Abstract
Tumor necrosis factors (TNF) alpha and beta are structurally related cytokines that mediate a wide range of immunological, inflammatory, and cytotoxic effects. During bacterial infection of the bloodstream (sepsis), TNF-alpha induction by bacterial endotoxin is thought to be a major factor contributing to the cardiovascular collapse and critical organ failure that can develop. Despite antibiotic therapy, these consequences of sepsis continue to have a high mortality rate in humans. Here we describe a potent TNF antagonist, a TNF receptor (TNFR) immunoadhesin, constructed by gene fusion of the extracellular portion of human type 1 TNFR with the constant domains of human IgG heavy chain (TNFR-IgG). When expressed in transfected human cells, TNFR-IgG is secreted as a disulfide-bonded homodimer. Purified TNFR-IgG binds to both TNF-alpha and TNF-beta and exhibits 6- to 8-fold higher affinity for TNF-alpha than cell surface or soluble TNF receptors. In vitro, TNFR-IgG blocks completely the cytolytic effect of TNF-alpha or TNF-beta on actinomycin D-treated cells and is markedly more efficient than soluble TNFR (24-fold) or monoclonal anti-TNF-alpha antibodies (4-fold) in inhibiting TNF-alpha. In vitro, TNFR-IgG prevents endotoxin-induced lethality in mice when given 0.5 hr prior to endotoxin and provides significant protection when given up to 1 hr after endotoxin challenge. These results confirm the importance of TNF-alpha in the pathogenesis of septic shock and suggest a clinical potential for TNFR-IgG as a preventive and therapeutic treatment in sepsis.
Full text
PDF
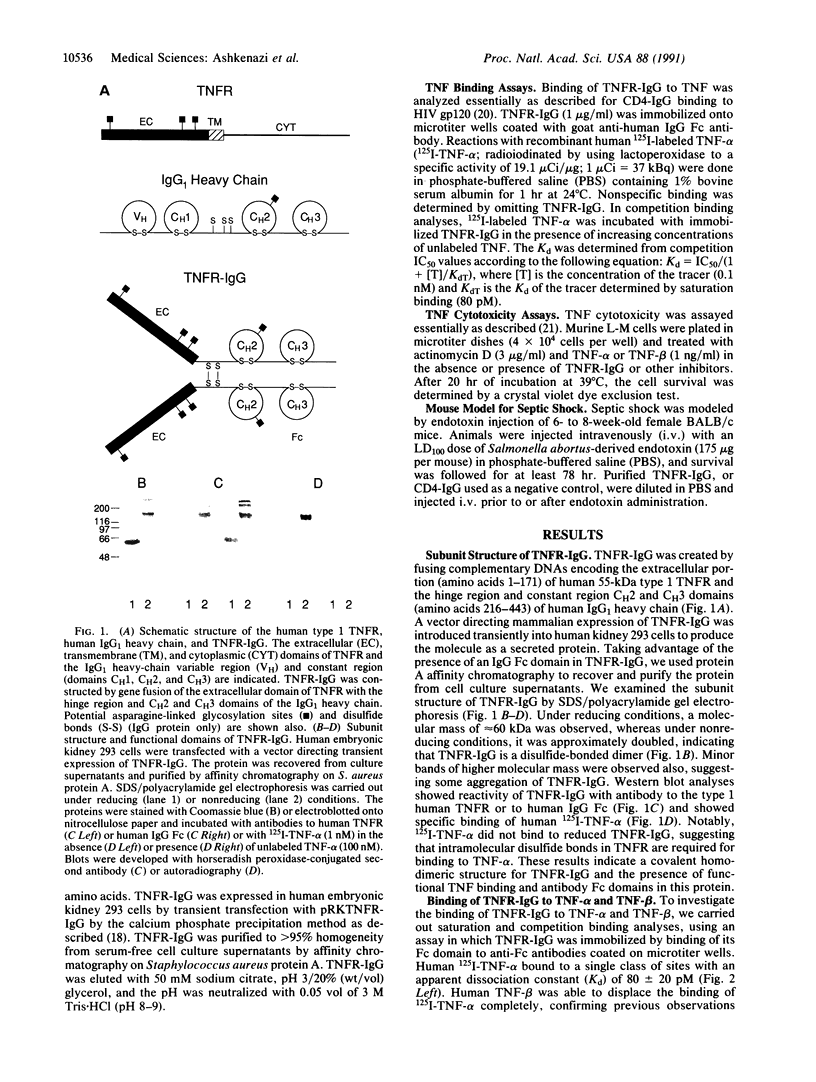
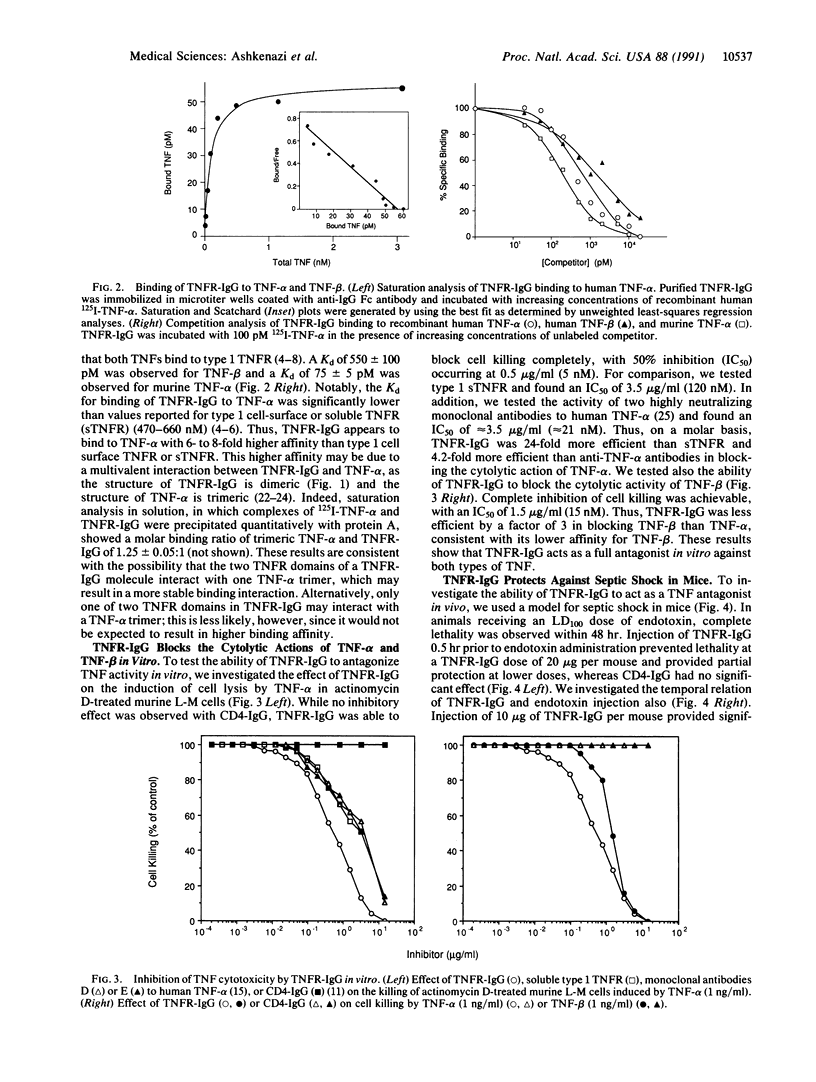
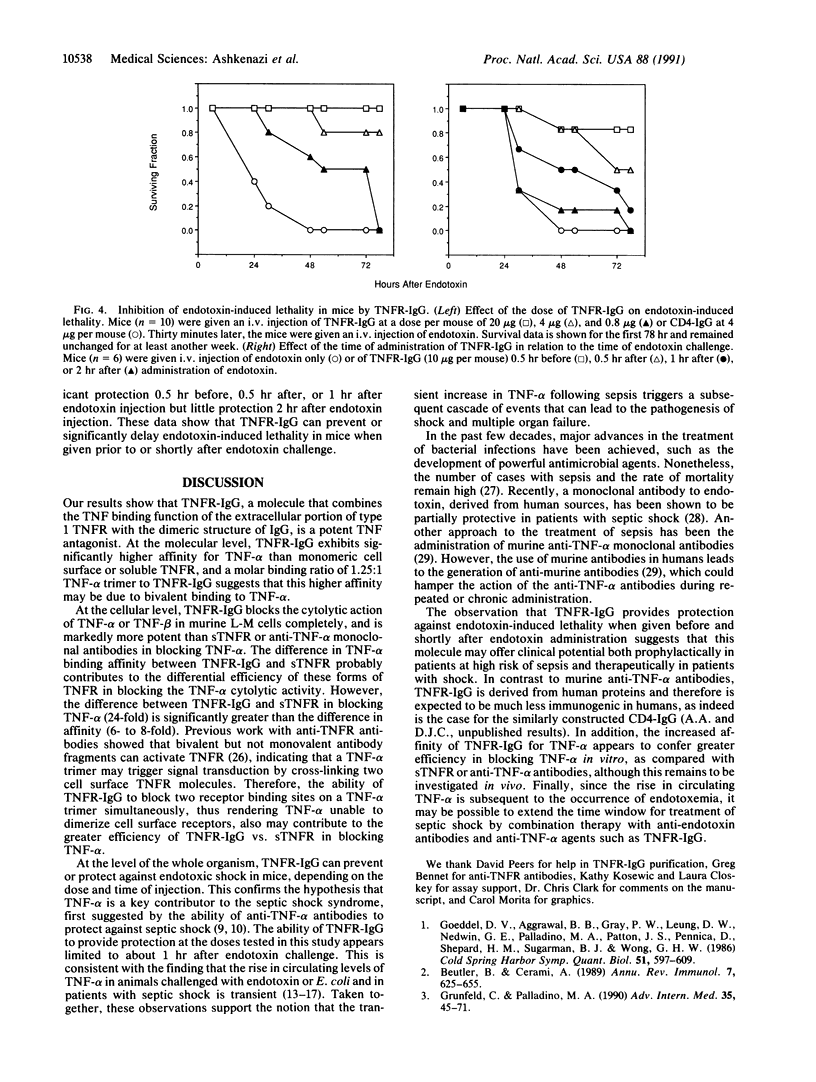

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkenazi A., Presta L. G., Marsters S. A., Camerato T. R., Rosenthal K. A., Fendly B. M., Capon D. J. Mapping the CD4 binding site for human immunodeficiency virus by alanine-scanning mutagenesis. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7150–7154. doi: 10.1073/pnas.87.18.7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Bringman T. S., Aggarwal B. B. Monoclonal antibodies to human tumor necrosis factors alpha and beta: application for affinity purification, immunoassays, and as structural probes. Hybridoma. 1987 Oct;6(5):489–507. doi: 10.1089/hyb.1987.6.489. [DOI] [PubMed] [Google Scholar]
- Byrn R. A., Mordenti J., Lucas C., Smith D., Marsters S. A., Johnson J. S., Cossum P., Chamow S. M., Wurm F. M., Gregory T. Biological properties of a CD4 immunoadhesin. Nature. 1990 Apr 12;344(6267):667–670. doi: 10.1038/344667a0. [DOI] [PubMed] [Google Scholar]
- Capon D. J., Chamow S. M., Mordenti J., Marsters S. A., Gregory T., Mitsuya H., Byrn R. A., Lucas C., Wurm F. M., Groopman J. E. Designing CD4 immunoadhesins for AIDS therapy. Nature. 1989 Feb 9;337(6207):525–531. doi: 10.1038/337525a0. [DOI] [PubMed] [Google Scholar]
- Debets J. M., Kampmeijer R., van der Linden M. P., Buurman W. A., van der Linden C. J. Plasma tumor necrosis factor and mortality in critically ill septic patients. Crit Care Med. 1989 Jun;17(6):489–494. doi: 10.1097/00003246-198906000-00001. [DOI] [PubMed] [Google Scholar]
- Eck M. J., Sprang S. R. The structure of tumor necrosis factor-alpha at 2.6 A resolution. Implications for receptor binding. J Biol Chem. 1989 Oct 15;264(29):17595–17605. doi: 10.2210/pdb1tnf/pdb. [DOI] [PubMed] [Google Scholar]
- Engelmann H., Holtmann H., Brakebusch C., Avni Y. S., Sarov I., Nophar Y., Hadas E., Leitner O., Wallach D. Antibodies to a soluble form of a tumor necrosis factor (TNF) receptor have TNF-like activity. J Biol Chem. 1990 Aug 25;265(24):14497–14504. [PubMed] [Google Scholar]
- Exley A. R., Cohen J., Buurman W., Owen R., Hanson G., Lumley J., Aulakh J. M., Bodmer M., Riddell A., Stephens S. Monoclonal antibody to TNF in severe septic shock. Lancet. 1990 May 26;335(8700):1275–1277. doi: 10.1016/0140-6736(90)91337-a. [DOI] [PubMed] [Google Scholar]
- Girardin E., Grau G. E., Dayer J. M., Roux-Lombard P., Lambert P. H. Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. N Engl J Med. 1988 Aug 18;319(7):397–400. doi: 10.1056/NEJM198808183190703. [DOI] [PubMed] [Google Scholar]
- Goeddel D. V., Aggarwal B. B., Gray P. W., Leung D. W., Nedwin G. E., Palladino M. A., Patton J. S., Pennica D., Shepard H. M., Sugarman B. J. Tumor necrosis factors: gene structure and biological activities. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):597–609. doi: 10.1101/sqb.1986.051.01.072. [DOI] [PubMed] [Google Scholar]
- Grunfeld C., Palladino M. A., Jr Tumor necrosis factor: immunologic, antitumor, metabolic, and cardiovascular activities. Adv Intern Med. 1990;35:45–71. [PubMed] [Google Scholar]
- Jones E. Y., Stuart D. I., Walker N. P. Structure of tumour necrosis factor. Nature. 1989 Mar 16;338(6212):225–228. doi: 10.1038/338225a0. [DOI] [PubMed] [Google Scholar]
- Kawade Y., Watanabe Y. Neutralization of interferon by antibody: appraisals of methods of determining and expressing the neutralization titer. J Interferon Res. 1984 Fall;4(4):571–584. doi: 10.1089/jir.1984.4.571. [DOI] [PubMed] [Google Scholar]
- Kohno T., Brewer M. T., Baker S. L., Schwartz P. E., King M. W., Hale K. K., Squires C. H., Thompson R. C., Vannice J. L. A second tumor necrosis factor receptor gene product can shed a naturally occurring tumor necrosis factor inhibitor. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8331–8335. doi: 10.1073/pnas.87.21.8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loetscher H., Pan Y. C., Lahm H. W., Gentz R., Brockhaus M., Tabuchi H., Lesslauer W. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990 Apr 20;61(2):351–359. doi: 10.1016/0092-8674(90)90815-v. [DOI] [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Michie H. R., Spriggs D. R., Manogue K. R., Sherman M. L., Revhaug A., O'Dwyer S. T., Arthur K., Dinarello C. A., Cerami A., Wolff S. M. Tumor necrosis factor and endotoxin induce similar metabolic responses in human beings. Surgery. 1988 Aug;104(2):280–286. [PubMed] [Google Scholar]
- Nophar Y., Kemper O., Brakebusch C., Englemann H., Zwang R., Aderka D., Holtmann H., Wallach D. Soluble forms of tumor necrosis factor receptors (TNF-Rs). The cDNA for the type I TNF-R, cloned using amino acid sequence data of its soluble form, encodes both the cell surface and a soluble form of the receptor. EMBO J. 1990 Oct;9(10):3269–3278. doi: 10.1002/j.1460-2075.1990.tb07526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall T. J., Lewis M., Koller K. J., Lee A., Rice G. C., Wong G. H., Gatanaga T., Granger G. A., Lentz R., Raab H. Molecular cloning and expression of a receptor for human tumor necrosis factor. Cell. 1990 Apr 20;61(2):361–370. doi: 10.1016/0092-8674(90)90816-w. [DOI] [PubMed] [Google Scholar]
- Silva A. T., Bayston K. F., Cohen J. Prophylactic and therapeutic effects of a monoclonal antibody to tumor necrosis factor-alpha in experimental gram-negative shock. J Infect Dis. 1990 Aug;162(2):421–427. doi: 10.1093/infdis/162.2.421. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Davis T., Anderson D., Solam L., Beckmann M. P., Jerzy R., Dower S. K., Cosman D., Goodwin R. G. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990 May 25;248(4958):1019–1023. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- Starnes H. F., Jr, Warren R. S., Jeevanandam M., Gabrilove J. L., Larchian W., Oettgen H. F., Brennan M. F. Tumor necrosis factor and the acute metabolic response to tissue injury in man. J Clin Invest. 1988 Oct;82(4):1321–1325. doi: 10.1172/JCI113733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Van Ostade X., Tavernier J., Prangé T., Fiers W. Localization of the active site of human tumour necrosis factor (hTNF) by mutational analysis. EMBO J. 1991 Apr;10(4):827–836. doi: 10.1002/j.1460-2075.1991.tb08015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987 Feb 14;1(8529):355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- Wolff S. M. Monoclonal antibodies and the treatment of gram-negative bacteremia and shock. N Engl J Med. 1991 Feb 14;324(7):486–488. doi: 10.1056/NEJM199102143240709. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J., Fisher C. J., Jr, Sprung C. L., Straube R. C., Sadoff J. C., Foulke G. E., Wortel C. H., Fink M. P., Dellinger R. P., Teng N. N. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N Engl J Med. 1991 Feb 14;324(7):429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]