11.1–17.4) | 9.3 (7.4–12.0) | <0.01 |
| H3K36me2d, relative % of total H3 | 1.44 (1.26–1.72) | 1.45 (1.26–1.76) | 1.43 (1.26–1.70) | 0.66 |
| H3K36me3e, relative % of total H3 | 1.61 (1.32–1.88) | 1.56 (1.26–1.84) | 1.63 (1.37–1.94) | 0.10 |
| H3K79me2f, relative % of total H3 | 1.27 (1.03–1.76) | 1.26 (1.05–1.74) | 1.29 (1.01–1.85) | 0.96 |
| Folate Deficientg (%) | 23.2 | 28.4 | 17.9 | 0.03 |
| Vitamin B12 Deficienth (%) | 24.4 | 24.1 | 24.7 | 0.90 |
| HHcys (%)i | 40.7 | 62.4 | 19.1 | <0.01 |
| Ever Smoked Cigarette (%) | 28.8 | 56.2 | 1.2 | <0.01j |
| Ever Used Betel Nut (%) | 26.9 | 29.0 | 24.8 | 0.40 |
| Education > 5 years (%) | 22.5 | 21.0 | 24.1 | 0.51 |
| Own TV (%) | 38.3 | 32.7 | 43.8 | 0.04 |
P was from Wilcoxon rank-sum test and Chi square test for sex difference for continuous and categorical variables, respectively
Whole sample, n = 315; Men, n = 160; Women, n = 155
Whole sample, n = 250; Men, n = 125 ; Women, n = 125
Whole sample, n = 318; Men, n = 159; Women, n = 159
Whole sample, n = 306; Men, n = 154; Women, n = 152
Whole sample, n = 321; Men, n = 162; Women, n = 159
Plasma folate < 9 nmol/L
Plasma vitamin B12 <151 pmol/L
Plasma Hcys ≥ 13 µmol/L
P was from Fisher’s exact test, since there were only two female smokers
Table 2.
Baseline characteristics of FACT participants with PTHM measures at baseline in 400 µg FA and placebo groups
| 400 µg FA (n = 107) | Placebo (n = 60) | ||
|---|---|---|---|
| Characteristic | Median (IQR) | Median (IQR) | Pa |
| Age (years) | 38 (34–46) | 38 (33–44) | 0.29 |
| BMI (kg/m2)b | 19.3 (17.8–21.0) | 19.5 (18.1–22.1) | 0.37 |
| RBC Folate (nmol/L)c | 424 (360–617) | 472 (357–548) | 0.74 |
| Plasma Folate (nmol/L) | 13 (8–19) | 13 (10–17) | 0.53 |
| Plasma Vitamin B12 (pmol/L) | 213 (154–296) | 220 (150–323) | 0.76 |
| Plasma Choline (µmol/L) | 11.0 (9.9–13.0) | 10.9 (9.7–13.1) | 0.86 |
| Plasma Betaine (µmol/L) | 42.4 (35.1–51.9) | 43.4 (34.1–54.7) | 0.84 |
| Plasma Hcys (µmol/L) | 11.4 (8.6–15.5) | 11.7 (8.9–16.0) | 0.77 |
| Blood Arsenic (µg/L) | 8.4 (6.0–12.7) | 8.7 (6.1–11.5) | 0.83 |
| bSe (µg/L) | 135 (122–152) | 136 (124–150) | 0.93 |
| uCr (mg/dL) | 46 (32–83) | 40 (20–62) | 0.03 |
| H3K36me2d, relative % of total H3 | 1.48 (1.28–1.86) | 1.56 (1.37–1.73) | 0.61 |
| H3K36me3e, relative % of total H3 | 1.62 (1.21–2.07) | 1.67 (1.38–2.00) | 0.51 |
| H3K79me2f, relative % of total H3 | 1.20 (1.06–1.68) | 1.16 (0.96–1.59) | 0.18 |
| Folate Deficient (%)g | 26.2 | 18.3 | 0.25 |
| Vitamin B12 Deficient (%)h | 23.4 | 26.7 | 0.63 |
| HHcys (%)i | 39.3 | 46.7 | 0.35 |
| Male (%) | 50.5 | 51.7 | 0.88 |
| Ever Smoker (%)j | 28.6 | 31.7 | 0.68 |
| Education > 5 years (%) | 22.4 | 16.7 | 0.37 |
| Own TV (%) | 42.1 | 40.0 | 0.80 |
P was from Wilcoxon rank-sum test and Chi Square test for difference between 400 µg FA and placebo groups for continuous and categorical variables, respectively
n = 104 for 400 µg FA, n = 59 for Placebo group
n = 103 for 400 µg FA group
n = 105 for 400 µg FA, n = 56 for Placebo group
n = 102 for 400 µg FA, n = 57 for Placebo group
n = 106 for 400 µg FA, n = 60 for Placebo group
Plasma folate < 9 nmol/L
Plasma vitamin B12 < 151 pmol/L
Plasma Hcys > 13 µmol/L
n = 104 for 400 µg FA
For most characteristics, study participants with PTHM measures were comparable to the rest of the FACT participants, although this subset of participants had significantly lower plasma folate and choline concentrations, were more likely to be folate deficient, and were more likely to own TVs (Supplementary Table S1). The subset of participants with RBC folate measures had lower plasma vitamin B12 concentrations and uCr concentrations and were more likely to own TVs, but were otherwise comparable to FACT participants without RBC folate measures (Supplementary Table S2).
Sex-Specific Associations between OCM Indices and PTHMs
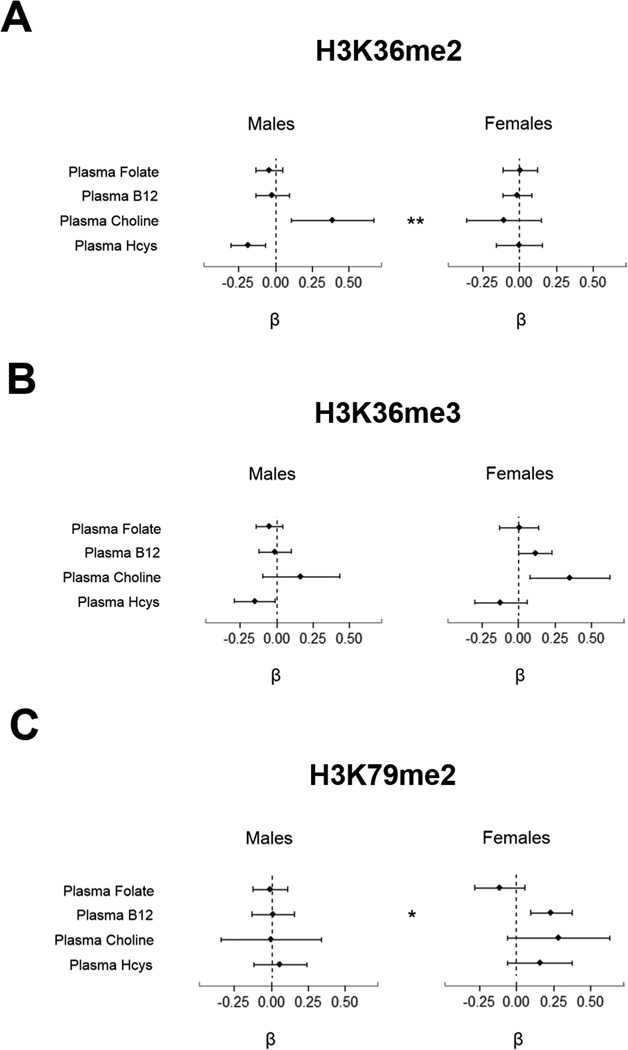
Sex-specific associations between OCM indices and PTHMs are shown in Figure 2. Alternative models, which replaced plasma folate with RBC folate, are shown in Supplementary Fig. S1. Plasma betaine was excluded from final models as it was not associated with any of the PTHMs in men or women after adjusting for plasma choline, and its addition to models did not alter coefficients for any of the other OCM indices.
Figure 2. Sex-Specific Associations between OCM Indices and PTHMs in FACT Participants.
Estimated regression coefficients and 95% confidence intervals for associations between each OCM index and (A) H3K36me2, (B) H3K36me3, and (C) H3K79me2 are shown separately by sex. The dashed line represents the null (β = 0). Associations with confidence intervals that do not cross the null are statistically significant (P < 0.05). Asterisks (*P < 0.05, **P < 0.01) indicate sex differences with p-values calculated from the Wald test. OCM indices were natural log-transformed and were included simultaneously in models. Models were adjusted for age, education, TV ownership, and ln-bSe. Analyses for H3K36me3 and H3K79me2 in men were additionally adjusted for cigarette smoking status. H3K36me2 was inverse-transformed and was modeled using a generalized linear model with an inverse-link function. H3K36me3 and H3K79me2 were natural log transformed and were modeled using linear models. Sample sizes for the main analyses were as follows H3K36me2: n = 159 for men, n = 159 for women; H3K36me3: n = 154 for men, n = 152 for women; H3K79me2: n = 162 for men, n = 159 for women.
H3K36me2
Although ln-RBC folate was negatively associated with H3K36me2 among men (β = −0.23; 95% CI, −0.41— −0.05), this was not statistically significant after adjusting for multiple tests (PFDR = 0.07). The associations between ln-choline and H3K36me2 differed significantly between men and women (Pdiff < 0.01), with ln-choline being positively associated with H3K36me2 among men (β = 0.39; 95% CI, 0.11— 0.66; PFDR = 0.03), but not women (β = −0.11; 95% CI, −0.36— 0.14; PFDR = 0.51). Although not statistically significant, there was also a suggestive difference by sex for the associations between ln-Hcys and H3K36me2 (Pdiff = 0.06). Ln-Hcys was inversely associated with H3K36me2 among men (β = −0.19; 95% CI, −0.30— −0.07; PFDR = 0.01), but not women (β = 0.00; 95% CI, −0.16— 0.15).
H3K36me3
After adjusting for multiple tests, plasma choline was positively and significantly associated with H3K36me3 among women only (β = 0.35; 95% CI, 0.08—0.62; PFDR = 0.03). There was also a suggestive inverse association between ln-Hcys and H3K36me3 among men (β = −0.15; 95% CI, −0.29— −0.02; PFDR = 0.08), with a similar trend among women (β = −0.12; 95% CI, −0.30— 0.05; PFDR = 0.25).
H3K79me2
Ln-vitamin B12 was positively associated with ln-H3K79me2 in women (β = 0.23; 95%CI, 0.09—0.37; PFDR < 0.01), but not men (β = 0.01; 95% CI, −0.14—0.15; PFDR = 0.94), and these associations differed significantly by sex (Pdiff < 0.05). Other OCM indices were not significantly associated with H3K79me2.
Sensitivity Analyses
Associations between OCM indices and PTHMs were very similar after additionally adjusting for BMI (Supplementary Table S3) and measures of arsenic exposure (Supplemental Table S4). Associations were also similar when OCM indices were examined individually, although some of the effects were slightly attenuated (Supplementary Table S5). Generally, the associations between OCM indices and PTHMs did not differ significantly by age or blood arsenic level (Supplementary Table S6 and S7). The only exception was the association between Hcys and H3K36me3, which differed significantly between men who were above vs. below the median age (38 years) (Pdiff < 0.01); among men greater than 38 years only, there was a negative association between Hcys and H3K36me3 (β = −0.28; 95% CI, −0.46— −0.10), which was statistically significant after adjusting for multiple tests (PFDR = 0.03) (Supplementary Table S6).
Effects of FA Supplementation on PTHMs
Baseline characteristics of participants with PTHM measures were comparable between the 400 µg FA and placebo treatment arms, except for uCr, which was higher in the 400 µg FA group (Table 2). However, uCr was not associated with any of the PTHMs at baseline or with the intra-person changes in PTHMs.
Compared with placebo, FA supplementation (400 µg/day for 12 weeks) did not alter the PTHMs (Table 3), and this was also true in sex-stratified analyses. Furthermore, changes in the OCM indices from baseline to week 12 were not significantly associated with changes in the PTHMs during the same period, after adjusting for multiple tests (Supplementary Table S8).
Table 3.
Median and inter-quartile range (IQR) within-person change in PTHM from baseline to week 12 in FACT participants by treatment arm
| PTHM | 400 µg FA | Placebo | Test for group difference |
|---|---|---|---|
| Median (IQR) | Median (IQR) | PFDRa | |
| All Participants | |||
| H3K36me2b | −0.05 (−0.39, 0.11) | −0.15 (−0.43, 0.11) | 0.80 |
| H3K36me3c | 0.02 (−0.28, 0.27) | 0.02 (−0.23, 0.30) | 0.80 |
| H3K79me2d | −0.06 (−0.28, 0.14) | −0.05 (−0.24, 0.04) | 0.80 |
| Males | |||
| H3K36me2e | −0.06 (−0.31, 0.11) | −0.07 (−0.44, 0.16) | 0.80 |
| H3K36me3f | 0.00 (−0.28, 0.23) | 0.05 (−0.23, 0.47) | 0.80 |
| H3K79me2g | −0.03 (−0.19, 0.21) | −0.03 (−0.28, 0.09) | 0.80 |
| Females | |||
| H3K36me2h | −0.05 (−0.44, 0.10) | −0.17 (−0.37, 0.04) | 0.80 |
| H3K36me3i | 0.04 (−0.31, 0.28) | 0.02 (−0.22, 0.12) | 0.80 |
| H3K79me2j | −0.13 (−0.47, 0.08) | −0.05 (−0.18, 0.04) | 0.80 |
P from Wilcoxon rank-sum test for treatment group difference
400 µg FA n = 103, Placebo n = 56
400 µg FA n = 98, Placebo n = 55
400 µg FA n = 97, Placebo n = 56
400 µg FA n = 52, Placebo n = 27
400 µg FA n = 50, Placebo n = 28
400 µg FA n = 50, Placebo n = 29
400 µg FA n = 51, Placebo n = 29
400 µg FA n = 48, Placebo n = 27
400 µg FA n = 47, Placebo n = 27
Discussion
Since lysine histone methyltransferases depend on SAM, many PTHMs are sensitive to nutritional methyl donors and other OCM indices [9, 24, 41–43]. However, few studies have examined the influences of nutritional methyl donors on PTHMs in human populations. In this study of arsenic-exposed Bangladeshi adults, we observed sex-dependent associations between several OCM indices and three PTHMs (H3K36me2, H3K36me3, and H3K79me2), which were selected because they are dysregulated in cancers [19–23] and are altered by nutritional methyl donors and/or arsenic in experimental studies [24–27].
Although most of the measured nutritional methyl donors and cofactors were positively associated with the PTHMs examined, this was not the case for folate. Plasma folate was not associated with these PTHMs, and supplementation with 400 µg FA/day for 12 weeks did not alter them. There is evidence that tetrahydrofolate may facilitate histone demethylation by accepting one-carbon groups as they are removed from histones [44, 45]. Thus, folate may have dual roles in regulating PTHMs, complicating predictions of its net effects on these marks. Polymorphisms in OCM genes may also modify the effects of folate on PTHMs. Previous studies have found that folate is only associated with DNA methylation among individuals with the C677T MTHFR polymorphism [46, 47]. However, in Bangladesh <2% of individuals are homozygous for this variant [48]. It is also possible that the 12 week duration of FA supplementation was too short to observe alterations in PTHMs. However, some smaller studies have shown that supplementation with the same, or lower, doses of FA for shorter durations can alter leukocyte DNA methylation patterns [49, 50]. Another important consideration is that all participants in the current study were subject to an arsenic-removal water filter intervention. We previously observed that H3K36me2 declined significantly with reductions in arsenic exposure [15]. Thus, arsenic-removal may have counteracted some FA treatment effects. It is also possible that FA supplementation altered PTHMs, but the effects were too small to detect given limited statistical power, particularly in sex-stratified analyses.
One limitation of this study relates to the fact that, by chance, the subset of participants with PTHM measures had slightly lower mean plasma folate and choline concentrations than FACT participants without PTHM measures. Similarly, the subset of participants with RBC folate measures on average had lower plasma vitamin B12 concentrations. We therefore cannot rule out the possibility that our findings may be more relevant to individuals with poor nutritional status. Likewise, our findings in Bangladesh may not be generalizable to other countries where nutritional deficiencies are less common.
There are several possible explanations for the sex differences observed in our study. Given that long range allosteric interactions normally regulate SAM concentrations, it is possible that PTHMs are only perturbed under conditions of nutritional deficiencies or excess. Consistent with other reports [4, 5], plasma choline, betaine, and Hcys concentrations were higher among men, and men were more likely to be folate deficient. There are also underlying sex differences in both the OCM pathway and epigenetic regulation. For example, phosphatidylethanolamine N-methyltransferase, which catalyzes phosphatidylcholine synthesis, is up-regulated by estrogen [51], and many histone methyltransferases and histone demethylases bind to androgen receptors [52]. Additionally, some histone demethylase genes reside exclusively on the Y chromosome [53].
Susceptibility to arsenic toxicity differs by sex, with some outcomes preferentially affecting males and others females [54]. It is possible that differential effects of arsenic and OCM indices on epigenetic marks, such as PTHMs, contribute to these differences. We have previously observed that arsenic exposure is associated with DNA methylation and PTHMs in a sex-dependent manner [14–17]. The findings from this study suggest that some of the OCM indices analyzed also influence certain PTHMs differentially by sex. For example, choline was positively, and Hcys negatively, associated with H3K36me2 among men. H3K36me2 plays an important role in repairing DNA double strand breaks, and there is evidence that an aberrant global increase in this mark is involved in oncogenic programming [55]. Among women, choline and vitamin B12 were positively associated with H3K36me3 and H3K79me2. A global loss of H3K36me3 has been associated with chromosomal instability and leukemia [56] and may exacerbate age-dependent changes in gene expression [57], while H3K79me2 has been shown to be essential for normal hematopoiesis and is dysregulated in certain types of leukemia [58]. Thus, choline and vitamin B12 may prevent aberrant alterations in several PTHMs that are implicated in cancer development. Importantly, we did not measure other nutrients in the OCM pathway (e.g., vitamin B6 and riboflavin). These nutrients may also influence PTHMs and should be evaluated in future studies.
Our findings also have potential implications for targeted clinical interventions. Many epigenetic therapies target the OCM pathway. For example, EPZ-5676, which is currently in a Phase I trial for the treatment of MLL-rearranged leukemia, is a SAM-competitive inhibitor of DOT1L, a histone methyltransferase which targets H3K79 [59]. Thus, it is possible that nutritional factors and/or the use of supplements that influence SAM concentrations (e.g., vitamin B12) could counteract the effects of EPZ-5676 and other epigenetic drugs. By analogy, there is evidence that antifolates, such as methotrexate, are more effective in patients with low baseline folate concentrations and less effective in individuals taking FA supplements (reviewed in [60]).
The data reported herein suggest that some OCM indices influence PTHMs in a sex-dependent manner and further demonstrate that FA supplementation, at least at a low dose of 400 µg/day for 12 weeks, does not influence PTHMs in PBMCs. Nevertheless, we cannot rule out the possibility that PTHMs in other target tissues, or other PTHMs, may have been influenced by FA supplementation. Thus, understanding the effects of FA and other nutritional donors on PTHMs in human populations merits additional study.
Supplementary Material
Acknowledgments
Financial Support: This study was funded by NIH grants P42 ES010349 (J.H. Graziano), RO1 CA133595 (M.V. Gamble), RO1 ES017875 (M.V. Gamble), F31 ES025100 (C.G. Howe), T32 ES007322 (J.H. Graziano), 5 P30 ES000260-51 (M. Costa), and P30 ES009089
Footnotes
Conflict of interest statement: The authors declare that they have no conflicts of interest.
ClinicalTrials.gov identifier and web-page: NCT01050556, https://clinicaltrials.gov/ct2/show/NCT01050556
References
- 1.Lee BWK, Sun HG, Zang T, Kim BJ, Alfaro JF, Zhou ZS. Enzyme-catalyzed transfer of a ketone group from an S-adenosylmethionine analogue: a tool for the functional analysis of methyltransferases. J Am Chem Soc. 2010;132:3642–3643. doi: 10.1021/ja908995p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mudd SH, Poole JR. Labile methyl balances for normal humans on various dietary regimens. Metabolism. 1975;24:721–735. doi: 10.1016/0026-0495(75)90040-2. [DOI] [PubMed] [Google Scholar]
- 3.Fukagawa NK, Martin JM, Wurthmann A, Prue AH, Ebenstein D, O’Rourke B. Sex-related differences in methionine metabolism and plasma homocysteine concentrations. Am J Clin Nutr. 2000;72:22–29. doi: 10.1093/ajcn/72.1.22. [DOI] [PubMed] [Google Scholar]
- 4.Gamble MV, Ahsan H, Liu X, Factor-Litvak P, Ilievski V, Slavkovich V, et al. Folate and cobalamin deficiencies and hyperhomocysteinemia in Bangladesh. Am J Clin Nutr. 2005;81:1372–1377. doi: 10.1093/ajcn/81.6.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konstantinova SV, Tell GS, Vollset SE, Nygård O, Bleie Ø, Ueland PM. Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. J Nutr. 2008;138:914–920. doi: 10.1093/jn/138.5.914. [DOI] [PubMed] [Google Scholar]
- 6.Zhang D, Wen X, Wu W, Guo Y, Cui W. Elevated homocysteine level and folate deficiency associated with increased overall risk of carcinogenesis: meta-analysis of 83 case-control studies involving 35,758 individuals. PLoS One. 2015;10:e0123423. doi: 10.1371/journal.pone.0123423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pogribny IP, Shpyleva SI, Muskhelishvili L, Bagnyukova TV, James SJ, Beland FA. Role of DNA damage and alterations in cytosine DNA methylation in rat liver carcinogenesis induced by a methyl-deficient diet. Mutat Res. 2009;669:56–62. doi: 10.1016/j.mrfmmm.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Pogribny IP, James SJ, Beland FA. Molecular alterations in hepatocarcinogenesis induced by dietary methyl deficiency. Mol Nutr Food Res. 2012;56:116–125. doi: 10.1002/mnfr.201100524. [DOI] [PubMed] [Google Scholar]
- 9.Pogribny IP, Tryndyak VP, Muskhelishvili L, Rusyn I, Ross SA. Methyl deficiency, alterations in global histone modifications, and carcinogenesis. J Nutr. 2007;137:216s–222s. doi: 10.1093/jn/137.1.216S. [DOI] [PubMed] [Google Scholar]
- 10.Zhou W, Alonso S, Takai D, Lu SC, Yamamoto F, Perucho M. Requirement of RIZ1 for cancer prevention by methyl-balanced diet. PLoS One. 2008;3:e3390. doi: 10.1371/journal.pone.0003390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pilsner JR, Liu X, Ahsan H, Ilievski V, Slavkovich V, Levy D, et al. Genomic methylation of peripheral blood leukocyte DNA: influences of arsenic and folate in Bangladeshi adults. Am J Clin Nutr. 2007;86:1179–1186. doi: 10.1093/ajcn/86.4.1179. [DOI] [PubMed] [Google Scholar]
- 13.Nohara K, Baba T, Murai H, Kobayashi Y, Suzuki T, Tateishi Y, et al. Global DNA methylation in the mouse liver is affected by methyl deficiency and arsenic in a sex-dependent manner. Arch Toxicol. 2011;85:653–661. doi: 10.1007/s00204-010-0611-z. [DOI] [PubMed] [Google Scholar]
- 14.Chervona Y, Hall MN, Arita A, Wu F, Sun H, Tseng HC. Associations between arsenic exposure and global posttranslational histone modifications among adults in Bangladesh. Cancer Epidemiol Biomarkers Prev. 2012;21:2252–2260. doi: 10.1158/1055-9965.EPI-12-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howe CG, Liu X, Hall MN, Slavkovich V, Ilievski V, Parvez F, et al. Associations between blood and urine arsenic concentrations and global levels of post-translational histone modifications in Bangladeshi men and women. Environ Health Perspect. 2016 doi: 10.1289/ehp.1510412. Epub Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niedzwiecki MM, Liu X, Hall MN, Thomas T, Slavkovich V, Ilievski V, et al. Sex-specific associations of arsenic exposure with global DNA methylation and hydroxymethylation in leukocytes: results from two studies in Bangladesh. Cancer Epidemiol Biomarkers Prev. 2015;24:1748–1757. doi: 10.1158/1055-9965.EPI-15-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pilsner JR, Hall MN, Liu X, Ilievski V, Slavkovich V, Levy D, et al. Influence of prenatal arsenic exposure and newborn sex on global methylation of cord blood DNA. PLoS One. 2012;7:e37147. doi: 10.1371/journal.pone.0037147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duns G, van den Berg E, van Duivenbode I, Osinga J, Hollema H, Hofstra RM, et al. Histone methyltransferase gene SETD2 is a novel tumor suppressor gene in clear cell renal cell carcinoma. Cancer Res. 2010;70:4287–4291. doi: 10.1158/0008-5472.CAN-10-0120. [DOI] [PubMed] [Google Scholar]
- 19.Fontebasso AM, Schwartzentruber J, Khuong-Quang DA, Liu XY, Sturm D, Korshunov A, et al. Mutations in SETD2 and genes affecting histone H3K36 methylation target hemispheric high-grade gliomas. Acta Neuropathol. 2013;125:659–669. doi: 10.1007/s00401-013-1095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He J, Nguyen AT, Zhang Y. KDM2b/JHDM1b, an H3K36me2-specific demethylase, is required for initiation and maintenance of acute myeloid leukemia. Blood. 2011;117:3869–3880. doi: 10.1182/blood-2010-10-312736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamagawa H, Oshima T, Numata M, Yamamoto N, Shiozawa M, Morinaga S, et al. Global histone modification of H3K27 correlates with the outcomes in patients with metachronous liver metastasis of colorectal cancer. Eur J Surg Oncol. 2013;39:655–661. doi: 10.1016/j.ejso.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 22.Bernt KM, Zhu N, Sinha AU, Vempati S, Faber J, Krivstov AV, et al. MLL-rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer Cell. 2011;20:66–78. doi: 10.1016/j.ccr.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Deng L, Chen F, Yao Y, Wu B, Wei L, et al. Inhibition of histone H3K79 methylation selectively inhibits proliferation, self-renewal and metastatic potential of breast cancer. Oncotarget. 2014;5:10665. doi: 10.18632/oncotarget.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bistulfi G, Vandette E, Matsui S, Smiraglia DJ. Mild folate deficiency induces genetic and epigenetic instability and phenotype changes in prostate cancer cells. BMC Biol. 2010;8:1741–7007. doi: 10.1186/1741-7007-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadhu MJ, Guan Q, Li F, Sales-Lee J, Iavarone AT, Hammond MC, et al. Nutritional control of epigenetic processes in yeast and human cells. Genetics. 2013;195:831–844. doi: 10.1534/genetics.113.153981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q, Xue P, Li H, Bao Y, Wu L, Chang S, et al. Histone modification mapping in human brain reveals aberrant expression of histone H3 lysine 79 dimethylation in neural tube defects. Neurobiol Dis. 2013;54:404–413. doi: 10.1016/j.nbd.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Zhou X, Sun H, Ellen TP, Chen H, Costa M. Arsenite alters global histone H3 methylation. Carcinogenesis. 2008;29:1831–1836. doi: 10.1093/carcin/bgn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters BA, Hall MN, Liu X, Parvez F, Sanchez TR, van Geen A, et al. Folic Acid and Creatine as Therapeutic Approaches to Lower Blood Arsenic: A Randomized Controlled Trial. Environ Health Perspect. 2015;123:1294–1301. doi: 10.1289/ehp.1409396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahsan H, Chen Y, Parvez F, Argos M, Hussain AI, Momotaj A, et al. Health Effects of Arsenic Longitudinal Study (HEALS): description of a multidisciplinary epidemiologic investigation. J Expo Sci Environ Epidemiol, 2006;16:191–205. doi: 10.1038/sj.jea.7500449. [DOI] [PubMed] [Google Scholar]
- 30.Food and Nutrition Board, Institute of Medicine. Dietary reference intakes for thiamine, riboflavin, niacin, vitamin B6, folate, vitamin B12, panthotenic acid biotin and choline. Washington, DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- 31.Holm PI, Ueland PM, Kvalheim G, Lien EA. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin Chem. 2003;49:286–294. doi: 10.1373/49.2.286. [DOI] [PubMed] [Google Scholar]
- 32.Yan J, Jiang X, West AA, Perry CA, Malysheva OV, Devapatla S, et al. Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am J Clin Nutr. 2012;95:1060–1071. doi: 10.3945/ajcn.111.022772. [DOI] [PubMed] [Google Scholar]
- 33.Pfeiffer CM, Huff DL, Gunter EW. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem. 1999;45:290–292. [PubMed] [Google Scholar]
- 34.Pruszkowski E, Neubauer K, Thomas R. An overview of clinical applications by inductively coupled plasma mass spectrometry. Atomic spectroscopy. 1998;19:111–115. [Google Scholar]
- 35.Slot C. Plasma creatinine determination a new and specific Jaffe reaction method. Scand J Clin Lab Invest. 1965;17:381–387. doi: 10.3109/00365516509077065. [DOI] [PubMed] [Google Scholar]
- 36.Howe CG, Gamble MV. Enzymatic cleavage of histone H3: a new consideration when measuring histone modifications in human samples. Clin Epigenetics. 2015;7:7. doi: 10.1186/s13148-014-0041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clogg CC, Petkova E, Haritou A. Statistical methods for comparing regression coefficients between models. Am J Sociol. 1995;100:1261–1293. [Google Scholar]
- 38.Uthus EO, Ross SA, Davis CD. Differential effects of dietary selenium (se) on folate on methyl metabolism in liver and colon of rats. Biol Trace Elem Res. 2006;109:201–214. doi: 10.1385/BTER:109:3:201. [DOI] [PubMed] [Google Scholar]
- 39.Xiang N, Rui Zhao, Song G, Zhong W. Selenite reactivates silenced genes by modifying DNA methylation and histones in prostate cancer cells. Carcinogenesis. 2008;29:2175–2181. doi: 10.1093/carcin/bgn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huu Bich T, Thi Quynh Nga P, Ngoc Quang L, Van Minh H, Ng N, Juvekar S, et al. Patterns of alcohol consumption in diverse rural populations in the Asian region. Glob Health Action. 2009;2 doi: 10.3402/gha.v2i0.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehedint MG, Niculescu MD, Craciunescu CN, Zeisel SH. Choline deficiency alters global histone methylation and epigenetic marking at the Re1 site of the calbindin 1 gene. FASEB J. 2010;24:184–195. doi: 10.1096/fj.09-140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davison JM, Mellott TJ, Kovacheva VP, Blusztajn JK. Gestational choline supply regulates methylation of histone H3, expression of histone methyltransferases G9a (Kmt1c) and Suv39h1 (Kmt1a), and DNA methylation of their genes in rat fetal liver and brain. J Biol Chem. 2009;284:1982–1989. doi: 10.1074/jbc.M807651200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lambrot R, Xu C, Saint-Phar S, Chountalos G, Cohen T, Paquet M, et al. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat Commun. 2013;4:2889. doi: 10.1038/ncomms3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luka Z, Pakhomova S, Loukachevitch LV, Calcutt MW, Newcomer ME, Wagner C. Crystal structure of the histone lysine specific demethylase LSD1 complexed with tetrahydrofolate. Protein Sci. 2014;23:993–998. doi: 10.1002/pro.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia BA, Luka Z, Loukachevitch LV, Bhanu NV, Wagner C. Folate Deficiency Affects Histone Methylation. Med Hypotheses. 2016;88:63–67. doi: 10.1016/j.mehy.2015.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friso S, Choi SW, Girelli D, Mason JB, Dolnikowski GG, Bagley PJ, et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acam Sci U S A. 2002;99:5606–5611. doi: 10.1073/pnas.062066299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stern LL, Mason JB, Selhub J, Choi SW. Genomic DNA hypomethylation, a characteristic of most cancers, is present in peripheral leukocytes of individuals who are homozygous for the C677T polymorphism in the methylenetetrahydrofolate reductase gene. Cancer Epidemiol Biomarkers Prev. 2000;9:849–853. [PubMed] [Google Scholar]
- 48.Ahsan H, Chen Y, Kibriya MG, Slavkovich V, Parvez F, Jasmine F, et al. Arsenic metabolism, genetic susceptibility, and risk of premalignant skin lesions in Bangladesh. Cancer Epidemiol Biomarkers and Prev. 2007;16:1270–1278. doi: 10.1158/1055-9965.EPI-06-0676. [DOI] [PubMed] [Google Scholar]
- 49.Pufulete M, Al-Ghnaniem R, Khushal A, Appleby P, Harris N, Gout S. Effect of folic acid supplementation on genomic DNA methylation in patients with colorectal adenoma. Gut. 2005;54:648–653. doi: 10.1136/gut.2004.054718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crider KS, Quinlivan EP, Berry RJ, Hao L, Li Z, Maneval D, et al. Genomic DNA methylation changes in response to folic acid supplementation in a population-based intervention study among women of reproductive age. PLoS One. 2011;6:e28144. doi: 10.1371/journal.pone.0028144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28:778–808. doi: 10.1210/er.2007-0019. [DOI] [PubMed] [Google Scholar]
- 53.Bellott DW, Hughes JF, Skaletsky H, Brown LG, Pytinkova T, Cho TJ, et al. Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature. 2014;508:494–499. doi: 10.1038/nature13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.NRC. Critical aspects of EPA's IRIS assessment of inorganic arsenic. National Research Council Interim Report; 2013. [Google Scholar]
- 55.Wagner EJ, Carpenter PB. Understanding the language of Lys 36 methylation at histone H3. Nat Rev Mol Cell Biol. 2012;13:115–126. doi: 10.1038/nrm3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu X, He F, Zeng H, Ling S, Chen A, Wang Y, et al. Identification of functional cooperative mutations of SETD2 in human acute leukemia. Nat Genet. 2014;46:287–293. doi: 10.1038/ng.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pu M, Ni Z, Wang M, Wang X, Wood JG, Helfand SL. Trimethylation of Lys36 on H3 restricts gene expression change during aging and impacts life span. Genes Dev. 2015;29:718–731. doi: 10.1101/gad.254144.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen AT, Zhang Y. The diverse functions of Dot1 and H3K79 methylation. Genes Dev. 2011;25:1345–1358. doi: 10.1101/gad.2057811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stein EM, Tallman MS. Mixed lineage rearranged leukaemia: pathogenesis and targeting DOT1L. Curr Opin Hematol. 2015;22:92–96. doi: 10.1097/MOH.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 60.Smith AD, Kim YI, Refsum H. Is folic acid good for everyone? Am J Clin Nutr. 2008;87:517–533. doi: 10.1093/ajcn/87.3.517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.