Abstract
IL-27, a multifunctional cytokine produced by antigen-presenting cells, antagonizes inflammation by affecting conventional dendritic cells (cDC), inducing IL-10, and promoting development of regulatory Tr1 cells. Although the mechanisms involved in IL-27 induction are well-studied, much less is known about the factors that negatively impact IL-27 expression. Prostaglandin E2 (PGE2), a major immunomodulatory prostanoid, acts as a pro-inflammatory agent in several models of inflammatory/autoimmune diseases, promoting primarily Th17 development and function. In this study, we report on a novel mechanism which promotes the pro-inflammatory function of PGE2. We showed previously that PGE2 inhibits IL-27 production in murine bone marrow derived DCs. Here, we show that, in addition to BMDCs, PGE2 inhibits IL-27 production in macrophages and in splenic cDC and we identify a novel pathway consisting of signaling through EP2/EP4→induction of cAMP→downregulation of IRF1 expression and binding to the p28 ISRE site. The inhibitory effect of PGE2 on p28 and irf1 expression does not involve endogenous IFNβ, STAT1 or STAT2, and inhibition of IL-27 does not appear to be mediated through PKA, EPAC, PI3K, or MAPKs. We observed similar inhibition of il27p28 expression in vivo in splenic DC following administration of dimethyl PGE2 in conjunction with LPS. Based on the anti-inflammatory role of IL-27 in cDC and through the generation of Tr1 cells, we propose that the PGE2-induced inhibition of IL-27 in activated cDC represents an important additional mechanism for its in vivo pro-inflammatory functions.
Introduction
Interleukin-27 (IL-27), a cytokine that belongs to the IL-12 family, is a p28/EBI3 heterodimer which signals through the IL-27Rα(WSX-1)/gp130 receptor (1, 2). The inclusion of IL-27 within the IL-12 cytokine family is based on the fact that the IL-27 subunits EBI3 and p28 are related to IL-12p40 and IL-12p35, respectively. The homology extends to the IL-27/IL-12 receptors with IL-27Rα/WSX-1 being homologous to IL12Rβ2 (3). Myeloid cells including dendritic cells (DC) are major IL-27 producers following activation through TLRs, CD40 and IFN type I and II receptors (3, 4). In a model using conditional IL27p28-deficient mice, CD11c+ DC were identified as the critical source of IL-27 (5). Expression of IL-27Rα is limited to immune cells, especially T and NK cells (2), with IL-27Rα increasing following T cell activation (6). Recently, cDCs were reported to express IL-27Rα at higher levels than plasmacytoid DC (7).
Although initial studies suggested a pro-inflammatory role for IL-27, models of infectious and autoimmune diseases in Il27ra deficient mice identified IL-27 as a negative regulator of T cell functions (3, 8). Both cDC and CD4 T cells are targets for IL-27-mediated negative regulation. Mascanfroni et al (7) reported recently that IL-27 signaling in cDC limits Th1 and Th17 differentiation and function. In addition, IL-27 was also reported to induce the expansion and differentiation of Tr1 cells, one of the two major types of regulatory CD4 T cells (8, 9). The Foxp3-IL-10+IFNγ+ Tr1 cells secrete high levels of IL-10, and both Tr1 differentiation and production of IL-10 are IL-27 dependent (10–13).
Although the induction of IL-27 production has been studied extensively, much less is known about mechanisms that limit IL-27 expression. Presently, only extracellular ATP acting on purinergic receptors and C5a acting through C5aR, were reported to inhibit IL-27 production (14, 15).
PGE2, a major prostanoid derived from arachidonic acid, is produced by immune cells including cDC in response to pro-inflammatory stimuli and signals through four EP receptors (EP1-4) which vary in affinity, signal duration and signaling pathways (16–19). The role of PGE2 in DC maturation and function has been studied extensively. In immature DC, PGE2 accelerates maturation and promotes a pro-inflammatory phenotype (20–22). In terms of DC cytokines and chemokines, PGE2 was reported to reduce IL-12, TNFα, CCL3 and CCL4 (23–25), while upregulating IL-23 and promoting Th17 function (26–30). The effects of PGE2 on Th1 differentiation depend on the experimental system. In the presence of APCs, PGE2 suppresses Th1 differentiation (23), whereas, when purified naïve CD4 T cells are differentiated in Th1 polarizing conditions, PGE2 promotes Th1 differentiation (31). This apparent discrepancy can be explained by the fact that, in the first system, PGE2 inhibits the APC release of IL-12, a required Th1 differentiation factor, whereas in the second system which includes exogenous IL-12, PGE2 upregulates the expression of IL-12Rβ2 in differentiating Th1 cells (31). The pro-inflammatory role of PGE2 has been confirmed in vivo in models of contact hypersensitivity, inflammatory bowel disease, rheumatoid arthritis, and experimental autoimmune encephalomyelitis (EAE), and shown to be mostly associated with increases in Th1/Th17 differentiation and/or function (18, 19, 32, 33).
Here we report on a novel pro-inflammatory function of PGE2, mediated through the inhibition of IL-27 production by cDCs. To date, little is known about the interaction between PGE2 and IL-27. Two publications, including one from our laboratory, reported previously that PGE2 inhibited LPS-induced IL-27p28 expression in BMDC (34) and THP-1 cells (35). In the present study we report that PGE2 inhibits IL-27 expression in bone marrow-derived DC (BMDC) and macrophages (BMDM), and in splenic DCs in vitro and in vivo. The inhibitory effect of PGE2 is mediated through EP2/EP4 receptors, cAMP induction, and inhibition of IRF1 expression and binding to the p28 promoter ISRE site.
Materials and Methods
Mice
C57BL/6 (6–10 weeks old) and B6.129S2-Irf1tm1Mak (Irf1−/−) mice were purchased from Jackson Laboratory. 129S6/SvEv-Stat1tm1Rds (Stat1−/−) and wild type (WT) mice (129S6/SvEv) were from Taconic Farms. C57BL/6 Stat2−/− and corresponding WT mice were provided by Dr. Ana Gamero (Temple University, Philadelphia, PA, USA). Mice were housed and maintained in accordance with protocols approved by IACUC at Temple University.
Reagents
Prostaglandin E2, LPS (Escherichia coli O55:B5), peptidoglycan (Staphylococcus aureus), poly I:C and DNase were purchased from Sigma-Aldrich. Granulocyte-macrophage colony-stimulating factor, macrophage colony-stimulating factor and IFNγ were from Peprotech, Inc. Dimethyl-PGE2, butaprost, sulprostone, misoprostol, the specific activator of the exchange protein activated by cAMP (EPAC), 8-pCPT-2′-O-Me-cAMP (8-CPT), and the PI3K inhibitor LY294002 were purchased from Cayman Chemical. PKI (5–24) was purchased from Santa Cruz Biotechnology Inc. PKI (6–22), U0126, JNK inhibitor II, EP receptor antagonists PF-04418948 and ONO-AE3-208, and dibutyryl-cAMP were from Calbiochem. Capture and biotinylated anti-mouse IL-27p28 antibody and recombinant mouse IL-27p28 were from R&D Systems. Capture and biotinylated anti-mouse IFN-β antibodies were from BioLegend (ELISA detection limit of). Streptavidin-HRP was purchased from BioLegend. Tetramethylbenzidine substrate reagent set was from BD Biosciences. Anti-IRF1 and IgG control antibodies for ChIP were from Santa Cruz Biotechnology Inc. Primary anti-mouse antibodies for Western blot were from Cell Signaling Technology (IRF1), Abcam (GAPDH) and BD Biosciences (β-actin). IFN-β was purchased from PBL Assay Science. APC-conjugated anti-mouse CD11c and PE-Cy5-conjugated anti-mouse MHCII antibodies were purchased from eBioscience. FITC-conjugated anti-mouse CD80, CD86, and CD40 were purchased from BD Biosciences.
DC and macrophage culture
Primary DCs were derived from bone marrow cells as previously described (36). Macrophages were derived from bone marrow cells cultured in the presence of 10 ng/ml macrophage colony-stimulating factor, and adherent cells were collected on day 7. Spleen DCs were isolated from spleens dissociated in the presence of 0.5 mg/ml liberase TL (Roche Diagnostics) and DNase, and magnetically sorted using CD11c MicroBeads per manufacturer’s instructions (Miltenyi Biotec). Purity was determined by FACS analysis (>93% CD11c+).
qRT-PCR
Gene expression analysis of il-10, Il12a(p35), Il12b(p40), Il23a(p19), ifnb, p28, irf1, β-actin and Gapdh was performed using SYBR-green based qRT-PCR. The following primer sets were used: Il10 sense 5′-CCTGGTAGAAGTGATGCCCC-3′ and antisense 5′-TCCTTGATTTCTGGGCCATG-3′; Il12a(p35) sense 5′-GAGGACTTGAAGATGTACAG-3′ and antisense 5′-TTCTATCTGT GTGAGGAGGGC-3′; Il12b(p40) sense 5′-GACCCTGCCGATTGAACTGGC-3′ and antisense 5′-CAACGTTGCATCCTAGGATCG-3′; Il23a(p19) sense 5′-TGCTGGATTGC AGAGCAGTAA-3′ and antisense 5′-ATGCAGAGATTCCGAGAGA-3′; ifnb sense 5′-CCCTATGGAGATGACGGAGA-3′ and antisense 5′-ACCCAGTGCTGGAGAAATTG-3′, p28 (in vitro experiments) sense 5′-TCTGGTACAAGCTGGTTCCTGG-3′ and antisense 5′-TAGCCCTGAACCT CAGAGAGCA-3′; p28 (in vivo studies) (37) forward 5′-ATCTCGATTGCCAGGAGTGA-3′ and antisense 5′-GTGGTAGCGAGGAAGCAGAGT-3′; irf1 sense 5′-CCCACAGAAGAG CATAGCAC-3′ and antisense 5′-AGCAGTTCTTTGGGAATAGG-3′; β-actin sense 5′-AGCTTCTTTGCAGCTCCTTCGTTGC-3′ and antisense 5′-ACCAGCGCAGCGATATCG TCA-3′; Gapdh sense 5′-GGAGCGAGACCCCACTAA-3′ and antisense 5′-ACATACT CAGCACCGGCCTC-3′. Expression levels of each gene were calculated using relative standard curves and normalized to either β-actin or Gapdh.
Focused PCR-array
BMDCs were treated with LPS (1μg) with or without PGE2 (10−6M) for 90 min. mRNA was extracted and reverse transcribed using the RT2 First Strand kit. Expression levels of transcription factors were determined using the RT2 Profiler PCR Array Mouse Transcription Factors.
FACS analysis
Cells were treated as indicated and incubated at 4°C for 30 min with anti-mouse CD11c, CD80, CD86, CD40, MCHII or corresponding isotype controls. Cells were washed three times and collected using BD FACSCalibur. For all experiments, stained cell preparations were compared to isotype controls in order to identify and gate on positive cell populations. Data was analyzed using FlowJo (Ashland, OR) and GraphPad Prism 5.0.
Cytokine ELISA
Cytokine levels in cell culture supernatants were quantified by sandwich ELISA. Cells were plated in 12-well culture plates (1–2 × 106/ml) and stimulated for 24hr. Detection limit was 5 pg/ml for IL-27 and 15 pg/ml for IFNβ.
Western blot analysis
BMDCs (2–3 × 106) were serum starved for 4 hours prior to treatment with LPS and PGE2. Samples were lysed, denatured (95°C, 5 minutes) and loaded on 10% Bis-Tris gels. Following electrophoresis, protein was transferred to PVDF membranes (Bio-Rad) and probed overnight with primary antibodies. Membranes were washed, incubated with HRP goat anti-rabbit Ig (BD Biosciences) then detected using Immobilon Western chemiluminescent HRP substrate (Millipore). Membranes were then stripped and re-probed for β-actin.
Chromatin Immunoprecipitation Assay (ChIP)
BMDCs (1x107) were stimulated for 3–4hr then fixed with 1% formaldehyde for 15 minutes. Cells were treated with 125 mM glycine for 5 min to stop fixation, washed twice with PBS containing protease inhibitors, lysed, sonicated using a sonic Dismembrator (Fisher Scientific) and processed as previously described (38). Precipitated DNA and 10% input was subjected to qPCR with primers specific to the ISRE site within the p28 promoter: sense 5′-GCTGAAAGTACAAGTAGGACAGAA-3′ and antisense 5′-AGCCATCTCCTGG GTAGG-3′. Results were analyzed using the ΔΔCT method.
In vivo experiment
C57BL/6 mice (n= 4–8 per group) were injected intraperitoneally with vehicle (0.4% DMSO in PBS) or dmPGE2 (200μg/kg) followed by a second injection 4hr later with vehicle or dmPGE2 in addition to LPS (10mg/kg). CD11c+ cells were purified from spleens 4hr after the second set of injections. mRNA was extracted and subjected to qRT-PCR for p28 expression.
Statistical analysis
Results represent mean ± SD. Comparisons between groups were performed using unpaired t-test or one way ANOVA followed by Bonferroni correction. Statistical significance was determined with P<0.05 (*P<0.05, **P<0.01, and ***P<0.001). Graphs were generated and statistical analysis performed using GraphPad Prism 5.0.
Results
PGE2 inhibits IL-27 production in CD11c+ DC and macrophages
The effect of PGE2 on IL-27 production was first tested in BMDCs stimulated with various concentrations of LPS (0.1, 0.5 and 1 μg/ml). We observed a similar inhibition pattern with more than 50% inhibition of IL-27 by PGE2 10−6 to 10−8M. In subsequent experiments we used LPS at a concentration of 1 μg/ml unless otherwise noted. The PGE2 concentrations were chosen in agreement with the concentrations reported in vivo in inflammatory conditions (10−6–10−7M) (39–41). PGE2 reduced IL-27 production in a dose-dependent manner in both LPS- and LPS+IFNγ stimulated BMDCs (Fig. 1A). A similar inhibitory pattern was observed in BMDCs stimulated with peptidoglycan or poly I:C (Fig. 1B–C), indicating that PGE2 affects both MyD88 and TRIF signaling pathways. The inhibition of IL-27 by PGE2 is not restricted to BMDCs. We observed a similar inhibitory pattern in splenic DCs (Fig. 1D) and in BMDM stimulated with LPS, LPS+IFNγ, and poly I:C (Fig. 1E–F).
Figure 1. PGE2 inhibits IL-27 production in CD11c+ DC and macrophages.
BMDC were stimulated with (A) LPS (TLR4; 100 ng/ml) or LPS+IFNγ (500 U/ml), (B) peptidoglycan (PGN, TLR2; 20 or 40 μg/ml), or (C) poly I:C (TLR3; 50 or 100 μg/ml) in the presence or absence of PGE2 for 24hr. One representative experiment of three is shown. (D) CD11c+ cells were isolated from the spleens of naïve C57BL/6 mice, plated for 1h, and stimulated with LPS (1 μg/ml) and PGE2 for 24hr. BMDM were stimulated with (E) LPS (1 μg/ml) or LPS+IFNγ (500U/ml), or (F) poly I:C (50 or 100 μg/ml), in the presence or absence of PGE2 for 24h. One representative experiment of three is shown. Supernatants were collected and analyzed by ELISA for IL-27. (G) BMDC were pretreated with NS-398 (10−5M, 10−6M) for 30 min, then stimulated with LPS (1μg/ml). Supernatants were collected at 24hr and analyzed by ELISA for IL-27 or PGE2. Each sample was tested in duplicate and results represent means ± SD. Statistics were calculated using one way ANOVA followed by Bonferroni correction; **P<0.01 and ***P<0.001.
Since various TLR ligands induce endogenous PGE2, we tested the effect of endogenous PGE2 on IL-27 production in BMDCs treated with LPS in the presence or absence of the COX2 inhibitor NS-398. Although there was some variability from experiment to experiment, the levels of IL-27 were increased in the presence of NS-398 concentrations which abolished the generation of endogenous PGE2 (Fig. 1G). These results support a role for endogenous PGE2 in the reduction of IL-27 production.
PGE2 reduced mRNA expression levels of p28, but not ebi3, in both LPS- and LPS+IFNγ-treated DCs (Fig. 2A) and in LPS-treated BMDM (Fig. 2B), indicating that the reduction in IL-27 is transcriptional through inhibition of p28 expression.
Figure 2. PGE2 inhibits IL-27p28 expression in DC and macrophages.
BMDC (A) and BMDM (B) were stimulated with LPS or LPS+IFNγ in the presence or absence of PGE2 for 6hr. p28 and ebi3 expression was determined by qRT-PCR. One representative experiment of three is shown. Results represent means ± SD. Statistics were calculated using one way ANOVA followed by Bonferroni correction; *P<0.05, **P<0.01 and ***P<0.001.
To determine if the effect of PGE2 on IL-27 was associated with a general reduction in DC activation, we assessed the effects of PGE2 on MHCII, co-stimulatory molecules and cytokine expression. PGE2 had minimal effects on surface MHCII, CD80 and CD86 expression in DCs treated with LPS or LPS+IFNγ, with the only statistically significant decrease in the percentage of CD40+ cells (Fig. S1A) and CD40 MFI (230 with PGE2 versus 390 without PGE2). As expected in terms of cytokine expression, PGE2 increased il-10 and il23a, and had a pronounced inhibitory effect on il12a and il12b expression (Fig. S1B).
PGE2 signals through EP2/EP4 and cAMP to reduce IL-27
To examine the role of EP receptors, BMDCs were treated with LPS in the presence of the receptor agonists butaprost (EP2), misoprostol (EP3, EP4>EP1), PGE1OH (EP4>EP3) and sulprostone (EP3>EP1). Butaprost, misoprostol and PGE1OH, but not sulprostone, inhibited IL-27 production (Fig. 3A), suggesting that EP1 and EP3 were not involved in the inhibition of IL-27. To confirm the role of EP2/EP4, DC were pretreated for 30 minutes with the selective receptor antagonists ONO-AE3-208 (EP4) and/or PF-04418948 (EP2), followed by treatment with LPS in the presence of PGE2. Addition of either antagonist had slight effects on the inhibition of IL-27, whereas addition of both EP2 and EP4 antagonists completely reversed the inhibitory effect of PGE2 (Fig. 3B), confirming the role of both EP2 and EP4.
Figure 3. PGE2 signals through EP2/EP4 and cAMP to inhibit IL-27 production.
(A) BMDC were stimulated with LPS in the presence or absence of PGE2, butaprost, misoprostol, PGE1OH and sulprostone (10−5M). (B) BMDC were pretreated for 30 min with selective receptor antagonists, ONO-AE3-208 (EP4) and/or PF-04418948 (EP2) (10−6M), followed by stimulation with LPS in the presence of 10−7M PGE2. (C) BMDC were stimulated with LPS and dbcAMP. (D) BMDC were stimulated with LPS and forskolin. Supernatants were collected at 24hr and analyzed by ELISA for IL-27. One representative experiment of three is shown. Results represent means ± SD. Statistics were calculated using one way ANOVA followed by Bonferroni correction; ***P<0.001.
Signaling through EP2 and EP4 is known to activate adenylate cyclase leading to increased levels of cAMP (42). To determine the involvement of cAMP in the inhibition of IL-27, we treated DCs with LPS in the presence of the stable, cell-permeable cAMP analog dibutyryl-cAMP (dbcAMP). We found that dbcAMP mimicked the effects of PGE2 inhibiting IL-27 production in a dose-dependent manner (Fig. 3C). Furthermore, addition of the adenylate cyclase activator forskolin also inhibited IL-27 production (Fig. 3D). These results indicate that PGE2 signals through cAMP to inhibit IL-27 production in LPS-stimulated DCs.
Increased intracellular cAMP levels lead to activation of a number of downstream signaling pathways, primarily through activation of EPAC and PKA (43, 44). To assess the involvement of EPAC, we treated DCs with LPS in the presence of the selective EPAC activator, 8-pCPT-2′-O-Me-cAMP (8-CPT). In contrast to PGE2, 8-CPT had no effect on LPS-induced production of IL-27 (Fig. 4A). To determine the role of PKA signaling, we pretreated DCs with the PKA inhibitors PKI (6–22), PKI (5–24), or H89 followed by LPS with or without PGE2. The PKA inhibitors did not affect IL-27 production in LPS-stimulated DCs, and did not reverse the inhibitory effect of PGE2 (Fig. 4B–C). These results strongly suggest that the inhibitory effect of PGE2 on IL-27, although cAMP-dependent, is not mediated through EPAC or PKA activation. We showed previously that PI3K activation mediated the inhibitory effect of PGE2 on CCL3/4 expression in DCs (45). However, PI3K does not appear to be involved in the inhibition of IL-27, since the PI3K inhibitor LY294002 alone or in combination with PKI (6–22) did not reverse the inhibitory effect of PGE2 (Fig. 4D). In terms of MAPK, none of the three major pathways (i.e. ERK1/2, JNK, and p38 MAPK) appear to be involved, since use of ERK and JNK inhibitors did not reverse the PGE2 inhibitory effect on p28 expression (Fig. 4E), and we did not observe changes in p38 MAPK phosphorylation upon treatment with PGE2 (results not shown).
Figure 4. Inhibition of IL-27 by PGE2 is not mediated through EPAC, PKA, PI3K, or MAPK pathways.
(A) BMDCs were stimulated with LPS in the absence or presence of the selective EPAC activator 8-CPT for 24hr. One of two independent experiments is shown. BMDCs were pretreated for 3hr with the PKA inhibitors PKI (5–24) or H89 (B), or PKI (6–22) (C) followed by LPS or LPS+PGE2 (10−7M) for 24hr. One of two independent experiments is shown. (D) BMDCs were pretreated for 30 min with the PKA inhibitor PKI (6–22), the PI3K inhibitor LY294002 (LY) or both, followed by LPS or LPS+PGE2 for 24hr. Supernatants were collected and subjected to IL-27 ELISA. One of two independent experiments is shown. (E) BMDCs were pretreated for 1h with the PI3K inhibitor LY294002 (LY), ERK1/2 inhibitor U0126, or JNK inhibitor II (JNK II), followed by stimulation with LPS or LPS+PGE2 for 3hr. p28 expression was determined by qRT-PCR. One of three independent experiments is shown. Results represent means ± SD. Statistics were calculated using one way ANOVA followed by Bonferroni correction; **P<0.01 and ***P<0.001.
Inhibition of IL-27 by PGE2 is mediated through effects on IRF1
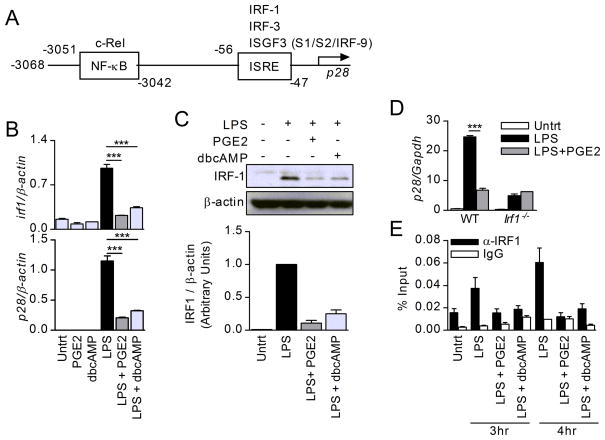
Molle and colleagues (37) reported that IL-27p28 expression following TLR4 signaling is regulated at transcriptional level in two stages. The first stage (2–4 hours) is responsible for the initial p28 expression and involves MyD88-dependent IRF1 and IRF3 activation. The second stage, responsible for sustained p28 expression (6–12 hours), depends on IFNAR signaling leading to STAT1-dependent sustained IRF1 activation and formation of the ISGF3 (STAT1/STAT2/IRF9) complex. Prior studies identified a proximal ISRE site within both human and mouse p28 promoters, and established that, in addition to the ISGF3 complex, both IRF1 and IRF3 act as essential p28 transactivating factors by binding to the ISRE site (Fig. 5A) (37, 46–50).
Figure 5. Effects of PGE2 on IRF1.
(A) Schematic of the murine p28 promotor with NF-κB and ISRE sites. (B) BMDC were stimulated with LPS in the presence of either PGE2 (10−6M) or dbcAMP (10−4M) for 2hr. Irf1 and p28 expression was determined by qRT-PCR. One of two independent experiments is shown. (C) BMDC were treated with LPS with or without PGE2 or dbcAMP for 3hr. Total cell lysates were collected and analyzed by Western blot for IRF1 and β-actin. One Western blot scan from three independent experiments is shown, with cumulative densitometry analysis below. (D) BMDC from WT and Irf1−/− were stimulated with LPS with or without PGE2 for 3hr and analyzed by qRT-PCR for p28. One representative experiment of two is shown. (E) BMDC were stimulated with LPS in the presence or absence of PGE2 or dbcAMP for the indicated times. Cells were fixed, lysed, sonicated and subjected to ChIP utilizing anti-IRF1 (black bars) and control IgG (white bars). Precipitated DNA was purified and analyzed by qPCR using primers specific for the ISRE site within the p28 promoter. One of three independent experiments is shown. Results represent means ± SD. Statistics were calculated using one way ANOVA followed by Bonferroni correction; ***P<0.001.
To identify transcription factors (TF) affected by PGE2 in LPS-stimulated DCs, we used an RT2 profiler PCR assay and compared early expression (90 minutes) of various TF in LPS-stimulated DCs treated with or without PGE2. Seven genes were differentially expressed (at levels higher than 2-fold difference), including Irf1 which was downregulated by PGE2 (Suppl. Table 1). Irf1 downregulation by PGE2 was confirmed by qRT-PCR (Fig. 5B), Western blot (Fig. 5C) and by flow cytometry (reduction from 71% IRF1+ cells in LPS-treated DCs to 53% following PGE2 treatment, data not shown). In addition, dbcAMP had a similar inhibitory effect as PGE2 on IRF1 mRNA and protein expression (Fig. 5B–C).
We further investigated the possible involvement of IRF1 in the PGE2 inhibition of IL-27 by comparing WT and irf1−/− DCs. As expected from the reported role of IRF1 in IL-27p28 expression, irf1−/− DCs expressed much lower levels of p28 mRNA than WT DCs. However, in contrast to WT DCs, the inhibitory effect of PGE2 was lost in irf1−/− DCs (Fig. 5D). Next, we used ChIP assays to determine the effect of PGE2 on IRF1 binding to the ISRE site within the mouse p28 promoter. Stimulation with LPS led to IRF1 binding to the ISRE site, and PGE2 and dbcAMP significantly reduced IRF1 binding (Fig. 5E). These results support IRF1 as a major mediator in the PGE2 inhibition of LPS-induced IL-27 expression. The fact that both PGE2 and dbcAMP have similar inhibitory effects on IL-27 production and on IRF1 binding to the p28 ISRE site supports the role of cAMP as mediator of the inhibitory effect of PGE2 on IL-27 production.
Inhibition of IL-27 and IRF1 by PGE2 is not mediated through effects on STAT1 or STAT2
In DCs, p28 expression has been shown to require the sequential recruitment of IRF1, IRF3 and ISGF3 to the endogenous promoter region (37). STAT1 and STAT2 are essential components of ISGF3 and participate in IRF1 expression (51–53). Therefore, we investigated whether the inhibitory effect of PGE2 on IL-27 is mediated through effects on STAT1 and/or STAT2. We first assessed the effects of PGE2 on STAT1 Tyr701 phosphorylation in LPS-treated DCs by Western blotting and flow cytometry. The results were inconsistent, with slight or no reductions in phosphorylated STAT1. Next, we addressed the role of STAT1/STAT2 by testing the effects of PGE2 in STAT1- and STAT2-deficient DCs. While WT DCs produced IL-27 in response to LPS and significantly increased IL-27 production upon LPS+IFNγ treatment, Stat1−/− DCs produced much lower levels of IL-27 (more than 90% reduction) and lost the ability to respond to IFNγ. However, PGE2 retained its inhibitory activity for IL-27 (Fig. 6A). In terms of irf1 expression, Stat1−/− DCs were unable to increase irf1 expression in response to IFNγ, but similar to the IL-27 data, PGE2 reduced irf1 in both WT and Stat1−/− DCs (Fig. 6B). These results strongly suggest that PGE2 inhibition of IL-27 and downregulation of irf1 expression are STAT1-independent.
Figure 6. The inhibitory effect of PGE2 on IL-27 and irf1 expression is not mediated through STAT1/STAT2.
BMDC from WT 129S6 mice and Stat1−/− mice (A) or WT C57BL/6 mice and Stat2−/− mice (C) were stimulated with LPS or LPS+IFNγ in the presence or absence of PGE2 for 24hr. Supernatants were collected and subjected to IL-27 ELISA. ELISA data are representative of two independent experiments for Stat1−/− data and three independent experiments for Stat2−/− data. BMDC from WT 129S6 mice and Stat1−/− mice (B) or WT C57BL/6 mice and Stat2−/− mice (D) were stimulated with LPS or LPS+IFNγ in the presence or absence of PGE2 for 1h. Irf1 expression was determined by qRT-PCR. One of two independent experiments is shown. Results represent means ± SD. Statistics were calculated using one way ANOVA followed by Bonferroni correction (A, C) or unpaired t-test (B, D); *P<0.05, **P<0.01 and ***P<0.001.
Stat2−/− DCs maintained their capacity to respond to IFNγ, although IL-27 production was reduced for both LPS and LPS+IFNγ treatment as compared to WT DCs (Fig. 6C). PGE2 retained its inhibitory capacity in the Stat2−/− DCs (Fig. 6C), and this was associated with a reduction in irf1 expression (Fig. 6D), suggesting that, similar to STAT1, the effects of PGE2 on IL-27 and IRF1 are STAT2-independent
Reduction in endogenous IFNβ by PGE2 plays a minor role in the inhibition of IL-27
Upon stimulation with LPS, DCs produce both IL-27 and IFNβ. Subsequently, IFNβ signaling through IFNAR results in the formation of the ISGF3 complex and sustained p28 expression (37). PGE2 suppresses IFNβ production in macrophages (54). In DC, LPS led to expression of both ifnb and p28 with peaks at 1 and 3h, respectively. PGE2 completely reduced p28 expression and IL-27 production, while ifnb expression and protein production were only partially reduced (Fig. 7A). To ascertain whether inhibition of IL-27 by PGE2 results from a reduction in DC-derived IFNβ, we supplemented the cultures with exogenous IFNβ. PGE2 retained most of its inhibitory activity (>80% for PGE 10−6M, and 50% for 10−8M) (Fig. 7B). We observed a similar pattern in irf1 expression, with PGE2 still reducing irf1 expression in DC treated with LPS in the presence of exogenous IFNβ (Fig. 7C).
Figure 7. Inhibition of IL-27 by PGE2 is not mediated by the reduction in endogenous IFNβ.
(A) BMDCs were stimulated with LPS with or without PGE2 for 1, 3, 6 and 8hr to analyze p28 and ifnb expression levels by qRT-PCR or for 3, 6 and 24hr to analyze IL-27 and IFNβ protein levels by ELISA. One of two independent experiments is shown. (B) BMDCs were treated with either LPS or LPS+IFN-β (1000U/ml) in the presence or absence of PGE2 (10−6M, 10−8M) for 24h. Supernatants were analyzed by IL-27 ELISA. Results are presented as percent inhibition compared to no PGE2 controls. One of four independent experiments is shown. (C) BMDCs were treated with either LPS or IFNβ in the presence or absence of PGE2 for 1h. Irf1 expression was determined by qRT-PCR. One of two independent experiments is shown. Results represent means ± SD. Statistics were calculated using one way ANOVA followed by Bonferroni correction; *P<0.05, **P<0.01 and ***P<0.001.
PGE2 reduces in vivo IL-27 expression in splenic DC
To determine whether PGE2 affects in vivo IL-27 production in splenic CD11c+ DC, we analyzed p28 expression in mice inoculated with LPS and PGE2. The control group was injected intraperitoneally with vehicle, whereas experimental groups received dmPGE2 (stable PGE2 analog), LPS, or LPS+dmPGE2 (Fig. 8A). Splenic CD11c+ cells were collected four hours later and p28 expression was analyzed by qRT-PCR. DCs from vehicle- and dmPGE2-treated mice did not express p28 mRNA. LPS administration resulted in an increase in p28 expression compared to the vehicle control, and co-administration of dmPGE2 reduced p28 expression (Fig. 8B).
Figure 8. In vivo effects of PGE2 on splenic DC p28 expression.
(A) Schematic of experimental timeline and groups. (B) C57BL/6 mice (n=4–8) were injected intraperitoneally with vehicle (0.4% DMSO in PBS) or dmPGE2 (0.2mg/kg) twice at 4hr interval. LPS (10mg/kg) was given intraperitoneally to groups 3 and 4 with the second vehicle or dmPGE2 inoculation. CD11c+ cells were purified from the spleen 4hr after the second set of injections. Expression of p28 was analyzed by qRT-PCR. Graph represents cumulative data from three experiments. Statistics were calculated using one way ANOVA followed by Bonferroni correction; **P<0.01.
Discussion
Recently, the regulatory function of IL-27 in both innate and adaptive immunity and its crucial role in the generation of Tr1 cells, received a lot of attention (3, 8, 55). Although the mechanisms involved in the induction of IL-27 in cDCs and macrophages have been studied extensively, little is known about factors and mechanisms that negatively impact IL-27 expression. We were the first to report that PGE2 inhibited IL-27 production by BMDCs (56), and a later publication reported on a similar finding in THP-1 cells (35). Here we report that PGE2 inhibits IL-27 production and p28 expression through a novel mechanism involving EP2/EP4-mediated cAMP increase, subsequent inhibition of irf1 expression and reduced IRF1 binding to the p28 promoter ISRE site. We also observed a similar reduction in p28 expression in vivo in splenic CD11c+ DCs following inoculation of dmPGE2 in conjunction with LPS.
In vivo, PGE2 has been described as a pro-inflammatory agent in models of contact hypersensitivity, inflammatory bowel disease, rheumatoid arthritis and EAE, primarily through EP4 signaling leading to Th1/Th17 differentiation (27, 31, 34, 56–59). In vitro pro-inflammatory effects of PGE2 include DC and CD4 T cells. PGE2 contributes to DC maturation and promotes migration to draining lymph nodes (20, 36, 60). PGE2 also stimulates IL-23 production in DCs and upregulates IL-23R in T cells, promoting the generation of pathogenic Th17 cells (22, 26–29, 61). Although previous reports indicated that PGE2 favors Th2 at the expense of Th1 differentiation primarily through its inhibitory effect on IL-12 production in APCs (23, 62), PGE2 was reported to also facilitate Th1 differentiation in polarizing conditions which include addition of exogenous IL-12 through the upregulation of IL-12Rβ2 and IFNγR1 in the differentiating naïve T cells (31).
In contrast to the effects on Th1, Th2, and Th17 differentiation, the PGE2 effects on regulatory T cells are less studied, with reports suggesting differences between tumor versus non-tumor microenvironments. Tumor-derived COX2/PGE2 was shown to promote Foxp3+Treg and Foxp3-Tr1 cells in cancer (63–67). In contrast, PGE2 inhibits the differentiation of non-tumor murine Foxp3+Treg and of CD46-induced Tr1 cells (68, 69).
IL-27, expressed and secreted by activated cDCs, was identified as the essential Tr1 differentiation and growth factor, and shown recently to also affect cDC function (7, 70–72). In DCs, IL-27 reduced costimulatory molecules, IL-12, IL-6 and IL-23, and inhibited DC capacity to generate Th1/Th17 cells (7). In addition, adoptive transfer of IL-27-treated DC reduced EAE and suppressed recall responses of Th1 and Th17 cells (7). In CD4 T cells, IL-27 promoted both Tr1 differentiation and IL-10 production through STAT1/STAT3-mediated upregulation of c-Maf, AhR, and Egr2→Blimp1 (73, 74).
Previously, we reported that PGE2 inhibited IL-27 expression in BMDCs (34), and Zhu et al (35) reported similar results in THP-1 cells. Here we report that PGE2 inhibition of IL-27 is similar in BMDCs, BMDMs, and splenic DCs, and that it occurs upon activation of various TLRs. Both EP2 and EP4 receptors, previously identified in DCs and shown to mediate most PGE2 effects in immune cells, were involved (25, 75, 76). The role of cAMP was confirmed by similar inhibitory effects of PGE2, dbcAMP and forskolin. However, neither EPAC nor PKA appeared to be involved. The lack of effect of PKA, PI3K, MAPK and JNK inhibitors and the lack of effect of PGE2 on p38 phosphorylation, suggests that none of these signaling pathways play an essential role in the PGE2-induced inhibition of IL-27.
Upon analyzing mRNA expression of IL-27 subunits we found that PGE2 inhibited p28, but not Ebi3. Expression of mouse p28 is partially dependent on c-Rel binding to the distal NF-κB site. However, binding of IRF1 to the proximal ISRE site represents the essential event for both human and mouse p28 expression (46–49). A temporal analysis of LPS-induced p28 expression showed that the initial burst in p28 transcription requires IRF1 and IRF3, with rapid nuclear translocation of IRF1 being MyD88-dependent (37). This is followed by TRIF/IRF3-mediated production of IFNβ which then upregulates and sustains STAT1-dependent IRF1 expression, and activates the ISGF3 complex (STAT1/STAT2/IRF9). The latter events serve to sustain p28 expression (37).
We investigated whether PGE2 inhibits IL-27 through effects on IFNβ, IRF1, IRF3, STAT1 and/or STAT2. The effect of PGE2 does not appear to be mediated through reduction in IFNβ. Although we observed PGE2 inhibition of endogenous IFNβ, it was only partial (approx. 50%) as compared to >90% inhibition of IL-27. Moreover, the inhibitory effect of PGE2 was maintained in the presence of exogenous IFNβ (at concentrations equivalent to, or higher than the endogenous levels observed in the absence of PGE2).
We identified IRF1 as the most important contributor to the effect of PGE2 on p28 expression. In DCs stimulated with LPS and treated with PGE2, PGE2 inhibited irf1 expression as early as two hours. A significant reduction in intracellular IRF1 protein was also observed by Western blot. In agreement with the crucial role of IRF1 in p28 expression, LPS-treated irf1−/− DC expressed low levels of p28. Moreover, PGE2 lost its suppressive activity for p28 expression in irf1−/− DCs, suggesting that the inhibitory effect was mediated primarily through reduction in IRF1. This was also supported by ChIP analysis, where PGE2 abolished LPS-induced IRF1 binding to the p28 ISRE site. Although STAT1 represents a major factor in the induction of IRF1, it does not play a role in the inhibitory effect of PGE2. In Stat1−/− DCs although IL-27 production was reduced, PGE2 still inhibited IL-27 and irf1 expression. Stat2−/− DCs expressed irf1 at higher levels than WT DCs, presumably due to an increase in STAT1 dimers, but again PGE2 was able to inhibit both IL-27 production and irf1 expression. These results suggest that the PGE2 inhibition of IRF1 and subsequently of p28 expression is not mediated through STAT1 or STAT2.
Most of the effects of PGE2 on immune cells are mediated through induction of cAMP (77, 78). Not surprisingly, we found that, similar to PGE2, dbcAMP and forskolin inhibited IL-27 production in cDCs. What was surprising however, was the lack of involvement of either PKA or EPAC, the classical signaling molecules downstream of cAMP. We established a link between cAMP and IRF1 by showing that similar to PGE2, dbcAMP inhibits irf1 expression, reduces the levels of intracellular IRF1 protein, and inhibits IRF1 binding to the ISRE site in the p28 promoter. The involvement of cAMP in IRF1 inhibition has been previously reported for two other cAMP inducing agents, e.g. cholera toxin and Bordetella pertussis, without identifying the intermediary signaling molecules (79, 80). We were able to eliminate a number of possible intermediaries such as PKA, EPAC, PI3K, MAPKs, STAT1 and STAT2. However, at the present time, the link between cAMP and IRF1 in the inhibition of IL-27 by PGE2 in cDC remains to be identified.
The relevance of our findings is reinforced by the fact that we observed similar inhibition of IL-27 in splenic DCs in vitro and in vivo following treatment with exogenous PGE2. In terms of the role of endogenous PGE2, the increase in IL-27 production in cDC stimulated with LPS in the presence of a selective COX2 inhibitor points to a role similar to the exogenous PGE2. This remains to be confirmed in vivo. Since the microsomal PGE2 synthase mPGES1 is the major contributor for the specific generation of inducible PGE2, mPGES1 deficient mice (58) are the most attractive target for investigating the role of endogenous PGE2 in modulating the IL-27 response. However, a more recent report cautions against the assumption that the genetic global deletion of mPGES1 would affect solely PGE2, since mPGES1−/− DC exhibit preferential shunting towards PGD2 production (81). Therefore, the development and use of a conditional temporal mPGES1 KO might be a better option.
In conclusion, our results indicate that PGE2 inhibits IL-27 production in TLR-activated cDC by reducing p28 expression through the EP2/EP4→cAMP→IRF1 pathway. The inhibition occurs also in the presence of type I and II IFNs, suggesting that the reduction in endogenous IFNβ is not a major contributing factor. In contrast, inhibition of IRF1 mRNA and protein expression and of IRF1 binding to the p28 ISRE site plays a major role in the PGE2 inhibition of IL-27. In an inflammatory milieu, early release of PGE2 due to the activation of inducible COX2 and mPGES1 could be a determining factor in promoting acute inflammation. Among the variety of PGE2-related physiological effects, inhibition of IL-27 in APCs could contribute to a reduction in the differentiation and possibly activation of Tr1 cells, and act as an additional proinflammatory mechanism.
Supplementary Material
Acknowledgments
This project was supported by NIH R01AI047325 (D.G).
We thank Dr. Italo Tempera and Dr. Kayla Martin (LKSOM, Temple University) for assistance with ChIP studies.
Abbreviations
- 8-CPT
8-pCPT-2′-O-Me-cAMP (EPAC activator)
- BMDC
bone marrow-derived dendritic cells
- BMDM
bone marrow-derived macrophages
- ChIP
chromatin immunoprecipitation
- COX2
cyclooxygenase 2
- dbcAMP
dibutyryl-cAMP
- EPAC
exchange protein activated by cAMP
- IRF
interferon regulatory factor
- ISGF3
interferon-stimulated gene factor 3
- ISRE
interferon-stimulated response element
- PGE2
prostaglandin E2
Footnotes
Author Contributions
K.M.H., J.-H.Y. and D.G. designed experiments; K.M.H., J.-H.Y., W.K., K.M.R. and P.-C.K. performed experiments. A.M.G. provided Stat2−/− mice and advice on proposed experiments. K.M.H. and D.G. wrote the manuscript.
Disclosure of Conflicts of Interest
All authors declare no conflicts of interest.
References
- 1.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 2.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal-Malefyt R, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida H, Hunter CA. The immunobiology of interleukin-27. Annu Rev Immunol. 2015;33:417–443. doi: 10.1146/annurev-immunol-032414-112134. [DOI] [PubMed] [Google Scholar]
- 4.Hall AO, Silver JS, Hunter CA. The immunobiology of IL-27. Adv Immunol. 2012;115:1–44. doi: 10.1016/B978-0-12-394299-9.00001-1. [DOI] [PubMed] [Google Scholar]
- 5.Zhang S, Liang R, Luo W, Liu C, Wu X, Gao Y, Hao J, Cao G, Chen X, Wei J, Xia S, Li Z, Wen T, Wu Y, Zhou X, Wang P, Zhao L, Wu Z, Xiong S, Gao X, Gao X, Chen Y, Ge Q, Tian Z, Yin Z. High susceptibility to liver injury in IL-27 p28 conditional knockout mice involves intrinsic interferon-g dysregulation of CD4+ T cells. Hepatology. 2013;57:1620–1631. doi: 10.1002/hep.26166. [DOI] [PubMed] [Google Scholar]
- 6.Villarino AV, Larkin J, Saris CJM, Caton AJ, Lucas S, Wong T, De Sauvage FJ, Hunter CA. Positive and negative regulation of the IL-27 receptor during lymphoid cell activation. J Immunol. 2005;174:7684–7691. doi: 10.4049/jimmunol.174.12.7684. [DOI] [PubMed] [Google Scholar]
- 7.Mascanfroni ID, Yeste A, Vieira SM, Burns EJ, Patel B, Sloma I, Wu Y, Mayo L, Ben-Hamo R, Efroni S, Kuchroo VK, Robson SC, Quintana FJ. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat Immunol. 2013;14:1054–1063. doi: 10.1038/ni.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meka RR, Venkatesha SH, Dudics S, Acharya B, Moudgil KD. IL-27-induced modulation of autoimmunity and its therapeutic potential. Autoimmun Rev. 2015;14:1131–1141. doi: 10.1016/j.autrev.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pot C, Apetoh L, Awasthi A, Kuchroo VK. Induction of regulatory Tr1 cells and inhibition of T(H)17 cells by IL-27. Semin Immunol. 2011;23:438–445. doi: 10.1016/j.smim.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pot C, Jin H, Awasthi A, Liu SM, Lai C, Madan R, Sharpe AH, Karp CL, Miaw S, Ho I, Kuchroo VK. Cutting Edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murugaiyan G, Mittal A, Lopez-Diego R, Maier LM, Anderson DE, Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol. 2009;183:2435–2443. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, Guo B, Herbert DR, Bulfone A, Trentini F, Di Serio C, Bacchetta R, Andreani M, Brockmann L, Gregori S, Flavell RA, Roncarolo MG. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med. 2013;19:739–746. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- 13.Gregori S, Passerini L, Roncarolo MG. Clinical outlook for Type-1 and FOXP3+ T regulatory cell-based therapy. Front Immunol. 2015;6:1–8. doi: 10.3389/fimmu.2015.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnurr M, Toy T, Shin A, Wagner M, Cebon J, Maraskovsky E. Extracellular nucleotide signaling by P2 receptors inhibits IL-12 and enhances IL-23 expression in human dendritic cells: a novel role for the cAMP pathway. Blood. 2005;205:1582–1589. doi: 10.1182/blood-2004-05-1718. [DOI] [PubMed] [Google Scholar]
- 15.Bosmann M, Haggadone MD, Hemmila MR, Zetoune FS, Sarma JV, Ward PA. Complement activation product C5a is a selective suppressor of TLR4-induced, but not TLR3-induced, production of IL-27(p28) from macrophages. J Immunol. 2012;188:5086–5093. doi: 10.4049/jimmunol.1102914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agard M, Asakrah S, Morici LA. PGE2 suppression of innate immunity during mucosal bacterial infection. Front Cell Infect Microbiol. 2013;3:1–11. doi: 10.3389/fcimb.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harizi H. The immunobiology of prostanoid receptor signaling in connecting innate and adaptive immunity. BioMed Res Int. 2013;2013:1–10. doi: 10.1155/2013/683405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez M, Domingo E, Municio C, Alvarez Y, Hugo E, Fernandez N, Crespo MS. Polarization of the innate immune response by prostaglandin E2: a puzzle of receptors and signals. Mol Pharmacol. 2014;85:187–197. doi: 10.1124/mol.113.089573. [DOI] [PubMed] [Google Scholar]
- 19.Kawahara K, Hohjoh H, Inazumi T, Tsuchiya S, Sugimoto Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim Biophys Acta. 2015;1851:414–421. doi: 10.1016/j.bbalip.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 21.Kalinski P, Schuitemaker JHN, Hilkens CMU, Kapsenberg ML. Prostaglandin E2 induces the final maturation of the IL-12-deficient CD1a+CD83+ dendritic cells: The levels of IL-12 are determined during the final dendritic cell maturation and are resisitant to futher modulation. J Immunol. 1998;161:2804–2809. [PubMed] [Google Scholar]
- 22.Khayrullina T, Yen JH, Jing H, Ganea D. In vitro differentiation of dendritic cells in the presence of prostaglandin E2 alters the IL-12/IL-23 balance and promotes differentiation of Th17 cells. J Immunol. 2008;181:721–735. doi: 10.4049/jimmunol.181.1.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalinski P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159:58–35. [PubMed] [Google Scholar]
- 24.Vassiliou E, Jing H, Ganea D. Prostaglandin E2 inhibits TNF production in murine bone marrow-derived dendritic cells. Cell Immunol. 2003;223:120–132. doi: 10.1016/s0008-8749(03)00158-8. [DOI] [PubMed] [Google Scholar]
- 25.Jing H, Vassiliou E, Ganea D. Prostaglandin E2 inhibits production of the inflammatory chemokines CCL3 and CCL4 in dendritic cells. J Leukoc Biol. 2003;74:868–879. doi: 10.1189/jlb.0303116. [DOI] [PubMed] [Google Scholar]
- 26.Sheibanie AF, Tadmori I, Jing H, Vassiliou E, Ganea D. Prostaglandin E2 induces IL-23 production in bone marrow-derived dendritic cells. FASEB J. 2004;18:1318–1320. doi: 10.1096/fj.03-1367fje. [DOI] [PubMed] [Google Scholar]
- 27.Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K, Sugimoto Y, Narumiya S. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med. 2009;15:633–640. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 28.Ma X, Aoki T, Narumiya S. Prostaglandin E2-EP4 signaling persistently amplifies CD40-mediated induction of IL-23 p19 expression through canonical and non-canonical NF-κB pathways. Cell Mol Immunol. 2016;13:240–250. doi: 10.1038/cmi.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qian X, Gu L, Ning H, Zhang Y, Hsueh EC, Fu M, Hu X, Wei L, Hoft DF, Liu J. Increased Th17 cells in the tumor microenvironment is mediated by IL-23 via tumor-secreted prostaglandin E2. J Immunol. 2013;190:5894–5902. doi: 10.4049/jimmunol.1203141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Q, Yin Z, Zhao B, Sun F, Yu H, Yin X, Zhang L, Wang S. PGE2 elevates IL-23 production in human dendritic cells via a cAMP dependent pathway. Mediators Inflamm. 2015;2015:1–7. doi: 10.1155/2015/984690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao C, Hirata T, Soontrapa K, Ma X, Takemori H, Narumiya S. Prostaglandin E2 promotes Th1 differentiation via synergistic amplification of IL-12 signalling by cAMP and PI3-kinase. Nat Commun. 2013;4:1–13. doi: 10.1038/ncomms2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia XY, Chang Y, Sun XJ, Dai X, Wei W. The role of prostaglandin E2 receptor signaling of dendritic cells in rheumatoid arthritis. Int Immunopharmacol. 2014;23:163–169. doi: 10.1016/j.intimp.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 33.Sakata D, Yao C, Narumiya S. Prostaglandin E2, an immunoactivator. J Pharmacol Sci. 2010;112:1–5. doi: 10.1254/jphs.09r03cp. [DOI] [PubMed] [Google Scholar]
- 34.Sheibanie AF, Yen JH, Khayrullina T, Emig F, Zhang CY, Tuma RF, Ganea D. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23-IL-17 axis. J Immunol. 2007;178:8138–8147. doi: 10.4049/jimmunol.178.12.8138. [DOI] [PubMed] [Google Scholar]
- 35.Zhu CL, Cao YH, Zhang R, Song Y, Liu WY, Pan F, Li Y, Zhu Y, Liu F, Wu JG. Stimulatory effect of LPS and feedback effect of PGE2 on IL-27 production. Scand J Immunol. 2010;72:469–475. doi: 10.1111/j.1365-3083.2010.02460.x. [DOI] [PubMed] [Google Scholar]
- 36.Yen JH, Khayrullina T, Ganea D. PGE2-induced metalloproteinase-9 is essential for dendritic cell migration. Blood. 2008;111:260–270. doi: 10.1182/blood-2007-05-090613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molle C, Goldman M, Goriely S. Critical role of the IFN-stimulated gene factor 3 complex in TLR-mediated IL-27p28 gene expression revealing a two-step activation process. J Immunol. 2010;184:1784–1792. doi: 10.4049/jimmunol.0902005. [DOI] [PubMed] [Google Scholar]
- 38.Yen JH, V, Kocieda P, Jing H, Ganea D. Prostaglandin E2 Induces Matrix Metalloproteinase 9 Expression in Dendritic Cells through Two Independent Signaling Pathways Leading to Activator Protein 1 (AP-1) Activation. J Biol Chem. 2011;286:38913–38923. doi: 10.1074/jbc.M111.252932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai CC, Hong YC, Chen CC, Wu YM. Measurement of prostaglandin E2 and leukotriene B4 in the gingival crevicular fluid. J Dent. 1998;26:97–103. doi: 10.1016/s0300-5712(96)00084-x. [DOI] [PubMed] [Google Scholar]
- 40.Goodson JM, Dewhirst FE, Brunetti A. Prostaglandin E2 levels and human periodontal disease. Prostaglandins. 1974;6:81–85. doi: 10.1016/s0090-6980(74)80043-2. [DOI] [PubMed] [Google Scholar]
- 41.Gelb H, Schumacher HR, Cuckler J, Baker DG. In vivo inflammatory response to polymethylmethacrylate particulate debris: effect of size, morphology, and surface area. J Orthop Res. 1994;12:83–92. doi: 10.1002/jor.1100120111. [DOI] [PubMed] [Google Scholar]
- 42.Yokoyama U, Iwatsubo K, Umemura M, Fujita T, Ishikawa Y. The prostanoid EP4 receptor and its signaling pathway. Pharmacol Rev. 2013;65:1010–1052. doi: 10.1124/pr.112.007195. [DOI] [PubMed] [Google Scholar]
- 43.Grandoch M, Roscioni SS, Schmidt M. The role of Epac proteins, novel cAMP mediators, in the regulation of immune, lung and neuronal function. Br J Pharmacol. 2010;159:265–284. doi: 10.1111/j.1476-5381.2009.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mosenden R, Tasken K. Cyclic AMP-mediated immune regulation - Overview of mechanisms of action in T cells. Cell Signal. 2011;23:1009–1016. doi: 10.1016/j.cellsig.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 45.Jing H, Yen JH, Ganea D. A novel signaling pathway mediates the inhibition of CCL3/4 expression by prostaglandin E2. J Biol Chem. 2004;279:55176–55186. doi: 10.1074/jbc.M409816200. [DOI] [PubMed] [Google Scholar]
- 46.Remoli ME, Gafa V, Giacomini E, Severa M, Lande R, Coccia EM. IFN-b modulates the response to TLR stimulation in human DC: Involvement of IFN regulatory factor-1 (IRF-1) in IL-27 gene expression. Eur J Immunol. 2007;37:3499–3508. doi: 10.1002/eji.200737566. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Qian X, Ning H, Yang J, Xiong H, Liu J. Activation of IL-27 p28 gene transcription by interferon regulatory factor 8 in cooperation with interferon regulatory factor 1. J Biol Chem. 2010;285:21269–21281. doi: 10.1074/jbc.M110.100818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pirhonen J, Siren J, Julkunen I, Matikainen S. IFN-a regulates Toll-like receptor-mediated IL-27 gene expression in human macrophages. J Leukoc Biol. 2007;82:1185–1192. doi: 10.1189/jlb.0307157. [DOI] [PubMed] [Google Scholar]
- 49.Liu J, Guan X, Ma X. Regulation of IL-27 p28 gene expression in macrophages through MyD88- and interferon-gamma-mediated pathways. J Exp Med. 2007;204:141–152. doi: 10.1084/jem.20061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Molle C, Nguyen M, Flamand V, Renneson J, Trottein F, De Wit D, Willems F, Goldman M, Goriely S. IL-27 synthesis induced by TLR ligation critically depends on IFN regulatory factor 3. J Immunol. 2007;178:7607–7615. doi: 10.4049/jimmunol.178.12.7607. [DOI] [PubMed] [Google Scholar]
- 51.Au-Yeung N, Mandhana R, Horvath CM. Transcriptional regulation by STAT1 and STAT2 in the interferon JAK STAT pathway. JAKSTAT. 2013;2:e23931–23931–23938. doi: 10.4161/jkst.23931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wesoly J, Szweykowska-Kulinska Z, Bluyssen HAR. STAT activation and differential complex formation dictate selectivity of interferon responses. Acta Biochim Pol. 2007;54:27–38. [PubMed] [Google Scholar]
- 53.Li X, Leung S, Qureshi S, Darnell JEJ, Stark GR. Formation of STAT-STAT2 heterodimers and their role in the activation of IRF-1 gene transcription by interferon-a. J Biol Chem. 1996;271:5790–5794. doi: 10.1074/jbc.271.10.5790. [DOI] [PubMed] [Google Scholar]
- 54.Xu XJ, Reichner JS, Mastrofrancesco B, Henry WL, Albina JE. Prostaglandin E2 suppresses lipopolysaccharide-stimulated IFN-beta production. J Immunol. 2008;180:2125–2131. doi: 10.4049/jimmunol.180.4.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aparicio-Siegmund S, Garbers C. The biology of interleukin-27 reveals unique pro- and anti-inflammatory functions in immunity. Cytokine Growth Factor Rev. 2015;26:579–586. doi: 10.1016/j.cytogfr.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 56.Sheibanie AF, Khayrullina T, Safadi FF, Ganea D. Prostaglandin E2 exacerbates collagen-induced arthritis in mice through the inflammatory interleukin-23/interleukin-17 axis. Arthritis Rheum. 2007;56:2608–2619. doi: 10.1002/art.22794. [DOI] [PubMed] [Google Scholar]
- 57.McCoy JM, Wicks JR, Audoly LP. The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J Clin Invest. 2002;110:651–658. doi: 10.1172/JCI15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trebino CE, Stock JL, Gibbons CP, Naiman BM, Wachtmann TS, Umland JP, Pandher K, Lapointe JM, Saha S, Roach ML, Carter D, Thomas NA, Durtschi BA, McNeish JD, Hambor JE, Jakobsson PJ, Carty TJ, Perez JR, Audoly LP. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. PNAS. 2003;100:9044–9049. doi: 10.1073/pnas.1332766100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Esaki Y, Li Y, Sakata D, Yao C, Segi-Nishida E, Matsuoka T, Fukuda K, Narumiya S. Dual roles of PGE2-EP4 signaling in mouse experimental autoimmune encelphalomyelitis. PNAS. 2010;107:12233–12238. doi: 10.1073/pnas.0915112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Helden SFG, Krooshoop KCM, Raymakers RAP, Figdor CG, van Leeuwen FN. A critical role for prostaglandin E2 in podosome dissolution and induction of high-speed migration during dendritic cell maturation. J Immunol. 2006;177:1567–1574. doi: 10.4049/jimmunol.177.3.1567. [DOI] [PubMed] [Google Scholar]
- 61.Kocieda VP, Adhikary S, Emig F, Yen JH, Toscano MG, Ganea D. Prostaglandin E2-induced IL-23p19 subunit is regulated by cAMP-responsive element-binding protein and C/AATT enhancer-binding protein beta in bone marrow-derived dendritic cells. J Biol Chem. 2012;287:36922–36935. doi: 10.1074/jbc.M112.402958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bao YS, Zhang P, Xie RJ, Wang M, Wang ZY, Zhou Z, Zhai WJ, Feng SZ, Han MZ. The regulation of CD4+ T cell immune responses toward Th2 cell development by prostaglandin E2. Int Immunopharmacol. 2011;11:1599–1605. doi: 10.1016/j.intimp.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 63.Stolina M, Sharma S, Lin Y, Dohadwala M, Gardner B, Luo J, Zhu L, Kronenberg M, Miller PW, Portanova J, Lee JC, Dubinett SM. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol. 2000;164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 64.Sharma S, Stolina M, Yang SC, Baratelli F, Lin JF, Atianzar K, Luo J, Zhu L, Lin Y, Huang M, Dohadwala M, Batra RK, Dubinett SM. Tumor cyclooxygenase 2-dependent suppression of dendritic cell function. Clin Cancer Res. 2003;9:961–968. [PubMed] [Google Scholar]
- 65.Yuan XL, Chen L, Li MX, Dong P, Xue J, Wang J, Zhang T, Wang X, Zhang FM, Ge HL, Shen LS, Xu D. Elevated expression of Foxp3 in tumor-infiltrating Treg cells suppresses T-cell proliferation and contributes to gastric cancer progression in a COX-2-dependent manner. Clin Immunol. 2010;134:277–288. doi: 10.1016/j.clim.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 66.Akasaka Y, Liu G, Chung NHC, Ehtesham M, Black KL, Yu JS. Induction of a CD4+ T regulatory type 1 response by cyclooxygenase-2-overexpressing glioma. J Immunol. 2004;173:4352–4359. doi: 10.4049/jimmunol.173.7.4352. [DOI] [PubMed] [Google Scholar]
- 67.Mandapathil M, Hilldorfer B, Szczepanski MJ, Czystowka M, Szajnik M, Ren J, Lang S, Jackson EK, Gorelik E, Whiteside TL. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J Biol Chem. 2010;285:7176–7186. doi: 10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen H, Qin J, Wei P, Zhang J, Li Q, Fu L, Li S, Ma C, Cong B. Effects of leukotriene B4 and prostaglandin E2 on the differentiation of murine Foxp3+ T regulatory cells and Th17 cells. Prostaglandins Leukot Essent Fatty Acids. 2009;80:195–200. doi: 10.1016/j.plefa.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 69.Kickler K, Maltby K, Ni Choileain S, Stephen J, Wright S, Hafler DA, Jabbour HN, Astier AL. Prostaglandin E2 affects T cell response through modulation of CD46 expression. J Immunol. 2012;188:5303–5310. doi: 10.4049/jimmunol.1103090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 71.Fitzgerald DC, Zhang GX, El-behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJM, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 72.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJM, O’Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 73.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL, Kuchroo VK. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iwasaki Y, Fujio K, Okamura T, Yanai A, Sumitomo S, Shoda H, Tamura T, Yoshida H, Charnay P, Yamamoto K. Egr-2 transcription factor is required for Blimp-1-mediated IL-10 production in IL-27-stimulated CD4+ T cells. Eur J Immunol. 2013;43:1063–1073. doi: 10.1002/eji.201242942. [DOI] [PubMed] [Google Scholar]
- 75.Narumiya S. Prostanoids in immunity: roles revealed by mice deficient in their receptors. Life Sci. 2003;74:391–395. doi: 10.1016/j.lfs.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 76.Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103:147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brudvik KW, Tasken K. Modulation of T cell immune functions by the prostaglandin E2-cAMP pathway in chronic inflammatory states. Br J Pharmacol. 2011;166:411–419. doi: 10.1111/j.1476-5381.2011.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.La Sala A, He J, Laricchia-Robbio L, Gorini S, Iwasaki A, Braun M, Yap GS, Sher A, Ozato K, Kelsall B. Cholera toxin inhibits IL-12 production and CD8alpha+ dendritic cell differentiation by cAMP-mediated inhibition of IRF8 function. J Exp Med. 2009;206:1227–1235. doi: 10.1084/jem.20080912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spensieri F, Fedele G, Fazio C, Nasso M, Stefanelli P, Mastrantonio P, Ausiello CM. Bordetella pertussis inhibition of interleukin-12 (IL-12) p70 in human monocyte-derived dendritic cells blocks IL-12 p35 through adenylate cyclase toxin-dependent cyclic AMP induction. Infect Immun. 2006;74:2831–2838. doi: 10.1128/IAI.74.5.2831-2838.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Monrad SU, Kojima F, Kapoor M, Kuan EL, Sarkar S, Randolph GJ, Crofford LJ. Genetic deletion of mPGES-1 abolishes PGE2 production in murine dendritic cells and alters the cytokine profile, but does not affect maturation or migration. Prostaglandins Leukot Essent Fatty Acids. 2011;84:113–121. doi: 10.1016/j.plefa.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.