Abstract
Background
Guidelines for prostate-specific antigen (PSA) screening in subgroups with increased risk of prostate cancer (PCa) diagnosis due to race or genotype are underdeveloped. Our goal was to investigate types of increased PCa risk and implications for targeted screening.
Methods
Computer simulation of subgroups with average and hypothetical increased risk(s) of onset of latent disease, progression, and/or cancer-specific death. For each subgroup, we predicted lifetime probabilities of overdiagnosis and life saved under more and less intensive PSA screening strategies. An application estimated risks of onset among BRCA1/2 mutation carriers in the IMPACT study using maximum likelihood.
Results
Our simulations implied PSA screening can save more lives among subgroups with increased risk than with average risk, but more intensive screening did not always improve harm-benefit tradeoffs. IMPACT data were consistent with increased risks of onset among BRCA1 and BRCA2 mutation carriers (HR=1.05, 95% CI 0.63–1.59 and HR=1.81, 95% CI 1.14–2.78, respectively). Our analysis suggests screening BRCA2 mutation carriers earlier and more frequently than the average-risk population, but a lower PSA threshold for biopsy is unlikely to improve outcomes.
Conclusions
Effective screening in men with increased PCa risk depends on the manner in which the risk is increased. More intensive screening is not always optimal.
Impact
Guidelines for screening men at increased PCa risk should consider the mechanism inducing the increased risk. While the benefit of screening may be greater in men with increased risks, more intensive screening is not always appropriate.
Keywords: Family history, germline mutations, prostatic neoplasms, prostate-specific antigen, targeted screening
Introduction
Among men in economically developed countries, prostate cancer (PCa) is the most frequently diagnosed tumor and the third most common cause of cancer death (1). Prostate-specific antigen (PSA) screening can advance the point of diagnosis and, when coupled with early treatment, can likely reduce the risk of cancer-specific death (2). However, PSA screening can also detect cancer that would never have presented clinically (3). Current guidelines either recommend against routine PSA-based screening (4, 5) or advocate shared and informed decision-making (6–8) in men with average risk.
Certain subgroups have increased risk for PCa diagnosis. African American men historically had PCa incidence that is 1.6 times that in Caucasian peers. Family history of PCa has also long been recognized as a risk factor (9). Recently, the Identification of Men with a genetic predisposition to ProstAte Cancer: Targeted screening in BRCA1/2 mutation carriers and controls (IMPACT) study found higher PCa incidence among BRCA1/2 carriers relative to non-carriers (10).
Higher observed incidence may be due to greater risk of disease onset or more aggressive cancer. Though still controversial, many believe that men of African descent are more likely to be diagnosed with advanced PCa and to die of the disease (11). Studies have indicated that BRCA1/2 carriers may have higher risk of metastasis and worse cancer-specific survival than non-carriers (12, 13).
A common reaction to identifying a subgroup with increased risk is to consider screening that subgroup more intensively than average-risk individuals. A recent editorial stated that since male germline BRCA2 mutation carriers have higher risks of early-onset, aggressive disease and higher PCa mortality, “screening intervals should be shorter and the PSA thresholds lower for them than for men in the general population” (14). However, connections between the ways that risk may be increased and appropriate screening approaches have not been systematically studied. Different types of increased risk, including higher frequency of onset of latent disease, more rapid progression to an advanced state, and shorter survival (e.g., due to poor response to standard therapies), may have different implications for targeted screening.
In this article, we investigate targeted screening in men with increased risk of PCa diagnosis. We adapt an established model of PCa natural history, detection, and survival to implement possible mechanisms of increased risk and use the resulting models to examine harm-benefit tradeoffs of different strategies. We demonstrate general lessons by considering screening decisions for men with germline BRCA mutations.
Materials and Methods
Modeling average and increased risks
To investigate targeted screening in subgroups with average and increased PCa risks, we used the Fred Hutchinson Cancer Research Center (FHCRC) model (15, 16). The model projects PSA growth before and after onset of latent disease using data from the placebo arm of the Prostate Cancer Prevention Trial (17). The risk of onset increases with age, and risks of progression to metastasis and to diagnosis increase with PSA levels. These risks were estimated so the model simulates PCa incidence under US screening patterns that matches incidence in the Surveillance, Epidemiology, and End Results (SEER) program.
To implement US screening patterns, we used a reconstruction (18) that combined data from the National Health Interview Survey and the SEER-Medicare database. Following common practice in the US, we assumed men with PSA >4.0 ng/mL were referred to biopsy. Receipt of biopsy was based on frequencies in the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial (19), which involved patient-physician decision-making and reflects real-world practice. Finally, we assumed biopsy sensitivity to detect latent cancer increased with calendar year to reflect dissemination of extended-core biopsy schemes (15).
In the absence of treatment, cancer-specific survival was derived from SEER untreated cases diagnosed in 1983–1986, just before PSA screening began. Early detection improves survival by detecting would-be metastatic cases while they are still localized and re-assigns survival accordingly. This “stage-shift” effect of screening is consistent with the mortality reduction in the European Randomized Study of Screening for Prostate Cancer (16) and is not contradicted by PLCO results (20). Frequencies of initial treatment were derived from SEER cases diagnosed in 2010 by age and tumor stage and grade. Efficacy of radical prostatectomy was based on the Scandinavian trial of prostatectomy vs watchful waiting (hazard ratio [HR] 0.56) (21), and we assumed similar efficacy for contemporary radiation therapy (22, 23). Non-cancer survival was based on US life tables (24), and overall survival was the earlier of cancer-specific and non-cancer survival.
The model allowed us to project disease prevalence, incidence, and PCa mortality in the average-risk population. Under a specified screening strategy, we estimated the lifetime probability of overdiagnosis as the frequency of screen-detected cancers that would not present clinically before non-cancer death and the lifetime probability of life saved as the difference between model-projected frequencies of PCa death with and without screening (16).
To represent hypothetical increased risks, we applied a multiplicative factor (HR=2.0) to risks of latent onset, progression to a symptomatic or metastatic state, and/or PCa death following diagnosis. For each type of increased risk, we simulated outcomes for 10 million men under all combinations of starting age (40, 45, or 50 yr), ending age (64 or 69 yr), frequency (annual or biennial), and PSA threshold for biopsy (1.0 or 3.0 ng/mL). We calculated lifetime probabilities of overdiagnosis and life saved, and we compared approaches using the additional number needed to diagnose to prevent one PCa death (NND), calculated as the ratio of these probabilities. We defined preferred strategies as those with NND≤5 as an example of a policy target; in specific settings other criteria could be used.
Men with germline BRCA mutations
To investigate how risk might be increased for men with germline BRCA mutations, we estimated the risk of latent onset corresponding to BRCA status using published results from the first screening round of the IMPACT study (10). IMPACT investigators recruited undiagnosed men aged 40–69 yr from families with known or suspected BRCA1 or BRCA2 mutations in 2005–2013 and assigned carrier status based on genetic testing. Participants were offered annual (biennial in the Netherlands) PSA screening with referral to biopsy for PSA >3.0 ng/mL.
We simulated BRCA1 and BRCA2 cohorts in the IMPACT study and identified increased risks of onset relative to the general population that best matched study results. Before entering the study, we assumed participants were screened according to patterns in the US before 1995 (18) with biopsy frequency and sensitivity as for average-risk men. After recruitment, we assumed 80% of men with PSA >3.0 ng/mL received a biopsy to match study results (10) and a biopsy had 90% sensitivity to detect cancer for consistency with the number of cores in the study protocol. We simulated each cohort 10,000 times over a range of values for the HR for onset; estimation was based on maximizing a Poisson likelihood for observed and projected cancers diagnosed, and 95% confidence intervals (CIs) were derived using the profile likelihood method (25).
Results
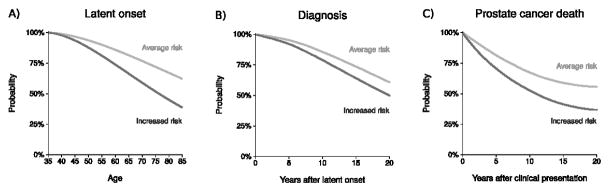
Figure 1 illustrates model-projected Kaplan-Meier curves for the specified endpoints among men at average and increased (doubled) risks of latent onset, diagnosis, and cancer-specific death. The risk of diagnosis is among men with onset in their lifetimes, and the risk of cancer death is among men who present clinically. All endpoints are in the absence of screening, treatment, or competing death.
Figure 1.
Average risks of prostate cancer A) latent onset, B) diagnosis, and C) death in the absence of screening, treatment, or competing mortality estimated by the FHCRC model, and hypothetical increased risks reflecting hazard ratios of 2.0.
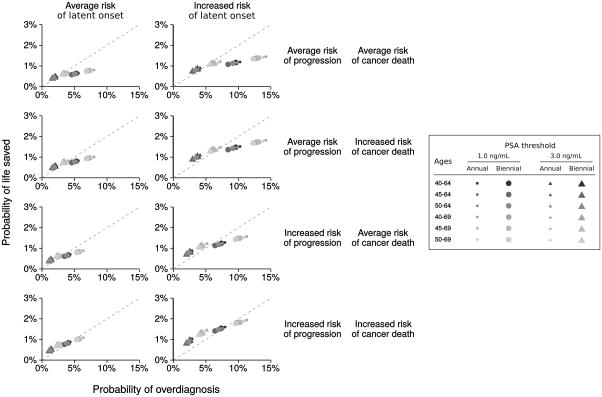
Figure 2 illustrates projected lifetime probabilities of overdiagnosis and life saved by screening for men with average and all combinations of increased risks. Dashed lines show NND=5; strategies above this line have fewer than 5 overdiagnoses per life saved.
Figure 2.
Lifetime probability of overdiagnosis and life saved by screening for men with average and increased risk(s) of onset of latent prostate cancer, progression to a symptomatic or metastatic state, and/or cancer death predicted by the FHCRC model.
In the average-risk population (upper left panel), biennial screening to age 64 yr with biopsy referral when PSA >3.0 ng/mL had the smallest NNDs (range across starting ages 4.1–4.4), changing to annual screening raised NNDs closer to 5, and screening to age 69 yr pushed NNDs above 5. All strategies with biopsy referral for PSA >1.0 ng/mL implied substantially higher NNDs (range 7.8–9.9) due to more overdiagnosis with few additional lives saved.
In men with increased risk of onset, the NNDs of all strategies had similar rank order as in average-risk men, but probabilities of overdiagnosis and life saved were both higher. In men with increased risk of progression, the probability of overdiagnosis was lower and the probability of life saved was higher compared to average-risk men under the same strategies. In men with increased risk of cancer death, the probability of overdiagnosis was similar and the probability of life saved was higher compared to average-risk men. These results match expectations.
In men with increased risks of onset and progression, a greater number of screening strategies yielded NND≤5 compared to average-risk men. Thus, if our goal was to achieve NND≤5 while maximizing benefit, we could consider more intensive strategies in men at higher risk. For example, annual screening to age 69 yr with PSA threshold 3.0 ng/mL could be considered in men with increased risks of onset and progression but not in average-risk men or in men with only increased risk of onset. This example shows that preferred strategies may differ across subgroups with different mechanisms of increased risk. In no subgroup did lowering the PSA threshold from 3.0 to 1.0 ng/mL improve outcomes, indicating that, under our model assumptions, these strategies would not be preferred.
Last, we examine men with germline BRCA1/2 mutations. Our assumptions about screening before the IMPACT study implied 35% of simulated participants received at least one PSA test, similar to the reported 37% of participants (10). Comparing observed and projected cancers diagnosed during a single round of screening, we estimated non-significantly increased risk of onset in BRCA1 carriers (HR=1.05, 95% CI 0.63–1.59) and significantly increased risk of onset in BRCA2 carriers (HR=1.81, 95% CI 1.14–2.78). Consequently, our general results about screening men at increased risk of onset may apply to BRCA2 mutation carriers. In particular, a wider range of screening ages may be acceptable, but lowering the PSA threshold for biopsy referral is likely to increase overdiagnosis with few additional lives saved.
Discussion
Risk stratification promises to reduce the costs and harms of screening by focusing resources on the subgroups most likely to benefit. However, realizing its potential requires that we understand the mechanisms by which the subgroups have increased risk. Is the increased risk due to earlier and more frequent disease onset, greater likelihood of progression to symptoms or metastasis, shorter survival, or some combination of these?
This article investigates ways in which the risk of PCa diagnosis might be increased and links the mechanism of increased risk to targeted screening strategies. Although it may seem intuitive that higher risk strata should be screened more intensively than those with average risk, we note that in certain cases, more intensive screening may achieve greater benefit only at the expense of much greater harm. A key conclusion of our investigation is that the way in which risk is increased should factor into the decision about whether and how to recommend screening.
We also offer the following specific conclusions. (1) Individuals with increased risk of onset are both more likely to be overdiagnosed and more likely to benefit from early detection, and thus appropriate screening ages and intervals depend on the tradeoffs deemed acceptable. (2) Individuals with increased risk of progression are less likely to be overdiagnosed, and a shorter interval between screens may be recommended. (3) Individuals with increased risk of cancer death are more likely to benefit from early detection, and a shorter interval between screens may also be recommended provided the survival benefit is greater with earlier detection (as assumed in this study). However, in general, we find that a lower PSA threshold for biopsy disproportionately increases overdiagnoses relative to additional lives saved. Therefore we do not recommend this unless the increased chance of overdiagnosis is considered to be acceptable given the additional lives saved.
It might seem surprising that men with increased risk of onset can be more likely to be overdiagnosed. This association is a direct consequence of representing this increased risk using a hazard ratio: a hazard ratio greater than 1 implies not only onset at an earlier age, on average, but also a higher cumulative prevalence of latent disease at every age, relative to the general population. In other words, under this mechanism of increased risk, the high-risk subgroup always has a larger pool of latent disease, and consequently greater absolute magnitudes of screening harms and benefits. However, the higher chance of overdiagnosis due to increased risk of onset may be partially or completely offset by increased risk of progression, so the net effect on the chance of overdiagnosis depends on the relative magnitudes of these increased risks.
This work was motivated by research linking the frequency of PCa, and particularly metastatic PCa, with germline mutations in BRCA2 and other DNA repair genes (26, 27). As genetic screening appears set to expand in these patients, counseling unaffected family members may become increasingly common. Within our model, we estimated that BRCA2 germline mutation carriers in the IMPACT study have 81% higher risk of onset than the general population. If BRCA2 is only associated with increased risk of onset, we would recommend a wider range of screening ages than in the average-risk population. However, there is accumulating evidence that men with germline BRCA1/2 mutations also exhibit varying degrees of more aggressive tumor features and worse baseline survival than average-risk men (12). A recent study estimated HR=2.36 for the risk of metastasis after treatment and HR=2.17 for cause-specific survival after treatment among all (BRCA1 and BRCA2) mutation carriers combined (13). These results suggest that BRCA2 carriers may resemble the highest-risk subgroup in Figure 2 and motivates considering an expanded range of screening ages and more frequent screen tests in these men. However, based on our analysis, lowering the PSA threshold for biopsy (e.g., using a PSA threshold of 1.0 ng/mL over a more accepted threshold like 3.0 ng/mL) would not be well-advised.
The problem of tailoring screening to subgroups with differing genetic risk has been recognized previously. In breast cancer, Kurian et al. considered women with BRCA1 and BRCA2 mutations and developed a tool to project disease incidence and mortality under different prevention and screening strategies (28, 29). Heijnsdijk et al. examined natural history in these women and evaluated mammography screening with and without magnetic resonance imaging (30). In PCa, Yen et al. considered risk stratification using single-nucleotide polymorphisms and presented suggestions for more intensive screening in men with higher risk to reduce mortality by a similar magnitude to that achievable in the average-risk population (31). Pashayan et al. projected implications of screening based on polygenic risk rather than age in breast and PCa (32). These studies also reflect the importance of understanding mechanisms and magnitudes of increased risk before developing targeted screening strategies.
In practice, developing an appropriate screening strategy in a particular subgroup requires prospectively collected serial screening and incidence data and a model that can estimate average and increased risks of disease onset and sojourn times (33). For PCa and other cancers, natural history has already been studied for the average-risk population and certain increased-risk subgroups (34). Once preferred screening strategies are determined in particular high-risk subgroups, further research is needed to investigate programs to identify the high-risk strata (e.g., genetic screening).
Like all modeling applications, ours is subject to limitations. The FHCRC model is a simplification of PSA growth and cancer progression designed to approximate the general male population. Because different components of the model were estimated using different data sources, its absolute projections may be less reliable than relative comparisons. Furthermore, although it closely approximates US PCa incidence trends with and without PSA screening by age, stage, and grade, the model abstracts a set of complex biological processes and interventions, and it may not generalize to particular subgroups. For example, increased risks may not be simple multiples of average risks. Our main data source for germline BRCA mutation carriers, the IMPACT study, is also limited. Importantly, carriers in this study may not be representative of the general carrier population. Further, it involves only one round of screening and no metastatic cases. Thus, we cannot use this study to determine precisely which mechanism of increased risk (onset or progression) explains the observed incidence in that study. Consequently, we also consider other studies that bear on the mechanism of increased risk among BRCA1/2 mutation carriers.
In conclusion, different mechanisms and magnitudes of increased PCa risks produce different levels of harms and benefits for a given screening approach. Consequently, screening guidelines for men with increased risk should take evidence about these factors into account. In general, while an efficacious screening test can produce greater benefit in subgroups with increased risk than in the average-risk population, more intensive screening does not always improve harm-benefit tradeoffs.
Acknowledgments
Support: This work was made possible by Grant Numbers U01 CA199338 (R. Gulati, R. Etzioni) and P50 CA097186 (all authors) from the National Cancer Institute (NCI). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCI. HHC received a 2015 Prostate Cancer Foundation Young Investigator Award.
Footnotes
The authors declare no potential conflict of interest.
Conflict of interest: None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Zappa M, Nelen V, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027–35. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Etzioni R, Penson DF, Legler JM, di Tommaso D, Boer R, Gann PH, et al. Overdiagnosis due to prostate-specific antigen screening: Lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94:981–90. doi: 10.1093/jnci/94.13.981. [DOI] [PubMed] [Google Scholar]
- 4.Moyer VA Force USPST. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–34. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 5.Bell N, Connor Gorber S, Shane A, Joffres M, Singh H, Dickinson J, et al. Canadian Task Force on Preventive Health Care: Recommendations on screening for prostate cancer with the prostate-specific antigen test. CMAJ. 2014;186:1225–34. doi: 10.1503/cmaj.140703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf AM, Wender RC, Etzioni RB, Thompson IM, D’Amico AV, Volk RJ, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60:70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]
- 7.Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, Greene KL, et al. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190:419–26. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qaseem A, Barry MJ, Denberg TD, Owens DK, Shekelle P for the Clinical Guidelines Committee of the American College of P. Screening for Prostate Cancer: A Guidance Statement From the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2013 doi: 10.7326/0003-4819-158-10-201305210-00633. [DOI] [PubMed] [Google Scholar]
- 9.Carter BS, Bova GS, Beaty TH, Steinberg GD, Childs B, Isaacs WB, et al. Hereditary prostate cancer: epidemiologic and clinical features. J Urol. 1993;150:797–802. doi: 10.1016/s0022-5347(17)35617-3. [DOI] [PubMed] [Google Scholar]
- 10.Bancroft EK, Page EC, Castro E, Lilja H, Vickers A, Sjoberg D, et al. Targeted Prostate Cancer Screening in BRCA1 and BRCA2 Mutation Carriers: Results from the Initial Screening Round of the IMPACT Study. Eur Urol. 2014;66:489–99. doi: 10.1016/j.eururo.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chornokur G, Dalton K, Borysova ME, Kumar NB. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate. 2011;71:985–97. doi: 10.1002/pros.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castro E, Goh C, Olmos D, Saunders E, Leongamornlert D, Tymrakiewicz M, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31:1748–57. doi: 10.1200/JCO.2012.43.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castro E, Goh C, Leongamornlert D, Saunders E, Tymrakiewicz M, Dadaev T, et al. Effect of BRCA Mutations on Metastatic Relapse and Cause-specific Survival After Radical Treatment for Localised Prostate Cancer. Eur Urol. 2015;68:186–93. doi: 10.1016/j.eururo.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Bratt O, Loman N. Clinical Management of Prostate Cancer in Men with BRCA Mutations. Eur Urol. 2015;68:194–5. doi: 10.1016/j.eururo.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Gulati R, Inoue L, Katcher J, Hazelton W, Etzioni R. Calibrating disease progression models using population data: a critical precursor to policy development in cancer control. Biostatistics. 2010;11:707–19. doi: 10.1093/biostatistics/kxq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gulati R, Gore JL, Etzioni R. Comparative effectiveness of alternative prostate-specific antigen-based prostate cancer screening strategies: Model estimates of potential benefits and harms. Ann Intern Med. 2013;158:145–53. doi: 10.7326/0003-4819-158-3-201302050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–24. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 18.Mariotto A, Etzioni R, Krapcho M, Feuer EJ. Reconstructing prostate-specific antigen (PSA) testing patterns among black and white men in the US from Medicare claims and the National Health Interview Survey. Cancer. 2007;109:1877–86. doi: 10.1002/cncr.22607. [DOI] [PubMed] [Google Scholar]
- 19.Andriole GL. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125–32. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gulati R, Tsodikov A, Wever EM, Mariotto AB, Heijnsdijk EA, Katcher J, et al. The impact of PLCO control arm contamination on perceived PSA screening efficacy. Cancer Causes Control. 2012;23:827–35. doi: 10.1007/s10552-012-9951-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bill-Axelson A, Holmberg L, Garmo H, Rider JR, Taari K, Busch C, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370:932–42. doi: 10.1056/NEJMoa1311593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boorjian SA, Karnes RJ, Viterbo R, Rangel LJ, Bergstralh EJ, Horwitz EM, et al. Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer. 2011;117:2883–91. doi: 10.1002/cncr.25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooperberg MR, Ramakrishna NR, Duff SB, Hughes KE, Sadownik S, Smith JA, et al. Primary treatments for clinically localised prostate cancer: a comprehensive lifetime cost-utility analysis. BJU Int. 2013;111:437–50. doi: 10.1111/j.1464-410X.2012.11597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Center for Health Statistics. Vital statistics of the United States, Volume II: Mortality, part A. Washington DC: Government Printing Office; various years. [Google Scholar]
- 25.Venzon DJ, HMS A method for computing profile-likelihood-based confidence intervals. Appl Stat. 1988;37:87–94. [Google Scholar]
- 26.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–28. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N Engl J Med. 2016 doi: 10.1056/NEJMoa1603144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurian AW, Sigal BM, Plevritis SK. Survival analysis of cancer risk reduction strategies for BRCA1/2 mutation carriers. J Clin Oncol. 2010;28:222–31. doi: 10.1200/JCO.2009.22.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Decision Tool for Women with BRCA Mutations. 2011 [cited 2016 June 28]; Available from: http://brcatool.stanford.edu/
- 30.Heijnsdijk EA, Warner E, Gilbert FJ, Tilanus-Linthorst MM, Evans G, Causer PA, et al. Differences in Natural History between Breast Cancers in BRCA1 and BRCA2 Mutation Carriers and Effects of MRI Screening-MRISC, MARIBS, and Canadian Studies Combined. Cancer Epidemiol Biomarkers Prev. 2012;21:1458–68. doi: 10.1158/1055-9965.EPI-11-1196. [DOI] [PubMed] [Google Scholar]
- 31.Yen AM, Auvinen A, Schleutker J, Wu YY, Fann JC, Tammela T, et al. Prostate cancer screening using risk stratification based on a multi-state model of genetic variants. Prostate. 2015;75:825–35. doi: 10.1002/pros.22964. [DOI] [PubMed] [Google Scholar]
- 32.Pashayan N, Duffy SW, Chowdhury S, Dent T, Burton H, Neal DE, et al. Polygenic susceptibility to prostate and breast cancer: implications for personalised screening. Br J Cancer. 2011;104:1656–63. doi: 10.1038/bjc.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen Y, Zelen M. Screening Sensitivity and Sojourn Time From Breast Cancer Early Detection Clinical Trials: Mammograms and Physical Examinations. J Clin Oncol. 2001;19:3490–9. doi: 10.1200/JCO.2001.19.15.3490. [DOI] [PubMed] [Google Scholar]
- 34.CISNET Model Registry. 2016 [cited 2016 June 28]; Available from: https://resources.cisnet.cancer.gov/registry/learn/