Abstract
The endocannabinoid system in the brain and periphery plays a major role in controlling food intake and energy balance. We reported that tasting dietary fats was met with increased levels of the endocannabinoids, 2-arachidonoyl-sn-glycerol (2-AG) and anandamide, in the rat upper small intestine, and pharmacological inhibition of this local signaling event dose-dependently blocked sham feeding of fats. We now investigated the contribution of peripheral endocannabinoid signaling in hyperphagia associated with chronic consumption of a western-style diet in mice ([WD] i.e., high fat and sucrose). Feeding patterns were assessed in male C57BL/6Tac mice maintained for 60 days on WD or a standard rodent chow (SD), and the role for peripheral endocannabinoid signaling at CB1Rs in controlling food intake was investigated via pharmacological interventions. In addition, levels of the endocannabinoids, 2-AG and anandamide, in the upper small intestine and circulation of mice were analyzed via liquid chromatography coupled to tandem mass spectrometry to evaluate diet-related changes in endocannabinoid signaling and the potential impact on food intake. Mice fed WD for 60 days exhibited large increases in body weight, daily caloric intake, average meal size, and rate of feeding when compared to control mice fed SD. Inhibiting peripheral CB1Rs with the peripherally-restricted neutral cannabinoid CB1 receptor antagonist, AM6545 (10mg/kg), significantly reduced intake of WD during a 6 h test, but failed to modify intake of SD in mice. AM6545 normalized intake of WD, average meal size, and rate of feeding to levels found in SD control mice. These results suggest that endogenous activity at peripheral CB1Rs in WD mice is critical for driving hyperphagia. In support of this hypothesis, levels of 2-AG and anandamide in both, jejunum mucosa and plasma, of ad-libitum fed WD mice increased when compared to SC mice. Furthermore, expression of genes for primary components of the endocannabinoid system (i.e., cannabinoid receptors, and endocannabinoid biosynthetic and degradative enzymes) was dysregulated in WD mice when compared to SC mice. Our results suggest that hyperphagia associated with WD-induced obesity is driven by enhanced endocannabinoid signaling at peripheral CB1Rs.
Keywords: endocannabinoid, 2-AG, anandamide, peripheral, western diet, obesity
1. INTRODUCTION
Significant scientific and clinical evidence suggests one major driver of obesity is chronic consumption of foods that contain large quantities of fats and sugars (i.e., the western diet) [1, 2]. Humans, rodents, and possibly other mammals detect dietary fats [3–7] and sugars [8] via receptors in the oral cavity and alimentary tract, which are critical in mediating preferences displayed for these high-energy foods [6, 9, 10]. Numerous signaling pathways play important roles in the control of food intake, energy balance, and reward [9, 11], including endocannabinoid (eCB) signaling at cannabinoid CB1Rs in the brain [12–23] and periphery [12, 24–34].
Recent evidence suggests that the intake of palatable foods may be controlled by peripheral eCB signaling [9]. For example, tasting emulsions containing mono- (i.e., oleic acid) or di-unsaturated fats (i.e., linoleic acid) – but not carbohydrate (i.e., sucrose) or protein – was met with large increases in eCB levels in the rat upper small intestine [27, 29]. Pharmacological inhibition of eCB signaling at peripheral CB1Rs blocked (i) the intake of dietary fats in sham feeding rats [27] and (ii) robust preferences for di-unsaturated fats in a sham feeding two-bottle choice test [29] [see [6, 35] for description of the sham feeding paradigm in rat, which isolates the cephalic phase of feeding from post-ingestive influence].
In addition to tasting dietary fats, we reported that fasting for up to 24 h is associated with increases in production of the eCB, 2-arachidonoyl-sn-glycerol (2-AG), in the upper small intestine of rats through a cholinergic-dependent mechanism that possibly involves the vagus nerve [28]. For these experiments, fasting-induced 2-AG biosynthesis in the jejunum mucosa was blunted in rats that received either full subdiaphragmatic vagotomy or local intraduodenal infusion of the subtype 3 muscarinic acetylcholine receptor (m3 mAChR) antagonist, DAU5884 [28]. Furthermore, pharmacological inhibition of small intestinal m3 mAChRs or CB1Rs blocked fasting-induced refeeding [28]. Thus, gut-brain eCB signaling is a proposed orexigenic signal that may promote feeding under several distinct behavioral and metabolic conditions.
Several studies in humans and rodents suggest that peripheral eCB levels are increased under conditions of obesity [36–42]; however, a role for peripheral eCB signaling in driving hyperphagia associated with a western-style diet (WD) is unknown. In the current study, we investigated the impact of chronic consumption of WD on eCB levels in circulation and the upper small intestinal epithelium of mice, the contribution of WD-induced enhancements in eCB signaling at peripheral CB1Rs in promoting hyperphagia associated with a WD, and expression of genes encoding key eCB system components in the small intestine.
2. MATERIALS AND METHODS
2.1. Animals
Eight-week old male C57BL/6 mice (Taconic, Oxnard, CA, USA) were group-housed with free access to water and food, unless otherwise noted for food deprivation studies, and maintained on a 12 hour light/dark cycle (lights off at 1800 h). Test diets consisted of standard lab rodent chow [(SD) Lab Diet 5001, St. Louis, MO, USA; 13.4 % kcal as fat, 56% kcal from carbohydrates, mostly starch], or western-style diet [(WD) Research Diets D12709B, New Brunswick, NJ, USA; 40% kcal as fat, 43% kcal from carbohydrates, mostly sucrose]. Five days prior to experimentation, animals were single-housed in cages with wire mesh inserts to prevent coprophagia during 24 h food deprivation experiments. For studies analyzing feeding behaviors, mice were single-housed in feeding chambers (TSE, Chesterfield, MO, USA) with free access to water and SD or WD (described further below in Feeding behavior). All procedures met the U.S. National Institute of Health guidelines for care and use of laboratory animals, and were approved by the Institutional Animal Care and Use Committee of the University of California, Riverside.
2.2. Feeding behavior
Animals were acclimated to feeding chambers for five days prior to experimentation, and tested following 60 days on their respective test diet. Feeding behavior was monitored for the subsequent 24 h to assess daily intake patterns, or for 6 h following drug treatments. Feeding parameters included total caloric intake, average meal size, average rate of intake (kcals from food per minute), average number of meals, average meal duration, and average post-meal interval.
2.3. Chemicals and administration schedule
The peripherally restricted Cannabinoid Receptor Type 1 (CB1) antagonist, AM6545 (Sigma, St. Louis, MO, USA), was administered by IP injection at 10mg/kg in 2mL/kg. Vehicle consisted of 7.5% dimethyl sulfoxide (DMSO, Sigma, St. Louis, MO, USA), 7.5% tween 80 (Chem Impex Intl Inc., Wood Dale, IL, USA), 85% sterile saline (warmed in a bath sonicator for 30 minutes) 30 minutes prior (16:30 h) to the onset of behavioral analysis in feeding chambers. All control conditions were identical, except without drug present in the vehicle. The pharmacokinetics and half-life of AM6545 are not well-established, thus we chose to evaluate intakes over a period of 6 h from time of administration after the onset of the dark phase. Mice maintained on SD or WD were split into two groups and analyzed across two sessions of behavioral testing such that each subgroup received either vehicle or drug with 72h between administration.
2.4. Tissue processing
2.4.1. Lipid extraction
Isofluorane was used to anesthetize animals at time of tissue harvest (0900 to 1100 h), following 24 h food deprivation or ad-libitum feeding. Blood was collected by cardiac puncture and stored in EDTA-lined tubes on ice, then plasma was obtained by centrifugation (1500 g for 10 minutes, maintained at 4°C). Jejunum was rapidly collected, washed with phosphate-buffered saline (PBS) on ice, sliced longitudinally on a stainless steel plate on ice, scraped with a glass slide to obtain mucosa, then snap-frozen in liquid N2. All samples were stored at −80°C until processing. Frozen tissues were weighed and subsequently homogenized in 1.0 mL of methanol solution containing the internal standard, [2H5] 2-AG and [2H4]-AEA (Cayman Chemical, Ann Arbor, MI, USA). Lipids were extracted with chloroform (2 mL) and washed with water (1 mL). Lipids were similarly extracted from plasma samples, with the exception of a 0.9 % saline wash replacing water (0.1 mL plasma at the expense of saline). Organic phases were collected and separated by open-bed silica gel column chromatography as previously described [28]. Eluate was gently dried under N2 stream (99.998% pure) and resuspended in 0.1 mL of methanol:chloroform (9:1), with 1μL injection for analysis by ultra-performance liquid chromatography coupled to tandem mass spectrometry (UPLC/MS/MS).
2.4.2. Measurement of 2-AG and anandamide
Data was collected using an Acquity I Class UPLC system coupled to a Xevo TQ-S Mass Spectrometer (Waters, Milford, MA, USA) with accompanying electrospray ionization (ESI) interface. Lipids were separated on an Acquity UPLC BEH C18 column (2.1 × 50 mm i.d., 1.7μm, Waters) with inline Acquity guard column (UPLC BEH C18 VanGuard Pre-column; 2.1 × 5 mm i.d., 1.7μm, Waters), and eluted by a gradient of methanol in water (0.25% acetic acid, 5mM ammonium acetate) according to the following gradient at a flow rate of 0.4 mL per min: 80% methanol 0.5 min, 80% to 100% methanol 0.5–2.5 min, 100% methanol 2.5–3 min, 100% – 80% methanol 3–3.1 min). Column temperature was maintained at 40° C, and samples were maintained in the sample manager at 10° C. Argon (99.998%) was used as collision gas. MS detection was in positive ion mode and capillary voltage set at 0.1 kV. Cone voltage and collision energy as follows, respectively: 2-AG = 30v, 12v; [2H5] 2-AG = 25v, 44v; anandamide = 30v, 14v; [2H4] anandamide = 26v, 16v. Lipids were quantified using a stable isotope dilution method detecting protonated adducts of the molecular ions [M+H]+ in the multiple reaction monitoring (MRM) mode. Acyl migration from 2-AG to 1-AG is known to occur [43], thus all reported values for 2-AG represent the sum of 2-AG and 1-AG. Tissue processing and LCMS analysis from an individual experiment occurred independently of other experiments. Extracted ion chromatograms were used to quantify 2-AG (m/z = 379.3>287.3) and anandamide (m/z = 348.3>62.0), and [2H5] 2-AG (m/z = 384.3 > 93.4) and [2H4] anandamide (m/z = 352.3>66.1), which were used as an internal standards. Our established lower limit of quantitation (LLOQ; signal-to-noise ratio of greater than 10) of analytes using our optimized UPLC/MS/MS methods are as follows: 2-AG, 0.5 pmol; AEA, 0.008 pmol.
2.5. Gene expression analysis
Total RNA was extracted from jejunal mucosa using a Trizol (Invitrogen, Carlsbad, CA) and RNeasy (Qiagen, Valencia, CA) combined method, and generated first-strand complementary DNA using M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA). All surfaces for tissue collection and processing were sanitized using 70% ethanol and then treated with an RNAse inhibitor (RNAse out, G-Biosciences, St. Louis, MO, USA) to maintain integrity of isolated RNA. Reverse transcription of total RNA (9.5 μg) was performed with random hexamers (Invitrogen, Carlsbad, CA) for 50 min at 37°C. qRT-PCR was carried out using PrimePCR Assays (Biorad, Irvine, CA, USA) with primers for cannabinoid receptor 1 (CNR1), cannabinoid receptor 2 (CNR2), diacylglycerol alpha and beta (DAGLA and DAGLB), monoacylglycerol lipase (MGLL), and fatty acid amide hydrolase (FAAH) using the preconfigured Sybr green assay (Biorad, Irvine, CA). Reactions were run in triplicate. Hprt was selected as a housekeeping gene for experimental conditions; no changes in its expression were found across conditions included in our analysis (Cq values for conditions, n = 4, two replicates: ad-libitum fed standard diet, 24.09±0.10; 24 h fasted standard diet, 24.29±0.13; ad-libitum fed western diet, 24.09±0.44; 24 h fasted western diet, 24.21±0.23; not significant).
3. STATISTICAL ANALYSES
Data was analyzed using Graphpad Prism6 software. Results are expressed as the mean ± S.E.M. Significant differences between groups were assessed using Student’s two-tailed t-test, and regular or repeated measures two-way analysis of variance (ANOVA) with Student-Newman-Keuls or Sidak post hoc test, respectively, for comparison of means. Differences were considered significant if P<0.05.
4. RESULTS
4.1. Western diet-induced obese mice are hyperphagic
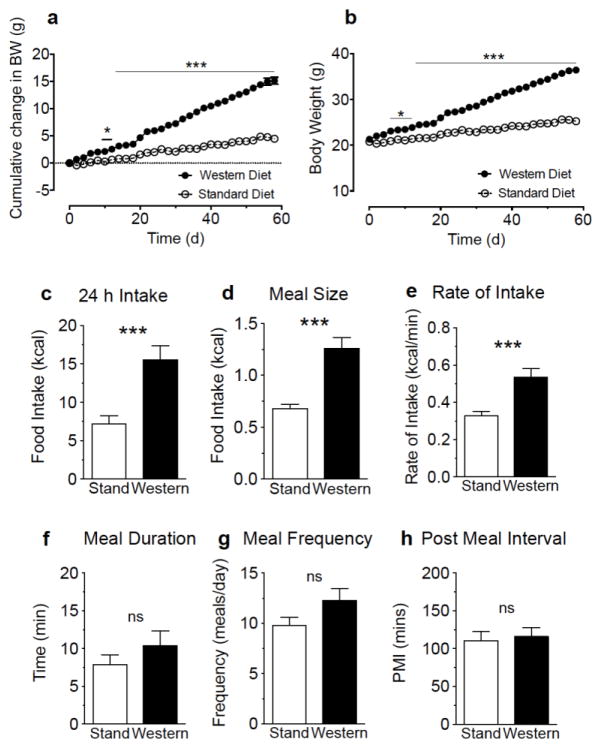
Mice fed WD ad-libitum for 60 days, when compared to mice maintained on standard diet (SD), rapidly gained body weight [Fig 1a, diet effect on cumulative change in body weight F(1,30)=125.6 p<0.001, and interaction between diet and time F(29,870)=157.3 p<0.001; Fig 1b, diet effect on cumulative gross body weight F(1,30)=91.74 p<0.001, and interaction between diet and time F(29,870)=157.3 p<0.001], and consumed substantially higher calories over a 24 h period (Fig 1c: t=3.89, p<0.001). This effect was met with increased average meal size (Fig 1d: t=4.75, p<0.001) and rate of food consumption (Fig 1e: t=3.77, p<0.001), and remained irrespective of body weight (SD versus WD: total calories, from 241.6±32.8 to 405±44.7 kcal per kg body weight, t=2.96, p=0.01; average meal size, from 23.4±1.5 to 33.0±2.7 kcal per kg body weight, t=2.78, p=0.01; average rate of intake, from 11.0±0.8 to 14.3±1.2 kcal per kg per minute, t=2.08, p=0.04; data from 8 animals per condition). Other feeding parameters including meal duration (Fig 1f: t=1.03, p=0.31), frequency (Fig 1g: t=1.69, p=0.11), and post-meal interval (Fig 1h: t=0.31, p=0.76) remained unchanged in WD mice when compared to SD mice. These results indicate that mice fed WD for 60 days exhibit hyperphagia due to larger meal size and rate of consumption.
Figure 1.
Chronic consumption of a western diet is associated with hyperphagia. Male mice maintained for 60 days on a western diet (Western) become obese (a, cumulative change in body weight; b, gross body weight) and display increases in 24 h caloric intake, meal size, and rate of intake (c–e) when compared to mice maintained on a standard chow diet (Stand). Meal duration, frequency, and post meal interval do not significantly differ between diets (f–h). Repeated measures two-way ANOVA, with Sidak’s multiple comparison post hoc test, *** = p<0.001 (a); unpaired Student’s t-test (two-tailed), *** = p<0.001 between Stand and Wetern. Results are expressed as means ± SEM; n=16/condition (a, b), n=8/condition (c–h).
4.2. Western diet intake is associated with increases in levels of endocannabinoids in jejunum epithelium and circulation
We previously reported that tasting dietary fats was met with increased levels of 2-AG and anandamide in the jejunum of rats, but in no other peripheral organ tested [27, 29]. Importantly, direct infusion of low doses of the CB1R antagonist, rimonabant (0.3mg/kg), into the jejunum, or peripheral administration of peripherally-restricted CB1R inhibitors that do not reach the brain (see for AM6545 [24, 30] and URB447 [26]) blocked fat sham feeding [27] and preferences for linoleic acid in a two-bottle choice sham feeding test [29]. These results suggest that tasting dietary fats drives eCB signaling in the upper small intestine, which in turn, generates positive feedback that promotes dietary fat intake [27, 29].
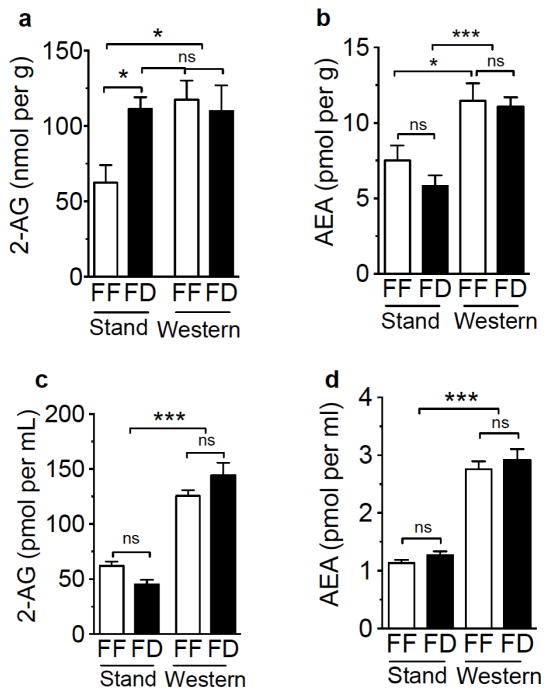
In order to elucidate the molecular underpinnings of WD-induced hyperphagia in the current study and evaluate the contribution of peripheral eCB signaling in these processes, we quantified small intestinal and circulating levels of the eCBs, 2-AG and anandamide, in mice maintained on WD or SD under free-feeding and 24 h fasting conditions. Free-feeding mice fed WD for 60 days (Fig 2a), when compared to free-feeding mice maintained on SD, displayed higher levels of 2-AG in jejunum mucosa that reached concentrations indistinguishable from fasted SD or fasted WD mice [Fig 2a, diet effect on 2-AG levels F(1,24)=4.52 p=0.04, and interaction between diet and feeding condition F(1,24)=4.98 p=0.03; multiple comparisons test of free-feeding SD versus fasted SD and both free-feeding and fasted WD p<0.05, multiple comparisons test of free-feeding WD versus both fasted SD and WD p=ns]. In addition, anandamide levels in jejunum of WD mice were elevated when compared to SD mice irrespective of feeding status [Fig 2b, diet effect on anandamide levels F(1,24)=25.88 p<0.001, and no interaction between diet and feeding condition F(1,24)=0.53 p=0.47; multiple comparisons test of FF SD versus FF WD, p<0.05; FD SD versus both FF and FD WD, p<0.001]. Furthermore, levels of 2-AG were greatly elevated in plasma of both free-feeding and 24 h fasted WD mice when compared to SD mice [Fig 2c; diet effect on 2-AG levels F(1,33)=141.3 p<0.001, and interaction between diet and feeding condition F(1,33)=6.62 p=0.02; multiple comparisons test of all SD feeding conditions versus WD, P<0.001], an effect also found for anandamide [Fig 2d, diet effect on anandamide levels F(1,33)=168.5 p<0.001, and no interaction between diet and feeding condition F(1,33)=0.01 p=0.94; multiple comparisons test of all SD feeding conditions versus WD, p<0.001]. Collectively, these data suggest that chronic ad-libitum consumption of a WD is associated with elevated levels of eCBs in mouse jejunal epithelium and plasma, which may in turn, activate peripheral CB1Rs and promote hyperphagic responses to WD.
Figure 2.
Mice fed a western diet display increases in levels of 2-AG and anandamide in jejunum mucosa and plasma. Mice maintained on a standard diet (Stand) that were fasted for 24 h (FD) display increases in 2-AG levels in jejunum mucosa, when compared to free feeding controls [FF (a)], and FF male mice maintained on western diet (Western) display increases in levels of 2-AG in jejunum mucosa to levels found in fasted Stand mice (a). Western mice display elevated anandamide in jejunum mucosa when compared to Stand mice, irrespective of feeding condition (b). Western mice display increases in plasma levels of 2-AG (c) and anandamide (d) when compared to Stand mice. Two-way ANOVA with Student-Newman-Keuls multiple comparison post hoc test. * = p<0.05, *** = p<0.001, ns = not significant. Results are expressed as means ± SEM; n=7/condition for jejunum, n=9–10/condition for plasma.
4.3. Inhibiting peripheral CB1Rs blocks hyperpagia in mice maintained on a western diet
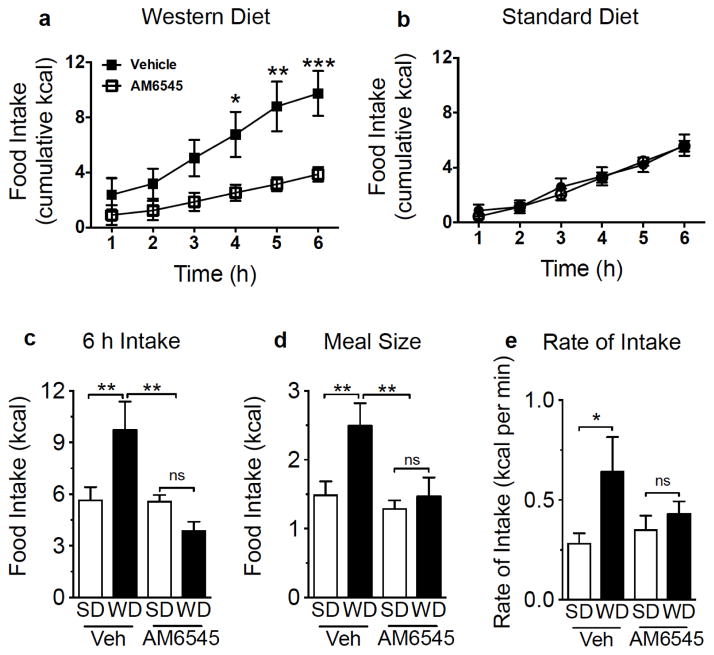
We next evaluated the contribution of heightened eCB signaling at peripheral CB1Rs (see Fig 2) in hyperphagia associated with WD-induced obesity. Mice fed ad-libitum WD or SD for 60 days were treated with the peripherally-restricted neutral CB1R antagonist, AM6545 ([25, 30] 10 mg per kg IP) or vehicle prior to a 6 h feeding test. When compared to vehicle treatment, AM6545 inhibited intake of WD [Fig 3a, drug effect on WD intake F(1,12)=7.45 p=0.018, and interaction between drug effect and time (F(5,60)=11.39 p<0.001; multiple comparisons test of AM6545 treatment versus vehicle, 4hr p<0.05, 5hr p<0.01, 6hr p<0.001)]. In contrast to WD, AM6545 failed to affect intake of SD at all time points [Fig 3b, no drug effect on WD intake F(1,14)=0.06 p=0.82, and no interaction between drug effect and time F(5,70=1.01 p=0.42]. Similar to results found for 24 h intakes of WD versus SD (Fig 1b–g), vehicle-treated WD mice, when compared to vehicle-treated SD mice, displayed increased 6 h caloric intake [Fig 3c, diet effect on intake F(1,26)=4.23 p=0.05; multiple comparisons test of SD versus WD p<0.01], average meal size [Fig 3d, diet effect on intake F(1,26)=5.41 p=0.02; multiple comparisons test of SD versus WD, p<0.01], and rate of feeding [Fig 3e, diet effect on intake F(1,26)=5.52 p=0.02; multiple comparisons test of SD versus WD, p<0.05]. Treatment with AM6545, when compared to vehicle, significantly decreased WD intake [Fig 3c, drug effect on intake F(1,26)=9.83 p=0.01, and interaction between drug and feeding condition F(1,26)=13.96 p=0.001; multiple comparisons test of AM6545 WD versus vehicle WD, p<0.01], and normalized intake to levels found for SC (Fig 3c; multiple comparisons test of AM6545 WD versus both AM6545 SD and vehicle SD, p=ns). Treatment with AM6545, when compared to vehicle, similarly decreased WD meal size [Fig 3d, drug effect on meal size F(1,26)=10.68 p=0.01, and interaction between drug and feeding condition F(1,26)=5.52 p=0.02; multiple comparisons test of AM6545 WD versus vehicle WD, p<0.01], and normalized intake to levels found for SC (Fig 3d; multiple comparisons test of AM6545 WD versus both AM6545 SD and vehicle SD, p=ns). Increased rate of intake found for vehicle WD versus vehicle SD was absent in AM6545-treated animals (Fig 3e; multiple comparisons test of WD versus SD in AM6545-treated mice, p=ns); however, there was high variability and a lack of group effect of drug [Fig 3e, drug effect on rate of intake F(1,26)=0.598 p=0.44, and no interaction between drug and feeding condition F(1,26)=2.25 p=0.14]. Treatment with AM6545 had no effect on any meal parameters included in our analysis in SD mice (Fig 3c–e; multiple comparisons test of AM6545 SD versus vehicle SD, p=ns). Our results suggest that elevated levels of endogenous eCB signaling at peripheral CB1Rs drives hyperphagia in WD-induced obesity.
Figure 3.
Inhibiting peripheral CB1Rs reduces food intake in mice fed a western diet, but not a standard diet, and normalizes intake and meal patterns in western diet fed mice. Pharmacological blockade of peripheral CB1Rs with AM6545, when compared to vehicle treatment (Veh), inhibits the caloric intake of male mice maintained for 60 days on western diet [WD (a)] during a 6 h test, but has no effect on caloric intake in mice maintained on standard chow diet [b (SD)]. Vehicle-treated WD mice display an increase in caloric intake (c), average meal size (d), and rate of feeding [e (kcal per minute of feeding)], when compared to Veh-treated SD mice during a 6 h test. Pharmacological blockade of peripheral CB1Rs with AM6545 reduces total caloric intake (c), meal size (d), and rate of intake (e) in WD mice to levels indistinguishable from SD control mice, and has no effect on meal parameters in SD mice (c–e). Repeated measures (a, b) or regular (c–e) two-way ANOVA, with Sidak’s or Student-Newman-Keuls, respectively, multiple comparison post hoc test, *= p<0.05, ** = p<0.01, *** = p<0.001. Results are expressed as means ± SEM; n=7–8/condition.
4.4. Western diet affects gene expression of eCB system components in jejunum
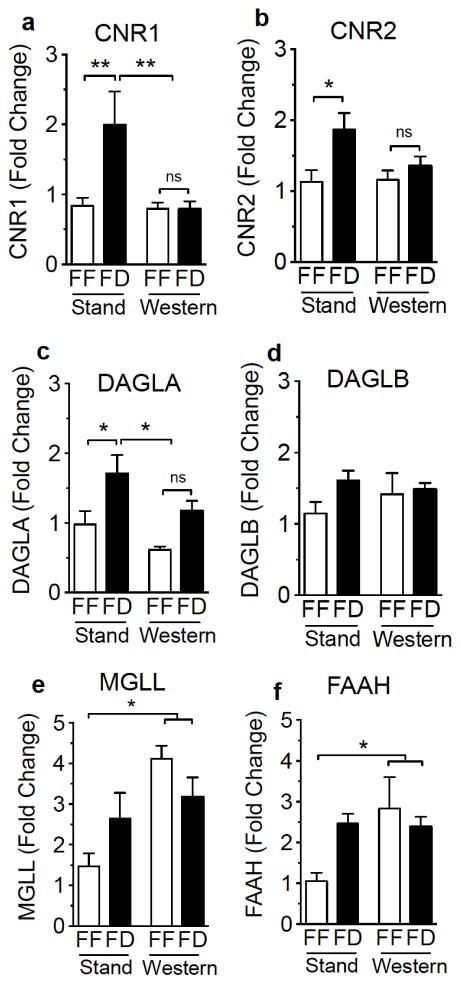
Expression of gene transcripts were increased in fasted SD mice when compared to free-feeding controls for cannabinoid receptor 1 [(CNR1) Fig 4a, feeding condition effect on expression F(1,41)=4.63, p=0.04; multiple comparisons test of free-feeding SD versus fasted SD, p<0.01), cannabinoid receptor 2 [(CNR2) Fig 4b, feeding condition effect on expression F(1,41)=6.83, p=0.01; multiple comparisons test of free-feeding SD versus fasted SD, p<0.05], and diacylglycerol lipase α [(DAGLA, 2-AG biosynthetic enzyme) Fig 4c, feeding condition effect on expression F(1,44)=13.21, p<0.001, multiple comparisons test of free-feeding SD versus fasted SD, p<0.05]. In addition, gene expression was significantly modified in WD-fed mice, when compared to SD-fed mice, such that fasting effects were absent for CNR1 [Fig 4a, diet effect on expression F(1,41)=5.39 p=0.03, and interaction between diet and feeding condition F(1,41)=4.698 p=0.04; multiple comparisons test of free-feeding WD versus fasted WD, ns; multiple comparisons test of both free-feeding and fasted WD versus fasted SD, p<0.01] and DAGLA [Fig 4c, diet effect on expression F(1,44)=6.42 p=0.02, and no interaction between diet and feeding condition F(1,44)=0.2481) p=0.63; multiple comparisons test of free-feeding WD versus fasted WD, p=ns; multiple comparisons test of both free-feeding and fasted WD versus fasted SD p<0.05]; however, a group diet effect was not found for CNR2 [Fig 4b, diet effect on expression F(1,41)=1.863, p=0.19], despite a lack of effect of fasting in WD mice (Fig 4b, multiple comparisons test of free-feeding WD mice versus fasted WD mice, p=ns).
Figure 4.
Expression of genes for components of the endocannabinoid system is modified in mice fed a western diet. Expression of mRNA encoding CB1R [a (CNR1)], CB2R [b (CNR2)], DAGL-α [c (DAGLA)], and FAAH (f) are elevated in standard chow-fed (Stand) 24 h fasted (FD) male mice, an effect absent in mice fed a western diet (Western). No changes are found under all conditions for DAGL-β [d (DAGLB)]. Expression of MAGL and FAAH genes are elevated in Western mice when compared to Stand (e, f). Two-Way ANOVA with Student-Newman-Keuls multiple comparison post hoc test. ** = p<0.01, * = p<0.05, ns = not significant. Results are expressed as means ± SEM; n=3–4/condition in triplicate.
Feeding status was not met with any significant changes in gene expression in SD and WD mice for diacylglycerol lipase β [DAGLB, 2-AG biosynthetic enzyme; Fig 4d, feeding condition effect on expression F(1,44)=2.1 p=0.154], monoacylglycerol lipase [(MGLL, 2-AG degradative enzyme) Fig 4e, feeding condition effect on expression F(1,44)=0.07 p=0.79], and fatty acid amide hydrolase [(FAAH, anandamide degradative enzyme) Fig 4f, feeding condition effect on expression F(1,41)=1.33 p=0.26]. There was, however, a significant group diet effect for expression of MGLL in WD-fed mice versus SD-fed mice for [Fig 4e, diet effect on expression F(1,44)=11.95 p=0.001, and interaction between diet and feeding condition F(1,44)=5.25 p=0.03; multiple comparisons test of both free-feeding and fasted WD mice versus free-feeding SD mice] and FAAH [Fig 4f, diet effect on expression F(1,41)=4.1 p=0.05, and interaction between diet and feeding status F(1,41)=4.81 p=0.03; multiple comparisons test of both free-feeding and fasted WD mice versus free-feeding SD mice]. Collectively, the results suggest a dysregulation in the expression of eCB components in WD mice; however, elevated levels of eCBS in jejunum and plasma are not entirely explained by these effects.
5. DISCUSSION
In 2013, the American Medical Association announced that obesity is a disease, which affects nearly one-third of American adults with significant negative impact on life expectancy [44]. Mounting evidence suggests that a primary contributing factor of these obesity rates is overconsumption of high-energy foods containing large quantities of fats and sugars, known as the “western diet” (WD [1, 2]). We utilized a mouse model of WD-induced obesity in the present study to investigate the role for peripheral eCB signaling in hyperphagia associated with consumption of western diet. Collectively, our results suggest that chronic consumption of a WD is associated with elevated levels of 2-AG and anandamide in jejunum mucosa and plasma of mice, which may in turn, activate peripheral CB1Rs and promote hyperphagic responses to WD.
Mice fed ad-libitum WD for 60 days displayed robust increases in caloric intake as a result of increased meal size, and rate of feeding (i.e., consummatory behaviors) when compared to control mice maintained on SD that contained low levels of fats and sugar (Fig 1). These data suggest that hyperphagia associated with WD-induced obesity may be driven by hedonically-positive feedback from specific constituents of the diet. Indeed, merely tasting dietary fats (i.e., corn oil) or carbohydrates (i.e., sucrose) induced dopamine outflow in the ventral striatum in rats [45, 46], which suggests that palatable food taste may promote intake by a mechanism that includes activating regions of the brain associated with food reward. Furthermore, we reported that tasting liquid diets containing dietary fats increased levels of the eCBs in the upper small intestine of rats, but no other organ tested, and pharmacological inhibition of this local signaling event with selective CB1R antagonists blocked intake and preferences for fat [27, 29]. These studies suggest a gut-brain eCB signaling axis that promotes palatable food intake based on its unique gustatory properties [6].
In the current study, hyperphagia observed in WD-induced obese mice was blocked by peripheral administration of the peripherally-restricted neutral CB1R antagonist, AM6545; however, AM6545 exerted no effect on the intake of SD during a 6 h test (Fig 2). The pharmacokinetics and half-life of AM6545 are not well-established, thus we evaluated intakes over a period of 6 h from the onset of the dark phase. Importantly, inhibiting peripheral eCB signaling at CB1Rs in WD mice normalized enhancements in meal size to levels found in SD mice and blocked increases in rate of feeding. These results suggest that heightened endogenous activity at peripheral CB1Rs is responsible for driving hyperphagia associated with chronic consumption of WD, an effect not present in mice fed SD, which might not display active eCB signaling during ad-libitum feeding conditions. Based on these results and our previous studies in rats that revealed increases in eCB signaling exclusively in the small intestine in response to tasting fats [27, 29] and fasting for 24 h [28], we asked whether eCB levels in the epithelium of the jejunum may also be elevated in WD mice and drive hyperphagia. Indeed, levels of 2-AG were significantly elevated in the epithelium of the jejunum in free-feeding mice fed WD for 60 days when compared to ad-libitum fed mice maintained on SD, and reached levels comparable to those in fasted mice maintained on SD (Fig 3a). This result is consistent with reports from other groups that show an enhancement of eCB levels in peripheral organs, namely in the upper small intestine [39, 47] and liver [40, 48], of mice maintained on high-fat diets for eight and 14 to 16 weeks, respectively. In contrast to fasting-induced increases in levels of jejunal 2-AG displayed by SD mice – which is an effect similar to results we previously reported in rats [28] – WD mice failed to display any further increases in 2-AG after a 24 h fast when compared to free-feeding conditions (Fig 3a). This result suggests a maximal level of 2-AG production in small intestinal epithelium, possibly due to a limited availability of 2-AG precursors (e.g., 1, stearoyl,2-arachidonoyl-sn-glycerol [28, 49]). Furthermore, in contrast to reports in rats [31, 39], we found no significant changes in anandamide levels in jejunum mucosa in response to fasting in SD mice, which might indicate species and/or strain differences among rodents. Similar to 2-AG, levels of anandamide were not affected by fasting in WD mice. Collectively, the results suggest that enhanced eCB activity at CB1Rs in the jejunum of WD mice may promote hyperphagia by increasing meal size and rate of feeding. Further studies that investigate discrete components of WD in these responses, however, are warranted.
Specific downstream pathways that communicate peripherally-derived eCB signals to the brain and promote feeding of WD remain to be determined; however, neural and endocrine mechanisms may play a prominent role. One potential downstream candidate mechanism includes enhanced eCB-mediated inhibition of cholecystokinin release from the upper small intestine in WD-induced obesity, which may in turn, act to increase meal size and rate of feeding of WD by delaying the activation of cholecystokinin-mediated satiation signaling to the brain carried by the afferent vagus nerve [50]. In support of this hypothesis, gene transcripts for CB1Rs have been identified in enteroendocrine I cells within the duodenum of rodents, which may act to inhibit cholecystokinin release [51]. In addition to enhanced local signaling in the upper small intestine of WD mice, elevated levels of circulating eCBs in WD mice may also act as an endocrine signal that directly interacts with feeding- and reward-related pathways in the brain, and thereby contribute to hyperphagia and WD-induced obesity found in our studies. Indeed, studies in normal weight and obese humans suggest that consumption of highly palatable foods (i.e., hedonic eating) is associated with elevated levels of 2-AG in plasma [52, 53]. Furthermore, when compared to normal weight human subjects, plasma levels of 2-AG were elevated in obese women [38], and both 2-AG and anandamide were elevated in saliva of obese male and female subjects [36]. It remains to be determined, however, if circulating eCBs may also directly interact with feeding- and reward-related pathways in the brain.
Analysis of gene expression of primary constituents of the eCB system suggests a dysregulation in the expression of key components of the eCB system following chronic consumption of WD; however, heightened eCB tone in WD obesity is not entirely explained by these results. We found that fasting for 24 h was met with increased expression of mRNA for CB1Rs, CB2Rs, and DAGLα in the jejunum mucosa of mice maintained on SD; however, WD mice failed to display a similar response (Fig 4a, b, c). Interestingly, there was group effect on the expression of DAGLAα such that WD mice had significantly lower expression when compared to SD. In light of elevated levels of 2-AG in the jejunum mucosa and plasma of mice fed a WD versus SD, this result is counter to what we expected for expression of this biosynthetic enzyme for 2-AG. Furthermore we found increases in the expression of mRNA for the 2-AG and anandamide degradative enzymes, MAGL and FAAH respectively, in mucosa of WD mice when compared to levels in SD mice (Fig 4e), which is counter to our expectation that eCB degradative enzyme expression would decrease given elevations in 2-AG and anandamide. This result may reflect an adaptive response to elevated levels of 2-AG and anandamide in WD mice. No changes were found in DAGLβ expression under all conditions (Fig 4d), which suggests that similar to results found in brain [49], the DAGLα isoform rather than DAGLβ, may be primarily involved in the biosynthesis of 2-AG in jejunum mucosa. Collectively, our gene expression results do not provide clear evidence that explains elevations in eCB levels in WD mice when compared to SD mice.
An analysis of levels of protein for all components of the eCB system is warranted, as well as analysis of the function of eCB biosynthetic and degradative enzymes via functional enzyme assays, which will help to address the underpinnings of eCB elevations in WD mice. Furthermore, it is plausible that that cholinergic signaling is enhanced under conditions of ad-libitum feeding of WD rather than any specific changes in the expression of eCB machinery. A test of these hypotheses remain. The latter scenario is supported by previous evidence from our group that suggests a key role for cholinergic signaling at small intestinal muscarinic m3 receptors, and likely the efferent vagus nerve, in driving 2-AG production and re-feeding after a fast in rats [28]. In further support of this hypothesis, we reported that tasting dietary fats drives production of the eCBs in the upper small intestine of rats, an effect that is absent in animals that received complete subdiaphragmatic vagotomy. This result suggests that critical signals from the oral cavity related to fat taste are transmitted to the small intestine via the vagus nerve [27, 29]. Future studies will be important to evaluate the contribution of cholinergic signaling in driving eCB production in the periphery that promotes hyperphagia associated with consumption of WD.
6. CONCLUSIONS
Our results suggest that mice fed a WD for 60 days are hyperphagic due to increases in meal size and rate of consumption, and that feeding responses are driven – at least in part – by increases in peripheral eCB tone in the upper small intestinal epithelium and circulation. Future studies will be critical to evaluate the specific downstream pathways that peripheral eCBs interact with to communicate with the brain in the control of feeding, including possible neural (e.g., vagal) and endocrine (i.e., circulation) mechanisms. In addition, it will be important to evaluate the interaction between a variety of species of lipids (e.g., high in mono- or di-unsaturated fats versus saturated and polyunsaturated fats) and carbohydrates (e.g., fructose, sucrose) on eCB signaling in the gut and brain, and their impact on hedonic feeding behaviors that can lead to obesity.
Together, our work suggests peripheral CB1Rs may be an effective therapeutic target for the treatment of western diet-induced obesity and eating disorders. This therapeutic approach has substantial advantage over traditional CB1Rs antagonists/inverse agonists (e.g., rimonabant) that cross the blood-brain barrier and interact with central brain mechanisms. For example, rimonabant was found to be effective for the treatment of obesity and metabolic syndrome in humans; however, benefits were met with severe psychiatric side effects, including depression and in some instances, suicide [54]. These outcomes precluded rimonabant from gaining FDA approval. On the other hand, the work in the present report as well as others [12, 24–34] suggests that targeting peripheral CB1Rs with antagonists (e.g., AM6545) that do not reach the brain may be an effective treatment strategy for metabolic syndrome and possibly eating disorders, without deleterious psychiatric side-effects inherent to brain-penetrant CB1R inhibitors.
Highlights.
Mice maintained on a western diet become obese and display hyperphagia
Hyperphagia results from increases in meal size and rate of consumption
Intestinal and plasma endocannabinoids increase in western diet-induced obese mice
Inhibiting peripheral endocannabinoid signaling normalizes feeding patterns
Acknowledgments
The authors gratefully acknowledge funding support from the National Institutes of Health grant DA034009 to NVD and Supplement to DA034009 to DAA. The authors report no conflicts of interest. The following students and staff are acknowledged for their technical assistance with experiments: Andrea Dillon, Victoria Dinh, Jaspreet Kaur, Kevin Mortazavi, Rishi Nanda, Pedro Anthony Perez, Jasmin Sanchez, Zoe Thompson, Doris Xie, and Mellonie Zhang.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Poti JM, Duffey KJ, Popkin BM. The association of fast food consumption with poor dietary outcomes and obesity among children: is it the fast food or the remainder of the diet? Am J Clin Nutr. 2014;99:162–71. doi: 10.3945/ajcn.113.071928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medina-RemOn A, Kirwan R, Lamuela-Raventos RM, Estruch R. Dietary Patterns and the Risk of Obesity, Type 2 Diabetes Mellitus, Cardiovascular Diseases, Asthma, and Mental Health Problems. Crit Rev Food Sci Nutr. 2016:0. doi: 10.1080/10408398.2016.1158690. [DOI] [PubMed] [Google Scholar]
- 3.Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, et al. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. The Journal of clinical investigation. 2005;115:3177–84. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, et al. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci. 2010;30:8376–82. doi: 10.1523/JNEUROSCI.0496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu P, Shah BP, Croasdell S, Gilbertson TA. Transient receptor potential channel type M5 is essential for fat taste. J Neurosci. 2011;31:8634–42. doi: 10.1523/JNEUROSCI.6273-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiPatrizio NV. Is fat taste ready for primetime? Physiology & behavior. 2014;136:145–54. doi: 10.1016/j.physbeh.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Running CA, Craig BA, Mattes RD. Oleogustus: The Unique Taste of Fat. Chem Senses. 2015;40:507–16. doi: 10.1093/chemse/bjv036. [DOI] [PubMed] [Google Scholar]
- 8.Mennella JA, Bobowski NK, Reed DR. The development of sweet taste: From biology to hedonics. Rev Endocr Metab Disord. 2016;17:171–8. doi: 10.1007/s11154-016-9360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiPatrizio NV, Piomelli D. The thrifty lipids: endocannabinoids and the neural control of energy conservation. Trends Neurosci. 2012;35:403–11. doi: 10.1016/j.tins.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sclafani A, Ackroff K. Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1119–33. doi: 10.1152/ajpregu.00038.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gatta-Cherifi B, Cota D. New insights on the role of the endocannabinoid system in the regulation of energy balance. International journal of obesity (2005) 2016;40:210–9. doi: 10.1038/ijo.2015.179. [DOI] [PubMed] [Google Scholar]
- 12.Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. The Journal of clinical investigation. 2003;112:423–31. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiPatrizio NV, Simansky KJ. Inhibiting parabrachial fatty acid amide hydrolase activity selectively increases the intake of palatable food via cannabinoid CB1 receptors. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1409–14. doi: 10.1152/ajpregu.90484.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiPatrizio NV, Simansky KJ. Activating parabrachial cannabinoid CB1 receptors selectively stimulates feeding of palatable foods in rats. J Neurosci. 2008;28:9702–9. doi: 10.1523/JNEUROSCI.1171-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–5. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 16.Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. British journal of pharmacology. 2002;136:550–7. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soria-Gomez E, Bellocchio L, Reguero L, Lepousez G, Martin C, Bendahmane M, et al. The endocannabinoid system controls food intake via olfactory processes. Nature neuroscience. 2014 doi: 10.1038/nn.3647. [DOI] [PubMed] [Google Scholar]
- 18.Soria-Gomez E, Massa F, Bellocchio L, Rueda-Orozco PE, Ciofi P, Cota D, et al. Cannabinoid type-1 Receptors in The Paraventricular Nucleus of The Hypothalamus Inhibit Stimulated Food Intake. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Soria-Gomez E, Matias I, Rueda-Orozco PE, Cisneros M, Petrosino S, Navarro L, et al. Pharmacological enhancement of the endocannabinoid system in the nucleus accumbens shell stimulates food intake and increases c-Fos expression in the hypothalamus. British journal of pharmacology. 2007;151:1109–16. doi: 10.1038/sj.bjp.0707313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson-Baker WC, McLaughlin CL, Baile CA. Oral and hypothalamic injections of barbiturates, benzodiazepines and cannabinoids and food intake in rats. Pharmacology, biochemistry, and behavior. 1979;11:487–91. doi: 10.1016/0091-3057(79)90030-3. [DOI] [PubMed] [Google Scholar]
- 21.Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. British journal of pharmacology. 2001;134:1151–4. doi: 10.1038/sj.bjp.0704379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verty AN, McGregor IS, Mallet PE. Paraventricular hypothalamic CB(1) cannabinoid receptors are involved in the feeding stimulatory effects of Delta(9)-tetrahydrocannabinol. Neuropharmacology. 2005;49:1101–9. doi: 10.1016/j.neuropharm.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 23.Cristino L, Busetto G, Imperatore R, Ferrandino I, Palomba L, Silvestri C, et al. Obesity-driven synaptic remodeling affects endocannabinoid control of orexinergic neurons. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E2229–38. doi: 10.1073/pnas.1219485110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randall PA, Vemuri VK, Segovia KN, Torres EF, Hosmer S, Nunes EJ, et al. The novel cannabinoid CB1 antagonist AM6545 suppresses food intake and food-reinforced behavior. Pharmacology, biochemistry, and behavior. 2010;97:179–84. doi: 10.1016/j.pbb.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cluny NL, Vemuri VK, Chambers AP, Limebeer CL, Bedard H, Wood JT, et al. A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. British journal of pharmacology. 2011;161:629–42. doi: 10.1111/j.1476-5381.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LoVerme J, Duranti A, Tontini A, Spadoni G, Mor M, Rivara S, et al. Synthesis and characterization of a peripherally restricted CB1 cannabinoid antagonist, URB447, that reduces feeding and body-weight gain in mice. Bioorg Med Chem Lett. 2009;19:639–43. doi: 10.1016/j.bmcl.2008.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiPatrizio NV, Astarita G, Schwartz G, Li X, Piomelli D. Endocannabinoid signal in the gut controls dietary fat intake. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12904–8. doi: 10.1073/pnas.1104675108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiPatrizio NV, Igarashi M, Narayanaswami V, Murray C, Gancayco J, Russell A, et al. Fasting stimulates 2-AG biosynthesis in the small intestine: role of cholinergic pathways. Am J Physiol Regul Integr Comp Physiol. 2015;309:R805–13. doi: 10.1152/ajpregu.00239.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DiPatrizio NV, Joslin A, Jung KM, Piomelli D. Endocannabinoid signaling in the gut mediates preference for dietary unsaturated fats. Faseb J. 2013;27:2513–20. doi: 10.1096/fj.13-227587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tam J, Vemuri VK, Liu J, Batkai S, Mukhopadhyay B, Godlewski G, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. The Journal of clinical investigation. 2010;120:2953–66. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, et al. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002;22:9612–7. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tam J, Cinar R, Liu J, Godlewski G, Wesley D, Jourdan T, et al. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 2012;16:167–79. doi: 10.1016/j.cmet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellocchio L, Soria-Gomez E, Quarta C, Metna-Laurent M, Cardinal P, Binder E, et al. Activation of the sympathetic nervous system mediates hypophagic and anxiety-like effects of CB1 receptor blockade. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4786–91. doi: 10.1073/pnas.1218573110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiPatrizio NV. Endocannabinoids in the Gut. Cannabis and Cannabinoid Research. 2016;1:67–77. doi: 10.1089/can.2016.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenberg D, Smith GP. The controls of fat intake. Psychosom Med. 1996;58:559–69. doi: 10.1097/00006842-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Matias I, Gatta-Cherifi B, Tabarin A, Clark S, Leste-Lasserre T, Marsicano G, et al. Endocannabinoids measurement in human saliva as potential biomarker of obesity. PLoS One. 2012;7:e42399. doi: 10.1371/journal.pone.0042399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matias I, Petrosino S, Racioppi A, Capasso R, Izzo AA, Di Marzo V. Dysregulation of peripheral endocannabinoid levels in hyperglycemia and obesity: Effect of high fat diets. Mol Cell Endocrinol. 2008;286:S66–78. doi: 10.1016/j.mce.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 38.Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Janke J, Batkai S, et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54:2838–43. doi: 10.2337/diabetes.54.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Izzo AA, Piscitelli F, Capasso R, Aviello G, Romano B, Borrelli F, et al. Peripheral endocannabinoid dysregulation in obesity: relation to intestinal motility and energy processing induced by food deprivation and re-feeding. British journal of pharmacology. 2009;158:451–61. doi: 10.1111/j.1476-5381.2009.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvheim AR, Malde MK, Osei-Hyiaman D, Lin YH, Pawlosky RJ, Madsen L, et al. Dietary linoleic acid elevates endogenous 2-AG and anandamide and induces obesity. Obesity (Silver Spring) 2012;20:1984–94. doi: 10.1038/oby.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alvheim AR, Torstensen BE, Lin YH, Lillefosse HH, Lock EJ, Madsen L, et al. Dietary linoleic acid elevates the endocannabinoids 2-AG and anandamide and promotes weight gain in mice fed a low fat diet. Lipids. 2014;49:59–69. doi: 10.1007/s11745-013-3842-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iannotti FA, Piscitelli F, Martella A, Mazzarella E, Allara M, Palmieri V, et al. Analysis of the “endocannabinoidome” in peripheral tissues of obese Zucker rats. Prostaglandins, leukotrienes, and essential fatty acids. 2013;89:127–35. doi: 10.1016/j.plefa.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–8. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 44.Kitahara CM, Flint AJ, Berrington de Gonzalez A, Bernstein L, Brotzman M, MacInnis RJ, et al. Association between class III obesity (BMI of 40–59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLoS Med. 2014;11:e1001673. doi: 10.1371/journal.pmed.1001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang NC, Hajnal A, Norgren R. Sham feeding corn oil increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1236–9. doi: 10.1152/ajpregu.00226.2006. [DOI] [PubMed] [Google Scholar]
- 46.Smith GP. Accumbens dopamine mediates the rewarding effect of orosensory stimulation by sucrose. Appetite. 2004;43:11–3. doi: 10.1016/j.appet.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Izzo AA, Sharkey KA. Cannabinoids and the gut: New developments and emerging concepts. Pharmacology & therapeutics. 2010;126:21–38. doi: 10.1016/j.pharmthera.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 48.Alvheim AR, Torstensen BE, Lin YH, Lillefosse HH, Lock EJ, Madsen L, et al. Dietary Linoleic Acid Elevates the Endocannabinoids 2-AG and Anandamide and Promotes Weight Gain in Mice Fed a Low Fat Diet. Lipids. 2013 doi: 10.1007/s11745-013-3842-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jung KM, Astarita G, Zhu C, Wallace M, Mackie K, Piomelli D. A key role for diacylglycerol lipase-alpha in metabotropic glutamate receptor-dependent endocannabinoid mobilization. Molecular pharmacology. 2007;72:612–21. doi: 10.1124/mol.107.037796. [DOI] [PubMed] [Google Scholar]
- 50.Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil. 2008;20(Suppl 1):64–72. doi: 10.1111/j.1365-2982.2008.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sykaras AG, Demenis C, Case RM, McLaughlin JT, Smith CP. Duodenal enteroendocrine I-cells contain mRNA transcripts encoding key endocannabinoid and fatty acid receptors. PLoS ONE. 2012;7:e42373. doi: 10.1371/journal.pone.0042373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monteleone AM, Di Marzo V, Monteleone P, Dalle Grave R, Aveta T, Ghoch ME, et al. Responses of peripheral endocannabinoids and endocannabinoid-related compounds to hedonic eating in obesity. European journal of nutrition. 2016;55:1799–805. doi: 10.1007/s00394-016-1153-9. [DOI] [PubMed] [Google Scholar]
- 53.Monteleone P, Piscitelli F, Scognamiglio P, Monteleone AM, Canestrelli B, Di Marzo V, et al. Hedonic eating is associated with increased peripheral levels of ghrelin and the endocannabinoid 2-arachidonoyl-glycerol in healthy humans: a pilot study. J Clin Endocrinol Metab. 2012;97:E917–24. doi: 10.1210/jc.2011-3018. [DOI] [PubMed] [Google Scholar]
- 54.Sam AH, Salem V, Ghatei MA. Rimonabant: From RIO to Ban. Journal of obesity. 2011;2011:432607. doi: 10.1155/2011/432607. [DOI] [PMC free article] [PubMed] [Google Scholar]