Abstract
Most cancer immunotherapies include activation of either innate or adaptive immune responses. We hypothesized that the combined activation of both innate and adaptive immunity will result in better antitumor efficacy. We have previously shown the synergy of an agonistic anti-CD40 monoclonal antibody (anti-CD40) and CpG-ODN in activating macrophages to induce tumor cell killing in mice. Separately, we have shown that a direct intratumoral injection of immunocytokine (IC), an anti-GD2 antibody linked to interleukin-2, can activate T and NK cells resulting in antitumor effects. We hypothesized that activation of macrophages with anti-CD40/CpG, and NK cells with IC, would cause innate tumor destruction, leading to increased presentation of tumor antigens and adaptive T cell activation; the latter could be further augmented by anti-CTLA-4 antibody to achieve tumor eradication and immunological memory. Using the mouse GD2+ B78 melanoma model, we show that anti-CD40/CpG treatment led to upregulation of T cell activation markers in draining lymph nodes. Anti-CD40/CpG + IC/anti-CTLA-4 synergistically induced regression of advanced subcutaneous tumors, resulting in cure of some mice and development of immunological memory against B78 and wild type B16 tumors. While the antitumor effect of anti-CD40/CpG did not require T cells, the antitumor effect of IC/anti-CTLA-4 was dependent on T cells. The combined treatment with anti-CD40/CpG + IC/anti-CTLA-4 reduced T regulatory cells in the tumors and was effective against distant solid tumors and lung metastases. We suggest that a combination of anti-CD40/CpG and IC/anti-CTLA-4 should be developed for clinical testing as a potentially effective novel immunotherapy strategy.
Introduction
Recent advances in cancer immunotherapy have shown it to be an effective strategy for treatment of certain cancers (1, 2). However, single agent immunotherapeutic approaches can have limited efficacy, whereas combining two or more immunotherapeutic strategies can be synergistic in inducing antitumor effects (3–5).
One of the activators of innate as well as adaptive immune responses is agonistic anti-CD40 antibody (anti-CD40), which can induce antitumor effects in mice and in cancer patients (6). The clinical potential of anti-CD40 has been demonstrated by regression of primary and metastatic adenocarcinomas in 4 of 21 patients with pancreatic cancer (2). This clinical and preclinical activity of anti-CD40 against pancreatic cancer confirms our earlier findings showing the antitumor effect of anti-CD40 via macrophage activation in several mouse models (7–9). We have also demonstrated that the antitumor effect of anti-CD40 can be greatly potentiated by CpG-ODN, a toll-like receptor 9 (TLR9) agonist, via synergistic activation of macrophages in mouse models of melanoma and neuroblastoma (10); however, complete responses were rarely achieved, suggesting that combining this approach with other immunotherapeutic modalities could be beneficial. Radiotherapy can convert tumor associated suppressive M2 macrophages into effector M1 macrophages in the tumor microenvironment, facilitating T cell immunotherapy (11). We showed that immunotherapy with anti-CD40/CpG similarly converts M2 pro-tumor macrophages into M1 antitumor effector macrophages (12), suggesting that this approach could also be effectively combined with T cell immunotherapy.
We have also shown that an intratumoral (IT) injection of immunocytokine (IC), which consists of an antitumor antibody linked to interleukin-2 (IL2), can serve as an in situ vaccine; it enhances local anti-tumor effects and can generate an adaptive T-cell response directed against distant tumors (13,14). These in situ vaccine effects involve T-cells as well as NK cells, and can result in T cell memory (13,14).
T cell activation and function in the tumor microenvironment of cancer patients are suppressed (15,16). Two inhibitory receptors on antitumor T cells, cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death 1 (PD-1), play an important role in T suppression by the tumor (17,18). Blockade of these inhibitory interactions, known as “immune checkpoint blockade”, with anti-CTLA-4, anti-PD-1, or both, counteracts the immunosuppression, results in augmenting endogenous tumor-specific T cell responses and provides clinical benefit, particularly in melanoma patients (19–21). CTLA-4 can synergize with nitric oxide produced by activated macrophages in inhibiting T cells via T regulatory cells (Treg) activation (22); anti-CTLA-4 antibody can deplete Treg in the tumor (23). Therefore, our overall hypothesis was that a synergistic activation of innate and adaptive immunity could be achieved by combining anti-CD40/CpG (to activate macrophages), IT-IC (to activate NK cells and T cells), and anti-CTLA-4 checkpoint inhibitor (to counteract T cell suppression), resulting in strong antitumor effects.
Material and Methods
Mice
Six to ten week old female C57BL/6 and nude mice were obtained from Taconic Farms (Germantown, NY) or from The Jackson Laboratory (Bar Harbor, ME). Mice were housed in the University of Wisconsin-Madison animal facilities at the Wisconsin Institutes for Medical Research. Mice were used in accordance with the Guide for Care and Use of Laboratory Animals (NIH publication 86-23, National Institutes of Health, Bethesda, MD, 1985).
Tumor cell lines
Mouse B16-F10 melanoma cells (further referred as B16) were transduced to express GD2 (B16-GD2) using a retroviral vector that encodes the GD2 mini-operon (MP9956:SFG.GD3synthase-2A-GD2synthase plasmid; a kind gift from Prof. Martin Pule from University College London). B16, B16-GD2, and B78 melanoma, a slow growing derivative of B16 which expresses GD2 (24), cell lines were grown in RPMI-1640 cell culture medium supplemented with 10% FBS (Sigma-Aldrich, St. Louis, MO), 2 mM L-glutamine, 100 U/ml penicillin/streptomycin, and 0.5 μM 2-ME (Invitrogen Life Technologies, Carlsbad, CA) at 37°C in a humidified 5% CO2 atmosphere.
In vivo tumor models
Subcutaneous (s.c.) tumors were established by injecting 2×106 (B78) or 1–5×105 (B16 or B16-GD2) cells in 0.1 ml of PBS. Tumor size is reported as tumor volume (mm3) by measuring perpendicular diameters of the tumor, and calculated as follows: (1/2) × tumor length × tumor width2, and expressed as mean volume ± SEM of tumor volumes for all mice of each experimental group.
Reagents
Agonistic anti-CD40 was obtained from ascites of nude mice injected with the FGK 45.5 hybridoma cells (a gift from Dr. F. Melchers, Basel Institute for Immunology, Basel, Switzerland) and enriched for IgG by ammonium sulfate precipitation. CpG1826 was purchased from TriLink Biotechnologies, San Diego, CA. The humanized hu14.18-IL2 IC (APN301, Apeiron Biologics, Vienna, Austria) was supplied by the NCI Biologics Resources Branch (Frederick, MD) via a collaborative relationship with Apeiron Biologics. Hu14.18-IL2 is an IC consisting of human interleukin-2 (IL2) genetically linked to the carboxyl-termini of each human IgG1 heavy chain of the GD2-specific hu14.18 mAb (25). Anti-CTLA-4 clone UC10-4F10-11, IgG2b, was a gift of Dr. Jeffrey Bluestone, UCSF, CA (26). This UC10-4F10-11 IgG2b was used in all experiments requiring anti-CTLA-4 antibody, unless stated otherwise. Anti-CTLA-4 clone 9D9, provided as both IgG2a and IgG2b isotypes, was obtained from Bristol-Myers Squibb, Redwood City, CA (23), and use of these 2 mAbs is clarified in the legend and labels. The anti-murine PD-1 mAb (clone 4H2), was obtained from Bristol-Myers Squibb, Redwood City, CA (27).
Immunotherapy
Tumor-bearing mice were treated with anti-CD40 mAb (0.25 mg/0.5 ml, unless indicated otherwise) i.p. and CpG (0.025 mg/0.1 ml, unless indicated otherwise) i.t. on different days as stated in figure legends. CpG was given 3 days after anti-CD40 as in our previous studies (10, 12) because the maximal upregulation of TLR9 on macrophages occurs 3 days after anti-CD40 injection (10). Hu14.18-IL2 IC was injected i.t. at doses of 5 or 25 mcg in 0.1 ml PBS (13). Anti-CTLA-4 (0.2 mg/0.2 ml of PBS) was injected i.p. every other day 3 times a week for 2 weeks. This combination is designated: anti-CD40/CpG + IC/anti-CTLA-4.
Flow cytometry of lymph node cells
C57BL/6 mice were injected s.c. on the left side of the abdomen with 2×106 B78 cells (day 0). Mice were injected with anti-CD40 on day 6 and CpG on day 9. Control tumor-bearing mice received rat IgG and PBS. Another group of control mice did not receive the tumor and treatments (“naïve”). On day 10, left inguinal (draining) lymph nodes and right inguinal (contralateral) lymph nodes (DLNs and CLNs, respectively) were removed and pooled from 3 mice per group. Lymph node cells were stained with anti-CD8-PE (clone 53-67), anti-CD69-APC (clone H1.2F3), anti-CD44-APC (clone IM7), anti-CD25-APC (clone PC61; all from BioLegend, San Diego, CA) and anti-CD4-FITC (clone GK1.5, eBioscience, San Diego, CA). Data acquisition was performed on FACSCalibur flow cytometer with CellQuest software (BD, Franklin Lakes, NJ). Flow cytometry analysis was performed on FlowJo software (TreeStar, Inc., Ashland, OR) by gating on CD8+ or CD4+ cells. Results are presented as percent of positive cells or mean fluorescence intensity (MFI) ratios.
Flow cytometry of tumor-infiltrating cells
B78 melanoma cells were injected s.c. into C57BL/6 mice on day 0. Anti-CD40 was injected i.p. on day 23; CpG was injected i.t. on days 26,28,30. 14.18-IL2 IC was given i.t. on days 26–30; anti-CTLA-4 was injected i.p. on day 26,28,30 and 33. Control tumor-bearing mice received no treatment. On day 34, tumors were harvested, cut into small pieces and incubated for 30 minutes at 37°C in dissociation solution containing HBSS supplemented with 5% FBS, 1mg/ml Collagenase type D and 100μg/ml DNase I (Sigma-Aldrich, St. Louis, MO).
For cell surface staining, the cells were preincubated with Mouse BD Fc Block™ purified anti-mouse CD16/CD32 (clone 2.4G2, BD Biosciences) for five minutes at 4°C. After blocking, cells were incubated with CD4-FITC (clone GK1.5; eBioscience), CD8a-PE (clone 53-6.7, BioLegend), F4/80-FITC (clone BM8, eBioscience), or CD49b-PE (clone DX5, BD Biosciences) at 4°C for 30 minutes. The stained cells were washed and resuspended in PBS/1% FBS, propidium iodide was added, and data were acquired on a BD FacsCalibur.
For T regulatory cell staining, the cells were first incubated with CD4-FITC (clone GK1.5; eBioscience), CD45 eFluor450 (clone 30-F11, eBioscience), CD25-APC (clone PC61, BioLegend) and Fixable viability dye 506 (FVD506, eBioscience) at 4°C for 30 min. The stained cells were fixed in the eBioscience Foxp3/ Transcription factor staining buffer set according to manufacturer’s manual. After fixation over night the cells were stained with Foxp3-PECy7 (clone FJK16s, eBioscience). Flow cytometry data was acquired using the MacsQuant Analyzer (Miltenyi Biotec) and analyzed using the software FlowJo version 10.1. Statistical analysis was performed using Graphpad Prism version 8.
Statistical analysis
An unpaired Student’s t-test and ANOVA test with either Dunnett’s post-test or Tukey post-test were used to determine significance of differences between experimental and relevant control values within each experiment.
Results
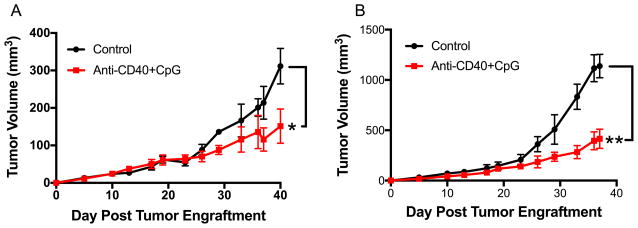
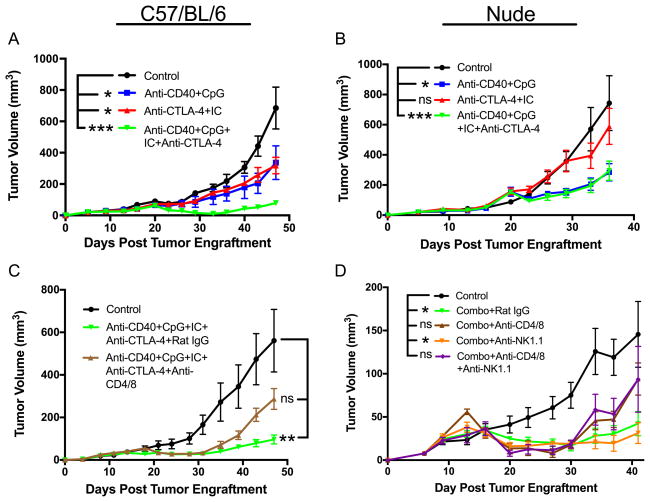
Systemic anti-CD40 combined with IT CpG induces T cell-independent antitumor effects which involve macrophages
In our previous studies we showed a strong antitumor synergy between anti-CD40 and CpG when both agents were injected systemically (i.p.). It was reported that CpG given IT can result in T cell activation enhanced by additional T cell activation modalities including anti-CTLA-4 (28). Because our goal was to facilitate T cell activation by the means of innate immunity, we thought to combine IT CpG with systemic anti-CD40 treatment. The results show that anti-CD40/CpG caused suppression of B78 melanoma growth in syngeneic C57BL/6 mice (Figure 1A) and in T-cell compromised nude mice (Figure 1B), indicating that the antitumor effect of systemic anti-CD40 combined with local CpG does not require T cells. To determine which cell population is responsible for the antitumor effect following anti-CD40/CpG therapy, C57BL/6 mice were injected s.c. with B16 cells (day 0) and with anti-CD40 i.p. on day 4. Three days later (day 7) peritoneal cells were obtained, stained, gated on CD11bhigh cells, sorted into 4 sub-populations based on their expression of CD11b and Gr-1 markers, and further characterized by histological staining and antitumor activity in vitro. Similarly to what we reported previously (29), antitumor effector cells were found to be CD11bhigh Gr-1− macrophages as was confirmed by morphology and secretion of nitric oxide (data not shown).
Figure 1.
Antitumor effect of anti-CD40 + CpG is T cell-independent. B78 melanoma cells (2×106) were injected s.c. into C57BL/6 (A) and nude mice (B) on day 0. Anti-CD40 (500 mcg) was given i.p. on day 23. CpG (25 mcg) was given i.t. on days 26, 28, 30. Data shown are means ± SEM of 5 mice per group. Statistics for all figures are depicted as follows: * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001. Unless stated otherwise, statistical differences for this and other figures are indicated for the last day on the graph. For all figures, the differences between the control vs. treatment groups are shown. In addition, the differences between a combined treatment vs. separate treatment groups are shown in Figures 3A and 4A.
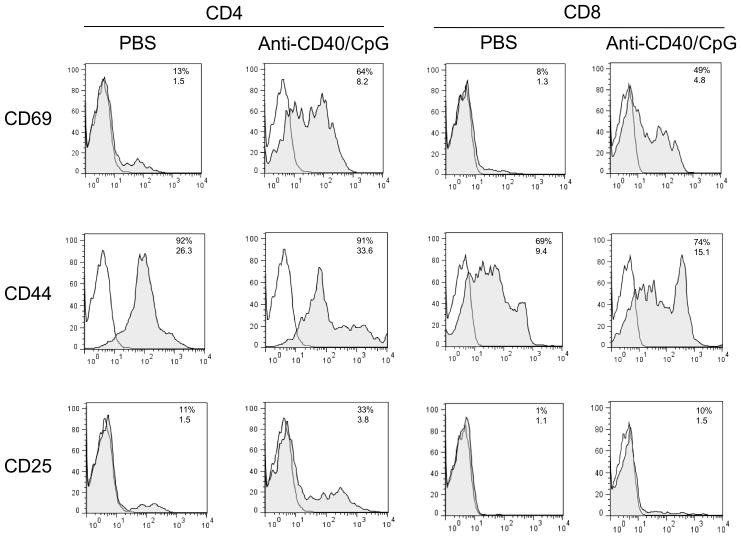
Anti-CD40/CpG treatment results in T cell activation
We hypothesized that T-cell independent anti-CD40/CpG therapy (Figure 1) resulting in tumor cell killing via macrophages can also activate T cells, presumably by enhancing antigen-presentation. To test this hypothesis, C57BL/6 mice were injected s.c. into the left side of the abdomen with B78 tumor cells (day 0), i.p with anti-CD40 (day 7) and i.t. with CpG (day 10). One day later, draining (left) and contralateral (right) inguinal lymph nodes were removed, pooled from three mice per group, and processed to single cell suspensions. The cells were stained with anti-CD4 and anti-CD8 mAbs and mAbs to T cell activation markers (CD69, CD44 and CD25). The results in Figure 2 show that anti-CD40 + CpG treatment upregulated the early T cell activation marker CD69 in both CD4 and CD8 T cells in draining lymph nodes. This upregulation was observed when anti-CD40 and CpG were given separately but was more pronounced when they were combined (data not shown). The upregulation of CD69 was more pronounced in draining lymph nodes (Figure 2), but was also observed in contralateral lymph nodes (Supplemental Figure 1), suggesting that this treatment induces both local and systemic activation of T cells. Similarly, anti-CD40 and CpG induced upregulation of other T cell activation markers, CD44 and CD25. These results suggest that anti-CD40/CpG therapy induced activation of T cells, although this activation was not essential for the early delay in B78 tumor growth as shown in Figure 1.
Figure 2.
Anti-CD40 and CpG induce activation of T cells in draining lymph nodes. C57BL/6 mice (3 mice per group) were injected s.c. into the left side of abdomen with 2×106 B78 cells (day 0). On day 7 the mice were injected i.p. with anti-CD40, and on day 10 they received CpG i.t. Control mice received PBS. On day 11, left inguinal lymph nodes were collected, pooled and stained with anti-CD4, anti-CD8, and with antibodies against T cell activation markers. The results are shown as histograms of viable lymph node cells gated on either CD4+ or CD8+ cells. Grey areas show staining with specific antibodies, white areas are isotype controls. Numbers above indicate percentage of positive cells and numbers below indicate mean fluorescence intensity.
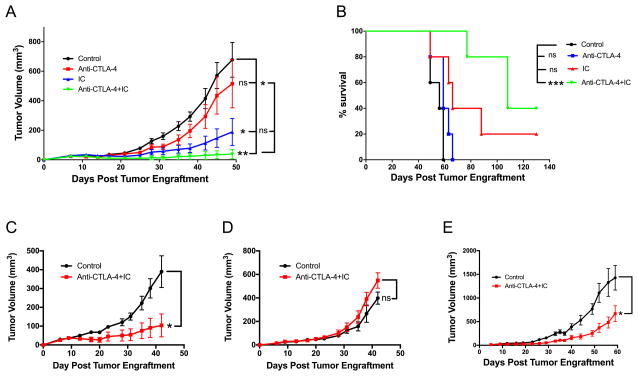
14.18-IL2 IC and anti-CTLA-4 synergize in inducing antitumor effects
Having established that anti-CD40 and CpG activate T cells, we thought to combine this therapy with another T cell-activating approach using IT treatment with 14.18-IL2 IC (14). As IT-IC has been shown to activate T cells (13,14), we first determined if checkpoint blockade in tumor-bearing mice would augment the antitumor effect of 14.18-IL2 IC. The results in Figure 3A show that, indeed, IT-IC and anti-CTLA-4 synergistically induced regression of a 7-day B78 melanoma resulting in survival of 40% of mice (Figure 3B). This antitumor effect was T cell-mediated as it was not observed in nude mice (Figure 3C vs. 3D). When the treatment with IT-IC and anti-CTLA-4 was used against more advanced tumors, i.e. starting on day 12 (Fig. 3E) post tumor cell implantation (vs. 7 days after tumor implantation, Figs. 3A–D), the antitumor effect was marginal with no mice rejecting the tumor. This lack of potency against slightly larger tumors indicates that the strength of the combination of IT-IC + anti-CTLA-4 is limited and not effective against more established tumors. We thus sought to test whether adding additional immunotherapy could be beneficial in this setting.
Figure 3.
Antitumor effect of the combination of 14.18-IL2 IC and anti-CTLA-4. A,B. B78 melanoma cells were injected s.c. into C57BL/6 mice (day 0). 14.18-IL2 IC (5 mcg/mouse) was injected i.t. daily on days 7–11. Anti-CTLA-4 i.p. (200 mcg/mouse) was injected i.p. on days 7,9,11,14,16,18. The data are shown as means ± SEM of tumor volumes (A) and survival (B) of 5 mice per group. C,D: Role of T cells. B78 melanoma cells were injected s.c. into C57BL/6 (C) and Nude (D) mice on day 0. 14.18-IL2 IC (5 mcg) was given i.t. on day 6–10. Anti-CTLA-4 (200 mcg) was given i.p. on day 6,8,10. Data shown are means ± SEM of 4–5 mice per group. E. Effect against advanced tumors. B78 cells were injected s.c. into C57BL/6 mice (day 0). Mice received 14.18-IL2 IC i.t. (5 mcg) on days 12–16, and anti-CTLA-4 i.p. on days 12,14,16,19,26 and 33. Data shown are means ± SEM of 4–5 mice per group.
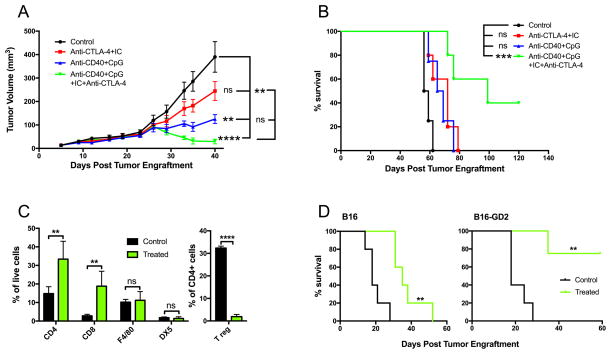
Synergistic effect of anti-CD40/CpG and IT-IC/anti-CTLA-4
We hypothesized that combining two different strategies - anti-CD40/CpG to activate innate immunity and IT-IC/anti-CTLA-4 to activate adaptive immunity - would result in an additive or synergistic antitumor effect against advanced B78 tumors. The results in Figure 4A show that when the treatment is started on day 23 post-tumor cell implantation (rather than on day 7 or 12, as in Figure 3), anti-CD40/CpG or IC/anti-CTLA-4 given separately had only a slight ability to slow growth of these advanced B78 tumors. In contrast, the combination of all these treatment strategies (anti-CD40/CpG + IC/anti-CTLA-4) not only slowed tumor growth, but caused their regression (Figure 4A), with 40% of animals becoming tumor-free and showing long term survival (Figure 4B). Examination of the cells in the tumor microenvironment revealed that the combined treatment resulted in the increase of CD4+ and CD8+ T cells, and dramatic reduction of T regulatory cells, whereas the percentage of NK cells and macrophages remained unchanged (Figure 4C).
Figure 4.
Synergistic antitumor effect of anti-CD40/CpG and 14.18-IL2 IC/anti-CTLA-4. B78 melanoma cells (2×106) were injected s.c. into C57BL/6 mice on day 0. Anti-CD40 (500 mcg) was injected i.p. on day 23; CpG (25 mcg) was injected i.t. on days 26,28,30. 14.18-IL2 IC (25 mcg) was given i.t. on days 26–30; anti-CTLA-4 (200 mg) was injected i.p. on day 26,28,30,33,35,40. The results are shown as means ± SEM of tumor volumes (A) and survival (B) of 5 mice per group. C. C57BL/6 mice bearing B78 tumors received combined treatment with anti-CD40/CpG and IC/anti-CTLA-4 (clone 9D9, IgG2a) as described in Figure 4A up to day 33. On day 34 tumors were removed, and single cell suspensions were evaluated for indicated immune cells by flow cytometry. The results are shown as means ± SEM of 5 mice per group. D. C57BL/6 mice were injected s.c. with 5×105 B16 or B16-GD2 cells. Anti-CD40 was injected i.p. on day 7; CpG was injected i.t. on days 10,12,14. 14.18-IL2 IC was given i.t. on days 10–14; anti-CTLA-4 (clone 9D9, IgG2a) was injected i.p. on day 10,12,14,17,19,21. Control mice received no treatment. The results are shown as survival of 5 mice per group.
Next we asked if GD2 expression on tumor cells is important for the antitumor activity of this combined treatment. As B16-derived GD2+ B78 melanoma grows much slower than B16, we have virally transduced B16 cells to express GD2. These B16-GD2 tumors grew in C57BL/6 mice at a rate similar to parental B16 tumors. We compared the effect of the combined treatment in B16 vs B16-GD2-bearing mice. The results in Figure 4D show that whereas anti-CD40/CpG + IC/anti-CTLA-4 therapy was effective in extending survival of mice with B16 tumors (P=0.002), all treated mice died prior to d60. In contrast, this same combined therapy was much more effective in mice bearing B16-GD2 tumors leading to 80% cure, suggesting that the anti-GD2 component of the IC plays a role in the antitumor effect of the combined treatment.
The antitumor effect of anti-CD40/CpG + IC/anti-CTLA-4 involves T cells
Next we determined the role of T cells in the antitumor effect observed with the combined anti-CD40/CpG + IC/anti-CTLA-4 regimen. C57BL/6 and nude mice were injected with B78 cells and given various treatments. B78 tumors initially shrank in all C57BL/6 mice, as a result of treatment with anti-CD40/CpG and IC/anti-CTLA-4 (Figure 5A, note the very small tumor volumes from day 28–35); however, in this experiment the tumors subsequently regrew. In contrast, no tumors shrank in nude mice treated with the anti-CD40/CpG and IC/anti-CTLA-4 combination (Figure 5B, note the larger mean tumor volumes on days 28–30 in these mice, compared to those in Figure 5A), although their growth was statistically slower than in untreated nude mice (Figure 5B). The antitumor effect of anti-CD40/CpG and IC/anti-CTLA-4 was more significant than all other treatments in C57BL/6 mice (Figure 5A), but was not different from the potency of anti-CD40/CpG (without IC/anti-CTLA-4) in nude mice (Figure 5B). These results were confirmed in antibody-depletion experiments. C57BL/6 mice were treated with anti-CD40/CpG and IC/anti-CTLA-4 between days 11 and 25 post-tumor cell implantation; one group was depleted of T cells by anti-CD4 and anti-CD8 mAbs while the other group received rat IgG. Both these groups showed similar antitumor efficacy (compared to the control mice not receiving anti-CD40/CpG and IC/anti-CTLA-4) up through day 31(Figure 5C). However, after day 40 the tumors in treated mice depleted of T cells were not significantly different from control, whereas the tumors in treated non-depleted mice were still significantly smaller (Figure 5C), indicating that T cells were not required for the antitumor effect during the initial 4 weeks, but were involved in sustaining the antitumor effect later on.
Figure 5.
Role of T cells but not NK cells in the antitumor effect of the combined treatment with anti-CD40/ CpG + 14.18-IL2 IC/anti-CTLA-4. C57BL/6 (A) and Nude mice (B) were injected s.c. with 2×106 B78 cells. Mice were treated with anti-CD40 (500 mg i.p.) on day 16 and CpG (25 mcg i.t.) on days 19, 21, 23; anti-CTLA-4 (200 mg i.p.) on days 19,21,23,26,28,30 and 14.18-IL2 IC (25 mcg i.t.) on days 19–23; combination of all four agents or PBS (control). The results are shown as means ± SEM of tumor volumes of 5 mice per group. C. C57BL/6 mice were injected s.c. with B78 cells. Treatment groups were injected with anti-CD40 on day 11, CpG on days 14, 16, 18, 14.18-IL2 IC on days 14–18, and anti-CTLA-4 on days 14,16,18,21,23,25. To deplete T cells, one group of treated mice received i.p. injections of both anti-CD4 and anti-CD8 (300 mcg each) on days 10,14,18,22,26, and another group of treated mice received rat IgG (600 mcg) as a control for T cell depletion. D. C57BL/6 mice were injected s.c. with B78 cells. All treatment groups were injected with anti-CD40 on day 10, CpG on days 13,15,17, anti-CTLA-4 on days 13,15,17,20,22,24, and hu14.18-IL2 IC on days 13–17 (designated as “Combo”). The mice received rat IgG, anti-CD4 + anti-CD8, anti-NK1.1 or a combination of anti-CD4/8 and anti-NK1.1 (all on days 9,13,17,21,25,29,33,37, and 41). The results are shown as means ± SEM of tumor volumes.
Given that anti-CD40 (7), CpG (30) and IC (14) can each activate NK cells, we next determined the role of NK cells in the antitumor effect of our combined treatment. Depletion of NK cells with anti-NK1.1 mAb did not reduce the antitumor effect of anti-CD40/CpG and IC/anti-CTLA-4. In fact, 1 of the 5 mice which received the combined treatment and anti-NK1.1 mAb rejected tumor and remained tumor-free. Furthermore, simultaneous depletion of both NK cells and T cells did not cause significant further reduction of the antitumor effect from the reduction of efficacy caused by depletion of T cells alone (Figure 5D). Together, these results suggest that some cell populations other than NK cells, likely macrophages, activated by anti-CD40/CpG (7,8,10) are responsible for the initial tumor killing, whereas T cells further activated by IT-IC and anti-CTLA-4 are responsible for the subsequent tumor cell eradication following the combined treatment in the non-NK/T depleted B78-bearing C57BL/6 mice (Fig. 5D).
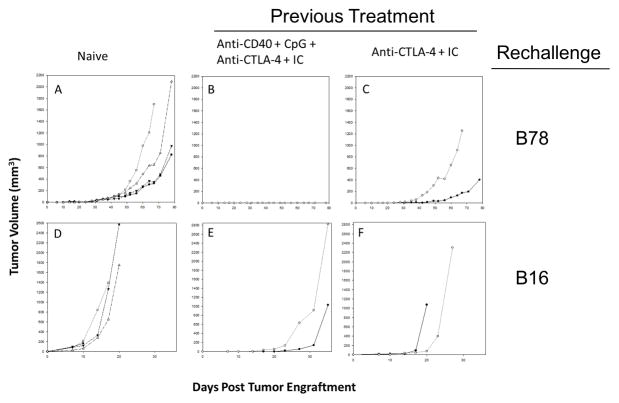
Anti-CD40/CpG + IC/anti-CTLA-4 induces immunological memory and systemic antitumor effects
We tested if the mice that were made tumor-free either by IC/anti-CTLA-4 (Figure 3B) or the combined treatment with anti-CD40/CpG + IC/anti-CTLA-4 (Figure 4B) had generated immunological memory and would be able to reject secondary tumor challenge given 2–7 months later. The results in Figure 6 show that mice which rejected their B78 tumors following the combined treatment with anti-CD40/CpG + IC/anti-CTLA-4 (Figure 6B) were resistant to rechallenge with B78 tumor cells, and mice which became tumor-free mice after IC/anti-CTLA-4 therapy were transiently resistant; they developed tumors after rechallenge (Figure 6C) but much later than the control mice (Figure 6C vs. 6A). The more aggressive, rapidly-growing, parental B16-F10, which in contrast to B78 does not express GD2, grew more slowly upon rechallenge in mice which previously rejected their B78 tumors following the combined treatment with anti-CD40/CpG + IC/anti-CTLA-4 (Figure 6E) compared to IC/anti-CTLA-4-treated (Figure 6F) and control mice (Figure 6D). The experiment using mice that rejected their B78 tumors following the combined treatment with anti-CD40/CpG + IC/anti-CTLA-4 was repeated, demonstrating similar results. These results indicate that this combination of anti-CD40/CpG, + IC/anti-CTLA-4 induces immunological memory in mice.
Figure 6.
Immunological memory in mice that rejected tumors following combined treatments. Naïve C57BL/6 mice (A,D) and C57BL/6 mice that rejected their B78 tumors following treatment with either 14.18-IL2 IC and anti-CTLA-4 (C,F,) or anti-CD40/CpG + 14.18-IL2 IC/anti-CTLA-4 (B,E), were injected s.c. with 2×106 B78 tumor cells (A–C) or 2×105 B16-F10 tumor cells (D–F). Tumor growth curves of individual mice are shown. The mice in group E (injected with B16-F10 cells) are the same mice as in group B that were rechallenged with B78 cells and did not develop tumors. The number of mice in each group shown are: A=4, B=2, C=2, D=4, E=2 and F=2. The time scales for D, E and F are smaller than for A, B and C because the B16-F10 grows far more quickly than does B78.
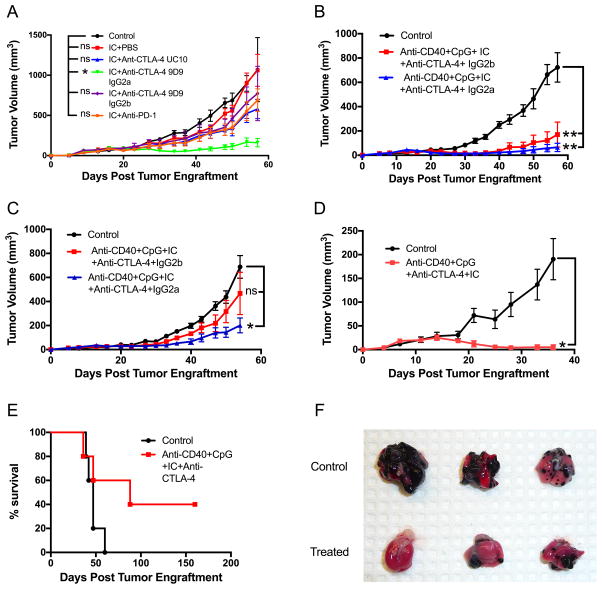
The experiments described above have demonstrated the antitumor effect against a locally treated tumor. Given the evidence that this combination of anti-CD40/CpG + IC/anti-CTLA-4 induces immunological memory (Figure 6), we hypothesized that this treatment may also be active against distant tumors. To potentially increase the antitumor effect, we first tested anti-CTLA-4 IgG2a (Figure 7), which was reported to be more effective than anti-CTLA-4 IgG2b (the isotype we used in Figures 3–6), likely by mediating a better reduction of T regulatory cells within a tumor (23). We found that, when combined with IC, anti-CTLA-4 IgG2a was more effective than anti-CTLA-4 IgG2b against B78 melanoma (Figure 7A). To test if anti-CD40/CpG + IC/anti-CTLA-4 immunotherapy is effective against a distant solid tumor, naïve C57BL/6 mice were injected on day 0 with B78 melanoma cells into both the left and right sides of abdomen. Treatment began on day 9 with IT injection of the tumor on the left side only with CpG and 14.18-IL2 IC, and with i.p. administration of anti-CD40 and anti-CTLA-4, either IgG2a or IgG2b. The results in Figure 7B show that anti-CD40/CpG + IC/anti-CTLA-4 IgG2a substantially suppressed growth of the tumor on the left side that had received the IT treatment. This same anti-CD40/CpG + IC/anti-CTLA-4 IgG2a treatment with IT CpG and IC to the left tumor also resulted in statistically significant reduction of the distant non-injected tumor on the right (Figure 7C). When the anti-CTLA-4 IgG2b was substituted for the IgG2a in this same regimen, there was no significant tumor reduction for the non-injected tumor on the right (Figure 7C). These results indicate that this anti-CD40/CpG + IC/anti-CTLA-4 IgG2a therapy had a systemic antitumor effect.
Figure 7.
Systemic antitumor and anti-metastatic effects of the combined therapy. A. Comparison of different checkpoint antibodies in combination with 14.18-IL2 IC against B78 melanoma. C57BL/6 mice were injected s.c. with 2×106 B78 cells. 14.18-IL2 IC (5 mcg/mouse) was injected i.t. on days 12–16. Various checkpoint antibodies (200 mcg/mouse) were injected i.p. on days 12,14,16,19,21 and 23. B,C. B78 melanoma cells (2×106) were injected s.c. into the right and left sides of the abdomen on day 0. Each tumor on the left was injected with CpG (25 mcg) on days 12,14,16 and with14.18-IL2 IC (25 mcg) on days 12–16. These treatments were given in combination with i.p. injections of anti-CD40 (500 mcg) on day 9 and anti-CTLA-4, IgG2a (200 mcg) on days 12,14,16,19,21,23. The results are shown as means ± SEM of volumes of the left side (treated) (B) and right side (untreated) (C) tumors. D-F: C57BL/6 mice were injected s.c. with 2×106 B78 melanoma cells (day 0) and i.v. with 1×105 B16-F10 melanoma cells (day 1). Mice were treated with anti-CD40 (0.5 mg) i.p. on day 8, with CpG (25 mcg) i.t. on days 11,13,15, with 14.18-IL2 IC (25 mcg) i.t. on days 11–15, and with anti-CTLA-4 IgG2a (200 mcg) i.p. on days 11,13,15,18,20,21. The results are shown as means ± SEM of s.c. tumor volumes (D) and survival (E) of 5 mice per group. Lung photographs taken on day 32 from a separate identical experiment are shown, contrasting visable metastases in control vs. treated mice (F).
To confirm this systemic effect of the combined treatment, we tested it in a metastatic model, using i.v. injection of B16-F10 tumor cells, which has been shown previously to induce numerous lung metastases (31). We used GD2− parental B16-F10 cells rather than GD2+ B78 cells for metastasis induction to exclude a direct role of the anti-GD2 14.18 mAb, a part of 14.18-IL2 IC, in the anti-metastatic effect. In mice that received B78 cells s.c. on day 0 and B16-F10 cells i.v. on day 1, the mice in the control (untreated group) showed progressive growth of their s.c. tumor, yet died of metastatic disease before the s.c. tumors grew large enough to require euthanasia. The combined treatment of these mice with CpG and 14.18-IL2 IC injected i.t. into the s.c. B78 tumor, and with anti-CD40 and anti-CTLA-4 (IgG2a) injected i.p., induced reduction of the primary tumors (Figure 7D) and also had an anti-metastatic effect, demonstrated by survival of 40% of treated mice whereas all control mice died (Figure 7E). Autopsies of dead mice confirmed the presence of metastases in the lungs or axillary lymph nodes. An additional identical experiment showed that the lungs removed on day 32 when some mice started dying exhibited melanoma metastases, which were more prevalent in the control group than the treatment group (Figure 7F).
Discussion
Most cancer immunotherapy strategies are focusing on activating adaptive immunity involving T cells. Some studies are targeting cells of the innate immune system; of these, most focus on activating NK cells (32). Some T-cell approaches have recently shown substantial clinical benefit, such as: CD19 - directed chimeric antigen receptor (CAR) - modified T cells for B cell malignancies (33) and the use of checkpoint blockade (CTLA-4 and PD-1) for melanoma and certain other malignancies (19–21). Even so, most patients with cancer are not currently receiving immunotherapy or benefitting from it. Preclinical data suggest that combining two or more immunotherapeutic approaches may enable greater antitumor efficacy than treatment with a single immunotherapeutic agent (3–5, 34,35). The majority of these combinatorial approaches, such as those adding STING (36) or FLT3 ligand (37) to other treatments, target T cell immunity, whereas some other approaches target innate immunity (38). In this study we tested the hypothesis that combining both immunological approaches, one targeting innate immunity and the other targeting T cells, will result in enhanced antitumor efficacy.
In previous studies we reported synergy between agonistic anti-CD40 and CpG via activation of innate immunity, mainly macrophages (10). This combination induced clear retardation of tumor growth but rarely resulted in complete tumor regression. We hypothesized that adding a strategy that enabled adaptive immune responses with T cell involvement would enhance antitumor efficacy and potentially induce immunological memory. We have previously developed an approach which involved tumor killing, partially by T cells, via i.t. administration of IC (13). However, before combining anti-CD40/CpG and IC, we considered ways to enhance the T cell-mediated antitumor effect of IC by using checkpoint blockade with anti-CTLA-4. Our results show a synergy between hu14.18-IL2 IC and anti-CTLA-4 that resulted in a T cell-dependent rejection of B78 tumors. These results are in agreement with a report by Schwager et al (39) showing that combining anti-CTLA-4 with a different IC, L19-IL2, induced a better antitumor effect than these two agents given separately.
In spite of the efficacy of IC combined with anti-CTLA-4 against small tumors (Fig. 3A–C), this treatment was less effective against larger tumors (Figure 3E). Therefore, we combined 14.18-IL2 IC and anti-CTLA-4 with anti-CD40 and CpG. Instead of injecting CpG i.p., as in our previous studies (10), we gave it here i.t. because it was shown that local treatment with CpG can induce both innate and adaptive immunity (28, 40). Similar to our previous studies (10), the antitumor effect of anti-CD40 given i.p. and CpG given i.t. was largely T cell-independent (Figure 1), suggesting that this combination activates macrophages, as shown in our previous studies (10, 12). The results of this study show that a combination of anti-CD40 and CpG (activating mostly innate immunity) with 14.18-IL2 IC and anti-CTLA-4 (activating mostly adaptive immunity) induced a substantial antitumor effect resulting in regression of advanced tumors and survival of 40% of mice (Figure 4A,B). This antitumor effect was systemic because a distant, untreated s.c. tumor (Figure 7C) or lung metastases (Figures 7E–F) were also inhibited. In addition, mice that became tumor-free, long-term survivors, exhibited tumor-reactive immunological memory (Figure 6B).
Checkpoint blockade treatment has shown clear clinical benefit, with FDA approval in several cancers (1, 21, 41–43). There is a growing enthusiasm for testing checkpoint blockade in combination with other approaches to augment immune-mediated antitumor effects (5, 39). Anti-CTLA-4 has been combined in preclinical studies with each of the separate types of agents used in our study. Enhanced antitumor effects of checkpoint blockade and local treatment with CpG have been reported (44, 45). A combinatorial therapy using anti-CTLA-4 and agonistic anti-CD40 induced a stronger T cell-mediated antitumor effect than either treatment given individually (46, 47). Anti-CTLA-4 was synergistic with the immunocytokine L19-IL2 (34). Here we show for the first time that a rational combination of all four of these immunomodulatory agents (anti-CD40, CpG, antibody-IL2 IC and anti-CTLA-4), each of which is either in clinical testing or already approved for clinical use, results in activation of innate and adaptive immunity and a synergistic antitumor effect resulting in more potent antitumor efficacy against well-established tumors. Addressing the mechanisms of this synergistic effect, we found that anti-CD40/CpG treatment of tumor-bearing mice induced local and systemic activation of T cells. This activation might be a result of local tumor destruction by activated macrophages and subsequent tumor antigen presentation. The mechanisms of anti-CTLA-4 augmenting T cell responses have been shown by others to be related to the blockade of the inhibitory activity of CTLA-4 on effector T cells (17,18) and also to the depletion of CD4+ T regulatory cells (46,48), particularly for the anti-CTLA-4 IgG2a isotype. When tested in combination with 14.18-IL2 IC against B78 melanoma, anti-CTLA-4 IgG2a was more effective than IgG2b, suggesting that better T regulatory cell depletion by anti-CTLA-4 IgG2a (23) is playing a beneficial role in our combined immunotherapy. We found that the combined treatment with anti-CD40/CpG + IC/anti-CTLA-4 substantially reduced T regulatory cells and increased the number of CD4+ and CD8+ T cells (Figure 4C); the relative contribution of individual treatments, and distinct T cell subpopulations, to this effect remains to be determined.
The combined treatment with anti-CD40/CpG + IC/anti-CTLA-4 induced much better antitumor effects in mice bearing GD2-expressing B16 tumor compared with parental B16 tumor, suggesting among other possibilities that 14.18 mAb in the IC plays a role in this antitumor effect. When 14.18-IL2 IC was given i.v. it had a much greater antitumor effect than a combination of IL2 and 14.18 mAb (49). In addition, when given IT as single agent treatment, an IC able to bind to the tumor via its mAb component is more effective than either IT administration of IL2 or IT administration of an IC consisting of a control mAb, unable to bind to the tumor (13). Even so, when these reagents are delivered in the tumor by IT injection, as in this study, the comparative efficacy of IC versus 14.18 mAb + IL2 versus IL2 alone, when given in combination with anti-CD40/CpG + anti-CTLA-4, is yet to be determined.
Overall, our results indicate that this combination of anti-CD40/CpG + IC/anti-CTLA-4 is more effective than its component parts, and activates responses via both innate and adaptive immune effects. These findings also provide the preclinical justification for further development of this form of combined cancer immunotherapy strategy in order to pursue early-phase clinical testing.
Supplementary Material
Acknowledgments
We thank Mrs. Lakeesha Carmichael for help with the statistical analysis of the data and Dr. Amy Erbe-Gurel for transfecting B16 cells with GD2.
Source of Funding: This work was supported by National Institutes of Health Grants CA032685, CA87025, CA166105, CA14520, CA197078, GM067386, and grants from the Midwest Athletes for Childhood Cancer Fund, The Crawdaddy Foundation, The Evan Dunbar Foundation, Hyundai Hope on Wheels Foundation, The University of Wisconsin-Madison Institute for Clinical and Translational Research Grant 1TL1RR025013-01, The Alex’s Lemonade Stand Foundation, The St. Baldrick’s Foundation, and Stand Up To Cancer – St. Baldrick’s Pediatric Dream Team Translational Research Grant (SU2C-AACR-DT1113). Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research.
Abbreviations
- anti-CD40
anti-CD40 monoclonal antibody
- IC
immunocytokine
- IT
intratumoral
Footnotes
Competing interests: Dr. H. Loibner declares employment and ownership interests in Apeiron Biologics Inc. Dr. S. Gilles declares employment and ownership interests in Provenance Biopharmaceuticals. Dr. A. Korman declares employment and equity interests in Bristol Myers Squibb. All other authors declare no conflicts of interest.
References
- 1.Couzin-Frankel J. Breakthrough of the year 2013: Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 2.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O’Dwyer PJ, Vonderheide RH. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rini B. Future approaches in immunotherapy. 2014. Semin Oncol. 41(Suppl 5):S30–40. doi: 10.1053/j.seminoncol.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Mahmood S, Upreti D, Sow I, Amari A, Nandagopal S, Kung SK. Bidirectional interactions of NK cells and dendritic cells in immunotherapy: current and future perspective. Immunotherapy. 2015;7:301–308. doi: 10.2217/imt.14.122. [DOI] [PubMed] [Google Scholar]
- 5.Zamarin D, Postow MA. Immune checkpoint modulation: rational design of combination strategies. Pharmacol Ther. 2015;150:23–32. doi: 10.1016/j.pharmthera.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Rakhmilevich AL, Alderson KL, Sondel PM. T cell-independent antitumor effects of CD40 ligation. Review. Int Rev Immunol. 2012;31:267–278. doi: 10.3109/08830185.2012.698337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buhtoiarov IN, Lum H, Berke G, Paulnock D, Sondel PM, Rakhmilevich AL. CD40 ligation induces antitumor reactivity of murine macrophages via an IFN gamma-dependent mechanism. J Immunol. 2005;174:6013–6022. doi: 10.4049/jimmunol.174.10.6013. [DOI] [PubMed] [Google Scholar]
- 8.Lum HD, I, Buhtoiarov N, Berke G, Paulnock DM, Sondel PM, Rakhmilevich AL. In vivo CD40 ligation can induce T cell-independent antitumor effects that involve macrophages. J Leuk Biol. 2006;79:1181–1192. doi: 10.1189/jlb.0405191. [DOI] [PubMed] [Google Scholar]
- 9.Rakhmilevich AL, I, Buhtoiarov N, Malkovsky M, Sondel PM. CD40 ligation in vivo can induce T cell independent antitumor effects even against immunogenic tumors. Cancer Immunol Immunother. 2008;57:1151–1160. doi: 10.1007/s00262-007-0447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buhtoiarov IN, Lum HD, Berke G, Sondel PM, Rakhmilevich AL. Synergistic activation of macrophages via CD40 and TLR9 results in T cell independent antitumor effects. J Immunol. 2006;176:309–318. doi: 10.4049/jimmunol.176.1.309. [DOI] [PubMed] [Google Scholar]
- 11.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, Klapproth K, Schäkel K, Garbi N, Jäger D, Weitz J, Schmitz-Winnenthal H, Hämmerling GJ, Beckhove P. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Buhtoiarov IN, Sondel PM, Wigginton JM, Buhtoiarova TN, Yanke EM, Mahvi DA, Rakhmilevich AL. Antitumor Synergy of Cytotoxic Chemotherapy and Anti-CD40 Plus CpG-ODN Immunotherapy Through Repolarization of Tumor Associated Macrophages. Immunol. 2011;132:226–239. doi: 10.1111/j.1365-2567.2010.03357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson EE, Lum HD, Rakhmilevich AL, Schmidt BE, Furlong M, Buhtoiarov IN, Hank JA, Raubitschek A, Colcher D, Reisfeld RA, Gillies SD, Sondel PM. Intratumoral Immunocytokine Treatment Results in Enhanced Antitumor Effects. Cancer Immunol Immunother. 2008;57:1891–902. doi: 10.1007/s00262-008-0519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang RK, Kalogriopoulos NA, Rakhmilevich AL, Ranheim EA, Seo S, Kim KM, Alderson KL, Gan J, Reisfeld RA, Gillies SD, Hank JA, Sondel PM. Intratumoral hu14.18-IL2 (IC) Induces Local and Systemic Antitumor Effects that Involve Both Activated T- and NK cells as well as Enhanced IC Retention. J Immunol. 2012;189:2656–2664. doi: 10.4049/jimmunol.1200934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker JC, Andersen MH, Schrama D, Thor Straten P. Immune-suppressive properties of the tumor microenvironment. Cancer Immunol Immunother. 2013;62:1137–1148. doi: 10.1007/s00262-013-1434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bazhin AV, Bayry J, Umansky V, Werner J, Karakhanova S. Overcoming immunosuppression as a new immunotherapeutic approach against pancreatic cancer. Oncoimmunol. 2013;2:e25736. doi: 10.4161/onci.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolchok JD, Saenger Y. The mechanism of anti-CTLA-4 activity and the negative regulation of T-cell activation. Oncologist. 2008;13(Suppl 4):2–9. doi: 10.1634/theoncologist.13-S4-2. [DOI] [PubMed] [Google Scholar]
- 18.Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer--preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 2010;37:430–439. doi: 10.1053/j.seminoncol.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Ramsay AG. Immune checkpoint blockade immunotherapy to activate anti-tumour T-cell immunity. Br J Haematol. 2013;162:313–325. doi: 10.1111/bjh.12380. [DOI] [PubMed] [Google Scholar]
- 20.Kyi C, Postow MA. Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett. 2014;588:368–376. doi: 10.1016/j.febslet.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 22.Deppong CM, Bricker TL, Rannals BD, Van Rooijen N, Hsieh CS, Green JM. CTLA4Ig inhibits effector T cells through regulatory T cells and TGF-β. J Immunol. 2013;191:3082–3089. doi: 10.4049/jimmunol.1300830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, Korman AJ. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1:32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 24.Straten PT, Guldberg P, Seremet T, Reisfeld RA, Zeuthen J, Becker JC. Activation of preexisting T cell clones by targeted interleukin 2 therapy. Proc Natl Acad Sci USA. 1998;95:8785–8790. doi: 10.1073/pnas.95.15.8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillies SD, Reilly EB, Lo KM, Reisfeld RA. Antibody-targeted interleukin 2 stimulates T-cell killing of autologous tumor cells. Proc Natl Acad Sci USA. 1992;89:1428–1432. doi: 10.1073/pnas.89.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mokyr MB, Kalinichenko T, Gorelik L, Bluestone JA. Realization of the Therapeutic Potential of CTLA-4 Blockade in Low-Dose Chemotherapy-treated Tumor-bearing Mice. Cancer Res. 1998;58:5301–5304. [PubMed] [Google Scholar]
- 27.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, Tangsombatvisit S, Grosso JF, Netto G, Smeltzer MP, Chaux A, Utz PJ, Workman CJ, Pardoll DM, Korman AJ, Drake CG, Vignali DA. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houot R, Levy R. T-cell modulation combined with intratumoral CpG cures lymphoma in a mouse model without the need for chemotherapy. Blood. 2009;113:3546–3552. doi: 10.1182/blood-2008-07-170274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van De Voort TJ, Felder MAR, Yang RK, Sondel PM, Rakhmilevich AL. Intratumoral delivery of low doses of anti-CD40 mAb combined with monophosphoryl lipid A induces T cell-independent, local and systemic antitumor effects in mice. J Immunother. 2013;36:29–40. doi: 10.1097/CJI.0b013e3182780f61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buhtoiarov IN, Sondel PM, Eickhoff JC, Rakhmilevich AL. Macrophages are essential for antitumor effects against weakly immunogenic murine tumors induced by class B CpG-ODN. Immunol. 2007;120:412–423. doi: 10.1111/j.1365-2567.2006.02517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fidler IJ, Nicolson GL. Organ selectivity for implantation survival and growth of B16 melanoma variant tumor lines. J Natl Cancer Inst. 1976;57:1199–202. doi: 10.1093/jnci/57.5.1199. [DOI] [PubMed] [Google Scholar]
- 32.Berrien-Elliott MM, Romee R, Fehniger TA. Improving natural killer cell cancer immunotherapy. Curr Opin Organ Transplant. 2015;20:671–680. doi: 10.1097/MOT.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heiblig M, Elhamri M, Michallet M, Thomas X. Adoptive immunotherapy for acute leukemia: New insights in chimeric antigen receptors. World J Stem Cells. 2015;7:1022–1038. doi: 10.4252/wjsc.v7.i7.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spranger S, Gajewski T. Rational combinations of immunotherapeutics that target discrete pathways. J Immunother Cancer. 2013;1:16. doi: 10.1186/2051-1426-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stone GW, Barzee S, Snarsky V, Santucci C, Tran B, Langer R, Zugates GT, Anderson DG, Kornbluth RS. Nanoparticle-delivered multimeric soluble CD40L DNA combined with Toll-Like Receptor agonists as a treatment for melanoma. PLoS One. 2009;4:e7334. doi: 10.1371/journal.pone.0007334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demaria O, De Gassart A, Coso S, Gestermann N, Di Domizio J, Flatz L, Gaide O, Michielin O, Hwu P, Petrova TV, Martinon F, Modlin RL, Speiser DE, Gilliet M. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc Natl Acad Sci U S A. 2015;112:15408–15413. doi: 10.1073/pnas.1512832112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013;73:6900–6912. doi: 10.1158/0008-5472.CAN-13-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manrique SZ, Dominguez AL, Mirza N, Spencer CD, Bradley JM, Finke JH, Lee JJ, Pease LR, Gendler SJ, Cohen PA. Definitive activation of endogenous antitumor immunity by repetitive cycles of cyclophosphamide with interspersed Toll-like receptor agonists. Oncotarget. 2016;7:42919–42942. doi: 10.18632/oncotarget.10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwager K, Hemmerle T, Aebischer D, Neri D. The immunocytokine L19-IL2 eradicates cancer when used in combination with CTLA-4 blockade or with L19-TNF. J Invest Dermatol. 2013;133:751–758. doi: 10.1038/jid.2012.376. [DOI] [PubMed] [Google Scholar]
- 40.Veenstra JJ, Gibson HM, Littrup PJ, Reyes JD, Cher ML, Takashima A, Wei WZ. Cryotherapy with concurrent CpG oligonucleotide treatment controls local tumor recurrence and modulates HER2/neu immunity. Cancer Res. 2014;74:5409–5420. doi: 10.1158/0008-5472.CAN-14-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janakiram M, Pareek V, Cheng H, Narasimhulu DM, Zang X. Immune checkpoint blockade in human cancer therapy: lung cancer and hematologic malignancies. Immunother. 2016;8:809–819. doi: 10.2217/imt-2016-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zibelman M, Ghatalia P, Geynisman DM, Plimack ER. Checkpoint inhibitors for renal cell carcinoma: current landscape and future directions. Immunother. 2016;8:785–798. doi: 10.2217/imt-2016-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia Y, Medeiros LJ, Young KH. Immune checkpoint blockade: Releasing the brake towards hematological malignancies. Blood Rev. 2016;30:189–200. doi: 10.1016/j.blre.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Mangsbo SM, Sandin LC, Anger K, Korman AJ, Loskog A, Tötterman TH. Enhanced tumor eradication by combining CTLA-4 or PD-1 blockade with CpG therapy. J Immunother. 2010;33:225–235. doi: 10.1097/CJI.0b013e3181c01fcb. [DOI] [PubMed] [Google Scholar]
- 45.Marabelle A, Kohrt H, Sagiv-Barfi I, Ajami B, Axtell RC, Zhou G, Rajapaksa R, Green MR, Torchia J, Brody J, Luong R, Rosenblum MD, Steinman L, Levitsky HI, Tse V, Levy R. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest. 2013;123:2447–2463. doi: 10.1172/JCI64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takeda K, Kojima Y, Uno T, Hayakawa Y, Teng MW, Yoshizawa H, Yagita H, Gejyo F, Okumura K, Smyth MJ. Combination therapy of established tumors by antibodies targeting immune activating and suppressing molecules. J Immunol. 2010;184:5493–5501. doi: 10.4049/jimmunol.0903033. [DOI] [PubMed] [Google Scholar]
- 47.Sckisel GD, Mirsoian A, Bouchlaka MN, Tietze JK, Chen M, Blazar BR, Murphy WJ. Late administration of murine CTLA-4 blockade prolongs CD8-mediated anti-tumor effects following stimulatory cancer immunotherapy. Cancer Immunol Immunother. 2015;64:1541–1552. doi: 10.1007/s00262-015-1759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, Peggs KS, Ravetch JV, Allison JP, Quezada SA. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lode HN, Xiang R, Varki NM, Dolman CS, Gillies SD, Reisfeld RA. Targeted interleukin-2 therapy for spontaneous neuroblastoma metastases to bone marrow. J Natl Cancer Inst. 1997;89:1586–1594. doi: 10.1093/jnci/89.21.1586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.